1. Introduction
Low plasma concentrations of endogenous 17β-estradiol in early life correlate with an atherogenic lipid profile and endothelial dysfunction in men [
1,
2], as well as with an increased risk of cardiovascular disease and, in particular, acute myocardial infarction (AMI) in women [
3,
4]. Furthermore, diseases characterized by hyperandrogenemia in women (e.g., polycystic ovary syndrome) and hypogonadism (low testosterone) in young men are predisposing factors for premature and extensive coronary artery disease [
5,
6].
In contrast, in cohorts with established cardiovascular disease, high serum estradiol levels in both men and women [
7,
8] and low serum testosterone in men predict the severity of coronary atherosclerosis [
7,
9]. The ratio of endogenous estradiol to testosterone (E2/T)—used as a surrogate marker of aromatase activity—indicates an adverse prognosis in older women with known cardiovascular disease [
10]. An allele variant of the enzyme aromatase converts testosterone to estradiol in various tissues and is a risk indicator for mortality in men with acute coronary syndrome [
11]. Aromatase activity varies throughout life, especially during acute illnesses (e.g., acute myocardial infarction), leading to increased endogenous estradiol concentrations [
10,
12,
13,
14]. The clinical implications of these fluctuations in endogenous sex hormone concentrations and aromatase activity in acute coronary disease remain uncertain.
Animal research indicates that bilateral ovariectomy increases ischemia–reperfusion (MI/R) injury of the myocardium. Estrogen treatment can mitigate this by inhibiting endoplasmic reticulum stress, reducing cardiomyocyte apoptosis, and resulting in smaller infarcts [
15,
16]. However, animal models do not adequately represent the extent of atherosclerosis in older humans, limiting their relevance. Studies involving human participants suggest that men with low serum testosterone levels undergoing primary percutaneous coronary intervention (PCI) for MI with ST elevation experience inferior myocardial reperfusion and myocardial systolic function [
17]. Conversely, elevated endogenous estradiol levels independently correlate with the risk of no-reflow in postmenopausal women [
18].
The regional inhomogeneities in ventricular refractoriness have been demonstrated to increase during myocardial ischemia and to decrease with the reversal of the ischemic state following successful angioplasty [
19]. Higher values of dispersion in ventricular repolarization are related to an increased incidence of ventricular arrhythmias and nonfatal myocardial infarction [
19]. In general, the sex-related differences in repolarization duration and dispersion can be explained by the cellular effects of sex steroids on action potential duration and autonomic control of the heart rate [
20,
21].
Our hypothesis is that the change in endogenous sex hormone concentrations and aromatase activity in acute MI is associated with myocardial damage, the inflammatory response to injury in the myocardium, and ventricular repolarization.
Purpose
The purpose of this study was to test for the significance of the sex-specific associations of gonadal hormones with the extent of the inflammatory response, myocardial damage, and ventricular arrhythmia risk in acute myocardial infarction (MI).
2. Materials and Methods
This single-center cohort study included 111 patients (37% women) diagnosed with AMI and admitted to the Clinic of Cardiology, University Hospital “Alexandrovska,” Sofia, between July 2011 and December 2013. Blood samples were drawn 48 h after symptom onset to measure the levels of sex steroids (total 17β-estradiol [E2], total testosterone [T], dehydroepiandrosterone sulfate), oxidized low-density lipoproteins (oxLDL), high-sensitivity C-reactive protein (hsCRP), white blood cell (WBC) counts, and cardiac enzymes (creatine kinase [CK], Muscle–Brain fraction of CK [CPK-MB], and high-sensitivity troponin T [hsTnT]). To measure coronary disease severity, we calculated the SYNTAX score for each patient with angiographically defined coronary atherosclerosis. The levels of E2 and T, as well as those of cardiac enzymes and inflammatory markers (CRP and WBC), were measured within six hours of PCI. In the group without catheter revascularization, hormones, enzymes, and inflammatory markers were measured within 48 h of symptom onset. If re-evaluated, the highest values were used for analysis. All the patients underwent standard echocardiography. Echocardiographic studies were performed in accordance with the recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging using the Sonos 5500 (Hewlett-Packard, Palo Alto, CA, USA) and Aloka ProSound 10 (Hitachi Aloka Medical, Hitachinaka, Japan) ultrasound systems. LV ejection fraction (EF) was measured using Simpson’s method.
We compared the E2 and T of the patients with AMI with the E2 and T of 15 men and women with stable coronary disease in order to test the hypothesis that endogenous E2 and T plasma concentrations change in the acute phase of myocardial infarction in parallel with the levels of inflammatory markers. Eighteen men and women of similar age as the studied cohort with coronary disease excluded via coronary angiography served as the control group.
Patients diagnosed with secondary hypogonadism or diseases of the adrenal and pituitary glands were excluded. Other exclusion criteria included acute infectious disease, chronic inflammatory disease, known or suspected neoplastic processes, surgical procedures, and trauma within two weeks before hospital admission. The participants had not used hormone or immunoreactive therapies six months before or during this study.
We adhered to the Declaration of Helsinki and received approval from the ethics committee of the Medical University of Sofia. All the participants provided written informed consent. This study was retrospectively registered in the UK’s Clinical Study Registry (ISRCTN) with study registration number ISRCTN62480360.
After a 12 h fast, venous blood samples were collected into EDTA sample tubes, centrifuged at 12,000 rpm for 20 min, and stored at −20 °C until analysis. The hsCRP concentrations were determined using a latex-enhanced immunoturbidimetric assay (Roche Diagnostics GmbH, Manheim, Germany) on the COBAS INTEGRA 700 analyzer. We assessed the levels of steroid hormones and hsTNT using an electrochemiluminescent immunoassay with Roche Diagnostics reagents on the Elecsys 2010 analyzer. These methods have been detailed elsewhere [
22,
23]. Plasma levels of oxLDL were quantified using the OxiSelect Human Oxidized LDL immunosorbent assay (ELISA; MDA-LDL) kit (Cell Biolabs, San Diego, CA, USA) and a sandwich ELISA [
24].
2.1. ECG Analysis
Standard 12-lead ECGs were recorded at a paper speed of 25 mm/s and a gain of 10 mm/mV. The QT interval was measured using a standardized technique and corrected with Bazett’s formula. Corrected QT dispersion was calculated as the difference between the maximum and minimum corrected QT intervals [
19] assessed using surface body electrocardiography (ECG).
2.2. Statistical Analysis
We checked variable distributions using the Kolmogorov–Smirnov and Shapiro–Wilk tests. We explored the associations between variables using both parametric (independent-samples t-test) and non-parametric (χ2 test, Fisher’s exact tests, and Mann–Whitney U test) methods, further validated by Cox proportional regression and univariate and multivariate analyses. The multivariable model which explored the predictors of the highest cardiac enzyme levels included estradiol, testosterone, the estradiol-to-testosterone ratio, white blood cell count, C-reactive protein, the extent and severity of coronary atherosclerotic plaques measured using the Syntax score, and age. The multivariable model testing the significance of the predictors of the highest levels of hormones and E2/T included white blood cell count, C-reactive protein, the extent and severity of coronary atherosclerotic plaques measured using the Syntax score, left ventricular (LV) ejection fraction (EF) as a measure of LV systolic function, and the age. The analyses were conducted using IBM SPSS Statistics for Windows, Version 19.0. (IBM Corp: Armonk, NY, USA). A two-tailed p-value less than 0.05 was deemed significant.
3. Results
A flowchart showing the steps in the enrollment of the patients studied is presented in
Figure 1.
Female patients were, on average, older than male patients (
Table 1) and had lower C-reactive protein (CRP) and cardiac enzyme levels. During the acute phase of MI, E2 levels were higher in male patients, while the E2-to-T ratio (E2/T) was lower in female patients (
Table 1). The groups did not differ substantially according to MI localization or atherosclerotic involvement of coronary arteries. Women were leaner than men but of similar BMI (
Table 1).
A total of 86% of the patients underwent PCI. Among the remaining 14%, some were diagnosed with non-obstructive coronary disease and were suitable for optimal conservative treatment, and the rest of these patients were diagnosed with three- and multi-vessel disease. The latter group was transferred for aorto-coronary by-pass surgery.
Plasma concentrations of E2 and T were higher in the patients with AMI compared to those with stable coronary disease, except there were no differences in E2 between the men with AMI and men with stable CAD (
Table 2). E2 levels were higher and T was lower in male patients with AMI compared to the men in the control group (
Table 2). Vice versa associations in E2 and T levels in the women with AMI versus female controls were found (
Table 2).
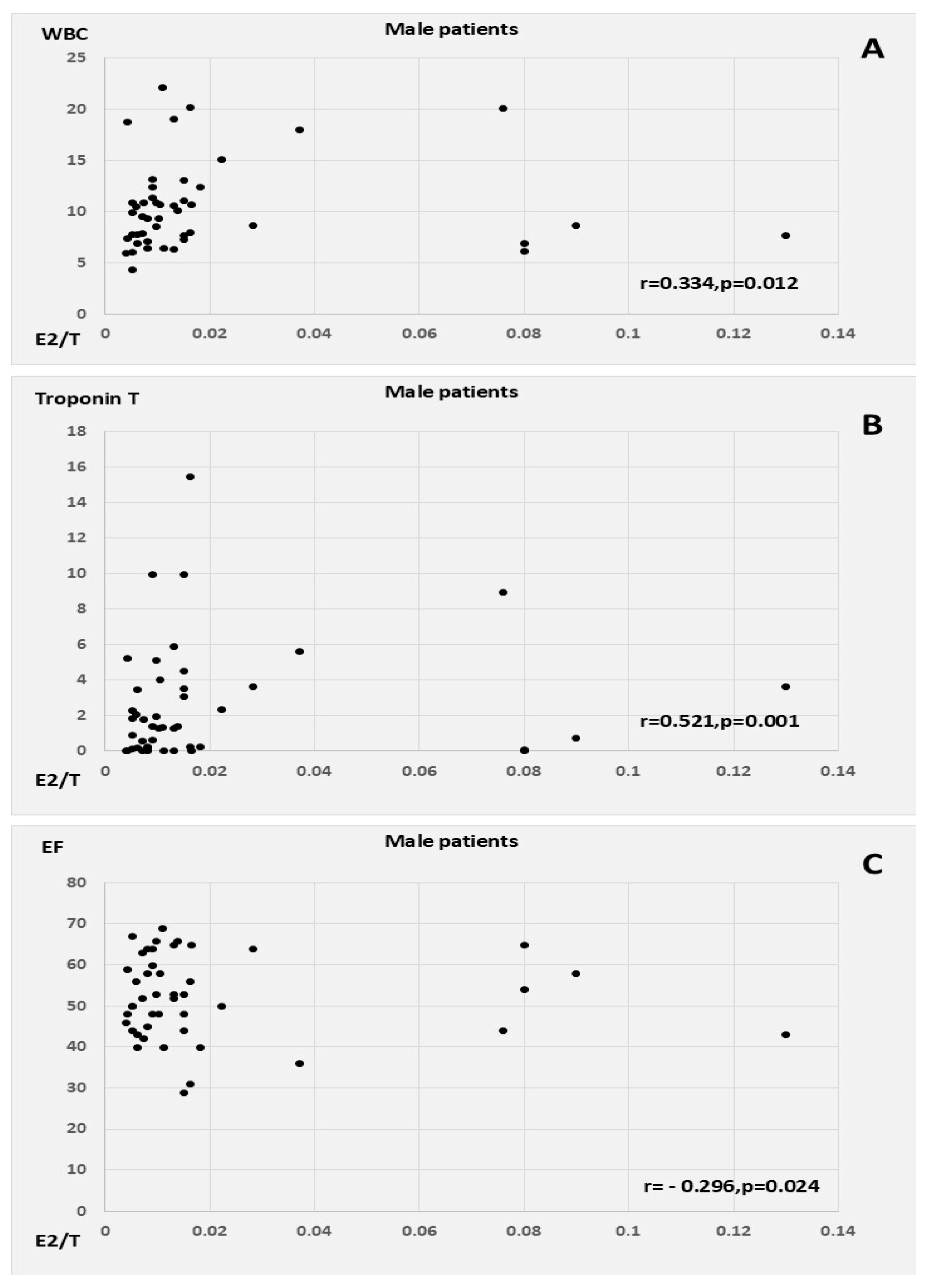
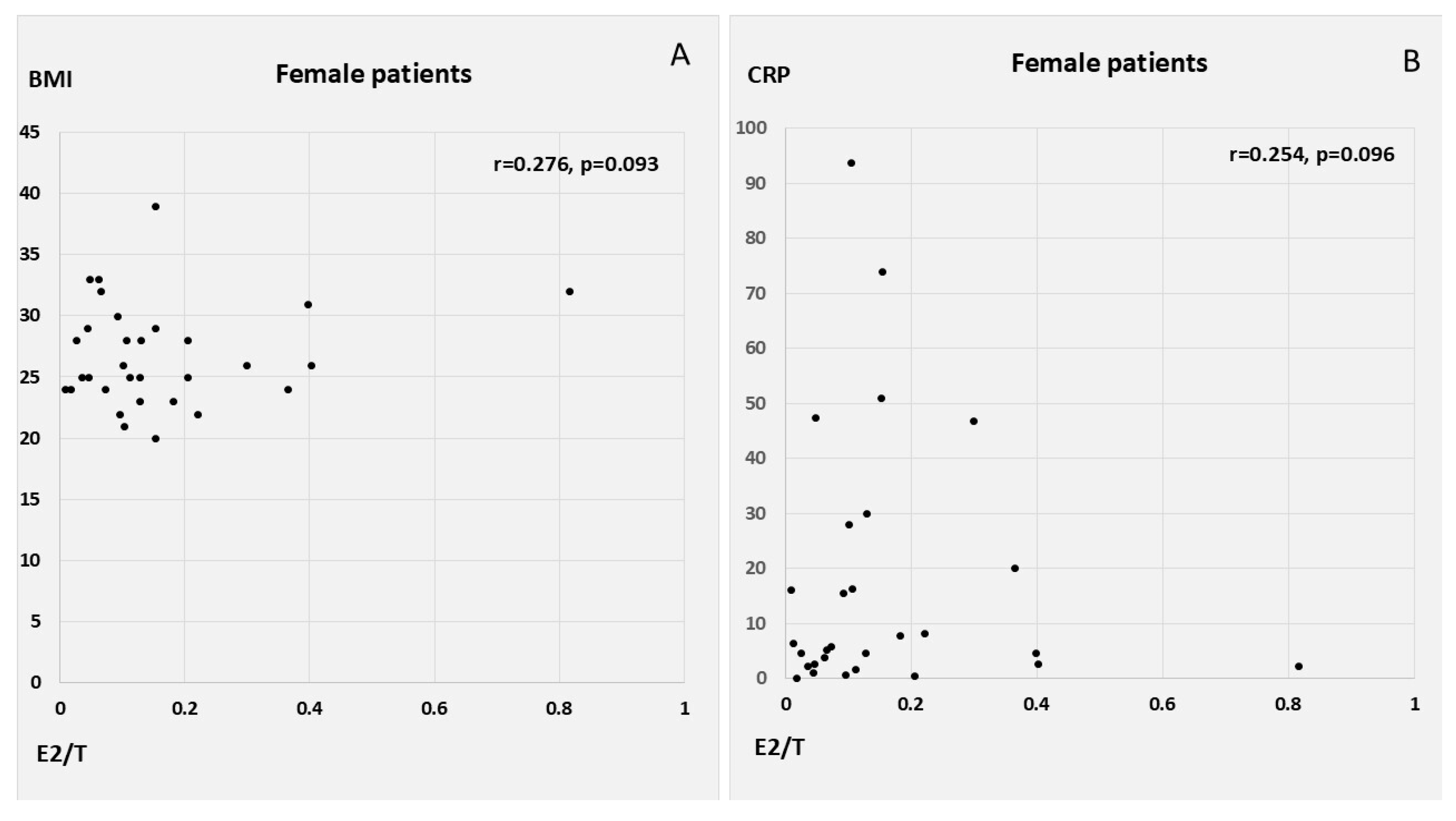
E2 correlated positively with CRP, WBC count, Syntax score, and QTc in the male patients with AMI, as well as with T, oxLDL, and MI size (peak cardiac enzymes) in the female AMI patients. E2 had a similar inverse correlation with LVEF in both men and women, in addition to showing a weak tendency for inverse association with age in women. T was associated with WBC count, myocardial injury, and lower EF in the female patients. E2/T was related to peak WBC count, peak troponin levels, and low EF among the male patients with AMI; conversely, in the female patients, E2/T was related to higher heart rates at admission and to a tendency towards higher BMI and CRP (
Table 3,
Figure 2 and
Figure 3).
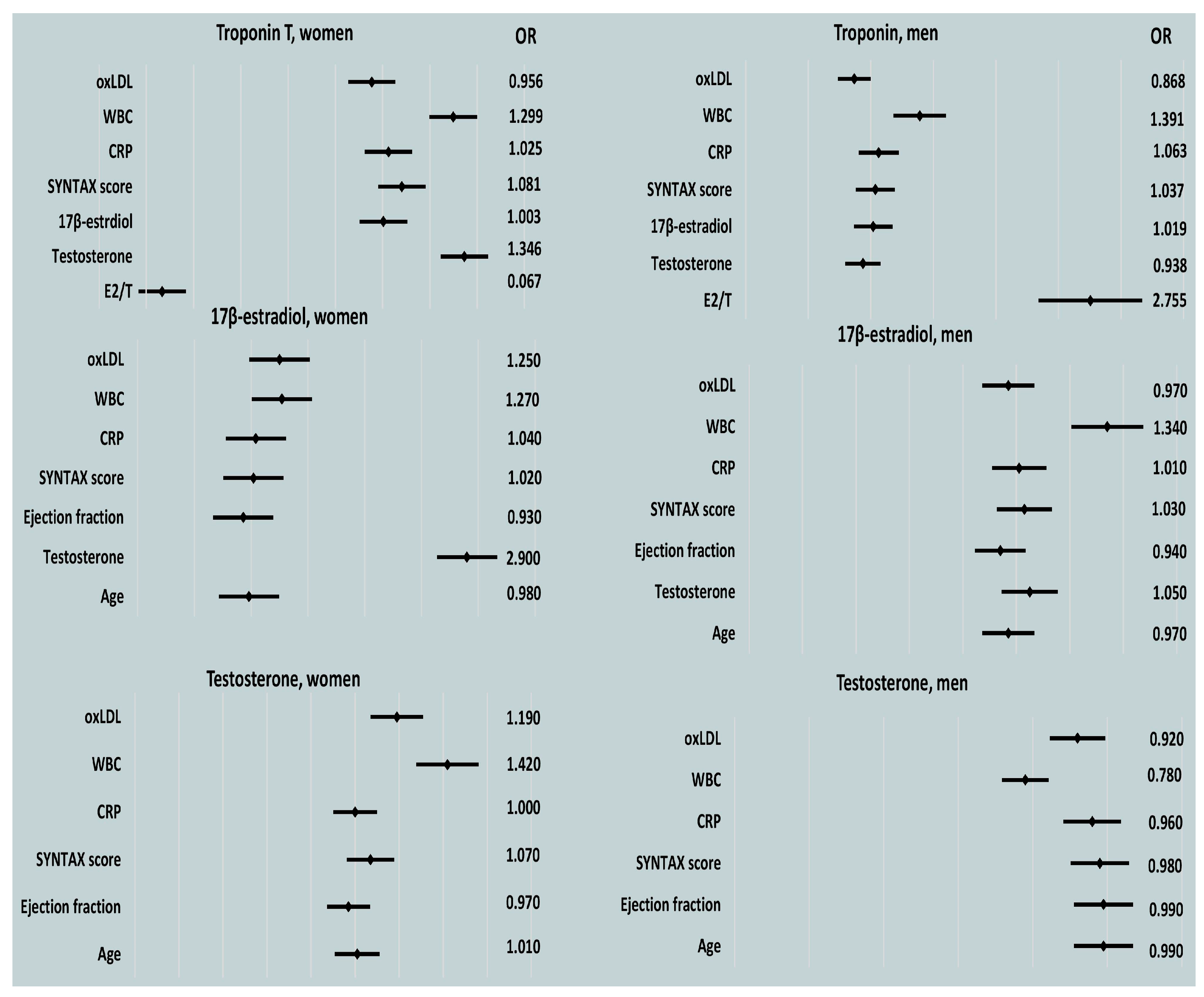
In the male patients, post-PCI E2, CRP, and WBC counts were predictors of peak cardiac enzyme levels, as determined by a univariable regression analysis. For the female patients, peak WBC count was a significant indicator of myocardial injury (
Table 4,
Table 5 and
Table 6 and
Figure 4).
For the male patients, high plasma concentrations of E2 and CRP were significant markers for an increase in Troponin T (TnT) levels. Peak WBC count was notably associated with the highest activities of both CK-MB and hs TnT in a multiple regression model (
Table 7).
An analysis of the correlations between gonadal steroid hormones and the E2/T ratio during the acute phase of MI revealed that increases in E2 were predicted by increases in CRP in both men and women. The lowest EFs tended to indicate women with the highest E2 levels in the acute phase of MI (
Table 8,
Figure 4).
In the multivariable model, neither EF nor the maximal elevation of CRP independently predicted peak levels of E2 in the female patients with AMI (EF 0.960, OR 0.890–1.050, p = 0.379; CRP 1.040, OR 0.990–1.090, p = 0.159).
CRP level and WBC count were tendency markers of a decrease in the plasma levels of total T in the male patients, while WBC count was inversely associated with T in the women with AMI (
Table 9). Among the male patients with AMI, peak CRP count showed a trend toward marking the lowest T levels in multivariable analysis (CRP 0.940, OR 0.880–1.000,
p = 0.053; WBC 1.210, OR 0.700–2.080,
p = 0.501).
Additionally, a trend suggested that high CRP levels were associated with a higher E2/T ratio (
Table 10).
E2 and T were not significantly related to extremely short or long repolarization periods after PCI in either men or women early in the course of MI in this analysis of data. The highest versus the lowest duration of cardiac repolarization at admission showed a relationship with the severity of coronary atherosclerosis, the extent of myocardial injury, and myocardial infarction size (reflected by the levels of cardiac enzymes), as well as with LV systolic function in the subset of male patients with myocardial infarction (
Table 11).
Lower ejection fractions of the left ventricle were independently predictive of prolonged repolarization, specifically for the male patients (EF: OR 0.810, 95% CI 0.650–1.010, p-0.0059; Syntax score: OR 1.030, p = 0.737; CK: OR 1.000, p = 680; CK-MB: OR 1.030, p = 0.243; troponin T OR 1.000, p = 0.998).
We found a significant, non-sex-specific association between the incidence of ventricular tachycardia (VT, 6.8%, n = 7) and left ventricular ejection fraction (43.6% ±12% vs. 53.2% ± 10.7%, p = 0.018), as well as a tendency with peak WBC count levels (9.7 × 109/L ± 3.7 × 109/L, p = 0.088), during the first week of AMI. Gonadal steroids were not associated with the incidence of VT in an analysis not adjusted for biological sex (E2: 125.9 ± 72.9 pmol/L vs. 131 ± 98.6 pmol/L, p = 0.886; T: 9.1 ± 5.6 nmol/L vs. 8.7 ± 7.7 nmol/L, p = 0.879; E2/T 0.49 ± 1.32 vs. 0.12 ± 0.3, p = 0.975).
4. Discussion
We found that during the acute phase of MI, the highest levels of endogenous estradiol and CRP were significantly related to the severity of cardiomyocyte necrosis (as evidenced by peak plasma TnT levels) in the male patients. Conversely, peak WBC count emerged as a sex-specific marker for acute myocardial damage in the women. Interestingly, peak plasma T in the women correlated with the rise in WBC count, while in the men with AMI, T was inversely related to the increase in CRP levels. Furthermore, a positive association was observed between CRP and the E2/T ratio in the male patients.
4.1. Sex-Specific Relationship of oxLDL and CRP with E2 and E2/T
According to basic research reports, oxLDL influences estradiol and testosterone production [
25,
26]. OxLDL, or oxidized low-density lipoprotein, refers to LDL particles that have been modified through oxidation. Oxidized LDL levels can increase after percutaneous coronary intervention, particularly in the acute phase following an MI. The degree of myocardial ischemia acts as a severe cellular stressor, resulting in oxidative stress with increased free radical generation and oxLDL formation. This increase is often transient and may be related to the mechanical disruption of plaque and the release of oxidized lipids during the procedure. However, no significant association of plasma oxLDL levels with inflammatory markers and E2 could be detected in our study, either due to the rapid immune clearance of oxLDL [
27], due to the blood samples being obtained too early, or because of a complex relationship which remained statistically undetectable in our small study group.
Many observational studies highlight the significance of the association between gonadal steroid hormones and markers of ongoing low-grade vascular inflammation in atherosclerosis in male patients with chronic coronary disease [
7]. Under nonacute conditions, excess adipose infiltration with macrophages leads to increased aromatase expression and elevated endogenous estradiol levels [
28]. Obesity might induce the production of chemokine ligand 2 (an MCP-1 receptor) in adipose tissue, thereby inducing inflammation and local aromatase, and subsequently amplifying estrogen levels [
29]. A relationship between certain cytokines (IL-6 and TNF-α) and E2 has been documented in male patients with metabolic syndrome [
30]. Notably, pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), induce the expression of the human aromatase gene in mammary adipose tissue [
31].
Recent observational studies show that the effect of exogenous estrogen (hormone replacement therapy) is a predisposing factor for adverse cardiovascular events in women who have had menopausal status for more than ten years [
32]. Experimental studies indicate that when atherosclerotic lesions are present, the altered expression of estrogen receptors on the vascular wall further exacerbates arterial wall damage [
32,
33]. In a low-grade inflammation state, such as established atherosclerosis, E2 can destabilize atherosclerotic plaques by inducing molecules like MCP-1 and matrix metalloproteinases (specifically MMP-2 and MMP-9) in endothelial cells [
32]. E2 induces different effects regarding atheromatous plaque instability through different ERs. The overexpression of ERα may result in E2-induced plaque instability by increasing MCP-1 protein expression and MMP-2 activity [
32]. In the absence of an inflammatory state, E2, at low concentrations, exerts atheroprotective effects, mainly by decreasing MMP-2 activity and increasing LOX expression via ERβ [
32].
During acute illness, a surge in aromatase in response to an elevated production of pro-inflammatory cytokines has been described [
34]. A critical illness produces endocrine responses, such as hypogonadotropic hypogonadism in male patients, associated with low luteinizing hormone, LH [
35]. Also, an increase in adipose aromatase content, decreased serum T levels, and a rise in estrone levels, with a trend toward a rise in E2, has been observed in both men and women [
36]. Aromatase expression in adipose tissue is primarily under the control of its promoter, which is regulated by cytokines and the stress-induced glucocorticoid cortisol [
36].
Importantly, several human studies have reported fluctuations in E2 and testosterone levels during acute coronary syndrome [
12,
13]. Persistently elevated E2 and declining testosterone have been associated with changes in hemostatic factors (notably, increased plasminogen activator inhibitor-1, PAI-1) in men with AMI [
12]. Elevated cardiac PAI-1 post-myocardial infarction may contribute to tissue remodeling and augmented cardiac fibrosis [
37], creating a prothrombotic profile in male AMI patients and in situ microthromboses.
4.2. Relationship of WBC Count with TnT in Female Patients
For the male AMI patients, the peak systemic inflammatory mediators predicted plasma 17β-estradiol levels and E2 correlated with infarct size, while testosterone levels did not emerge as a significant marker of myocardial injury. In contrast, WBC count was associated with T levels and myocardial injury severity specifically in the group of female patients.
According to observational data, in middle-aged women, a higher WBC count indicates an underlying inflammatory state associated with obesity, hyperandrogenemia, and polycystic ovary syndrome (PCOS) [
38]. Moreover, testosterone has been directly linked to increased platelet thromboxane A receptor density and maximal platelet aggregation [
39]. In particular conditions in which tissue factor biological action is crucial, such as acute myocardial infarction, the neutrophil count increases substantially [
40]. Tissue factor is expressed by endothelial cells, platelets, and neutrophils [
40]. Inflammatory molecules such as P-selectin and TNF-α share the ability to induce tissue factor synthesis on the neutrophil surface [
40]. A testosterone-rich environment may intensify platelet–leukocyte interactions, potentially leading to increased fibrin deposition and extensive coronary thrombus formation, which could be a potential source of distal embolization. Testosterone’s associations with thrombin generation parameters (like fibrinogen, factor VIIc, PAI-1) have been documented in young women with PCOS [
41] and middle-aged women [
42].
4.3. Lack of Overt Relationship of E2 with Myocardial Injury in Women with AMI
Interestingly, only E2 emerged as a predictor of myocardial injury in our study, and specifically in the men. In contrast, for the female AMI patients, troponin levels were associated with higher WBC counts. Notably, earlier population-based studies have proved that the plasma concentration of estradiol and its production rate were significantly higher in healthy men than in age-matched postmenopausal women without cardiovascular disease [
43]. The levels of E2 and T measured in our study coincided in time with peak plasma levels of inflammatory and myocardial injury markers (measured within six hours of PCI). Also, E2 and E2/T were significantly associated with the inflammatory response in the acute phase of MI and, in the case of E2, also with myocardial damage, suggesting a potential significance of E2 as a male-specific marker of myocardial injury in small AMI cohorts.
4.4. Lack of Significant Association of E2/T and E2 with Body Mass Index
Most E2 production in men arises from the extragonadal aromatization of adrenal androgens and testosterone in muscle and adipose tissues. The rate of testosterone conversion to estradiol is lower than that of androstenedione aromatization to estrone [
10,
44]. However, the estradiol/testosterone ratio is accepted as a measure of the aromatization of T into E2 in large studies of male patients [
45]. The aromatization of C19 steroids in peripheral tissues (adipose and skin fibroblasts) by enzyme aromatase is the primary mechanism for estrogen formation in postmenopausal women [
10]. The androstenedione-to-estrone ratio is used as a marker of aromatase enzyme activity in postmenopausal women [
10]. Nevertheless, the median value of estrone-to-androstenedione concentrations correlated significantly with the estradiol/testosterone ratio in postmenopausal women [
10].
Our analysis revealed that body mass index (BMI) did not significantly correlate with the levels of gonadal steroid hormones, E2/T, or inflammatory markers. Other studies have emphasized a stronger relationship between abdominal adipose tissue thickness (rather than BMI) and the levels of inflammatory mediators and sex hormones [
46]. Inflammatory pathways are activated soon after high-fat diet consumption, leading to inflammatory macrophage accumulation in white adipose tissue even before overt obesity manifests [
47]. The association of sex hormones and inflammatory markers with BMI as a measure of overall adiposity is obviously far too weak compared to the correlation of abdominal adipose tissue thickness with inflammatory mediators and sex hormones.
4.5. Association of QTc with EF in Male Patients and of QTc with CRP and Testosterone in Female Patients
The incidence of ventricular tachycardia detected until the end of the first week of AMI was non-sex-specifically associated with left ventricular ejection fraction and, as a tendency, with peak WBC count. In the male patients, the longer duration of cardiac repolarization (QTc) prior to PCI was related to the severity of coronary disease, infarction size (peak cardiac enzymes), and EFs. Specifically in the male patients, the reduction in LV systolic function showed a significant independent relationship with longer QTc in the multivariable model. In the subset of female patients with AMI, QTc showed a trend for association with younger age, lower CRP, and higher total testosterone levels but was not independently predicted by any of these variables. These results showed that regarding the women with acute MI, factors, such as higher endogenous plasma T levels, might be implicated in the predisposition to ventricular arrhythmia via mechanisms not directly involving acute myocardial injury.
Among the possible mechanisms involved in the deleterious actions of supraphysiological doses of testosterone in female individuals are unfavorable changes in the lipid profile, procoagulatory effects, activation of the sympathetic nervous system and renin–angiotensin–aldosterone system, and insulin resistance. These indirect actions of testosterone can affect the vasculature. Among its direct effects, the stimulation of pro-inflammatory enzymes in the vasculature [e.g., thromboxane synthase, cyclooxygenase-1 (COX-1), and COX-2] and reactive oxygen species generation in vascular smooth muscle cells may decrease nitric oxide (NO) bioavailability and lead to increased vasoconstriction, increased blood pressure, and renal dysfunction [
48].
In women, low testosterone levels may be linked to an increased risk of ventricular arrhythmias, including sudden cardiac death by prolonging ventricular repolarization [
48]. However, oxidative stress can also elevate the risk of arrhythmias in both sexes. Testosterone upregulates the expression of NADP oxidase (NOX4) and increases oxidative stress [
48]. Increased levels of mitochondrial NOX4 during aging lead to a higher inducibility of self-limiting ventricular arrhythmias [
49]. Transgenic mice with mitochondria-targeted Nox4 overexpression showed a significantly higher incidence of pacing-induced VT, associated with shorter action potential duration due to increased transient outward potassium currents. Fractional sarcoplasmic reticulum Ca
2+ release and Ca
2+ leak remained intact despite these changes [
49].
4.6. Significance and Limitations
The results of this study contribute to scientific theory beyond the pathophysiology of coronary disease. Incomplete gonadal hormone suppression is involved in resistance to adjuvant therapy in different hormone-sensitive neoplasms (breast cancer and prostate cancer). The knowledge that variations in endogenous 17β-estradiol, testosterone, and E2/T associated with systemic increases in inflammatory markers can occur in pathologic settings (e.g., acute myocardial infarction) is important for discerning resistant malignancies and for developing optimal protocols for therapy, including adjuvant antitumor therapy.
This study has some limitations. First, the hormone levels were measured once. The changes in hormone levels after the infarction are likely to better characterize the observed relationships of inflammatory markers with E2 and E2/T. Second, estrone levels are higher than estradiol in postmenopausal women, and the ratio of androstenedione to estrone (not the E2/T ratio) directly characterizes aromatase activity. Third, we did not have any measurement of fat mass except BMI. The thickness of abdominal adipose tissue measured by ultrasound indicates the effects of obesity with higher accuracy. Finally, in up to 14% of the patients, PCI was not performed, mainly because of non-obstructive coronary disease. In these patients, the peak levels of cardiac enzymes, and thus the size of the infarction, may not have been precisely evaluated due to the blood samples being obtained too early.