Abstract
In humans, heart failure (HF) and cancer are among the leading causes of morbidity and mortality. A growing body of evidence highlights a bidirectional relationship between these conditions, underpinned by shared risk factors and overlapping pathophysiological pathways. This review aims to explore the emerging role of the intestinal microbiome as a common mechanistic link between HF and cancer. Specifically, we examine how microbial dysbiosis and its metabolic products—such as trimethylamine N-oxide (TMAO), short-chain fatty acids (SCFAs), bile acids, lipopolysaccharides (LPS), and branched-chain amino acids (BCAAs)—contribute to inflammation, immune dysregulation, oxidative stress, and metabolic dysfunction. These mechanisms promote multiorgan impairment and establish a vicious cycle that fuels both tumorigenesis and cardiac deterioration. HF, cancer, and the gut microbiome are not isolated entities but are deeply interconnected through shared biological mechanisms—including chronic inflammation, microbial dysbiosis, immune and neurohumoral modulation, and metabolic derangement. These findings support the concept of a microbiome-centered axis involving the gut, heart, and tumors, which may underlie many chronic disease processes. Understanding these interactions may provide novel insights into disease pathogenesis and uncover promising therapeutic targets that leverage microbiome modulation to prevent or treat HF, cancer, and other systemic diseases.
1. Introduction
Heart failure (HF) and cancer are two of the most pressing global health challenges of the 21st century. HF affects over 64 million people worldwide and remains a leading cause of hospitalization and death, particularly among the aging population. Its global burden continues to rise due to increased longevity and improved survival after acute cardiovascular events. One-year mortality rates following hospitalization for acute HF reach 20–30%, and the condition is associated with high readmission rates and reduced quality of life [1].
Cancer is responsible for nearly 10 million deaths annually, with projections estimating over 28 million new cases worldwide by 2040. This increase is driven by aging populations and lifestyle-related risk factors [2]. In Europe, cancer accounts for approximately one in four deaths, making it the second leading cause of death after cardiovascular diseases. In 2020, cancer caused nearly 1.9 million deaths across the continent, representing about 25% of all deaths [3].
Notably, a growing number of patients are being diagnosed with both HF and cancer—either sequentially or concurrently—suggesting shared pathophysiological mechanisms and a potential bidirectional relationship [4]. Patients with HF may develop cancer [4,5,6], while cancer patients—especially those undergoing antitumor treatments—are at increased risk of developing HF [7,8]. Therefore, long-term follow-up of both groups is recommended [9,10].
These two conditions share common risk factors, including smoking, hypertension, metabolic imbalance, and genetic alterations. They also follow similar pathophysiological mechanisms, such as activation of the neurohumoral system (including the sympathetic and parasympathetic nervous systems and the renin-angiotensin-aldosterone system), heightened inflammation, and increased production of free radicals. Dysregulation of these pathways disrupts homeostasis, impairs structural and functional integrity, and weakens cellular and tissue defense mechanisms.
The term microbiota refers to various microorganisms, including bacteria, viruses, and fungi, that inhabit different parts of the human body, such as the skin, oral and nasal cavities, stomach, and especially the intestines. In contrast, the microbiome encompasses both the microbiota and their interactions with the host [11]. These microorganisms are essential to human health, supporting metabolic, immune, and inflammatory balance, as well as regulating obesity-related disorders [12,13,14].
In patients with HF and/or cancer, the microbiota and its metabolic products are significantly altered due to an impaired intestinal environment. This disruption, known as dysbiosis, is marked by structural and functional changes and increased intestinal permeability [15,16]. These alterations are not incidental; they are increasingly recognized as active contributors to disease development. The microbiome influences host physiology through immune modulation, metabolic regulation, and communication with the nervous and endocrine systems [17,18,19]. Many of these pathways such as chronic inflammation, oxidative stress, and immune dysfunction are common to both HF and cancer. Given its regulatory role and ability to affect distant organs through microbial metabolites, the microbiome is emerging as a compelling common link between the two diseases.
Thus, HF, cancer, and an altered microbiome are interconnected, sharing common pathophysiological mechanisms such as systemic inflammation, neurohumoral activation, oxidative stress, insulin resistance, and metabolic dysregulation. These processes are often driven by harmful microbial metabolites, including trimethylamine N-oxide (TMAO), lipopolysaccharides (LPS), branched-chain amino acids (BCAAs), and secondary bile acids, which promote endothelial dysfunction, immune activation, mitochondrial stress, and tissue remodeling. By influencing these shared mechanisms, a disrupted microbiome plays a pivotal role in the onset and progression of both cardiovascular and oncologic diseases.
The objective of this review is to explore the microbiome as a shared mechanistic link between HF and cancer, emphasizing the role of microbial metabolites and common pathophysiological pathways such as inflammation, dysbiosis, immune modulation, and metabolic dysfunction.
2. The Microbiome in Immunological, Metabolic, and Cardiovascular Homeostasis
The gut microbiome is a key regulator of human health, exerting significant influence on immune, metabolic, and cardiovascular homeostasis. It plays a crucial role in educating and modulating the host immune system by promoting the development of gut-associated lymphoid tissue (GALT), maintaining mucosal barrier integrity, and regulating inflammatory responses [20,21,22]. Commensal microorganisms help maintain immune tolerance to non-pathogenic antigens while ensuring robust defense mechanisms against harmful pathogens.
From a metabolic perspective, the microbiome enhances nutrient absorption, synthesizes essential vitamins, and produces important metabolites such as short-chain fatty acids (SCFAs), bile acids, and amino acid derivatives [23]. Among SCFAs, butyrate and propionate are particularly notable for their anti-inflammatory properties, ability to improve insulin sensitivity, and role in supporting energy balance [24]. Through these functions, the gut microbiota helps regulate body weight, glucose metabolism, and lipid levels.
Cardiovascular health is also strongly influenced by the microbiome. Microbial metabolites affect endothelial function, vascular tone, and the risk of atherosclerosis [25,26]. SCFAs contribute to vascular protection and help lower blood pressure [27], whereas dysregulated production of other metabolites, such as TMAO, can promote vascular inflammation and atherogenesis [28]. By maintaining metabolic balance, reducing oxidative stress, and modulating systemic inflammation, a healthy microbiome supports cardiovascular resilience.
Understanding these homeostatic functions is essential for appreciating how microbiome disturbances contribute to the development and progression of complex diseases such as HF and cancer.
Although experimental studies have described the role of the gut microbiome in maintaining immune, metabolic, and cardiovascular balance, longitudinal studies in humans remain limited. Future research should aim to characterize microbial profiles across diverse populations and investigate how specific microbiome configurations influence disease risk or resilience over time.
3. The Microbiome Interplay
The microbiome can exert both oncogenic and tumor-suppressive effects. Its oncogenic potential is well documented in several types of cancer. For example, Helicobacter pylori is implicated in gastric cancer; hepatitis B and C viruses in liver cancer; human papillomavirus in cervical and vaginal cancers; Epstein-Barr virus in nasopharyngeal carcinoma and lymphoma; and Escherichia coli in colorectal cancer, among others [28]. Conversely, some microbiota—such as butyrate-producing bacteria—have demonstrated protective effects by inhibiting the progression of colorectal cancer [29].
Regardless of whether its effects are oncogenic or protective, the microbiome clearly exerts a profound influence on immune cells and inflammatory processes. This is especially relevant given that the gastrointestinal tract contains the majority of the body’s immune cells. Moreover, microbial metabolites can have effects beyond the gut by entering the enterohepatic circulation, thereby influencing distant organ systems [28].
In HF, reduced cardiac output leads to gut ischemia and congestion—observed in both HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF) [30]. These changes disrupt the intestinal microbiota and increase intestinal permeability [16,31]. The resulting alterations: (a) activate immune and inflammatory responses, and (b) stimulate the neurohumoral axis, which contributes to left ventricular remodeling and myocardial fibrosis [30,32]. Additionally, these changes are linked to insulin resistance, obesity, metabolic syndrome [33], and impaired mitochondrial energy metabolism [15,19].
Taken together, these findings highlight how intestinal dysbiosis—characterized by disruption of the normal microbiota and its metabolic products—affects core pathophysiological mechanisms common to both cancer and HF. These include neurohumoral overactivation, chronic inflammation, and disturbances in metabolism and energy production [31,34].
There is a clear bidirectional relationship between the microbiome, HF, and cancer. Each condition can alter the composition and function of the gut microbiota, which in turn contributes to the development and progression of these diseases, establishing a self-perpetuating cycle (Figure 1).
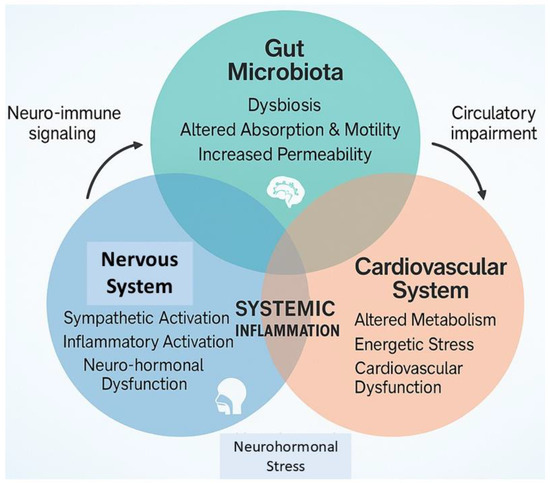
Figure 1.
The reciprocal interplay between gut microbiota and the nervous and cardiovascular systems. The altered intestinal homeostatic status initiates neuro-humoral, nervous, and immune system dysfunction, pillars of both cancer and cardiovascular disease pathophysiology.
While compelling evidence links microbiota-derived metabolites to both oncogenesis and cardiovascular dysfunction, much of the current data stems from preclinical studies. Translational research and human interventional trials are urgently needed to validate these causal relationships and to explore strategies for therapeutic modulation of the microbiome.
Table 1 summarizes key microbiome-derived carcinogenic metabolites, their bacterial sources, the pathways they activate, their effects on antitumor immunity, the associated cancer types, and their relationships with cardiovascular dysfunction and heart failure.

Table 1.
Microbiome-derived carcinogenic metabolites, responsible bacteria, involved pathways, effects on the immune system, associated cancers, and links with heart failure.
3.1. Shared Microbial Mechanisms in HF and Cancer
Dysbiosis refers to an imbalance or disruption in the normal composition, diversity, or function of the microbiome. It may involve a reduction in beneficial commensal microbes, an overgrowth of pathogenic species, or a loss of overall microbial diversity. Dysbiosis can be triggered by a variety of factors, including dietary changes, infections, antibiotic use, chronic diseases, and environmental exposures [35,36]. It plays a central role in the pathogenesis of both HF and cancer through shared biological pathways. Dysbiosis is marked by a reduction in beneficial commensals, an overgrowth of pathogenic species, and altered microbial metabolism, which together promote systemic inflammation, oxidative stress, and immune dysfunction [15,35,36,37].
Key microbial metabolites such as TMAO, LPS, SCFAs, secondary bile acids, BCAAs, and phenylacetylglutamine (PAGln) exert profound systemic effects. For example, TMAO, derived from dietary choline and carnitine by gut bacteria and converted in the liver via flavin-containing monooxygenases, promotes vascular inflammation, endothelial dysfunction, platelet hyperreactivity, and adverse cardiac remodeling [38,39,40,41,42,43,44,45,46]. It activates inflammatory pathways including NF-κB and the NLRP3 inflammasome, and has been associated with an increased risk of atherosclerosis, HF, and cancer progression [39,40,43,45].
Similarly, LPS, a product of Gram-negative bacteria such as Enterobacteriaceae, crosses a compromised intestinal barrier and enters systemic circulation. It activates Toll-like receptor 4 (TLR4), induces macrophage polarization, and drives the release of proinflammatory cytokines—key processes that contribute to myocardial inflammation, vascular dysfunction, and tumor-promoting immune modulation [37,47,48,49,50].
While SCFAs such as butyrate and propionate normally exert anti-inflammatory, antitumor, and insulin-sensitizing effects, their production is often diminished in dysbiotic states [23,24,51,52]. This reduction in protective metabolites, coupled with an increase in harmful ones, results in immune dysregulation, metabolic disturbance, mitochondrial stress, and chronic low-grade inflammation—all of which are common to both cancer and cardiovascular pathology [12,14,15,19,53,54,55].
The pathophysiological impact of these metabolites is not unidirectional. In both HF and cancer, impaired intestinal perfusion, systemic inflammation, and the effects of chemotherapeutic or cardiovascular therapies further exacerbate dysbiosis, creating a vicious cycle of mutual reinforcement [16,30,31,32]. Thus, the gut microbiota is not merely a bystander but an active participant in disease propagation, forming the foundation of a bidirectional, metabolite-driven feedback loop.
This unified perspective underscores the microbiome as a mechanistic bridge between oncologic and cardiovascular disease, and highlights shared therapeutic opportunities. Targeting microbiome-derived metabolites through diet, probiotics, or pharmacological agents may represent a promising strategy to attenuate systemic inflammation, restore metabolic balance, and interrupt disease progression.
While mechanistic pathways involving TMAO, LPS, and SCFAs have been well characterized in preclinical models, their direct causal roles in human disease progression, particularly in the context of concurrent HF and cancer, remain an area of active investigation.
3.2. Metabolic/Energetic Status
The intestinal microbiome plays a critical role in regulating host energy balance, metabolic homeostasis, and nutrient utilization. Under normal conditions, commensal microbes enhance carbohydrate fermentation, producing beneficial SCFAs such as butyrate and propionate. These SCFAs serve as energy substrates, maintain epithelial integrity, and exhibit anti-inflammatory and insulin-sensitizing properties [12,14,23,24,51,52]. The microbiome also participates in bile acid metabolism, modulates glucose and lipid profiles, and influences the bioavailability of micronutrients and neurotransmitters [15,23,56,57,58].
In dysbiotic states—commonly observed in both HF and cancer—the microbial production of protective SCFAs diminishes, while harmful byproducts such as TMAO, LPS, and BCAAs increase [15,53,59,60,61]. These changes promote insulin resistance, metabolic inflammation, mitochondrial dysfunction, and impaired oxidative metabolism [15,19,62,63,64].
Dietary composition significantly shapes the metabolic output of the microbiota. High-fat and high-cholesterol diets favor the generation of LPS and TMAO—metabolites strongly associated with atherosclerosis, cardiac fibrosis, and tumor progression [38,39,40,41,42]. Conversely, fiber-rich diets support SCFA production and metabolic resilience [15,51,52]. The microbiome’s metabolic output is also influenced by local factors such as intestinal pH, oxygen levels, and motility, as well as systemic factors including bile and pancreatic secretions, hormonal status, and host genetics [15,65,66,67,68,69].
Thus, the microbiome functions as a metabolic integrator between the gut and peripheral organs. In HF and cancer, its dysregulation contributes to energetic depletion, oxidative stress, and chronic inflammation—hallmarks that accelerate disease progression [15,53,54,55]. Interventions aimed at modulating microbial metabolism—such as dietary modification, probiotics, or targeted pharmacologic agents—may offer novel strategies to restore systemic metabolic balance.
3.3. Inflammation/Free Radical Production
The gut microbiota closely interacts with the host immune system and plays a critical role in regulating inflammatory responses at both local and systemic levels. Under normal physiological conditions, the microbiome supports the maturation of lymphoid tissues, maintains epithelial barrier integrity, and promotes immune tolerance by modulating T-cell responses and dendritic cell function [70,71,72,73]. Commensal bacteria also stimulate the expansion of cytotoxic CD8+ T cells, which possess anti-tumor activity, while preserving mucosal immune homeostasis [74,75].
However, during dysbiosis, pathogenic microbes and their toxic metabolites—such as lipopolysaccharides (LPS) and reactive nitrogen species—can dominate the gut environment. This shift impairs immune defense mechanisms and initiates chronic inflammation. The resulting disruption of mucosal immunity increases cytokine production and promotes abnormal epithelial cell proliferation, contributing to carcinogenesis [76,77,78]. Dysbiosis has also been linked to the development of cancers beyond the gut through systemic inflammatory pathways and microbial translocation [79,80,81].
Importantly, microbes originating in the gut have been identified within tumor tissues, forming what is known as the intratumoral microbiota. These microbes, often residing within immune cells, interfere with autophagy and immune surveillance, thereby promoting tumor progression [82,83,84]. At the same time, inflammation and microbial imbalance trigger systemic endocrine and neurohormonal changes—including insulin resistance, activation of the renin-angiotensin-aldosterone system (RAAS), and increased oxidative stress—all of which are key contributors to the development of both HF and cancer [33,85,86,87,88].
Free radicals, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), are major mediators of inflammation and cellular injury. While they serve important roles in signaling and immune defense at low concentrations, excessive production—common in dysbiotic states—results in lipid peroxidation, protein oxidation, and DNA damage [89,90,91]. In the heart, oxidative stress promotes myocardial cell apoptosis, necrosis, fibrosis, and mitochondrial dysfunction. These changes impair cardiac contractility and drive the progression of HF [91,92,93,94]. In cancer, free radicals contribute to genomic instability, damage tumor suppressor genes, and promote a tumor-friendly microenvironment, particularly under hypoxic conditions [95,96,97,98].
These interconnected mechanisms—chronic inflammation, immune dysregulation, and oxidative stress—represent a shared pathogenic link between dysbiosis and both cardiovascular and oncologic diseases. Although these associations are supported by both experimental and clinical data, the exact sequence of events and causal relationships remain uncertain. Further longitudinal studies are needed to determine whether targeting the microbiome can reduce oxidative and inflammatory damage in HF and cancer.
Although dysbiosis-induced immune dysregulation and oxidative stress are strongly implicated in preclinical models of both cancer and cardiovascular disease [89,90,91,92,93], the temporal sequence and clinical causality of these processes in humans are not yet fully delineated.
3.4. The Multiciliary Axis
The microbiome plays a regulatory role in neurogenesis, myelination, glial cell function, synaptic pruning, and blood–brain barrier permeability [18]. Communication with the central nervous system (CNS) is bidirectional, involving metabolic, endocrine, neurological, and immune pathways that influence both the onset and progression of various diseases [18,99]. This communication—often referred to as the “gut–brain axis”—occurs via both neural and humoral routes. Microbiome-derived signals reach the brain through stimulation of the enteric nervous system and the vagus nerve [100], or through the systemic circulation, which transports microbial metabolites—both beneficial and harmful—across the blood–brain barrier [101,102] (Figure 2).
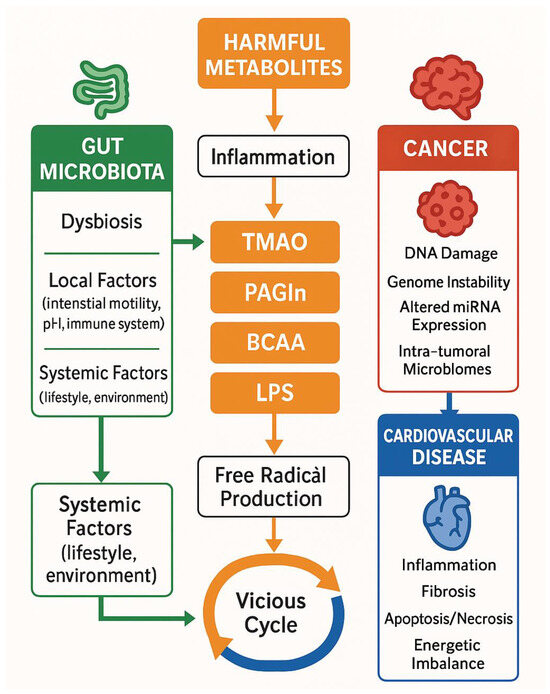
Figure 2.
Pathophysiological concepts for microbiome—cancer—cardiovascular diseases interplay. BCAA: branched-chain amino acids, CV: cardiovascular diseases, LPS: lipopolysaccharides, PAGLn: phenylacetylglutamine, TMAO: Trimethylamine N-Oxide.
Increased intestinal permeability allows microbial products such as LPS and SCFAs to activate both peripheral and central immune cells, promote cytokine release, and induce neuroinflammation. These processes affect CNS function and contribute to disease pathophysiology. Microbial products, including SCFAs, bile acid derivatives, neurotransmitter agonists, tryptophan metabolites, serotonin, and catecholamines, can modulate host metabolic and inflammatory responses, contributing to the development and progression of both cancer and cardiovascular diseases [103,104,105,106]. The inflammatory response triggered by harmful microbial stimuli activates immune cells and promotes cytokine release, which directly impacts CNS function [99]. This axis highlights an indirect mechanism by which the microbiome influences the nervous system and broader human physiology.
According to the International Cancer Microbiome Consortium, there is currently no direct evidence that the human commensal microbiome is a key determinant in the etiology of cancer [107]. While some cancers such as those associated with bacterial vaginosis and co-infection with HIV or human papillomavirus may involve a more direct microbial role, it appears that toxic products from a dysregulated microbiome are the primary contributors to cancer development. These metabolites disrupt host homeostasis, promote systemic inflammation and neurohumoral activation, and drive pathological processes that can facilitate cancer progression even at distant sites.
In HF, the nervous system is also significantly affected. Chronic cerebral hypoperfusion, inflammation, oxidative stress, and overactivation of the RAAS are key factors. RAAS is active in several organs, including the brain, heart, lungs, and intestines, and functions in an integrated manner to regulate homeostatic processes such as glycemic control and electrolyte balance [108]. When the gut microbiota and its metabolic outputs are pathologically altered, these regulatory mechanisms are disrupted. This disruption promotes cardiac fibrosis, cellular apoptosis and necrosis, and ultimately, the progression of HF [109,110] (Figure 3).
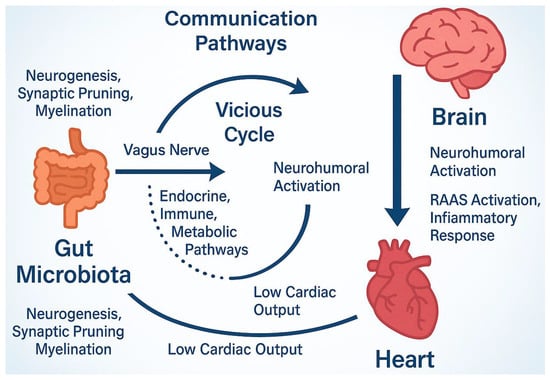
Figure 3.
Microbiome nervous system communication through the gut–brain axis. This axis using metabolic, endocrine and other pathways interfere with neurogenesis, myelination etc. The altered microbiota environments activate RAAS system that, in turn, promote free radical production and inflammatory response, the basic concept of HF syndrome. Accordingly, low cardiac output further aggravates these actions and stimulate central nervous system constituting thus a vicious cycle with harmful effects. RAAS; Renin-angiotensin-aldosterone system.
In addition, conditions such as insulin resistance, obesity, and metabolic syndrome [33], which are central to cardiovascular disease pathophysiology [19,31], are frequently present. In HF, reduced cardiac output and venous congestion impair intestinal function and promote dysbiosis. This microbial imbalance leads to excessive LPS production, which further increases intestinal permeability. The resulting endotoxemia drives systemic inflammation and activates immune and neurohumoral pathways [48,49,50], thereby exacerbating the severity of HF.
Clearly, a vicious cycle exists between the microbiome, HF, and the nervous system. These systems share overlapping pathophysiological mechanisms [100], and their dysfunction contributes to complications such as cognitive impairment [111]. Interestingly, the microbiome can also interact with the host’s reward pathways (e.g., the mesolimbic system) and modulate the effects of noradrenaline on the bone marrow, thereby enhancing antitumor immunity. Furthermore, the use of probiotics as adjunctive cancer therapy has shown potential to modulate the microbiome, improve psychological well-being, and slow cancer progression [112,113].
There is little doubt that a dynamic, reciprocal interplay exists among the brain, gut, and heart, forming a regulatory axis that governs nutrient absorption, gut motility, intestinal permeability, and broader biochemical, metabolic, and neurohormonal balance. Disruption of this axis compromises these homeostatic processes and may trigger disorders affecting the brain, gut, heart, and even cancer development [100].
Remarkably, this axis appears to be part of a broader, multicentric regulatory network involving multiple organ systems, including the brain (e.g., anxiety, depression), endocrine system (metabolic and hormonal disorders), cardiovascular system (heart disease, thrombosis), lungs (chronic obstructive pulmonary disease), liver (cirrhosis), pancreas (diabetes), and bones (osteoporosis), among others. Dysregulation within this network contributes to the pathogenesis of a wide range of systemic diseases [15].
Although the role of the gut–brain axis in disease is increasingly recognized, the precise pathways through which microbiome-induced neuroinflammation contributes to cancer and HF remain incompletely understood. Advanced imaging techniques and biomarker-based studies are needed to clarify the temporal and mechanistic aspects of this complex interplay.
Much of the current understanding of the gut–brain–heart axis stems from animal studies and observational human data. The precise pathways through which microbiome-driven neuroinflammation influences cancer and HF progression in humans remain largely hypothetical and warrant further clinical validation.
Is there a connection among these clinical states and genomic alterations?
4. Microbiome Relation to Genomic Mutation and Instability
The connection between cancer and an altered microbiome or metabolic environment, specifically dysbiosis, is well recognized [98,114]. Three key mechanisms explain the interplay between the microbiome, its metabolites, and the initiation and progression of cancer: inflammation, impaired intestinal permeability, and genomic damage. The first two mechanisms have been discussed previously. The third involves direct interactions between the microbiome and host DNA, as dysbiotic microbial communities and their toxic metabolites promote DNA damage, a phenomenon observed in both cancer and cardiovascular diseases.
DNA is a dynamic molecule that constantly undergoes replication and recombination. The fidelity of these processes depends on the cell’s ability to detect and repair abnormalities. However, when the frequency or intensity of DNA-damaging factors exceeds the repair capacity, lesions such as base mismatches, single- or double-strand breaks, and DNA adducts can occur. These abnormalities lead to defective sequences and the production of dysfunctional proteins. The accumulation of such DNA damage is a central driver of cellular mutations and, ultimately, tumorigenesis [114,115].
Microbes residing in various organs, especially the gastrointestinal tract, have also been detected within tumors, forming what is known as intratumoral microbiota. These microbial populations are closely associated with cancer development [116]. Intratumoral microbiomes, particularly those located near human leukocyte antigens (HLA-I and HLA-II), differ significantly from the microbiota of adjacent healthy tissue and vary across different tumor types [117,118,119].
Microbiota-induced DNA damage can occur either directly, as described, or indirectly through increased production of free radicals [120]. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) can modify DNA bases; for instance, through the formation of 8-hydroxy-deoxyguanosine, which results in G→T transversions. These mutations contribute to malignancy by disrupting tumor suppressor genes such as p53, stabilizing hypoxia-inducible factors (HIFs), and activating transcription factors like nuclear factor kappa B (NF-κB) and activator protein-1 (AP-1) [121].
Beyond genetic mutations, microbiome dysbiosis can also promote disease through epigenetic modifications. These include chemical alterations to DNA and histones, such as methylation and acetylation, that influence gene expression without altering the DNA sequence itself [122,123,124]. Certain microbial metabolites, particularly SCFAs (e.g., butyrate) and secondary bile acids, can modify the activity of DNA methyltransferases (DNMTs) and histone deacetylases (HDACs), thereby altering DNA methylation patterns [125]. Chronic inflammation and oxidative stress triggered by dysbiosis may promote hypermethylation of tumor suppressor gene promoters or global DNA hypomethylation—both hallmarks of cancer development [126,127]. Changes in histone acetylation can further affect chromatin accessibility and gene transcription, influencing key processes such as cell cycle regulation, apoptosis, and inflammation [127].
Although the role of microbiome-induced epigenetic alterations in HF is less well understood, emerging evidence suggests that oxidative and inflammatory environments driven by dysbiosis can induce epigenetic reprogramming in cardiac tissue. This may contribute to myocardial remodeling, fibrosis, and progression of HF [122,124]. Thus, epigenetic changes represent a crucial mechanistic link between microbiome dysbiosis, oncogenesis, and cardiac dysfunction.
In the context of cardiovascular disease, although the underlying mechanisms remain incompletely defined, gene mutations resulting in abnormal protein expression are increasingly recognized as contributing factors [128,129]. Each individual carries unique genomic variants, including single nucleotide polymorphisms and larger chromosomal abnormalities, which account for approximately 85% of the known genetic variation associated with disease susceptibility [130,131].
These variants can shape disease phenotypes and, conversely, the phenotype may influence microbiome composition. Altered gene expression can directly or indirectly impact microbial populations, which may, in turn, influence disease manifestation. This highlights a strong association between genetic variation and the development of diseases such as cancer and cardiovascular conditions [132].
Notably, associations have been documented between gut microbiota or their metabolites and HF, as well as with its major risk factors, including diabetes, hypertension, myocarditis, myocardial ischemia, arrhythmias, and both hypertrophic and dilated cardiomyopathies [133]. MicroRNAs (miRNAs), small, non-coding RNAs that regulate gene expression by modulating mRNA degradation and translational repression, play vital roles in processes such as cell differentiation, proliferation, and apoptosis [134]. A bidirectional relationship exists between miRNAs and the microbiome: miRNAs can influence microbiome composition and activity, while the microbiome can affect host miRNA expression through its metabolic and inflammatory effects [120,135].
This reciprocal interaction significantly shapes the development of various diseases, including cancer and cardiovascular disorders.
While the epigenetic influence of the microbiome on cancer development is becoming clearer, its role in HF remains underexplored. Future research should focus on elucidating how microbiome-driven DNA methylation and histone modifications contribute to cardiovascular disease. Epigenome-wide association studies conducted in well-characterized patient cohorts could offer valuable insights.
5. Insulin Resistance, Hyperinsulinemia, and Their Link to the Microbiome
Insulin resistance, characterized by impaired glucose uptake and utilization, is a central feature of metabolic syndrome, which is strongly linked to both cancer and cardiovascular disease [136]. Hyperinsulinemia—resulting from compensatory pancreatic insulin secretion—fosters a pro-inflammatory, pro-oxidant, and pro-growth environment, conducive to tumor progression and myocardial remodeling. Emerging evidence highlights the gut microbiome as a key modulator of insulin sensitivity, through direct and indirect mechanisms involving microbial metabolites, immune activation, and intestinal permeability [137].
The gut microbiota influences systemic metabolism via SCFAs, LPS, TMAO, and BCAAs. For example, SCFAs such as butyrate have anti-inflammatory and insulin-sensitizing properties [138], while BCAAs and LPS are associated with metabolic inflammation and IR [139,140]. In HF, congestion and gut ischemia promote dysbiosis, favoring Gram-negative bacterial overgrowth and LPS translocation, which activates TLR4 signaling and contributes to systemic insulin resistance [141,142].
In cancer, hyperinsulinemia supports tumor growth by activating insulin and IGF-1 receptors, promoting mitogenic signaling via PI3K/Akt and MAPK pathways [143,144,145]. In parallel, IR impairs mitochondrial efficiency and oxidative metabolism, both of which are disrupted in cancerous and failing myocardial cells. This metabolic derangement is exacerbated by dysbiosis-induced systemic inflammation and oxidative stress, creating a vicious cycle where microbiota, metabolism, and immune responses reinforce disease progression.
Hyperinsulinemia may modulate the tumor microenvironment, impair immune surveillance, and alter drug metabolism—effects that are influenced by microbiota-derived metabolites [146]. For instance, TMAO, produced from dietary choline by gut microbes, is elevated in IR states and is associated with both atherosclerosis and tumor angiogenesis [147].
Intervening on the gut microbiome may attenuate insulin resistance and, by extension, mitigate progression of HF and cancer. Prebiotics, probiotics, and dietary interventions (e.g., Mediterranean diet, fiber-rich intake) have been shown to restore microbial balance and improve insulin sensitivity [148]. Furthermore, emerging strategies such as fecal microbiota transplantation (FMT) and targeted microbial metabolite modulation (e.g., SCFA enhancement, LPS inhibition) may hold promise [149].
Metformin—a cornerstone antidiabetic drug—also exerts microbiome-modulating effects, increasing the abundance of Akkermansia muciniphila, a bacterium associated with improved metabolic outcomes [150]. Notably, metformin has shown antitumor effects in epidemiological studies, further linking insulin pathways, microbiota, and cancer biology.
Although associations between dysbiosis and insulin resistance are well-documented, it remains uncertain whether microbiome modulation can consistently reverse insulin resistance in clinical settings. Interventional studies employing probiotics, dietary fiber, or fecal microbiota transplantation are needed to test this therapeutic potential.
6. Microbiome Stabilizing Strategies
Several pharmacological and non-pharmacological interventions are essential in the management of both cancer and cardiovascular diseases. Non-pharmacological strategies, such as exercise training, lifestyle modifications, and adherence to a Mediterranean diet, have demonstrated beneficial effects on these conditions [151]. While their positive impact is well documented, potential adverse effects have also been reported [152,153]. These interventions can influence the composition and bioavailability of the intestinal microbiome, thereby affecting metabolic processes, immune cell function, and other host responses.
For example, excessive consumption of high-fat foods can increase the levels of harmful microbial metabolites such as TMAO, LPS, PAGIn, and phenylacetylglycine, all of which are associated with adverse health outcomes [154,155,156,157,158].
Beyond lifestyle interventions, both cardiovascular disease and cancer require pharmacological treatment. However, it is important to recognize that an altered gut microbiome can interfere with drug pharmacokinetics and pharmacodynamics [159,160,161,162]. For instance, the effectiveness of β-blockers [162,163], sodium-glucose cotransporter-2 (SGLT2) inhibitors [164,165], and RAAS inhibitors [110,166] may be reduced in the presence of dysbiosis, potentially leading to suboptimal therapeutic outcomes.
A similar phenomenon is observed with many anticancer therapies. The microbiome and its metabolites—through metabolic, immune (both innate and adaptive), epigenetic, and inflammatory pathways—can influence the effectiveness of immunotherapy [167]. As noted, “the gut microbiota may interact with oncogenic pathways, including epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and Kirsten rat sarcoma viral oncogene homolog (KRAS)” [168]. This highlights how gut microbiome alterations can affect not only cancer development and progression but also the response to cancer treatment.
Changes in the microbiome have also been linked to drug resistance. This may occur through mechanisms involving DNA damage, altered drug metabolism, and modifications of the tumor microenvironment [169]. As a result, toxic microbial byproducts may reduce the efficacy of targeted therapies and contribute to tumor growth and progression [170,171]. Specific examples include:
- Irinotecan, a pro-drug used to treat colorectal cancer, is metabolized into the active compound SN-38, a topoisomerase inhibitor. SN-38 can cause DNA damage and severe, potentially life-threatening toxicity [172].
- Gemcitabine, a nucleoside analog used in multiple cancers, can be inactivated by microbial enzymes that convert it into 2′,2′-difluorodeoxyuridine, thereby reducing its therapeutic effectiveness [173].
- Cyclophosphamide, a widely used chemotherapeutic agent, exerts some of its effects through immune modulation—a process also influenced by the gut microbiome [174].
The interaction between the microbiome and pharmacological agents is now well established, prompting growing interest in stabilizing the microbiome to optimize drug responses. Notably, different tumor types have been associated with distinct microbial profiles [175,176], emphasizing the need for personalized treatment strategies based on an individual’s microbiome composition.
In this context, antibiotic therapy targeting specific bacterial species has shown promise. For example, antibiotics targeting Bacteroides species have been linked to improved survival in patients with metastatic renal cell carcinoma receiving first-line VEGF tyrosine kinase inhibitors [177]. However, contradictory results in other cancer types suggest that the broad use of antibiotics may not always be beneficial [178].
An emerging area of interest is the use of probiotics and prebiotics to stabilize the gut microbiota. Probiotics—live microorganisms that support health—can help maintain mucosal integrity, regulate intestinal motility, and suppress pathogenic bacteria [11,179]. For instance:
- Lactobacilli produce antioxidant and anti-angiogenic compounds, reduce DNA damage, and mitigate inflammation [180].
- Bifidobacterium species have demonstrated the ability to activate the innate immune system and exhibit anti-cancer properties, particularly in lung, cervical, and breast cancers [180].
- Inulin and galacto-oligosaccharides (GOS) can stimulate immune responses and have shown anticancer potential [181].
Prebiotics, which promote the growth of beneficial gut bacteria, also exert protective effects by modulating intestinal metabolism [179]. A notable example is GOS, a class of carbohydrates found naturally in breast milk. These compounds enhance the production of beneficial cytokines such as interleukin-8 (IL-8), interleukin-10 (IL-10), and C-reactive protein, while reducing harmful ones such as interleukin-1β (IL-1β) [179].
Recent advances in microbiome research highlight the potential benefits of personalizing interventions based on individual microbial profiles to improve the treatment of both cancer and HF. In the context of HF, targeting the gut microbiome may offer an adjunctive strategy to modulate systemic inflammation, improve metabolic parameters, and restore intestinal barrier function. Dietary interventions such as increased fiber intake and adherence to a Mediterranean diet have been associated with enhanced SCFA production, improved endothelial function, and reduced cardiovascular risk [148,151]. Probiotic supplementation, particularly with Lactobacillus and Bifidobacterium species, has shown promise in small clinical studies by attenuating inflammatory cytokine release and improving left ventricular function [180,182]. Fecal microbiota transplantation, though still experimental in cardiovascular settings, offers a way to re-establish a eubiotic microbial ecosystem and has been shown to reduce insulin resistance and systemic inflammation in metabolic disease models. Integration of these interventions into HF management may be especially relevant for patients with comorbid metabolic syndrome, obesity, or gut-derived inflammation, further helping to attenuate systemic inflammation, improve metabolic profiles, and slow cardiac remodeling [182]. In oncology, specific microbial signatures have been associated with responses to immune checkpoint inhibitors and chemotherapy, suggesting that modulating the microbiome could enhance antitumor immunity and reduce drug resistance [183]. Precision microbiome-based interventions offer the possibility of reducing treatment-related toxicity, enhancing therapeutic efficacy, and preventing disease progression by restoring a balanced host–microbiome interaction tailored to each patient’s unique microbial ecosystem.
Although microbiome-targeted therapies are promising, the optimal strategies for specific patient populations remain undefined. Personalized microbiome interventions, tailored to an individual’s microbial profile and disease phenotype, should be a major focus of future clinical trials.
7. Challenges to Be Addressed and Strengths of the Manuscript
Although numerous studies support a link between the intestinal microbiome environment and both cancer and cardiovascular diseases, several critical challenges remain. There is a pressing need to generate robust, high-quality evidence to better understand this relationship. Specifically, longitudinal studies are required to determine whether alterations in the microbiome are a cause or a consequence of cancer and/or cardiovascular diseases.
To draw such conclusions, advancements in diagnostic techniques for accurately characterizing microbiome composition are essential. Current methods, such as analyzing blood or fecal samples to identify microbial biomarkers, are widely used but carry notable limitations. In order to improve accuracy and interpretation, it is imperative to first establish a clear definition of what constitutes normal microbiota [184], and to further stratify microbiome profiles by sex and age [185]. Additionally, expanding and refining existing microbiome databases is necessary to overcome current limitations in reference datasets [186].
Although the gut microbiome has been extensively studied in relation to individual disease states such as HF or cancer, its role as a shared pathophysiological bridge between these two major conditions remains underexplored. This review provides a novel and integrative perspective by examining how microbiome-derived metabolites such as TMAO, LPS, and SCFAs, influence common biological pathways including inflammation, immune modulation, metabolic dysfunction, and oxidative stress. We introduce the concept of a “gut–heart–tumor” axis and propose that microbial dysbiosis represents a unifying mechanism driving both cardiac and oncologic disease progression. Furthermore, this review synthesizes emerging evidence on the bidirectional interplay among the gut, heart, and brain, and explores therapeutic opportunities through microbiome modulation. By bridging cardiology, oncology, and microbiome science, our review offers a comprehensive framework that advances current understanding and suggests novel avenues for prevention and treatment in comorbid disease states.
8. Conclusions
In conclusion, HF, cancer, and the gut microbiome are not isolated entities but are deeply interconnected through shared biological mechanisms, including chronic inflammation, microbial dysbiosis, immune and neurohumoral modulation, and metabolic derangement. These findings support the concept of a microbiome-centered axis involving the gut, heart, and tumors, which may underlie many chronic disease processes. Recognizing the microbiome as a dynamic contributor to both cardiac and oncological health opens new frontiers for targeted interventions. Although preclinical studies have established compelling mechanistic links between microbial metabolites and both cardiovascular and oncologic pathology, translation into clinical practice remains in its early stages. Most human data are associative or observational, and causality has not been firmly established. Rigorous longitudinal studies, interventional trials, and multi-omics approaches are essential in order to validate microbiome-derived biomarkers and personalize prevention and treatment strategies in patients with concurrent HF and cancer. Modulating the gut microbiota through diet, probiotics, or pharmacological agents may offer a promising therapeutic avenue to simultaneously address the burden of cancer and cardiovascular disease.
Author Contributions
Conceptualization, I.P.; methodology, I.P. and C.K.; investigation, E.T. and C.K.; original draft preparation, I.P.; writing—review and editing, I.P., C.K. and E.T.; supervision, I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AP-1 | Activator Protein-1 |
| BCAA | Branched-Chain Amino Acids |
| CNS | Central Nervous System |
| EGFR | Epidermal Growth Factor Receptor |
| GOS | Galacto-Oligosaccharides |
| HF | Heart Failure |
| HIF | Hypoxia-Inducible Factors |
| HIV | Human Immunodeficiency Virus |
| HLA | Human Leukocyte Antigens |
| IL | Interleukin |
| KRAS | Kirsten Rat Sarcoma |
| LPS | Lipopolysaccharides |
| miRNAs | MicroRNAs |
| NF-κB | Nuclear Factor Kappa B |
| PAGIn | Phenylacetylglutamine |
| RAAS | Renin-Angiotensin-Aldosterone System |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SCFAs | Short-Chain Fatty Acids |
| SGLT2 | Sodium-Glucose Cotransporter-2 |
| TMAO | Trimethylamine N-oxide |
| VEGF | Vascular Endothelial Growth Factor |
References
- Crespo-Leiro, M.G.; Anker, S.D.; Maggioni, A.P.; Coats, A.J.; Filippatos, G.; Ruschitzka, F.; Ferrari, R.; Piepoli, M.F.; Delgado Jimenez, J.F.; Metra, M.; et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Rinde, L.B.; Smabrekke, B.; Hald, E.M.; Brodin, E.E.; Njolstad, I.; Mathiesen, E.B.; Lochen, M.L.; Wilsgaard, T.; Brekkan, S.K.; Vik, A.; et al. Myocardial infarction and future risk of cancer in the general population—The Tromso Study. Eur. J. Epidemiol. 2017, 32, 193–201. [Google Scholar] [CrossRef]
- Banke, A.; Schou, M.; Videbaek, L.; Moller, J.E.; Torp-Pedersen, C.; Gustafsson, F.; Dahl, J.S.; Kober, L.; Hildebrandt, P.R.; Gislason, G.H. Incidence of cancer in patients with chronic heart failure: A long-term follow-up study. Eur. J. Heart Fail. 2016, 18, 260–266. [Google Scholar] [CrossRef]
- Berton, G.; Cordiano, R.; Cavuto, F.; Bagato, F.; Segafredo, B.; Pasquinucci, M. Neoplastic disease after acute coronary syndrome: Incidence, duration, and features: The ABC-4* study on heart disease. J. Cardiovasc. Med. 2018, 19, 546–553. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Suter, T.; Plataniotis, G.; De Azambuja, E.; Sandri, M.T.; Criscitiello, C.; Goldhirsch, A.; Cipolla, C.; Roila, F. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann. Oncol. 2012, 23 (Suppl. S7), vii155–vii166. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology. Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Herrmann, J.; Lerman, A.; Sandhu, N.; Villarraga, H.; Mulvagh, S.; Kohli, M. Evaluation and management of patients with heart disease and cancer: Cardio-oncology. Mayo Clin. Proc. 2014, 89, 1287–1306. [Google Scholar] [CrossRef]
- Qasem, H.H.; El-Sayed, W.M. The bacterial microbiome and cancer: Development, diagnosis, treatment, and future directions. Clin. Exp. Med. 2025, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, A.; Cicchinelli, S.; Valletta, F.; De Luca, G.; Longhitano, Y.; Candelli, M.; Ojetti, V.; Sardeo, F.; Navarra, S.; Covino, M.; et al. Gut Microbiota and Autoimmune Diseases: A Charming Real World Together with Probiotics. Curr. Med. Chem. 2022, 29, 3147–3159. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Kitano, S.; Puah, G.R.Y.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies. Chem. Rev. 2023, 123, 31–72. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Di Ciaula, A.; Mahdi, L.; Jaber, N.; Di Palo, D.M.; Graziani, A.; Baffy, G.; Portincasa, P. Unraveling the Role of the Human Gut Microbiome in Health and Diseases. Microorganisms 2024, 12, 2333. [Google Scholar] [CrossRef]
- Lian, W.S.; Wang, F.S.; Chen, Y.S.; Tsai, M.H.; Chao, H.R.; Jahr, H.; Wu, R.W.; Ko, J.Y. Gut Microbiota Ecosystem Governance of Host Inflammation, Mitochondrial Respiration and Skeletal Homeostasis. Biomedicines 2022, 10, 860. [Google Scholar] [CrossRef]
- Paraskevaidis, I.; Xanthopoulos, A.; Tsougos, E.; Triposkiadis, F. Human Gut Microbiota in Heart Failure: Trying to Unmask an Emerging Organ. Biomedicines 2023, 11, 2574. [Google Scholar] [CrossRef]
- Calabrò, S.; Kankowski, S.; Cescon, M.; Gambarotta, G.; Raimondo, S.; Haastert-Talini, K.; Ronchi, G. Impact of Gut Microbiota on the Peripheral Nervous System in Physiological, Regenerative and Pathological Conditions. Int. J. Mol. Sci. 2023, 24, 8061. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, Y.; Zhong, H.; Liu, Z.; Geng, J.; Wang, H.; Wang, W. Gut microbes in central nervous system development and related disorders. Front. Immunol. 2024, 14, 1288256. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Jin, X.; Li, D.; Lu, J.; Wang, X.; Wu, M. Mitochondria as novel mediators linking gut microbiota to atherosclerosis that is ameliorated by herbal medicine: A review. Front. Pharmacol. 2023, 14, 1082817. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of short-chain fatty acids in inflammatory bowel disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef]
- Datta, S.; Pasham, S.; Inavolu, S.; Boini, K.M.; Koka, S. Role of gut microbial metabolites in cardiovascular diseases—Current insights and the road ahead. Int. J. Mol. Sci. 2024, 25, 10208. [Google Scholar] [CrossRef]
- Amedei, A.; Morbidelli, L. Circulating metabolites originating from gut microbiota control endothelial cell function. Molecules 2019, 24, 3992. [Google Scholar] [CrossRef]
- Wang, Y.; Dou, W.; Qian, X.; Chen, H.; Zhang, Y.; Yang, L.; Wu, Y.; Xu, X. Advancements in the study of short-chain fatty acids and their therapeutic effects on atherosclerosis. Life Sci. 2025, 369, 123528. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The Role of the Microbiome in Cancer Development and Therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef]
- Bultman, S.J. Emerging roles of the microbiome in cancer. Carcinogenesis 2014, 35, 249–255. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, Y.; Xu, H.; Zhou, Y. The Interaction of Gut Microbiota and Heart Failure with Preserved Ejection Fraction: From Mechanism to Potential Therapies. Biomedicines 2023, 11, 442. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, S.; Forslund, S.K.; Chechi, K.; Aron-Wisnewsky, J.; Chakaroun, R.; Nielsen, T.; Tremaroli, V.; Ji, B.; Prifti, E.; Myridakis, A.; et al. Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat. Med. 2022, 28, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, T.; Moens de Hase, E.; Van Hul, M.; Paquot, A.; Pelicaen, R.; Regnier, M.; Depommier, C.; Druart, C.; Everard, A.; Maiter, D.; et al. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice. Gut 2022, 71, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.L.; Backhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef]
- Hrncir, T. Gut microbiota dysbiosis: Triggers, consequences, diagnostic and therapeutic options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.U.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Casso, A.G.; VanDongen, N.S.; Ziemba, B.P.; Sapinsley, Z.J.; Richey, J.J.; Zigler, M.C.; Neilson, A.P.; Davy, K.P.; et al. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 2020, 76, 101–112. [Google Scholar] [CrossRef]
- Oktaviono, Y.H.; Lamara, D.A.; Saputra, P.B.T.; Arnindita, J.N.; Pasahari, D.; Saputra, M.E.; Suasti, N.M.A. The roles of trimethylamine-N-oxide in atherosclerosis and its potential therapeutic aspect: A literature review. Biomol. Biomed. 2023, 23, 936–948. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How gut microbiota contributes to heart failure. Transl. Res. 2021, 228, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Y.; Zhou, J.; Chen, R.; Li, J.; Zhao, X.; Zhou, P.; Liu, C.; Chen, Y.; Song, L.; et al. Association between the changes in trimethylamine N-oxide-related metabolites and prognosis of patients with acute myocardial infarction: A prospective study. J. Cardiovasc. Dev. Dis. 2022, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Yang, P.; Liu, X.; Lu, L.; Chen, Y.; Zhong, X.; Li, Z.; Liu, H.; Ou, C.; et al. Trimethylamine-N-oxide promotes vascular calcification through activation of NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome and NF-κB (nuclear factor κB) signals. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 751–765. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Zhang, W.; Liu, X. Mitochondrial dysfunction and mitochondrial therapies in heart failure. Pharmacol. Res. 2022, 175, 106038. [Google Scholar] [CrossRef]
- Gąsecka, A.; Fidali, O.; Kłębukowska, A.; Jasińska-Gniadzik, K.; Szwed, P.; Witkowska, K.; Eyileten, C.; Postuła, M.; Grabowski, M.; Filipiak, K.J.; et al. Plasma concentration of TMAO is an independent predictor of adverse outcomes in patients after acute myocardial infarction. Postep. Kardiol. Interwencyjnej 2023, 19, 31–39. [Google Scholar] [CrossRef]
- Jaworska, K.; Kopacz, W.; Koper, M.; Ufnal, M. Microbiome-derived trimethylamine N-oxide (TMAO) as a multifaceted biomarker in cardiovascular disease: Challenges and opportunities. Int. J. Mol. Sci. 2024, 25, 12511. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Barra, N.G.; Cavallari, J.F.; Henriksbo, B.D.; Schertzer, J.D. Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep. 2021, 36, 109691. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef]
- Lightfoot, Y.L.; Yang, T.; Sahay, B.; Mohamadzadeh, M. Targeting aberrant colon cancer-specific DNA methylation with lipoteichoic acid-deficient Lactobacillus acidophilus. Gut Microbes 2013, 4, 84–88. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the holobiont: How microbial metabolites affect human health and shape the immune system. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef]
- Van Kessel, S.P.; Frye, A.K.; El-Gendy, A.O.; Castejon, M.; Keshavarzian, A.; van Dijk, G.; El Aidy, S. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat. Commun. 2019, 10, 310. [Google Scholar] [CrossRef]
- McCarville, J.L.; Chen, G.Y.; Cuevas, V.D.; Troha, K.; Ayres, J.S. Microbiota Metabolites in Health and Disease. Annu. Rev. Immunol. 2020, 38, 147–170. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Ding, R.X.; Goh, W.R.; Wu, R.N.; Yue, X.Q.; Luo, X.; Khine, W.W.T.; Wu, J.R.; Lee, Y.K. Revisit gut microbiota and its impact on human health and disease. J. Food Drug Anal. 2019, 27, 623–631. [Google Scholar] [CrossRef]
- Wen, Y.; Sun, Z.; Xie, S.; Hu, Z.; Lan, Q.; Sun, Y.; Yuan, L.; Zhai, C. Intestinal flora derived metabolites affect the occurrence and development of cardiovascular disease. J Multidiscip. Healthc. 2022, 15, 2591–2603. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.; Huang, J.; Wang, J.; Cao, H.; Bai, Y.; Guo, J.; Su, Z. Connection between gut microbiome and the development of obesity. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Marchesi, N.; Govoni, S.; Coppola, A.; Gazzaruso, C. The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: New insights into old diseases. Curr. Opin. Pharmacol. 2019, 49, 1–5. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Silva, J.S.C.; Seguro, C.S.; Naves, M.M.V. Gut microbiota and physical exercise in obesity and diabetes—A systematic review. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 863–877. [Google Scholar] [CrossRef]
- Fluhr, L.; Mor, U.; Kolodziejczyk, A.A.; Dori-Bachash, M.; Leshem, A.; Itav, S.; Cohen, Y.; Suez, J.; Zmora, N.; Moresi, C.; et al. Publisher Correction: Gut microbiota modulates weight gain in mice after discontinued smoke exposure. Nature 2022, 603, E35. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Banaee, M.; Vazzana, I.; Betancourt-Lozano, M.; Gonzalez-Mille, D.J.; Aliko, V.; Faggio, C.; Ilizaliturri-Hernandez, C.A. Effect of emerging pollutants on the gut microbiota of freshwater animals: Focusing on microplastics and pesticides. Sci. Total Environ. 2024, 948, 174809. [Google Scholar] [CrossRef]
- Teffera, M.; Veith, A.C.; Ronnekleiv-Kelly, S.; Bradfield, C.A.; Nikodemova, M.; Tussing-Humphreys, L.; Malecki, K. Diverse mechanisms by which chemical pollutant exposure alters gut microbiota metabolism and inflammation. Environ. Int. 2024, 190, 108805. [Google Scholar] [CrossRef]
- Van Pee, T.; Nawrot, T.S.; van Leeuwen, R.; Hogervorst, J. Ambient particulate air pollution and the intestinal microbiome; a systematic review of epidemiological, in vivo and, in vitro studies. Sci. Total Environ. 2023, 878, 162769. [Google Scholar] [CrossRef]
- Behary, J.; Amorim, N.; Jiang, X.T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef]
- Radojevic, D.; Tomic, S.; Mihajlovic, D.; Tolinacki, M.; Pavlovic, B.; Vucevic, D.; Bojić, S.; Golić, N.; Čolić, M.; Đokić, J. Fecal microbiota composition associates with the capacity of human peripheral blood monocytes to differentiate into immunogenic dendritic cells in vitro. Gut Microbes 2021, 13, 1921927. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.Y.; Hong, S.; Nam, Y.-D. Understanding the role of the gut microbiome in solid tumor responses to immune checkpoint inhibitors for personalized therapeutic strategies: A review. Front. Immunol. 2025, 15, 1512683. [Google Scholar] [CrossRef] [PubMed]
- Aspesi, A.; La Vecchia, M.; Sala, G.; Ghelardi, E.; Dianzani, I. Study of Microbiota Associated to Early Tumors Can Shed Light on Colon Carcinogenesis. Int. J. Mol. Sci. 2024, 25, 13308. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2020, 19, 55–71. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- White, M.T.; Sears, C.L. The Microbial Landscape of Colorectal Cancer. Nat. Rev. Microbiol. 2024, 22, 240–254. [Google Scholar] [CrossRef]
- He, R.; Qi, P.; Shu, L.; Ding, Y.; Zeng, P.; Wen, G.; Xiong, Y.; Deng, H. Dysbiosis and extraintestinal cancers. Exp. Clin. Cancer Res. 2025, 44, 44. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Alvarez-Mercado, A.I.; Ruiz-Marin, C.M.; Reina-Perez, I.; Perez-Alonso, A.J.; Sanchez-Andujar, M.B.; Torné, P.; Gallart-Aragón, T.; Sánchez-Barrón, M.T.; Lartategu, S.R.; et al. Association of breast and gut microbiota dysbiosis and the risk of breast cancer: A case-control clinical study. BMC Cancer 2019, 19, 495. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.; Guan, Y.; Lou, Y.; Chen, H.; Xu, M.; Deng, D.; Chen, J.; Ni, B.; Zhao, L.; et al. Dysbiosis of the Gut Microbiome is associated with Tumor Biomarkers in Lung Cancer. Int. J. Biol. Sci. 2019, 15, 2381–2392. [Google Scholar] [CrossRef]
- Thomas, R.M.; Gharaibeh, R.Z.; Gauthier, J.; Beveridge, M.; Pope, J.L.; Guijarro, M.V.; Yu, Q.; He, Z.; Ohland, C.; Newsome, R.; et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis 2018, 39, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.P.; Poutahidis, T.; Fox, J.G.; Erdman, S.E. Breast cancer: Should gastrointestinal bacteria be on our radar screen? Cancer Res. 2007, 67, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tan, Q.; Fu, Q.; Zhou, Y.; Hu, Y.; Tang, S.; Zhou, Y.; Zhang, J.; Qiu, J.; Lv, Q. Gastrointestinal microbiome and breast cancer: Correlations, mechanisms and potential clinical implications. Breast Cancer 2016, 24, 220–228. [Google Scholar] [CrossRef]
- Guo, C.; An, Q.; Zhang, L.Y.; Wei, X.D.; Xu, J.; Yu, J.Y.; Wu, G.J.; Ma, J. Intratumoral microbiota as cancer therapeutic target. Aging Med. 2024, 7, 636–644. [Google Scholar] [CrossRef]
- Vezza, T.; Abad-Jimenez, Z.; Marti-Cabrera, M.; Rocha, M.; Victor, V.M. Microbiota-Mitochondria Inter-Talk: A Potential Therapeutic Strategy in Obesity and Type 2 Diabetes. Antioxidants 2020, 9, 848. [Google Scholar] [CrossRef]
- Paraskevaidis, I.; Farmakis, D.; Papingiotis, G.; Tsougos, E. Inflammation and Heart Failure: Searching for the Enemy—Reaching the Entelechy. J. Cardiovasc. Dev. Dis. 2023, 10, 19. [Google Scholar] [CrossRef]
- Vasan, R.S.; Sullivan, L.M.; Roubenoff, R.; Dinarello, C.A.; Harris, T.; Benjamin, E.J.; Sawyer, D.B.; Levy, D.; Wilson, P.W.F.; D’Agostino, R.B. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The Framingham Heart Study. Circulation 2003, 10, 1486–1491. [Google Scholar] [CrossRef]
- Edelmann, F.; Holzendorf, V.; Wachter, R.; Nolte, K.; Schmidt, A.G.; Kraigher-Krainer, E.; Duvinage, A.; Unkelbach, I.; Düngen, H.-D.; Tschöpe, C.; et al. Galectin-3 in patients with heart failure with preserved ejection fraction: Results from the Aldo-DHF trial. Eur. J. Heart Fail. 2015, 17, 214–223. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Higashi, Y. Roles of oxidative stress and inflammation in vascular endothelial dysfunction—Related disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor initiation and early tumorigenesis: Molecular mechanisms and interventional targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The role of oxidative stress in cardiac disease: From physiological response to injury factor. Oxidative Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Oxidative stress in cell death and cardiovascular diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9030563. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, X.; Xiong, Z.; Ihsan, A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.R.; Anadón, A.; Wang, X.; et al. Cancer metabolism: The role of ROS in DNA damage and induction of apoptosis in cancer cells. Metabolites 2023, 13, 796. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The role of gut microbiota and gut-brain interplay in selected diseases of the central nervous system. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Kyle Pellegrino, K.; Mármo, J.D.; Castro, T.B.R.; Furuich, M.; et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 2020, 583, 441–446. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The gut-brain axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.M.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: Disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1869501. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.F.; Dinan, T.G.; Stanton, C. Bacterial neuroactive compounds produced by psychobiotics. Adv. Exp. Med. Biol. 2014, 817, 221–239. [Google Scholar]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Logsdon, A.F.; Erickson, M.A.; Rhea, E.M.; Salameh, T.S.; Banks, W.A. Gut reactions: How the blood-brain barrier connects the microbiome and the brain. Exp. Biol. Med. 2018, 243, 159–165. [Google Scholar] [CrossRef]
- Scott, A.J.; Alexander, J.L.; Merrifield, C.A.; Cunningham, D.; Jobin, C.; Brown, R.; Alverdy, J.; O’Keefe, S.J.; Rex Gaskins, H.R.; Teare, J.; et al. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut 2019, 68, 1624–1632. [Google Scholar] [CrossRef]
- Jaworska, K.; Koper, M.; Ufnal, M. Gut microbiota and renin-angiotensin system: A complex interplay at local and systemic levels. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 321, G355–G366. [Google Scholar] [CrossRef]
- Alhajri, N.; Khursheed, R.; Ali, M.T.; AbuIzneid, T.; Al-Kabbani, O.; Al-Haidar, M.B.; Al-Hemeiri, F.; Alhashmi, M.; Pottoo, F.H. Cardiovascular Health and the Intestinal Microbial Ecosystem: The Impact of Cardiovascular Therapies on the Gut Microbiota. Microorganisms 2021, 9, 2013. [Google Scholar] [CrossRef]
- Karbach, S.H.; Schonfelder, T.; Brando, I.; Wilms, E.; Hormann, N.; Jackel, S.; Schuler, R.; Finger, S.; Knorr, M.; Lagrange, J.; et al. Gut microbiota promote angiotensin II–induced arterial hypertension and vascular dysfunction. J. Am. Heart Assoc. 2016, 5, e003698. [Google Scholar] [CrossRef]
- AlRawili, N.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Abdel-Fattah, M.M.; Al-Harchan, N.A.; Alruwaili, M.; Papadakis, M.; Alexiou, A.; EI-Saber Batiha, G. Trajectory of Cardiogenic Dementia: A New Perspective. J. Cell. Mol. Med. 2025, 29, e70345. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shaanan, T.L.; Schiller, M.; Azulay-Debby, H.; Korin, B.; Boshnak, N.; Koren, T.; Krot, M.; Shakya, J.; Rahat, M.A.; Hakim, F.; et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat. Commun 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.B.; Young, V.B.; Skufca, J.; Ginty, F.; Testerman, T.; Pearson, A.T.; Macklin, P.; Mitchell, A.; Shmulevich, I.; Xie, L.; et al. The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer 2020, 6, 192–204. [Google Scholar] [CrossRef]
- El Tekle, G.; Garrett, W.S. Bacteria in cancer initiation, promotion and progression. Nat. Rev. Cancer 2023, 23, 600–618. [Google Scholar] [CrossRef]
- Escriva, P.P.; Bernardino, C.C.T.; Letellier, E. De-coding the complex role of microbial metabolites in cancer. Cell Rep. 2025, 28, 115358. [Google Scholar] [CrossRef]
- Ding, T.; Liu, C.; Li, Z. The mycobiome in human cancer: Analytical challenges, molecular mechanisms, and therapeutic implications. Mol. Cancer 2025, 24, 18. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Kalaora, S.; Nagler, A.; Nejma, D.; Alon, M.; Barbolin, C.; Barnea, E.; Ketelaars, S.L.C.; Cheng, K.; Vervier, K.; Shental, N.; et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 2021, 592, 138–143. [Google Scholar] [CrossRef]
- Battaglia, T.W.; Mimpen, I.L.; Traets, J.J.H.; van Hoeck, A.; Zeverijn, L.J.; Geurts, B.S.; de Wit, G.F.; Noe, M.; Hofland, I.; Vos, J.L.; et al. A pan-cancer analysis of the microbiome in metastatic cancer. Cell 2024, 187, 2324–2335. [Google Scholar] [CrossRef]
- Drago, L.; De La Motte, L.R.; Deflorio, L.; Sansico, D.F.; Salvatici, M.; Micaglio, E.; Biazzo, M.; Giarritiello, F. Systematic review of bidirectional interaction between gut microbiome, miRNAs, and human pathologies. Front. Microbiol. 2025, 16, 1540943. [Google Scholar] [CrossRef]
- Santibáñez-Andrade, M.; Quezada-Maldonado, E.M.; Rivera-Pineda, A.; Chirino, Y.I.; García-Cuellar, C.M.; Sánchez-Pérez, Y. The road to malignant cell transformation after particulate matter exposure: From oxidative stress to genotoxicity. Int. J. Mol. Sci. 2023, 24, 1782. [Google Scholar] [CrossRef]
- Matacchione, G.; Piacenza, F.; Pimpini, L.; Rosati, Y.; Marcozzi, S. The role of the gut microbiota in the onset and progression of heart failure: Insights into epigenetic mechanisms and aging. Clin. Epigenetics 2024, 16, 175. [Google Scholar] [CrossRef]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.; Salvador, C.; Skibola, C.; Tollefsbol, T.O. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin. Epigenetics 2015, 7, 112. [Google Scholar] [CrossRef]
- Mehta, V.; Nagu, P.; Inbaraj, B.S.; Sharma, M.; Parashar, A.; Sridhar, K. Epigenetics and gut microbiota crosstalk: A potential factor in pathogenesis of cardiovascular disorders. Bioengineering 2022, 9, 798. [Google Scholar] [CrossRef]
- Stein, R.A.; Riber, L. Epigenetic effects of short-chain fatty acids from the large intestine on host cells. Microlife 2023, 4, uqad032. [Google Scholar] [CrossRef]
- Wu, Q.; Ni, X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr. Drug Targets 2015, 16, 13–19. [Google Scholar] [CrossRef]
- Lee, H.T.; Oh, S.; Ro, D.H.; Yoo, H.; Kwon, Y.W. The key role of DNA methylation and histone acetylation in epigenetics of atherosclerosis. J. Lipid Atheroscler. 2020, 9, 419–434. [Google Scholar] [CrossRef]
- Shah, R.A.; Asatryan, B.; Sharaf Dabbagh, G.; Aung, N.; Khanji, M.Y.; Lopes, L.R.; Van Duijvenboden, S.; Holmes, A.; Muser, D.; Landstrom, A.P.; et al. Genotype-first approach I. Frequency, penetrance, and variable expressivity of dilated cardiomyopathy associated putative pathogenic gene variants in UK Biobank participants. Circulation 2022, 146, 110–124. [Google Scholar] [CrossRef]
- Palmieri, G.; D’Ambrosio, M.F.; Correale, M.; Brunetti, N.D.; Santacroce, R.; Iacoviello, M.; Margaglione, M. The Role of Genetics in the Management of Heart Failure Patients. Int. J. Mol. Sci. 2023, 24, 15221. [Google Scholar] [CrossRef]
- Conrad, D.F.; Keebler, J.E.; DePristo, M.A.; Lindsay, S.J.; Zhang, Y.; Casals, F.; Idaghdour, Y.; Hartl, C.L.; Torroja, C.; Garimella, K.V.; et al. Variation in genome-wide mutation rates within and between human families. Nat. Genet. 2011, 43, 712–714. [Google Scholar]
- 1000 Genomes Project Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.R.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [PubMed]
- Bubier, J.A.; Chesler, E.J.; Weinstock, G.M. Host genetic control of gut microbiome composition. Mamm. Genome 2021, 32, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Hu, Y.; Chen, X.; Luo, Y.; Chen, J.; Wang, H. Effects of Gut Microbiota and Metabolites on Heart Failure and Its Risk Factors: A Two-Sample Mendelian Randomization Study. Front. Nutr. 2022, 9, 899746. [Google Scholar] [CrossRef]
- Dong, J.; Tai, J.W.; Lu, L.F. miRNA-microbiota interaction in gut homeostasis and colorectal Cancer. Trends Cancer 2019, 5, 666–669. [Google Scholar] [CrossRef]
- Viennois, E.; Zhao, Y.; Han, M.K.; Xiao, B.; Zhang, M.; Prasad, M.; Wang, L.; Merlin, D. Serum miRNA signature diagnoses and discriminates murine colitis subtypes and predicts ulcerative colitis in humans. Sci. Rep. 2017, 7, 2520. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef]
- Huang, R. Gut Microbiota: A Key Regulator in the Effects of Environmental Hazards on Modulates Insulin Resistance. Front. Cell Infect. Microbiol. 2022, 11, 800432. [Google Scholar] [CrossRef]
- Yu, W.; Sun, S.; Fu, Q. The role of short-chain fatty acid in metabolic syndrome and its complications: Focusing on immunity and inflammation. Front. Immunol. 2025, 16, 1519925. [Google Scholar]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Huang, X.; Yan, D.; Xu, M.; Li, F.; Ren, M.; Zhang, J.; Wu, M. Interactive association of lipopolysaccharide and free fatty acid with the prevalence of type 2 diabetes: A community-based cross-sectional study. J. Diabetes Investig. 2019, 10, 1438–1446. [Google Scholar] [CrossRef]
- Lupu, V.V.; Adam Raileanu, A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Macerola, N.; Favuzzi, A.M.; Nicolazzi, M.A.; Gasbarrini, A.; Montalto, M. The Gut in Heart Failure: Current Knowledge and Novel Frontiers. Med. Princ. Pract. 2022, 31, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.W.; Rossi, E.L.; O’Flanagan, C.H.; deGraffenried, L.A.; Hursting, S.D. The Role of the Insulin/IGF System in Cancer: Lessons Learned from Clinical Trials and the Energy Balance-Cancer Link. Front. Endocrinol. 2015, 6, 77. [Google Scholar] [CrossRef]
- Cao, J.; Yee, D. Disrupting Insulin and IGF Receptor Function in Cancer. Int. J. Mol. Sci. 2021, 22, 555. [Google Scholar] [CrossRef]
- Leitner, B.P.; Siebel, S.; Akingbesote, N.D.; Zhang, X.; Perry, R.J. Insulin and cancer: A tangled web. Biochem. J. 2022, 479, 583–607. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, Y.J.; Xue, C.D.; Li, S.; Gao, Z.N.; Qin, K.R. Effects of T2DM on cancer progression: Pivotal precipitating factors and underlying mechanisms. Front. Endocrinol. 2024, 15, 1396022. [Google Scholar] [CrossRef]
- Shanmugham, M.; Bellanger, S.; Leo, C.H. Gut-Derived Metabolite, Trimethylamine-N-oxide (TMAO) in Cardio-Metabolic Diseases: Detection, Mechanism, and Potential Therapeutics. Pharmaceuticals 2023, 16, 504. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, L.; Tang, H.; Qu, J.; Rao, B. Probiotics and Prebiotics as Dietary Supplements for the Adjunctive Treatment of Type 2 Diabetes. Pol. J. Microbiol. 2023, 72, 3–9. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Zhang, L.; Zhang, N. The role of fecal microbiota transplantation in type 2 diabetes mellitus treatment. Front. Endocrinol. 2024, 15, 1469165. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, X.; Cong, B. Advances in the mechanism of metformin with wide-ranging effects on regulation of the intestinal microbiota. Front. Microbiol. 2024, 15, 1396031. [Google Scholar] [CrossRef]
- Wu, R.Y.; Abdullah, M.; Maattanen, P.; Pilar, A.V.C.; Scruten, E.; Johnson-Henry, K.C.; Napper, S.; O’Brien, C.; Jones, N.L.; Sherman, P.M. Protein Kinase Cσ Signaling Is Required for Dietary Prebiotic-Induced Strengthening of Intestinal Epithelial Barrier Function. Sci. Rep. 2017, 7, 40820. [Google Scholar]
- Young, D.R.; Hivert, M.F.; Alhassan, S.; Camhi, S.M.; Ferguson, J.F.; Katzmarzyk, P.T.; Lewis, C.E.; Owen, N.; Perry, C.K.; Siddique, J.; et al. Sedentary behavior and cardiovascular morbidity and mortality: A science advisory from the American heart association. Circulation 2016, 134, e262–e279. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.F.; Allayee, H.; Gerszten, R.E. Nutrigenomics, the microbiome, and gene-environment interactions: New directions in cardiovascular disease research, prevention, and treatment: A scientific statement from the American heart association. Circ. Cardiovasc. Genet. 2016, 9, 291–313. [Google Scholar] [CrossRef]
- Organ, C.L.; Otsuka, H.; Bhushan, S.; Wang, Z.; Bradley, J.; Trivedi, R.; Polhemus, D.J.; Wilson Tang, W.H.; Wu, Y.; Hazen, S.L.; et al. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2016, 9, e002314. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Zong, X.; Fan, Q.; Yang, Q.; Pan, R.; Zhuang, L.; Tao, R. Phenylacetylglutamine as a risk factor and prognostic indicator of heart failure. ESC Heart Fail. 2022, 9, 2645–2653. [Google Scholar] [CrossRef]
- Romano, K.A.; Nemet, I.; Saha, P.P.; Haghikia, A.; Li, X.S.; Mohan, M.L.; Lovano, B.; Castel, L.; Witkowski, M.; Buffa, J.A.; et al. Gut Microbiota-Generated Phenylacetylglutamine and Heart Failure. Circ. Heart Fail. 2023, 16, e009972. [Google Scholar] [CrossRef]
- Fang, C.; Zuo, K.; Jiao, K.; Zhu, X.; Fu, Y.; Zhong, J.; Xu, L.; Yang, X. PAGln, an Atrial Fibrillation-Linked Gut Microbial Metabolite, Acts as a Promoter of Atrial Myocyte Injury. Biomolecules 2022, 12, 1120. [Google Scholar] [CrossRef]
- Sharma, A.; Buschmann, M.M.; Gilbert, J.A. Pharmacomicrobiomics: The holy grail to variability in drug response? Clin. Pharmacol. Ther. 2019, 106, 317–328. [Google Scholar] [CrossRef]
- Weersma, K.R.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Tuteja, S.; Ferguson, J.F. The Gut Microbiome and Response to Cardiovascular Drugs. Circ. Genom. Precis. Med. 2019, 12, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.N.; Velenosi, T.; Flaten, H.K.; McWilliams, G.; McDaniel, K.; Shelton, S.K.; Saben, J.; Krausz, K.W.; Gonzalez, F.J.; Monte, A.A. Metabolomic profiling of metoprolol hypertension treatment reveals altered gut microbiota-derived urinary metabolites. Hum. Genom. 2020, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.Y.A.; Rodgers, T.; Aarons, L.; Gueorguieva, I.; Dickinson, G.L.; Murby, S.; Brown, C.; Collins, B.; Rowland, M. Whole body physiologically based modelling of β-blockers in the rat: Events in tissues and plasma following an i.v. bolus dose. Br. J. Pharmacol. 2018, 175, 67–83. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Guo, T.; Gong, S.; Zjang, C. Effect of Dapagliflozin on Intestinal Flora in MafA-deficient Mice. Curr. Pharm. Des. 2018, 24, 3223–3231. [Google Scholar] [CrossRef]
- Van Bommel, E.J.M.; Herrema, H.; Davids, M.; Kramer, M.H.H.; Nieuwdorp, M.; van Raalte, D.H. Effects of 12-week treatment with dapagliflozin and gliclazide on faecal microbiome: Results of a double-blind randomized trial in patients with type 2 diabetes. Diabetes Metab. 2020, 46, 164–168. [Google Scholar] [CrossRef]
- Cheema, M.U.; Pluznick, J.L. Gut microbiota plays a central role to modulate the plasma and fecal metabolomes in response to angiotensin II. Hypertension 2019, 74, 184–193. [Google Scholar] [CrossRef]
- Mahmoudian, F.; Gheshlagh, S.R.; Hemati, M.; Farhadi, S.; Eslami, M. The influence of microbiota on the efficacy and toxicity of immunotherapy in cancer treatment. Mol. Biol. Rep. 2025, 52, 86. [Google Scholar] [CrossRef]
- Gong, L.; Yang, S.; Huang, J.; Li, Y. Modulation of gut microbiota in targeted cancer therapy: Insights on the EGFR/VEGF/KRAS pathways. Cancer Biol. Med. 2024, 21, 1141–1155. [Google Scholar] [CrossRef]
- Ke, X.; Shen, L. Molecular targeted therapy of cancer: The progress and future prospect. Front. Lab. Med. 2017, 1, 69–75. [Google Scholar] [CrossRef]
- Wei, J.; Zheng, Z.; Hou, X.; Jia, F.; Yuan, Y.; Yuan, F.; He, F.; Hu, L.; Zhao, L. Echinacoside inhibits colorectal cancer metastasis via modulating the gut microbiota and suppressing the PI3K/AKT signaling pathway. J. Ethnopharmacol. 2024, 318, 116866. [Google Scholar] [CrossRef]
- Ianiro, G.; Rossi, E.; Thomas, A.M.; Schinzari, G.; Masucci, L.; Quaranta, G.; Settanni, C.R.; Lopetuso, L.R.; Armanini, F.; Blanco-Miguez, A.; et al. Faecal microbiota transplantation for the treatment of diarrhoea induced by tyrosine-kinase inhibitors in patients with metastatic renal cell carcinoma. Nat. Commun. 2020, 11, 4333. [Google Scholar] [CrossRef] [PubMed]
- Palko-Łabuz, A.; Maksymowicz, J.; Sobieszczańska, B.; Wikiera, A.; Skonieczna, M.; Wesołowska, O.; Środa-Pomianek, K. Newly obtained apple pectin as an adjunct to irinotecan therapy of colorectal cancer reducing E. coli adherence and β-glucuronidase activity. Cancers 2021, 13, 2952. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Wong, C.C.; Yu, J. Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef]
- Deng, H.; Fan, X. The role of intestinal microbiota in tumor occurrence, development and immunotherapy: A review. Chin. J. Biotechnol. 2022, 38, 2105–2119. [Google Scholar]
- Hahn, A.W.; Froerer, C.; VanAlstine, S.; Rathi, N.; Bailey, E.B.; Stenehjem, D.D.; Agarwalet, N. Targeting bacteroides in stool microbiome and response to treatment with first-line VEGF tyrosine kinase inhibitors in metastatic renal-cell carcinoma. Clin. Genitourin. Cancer 2018, 16, 365–368. [Google Scholar] [CrossRef]
- Tinsley, N.; Zhou, C.; Nahm, S.; Rack, S.; Tan, G.C.; Lorigan, P.; Blackhall, F.; Cook, N. Antibiotic use reduces efficacy of tyrosine kinase inhibitors in patients with advanced melanoma and non-small-cell lung cancer. ESMO Open. 2022, 7, 100430. [Google Scholar] [CrossRef]
- Samanta, S. Potential impacts of prebiotics and probiotics on cancer prevention. Anti-Cancer Agents Med. Chem. 2022, 22, 605–628. [Google Scholar] [CrossRef]
- Eslami, M.; Yousefi, B.; Kokhaei, P.; Hemati, M.; Nejad, Z.R.; Arabkari, V.; Namdar, A. Importance of probiotics in the prevention and treatment of colorectal cancer. J. Cell. Physiol. 2019, 234, 17127–17143. [Google Scholar] [CrossRef]
- Ting, N.L.N.; Lau, H.C.H.; Yu, J. Cancer pharmacomicrobiomics: Targeting microbiota to optimise cancer therapy outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Epelde, F. The role of the gut microbiota in heart failure: Pathophysiological insights and future perspectives. Medicina 2025, 61, 720. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Lau, H.C.; Yu, J. Modulating gut microbiome in cancer immunotherapy: Harnessing microbes to enhance treatment efficacy. Cell Rep. Med. 2024, 5, 101478. [Google Scholar] [CrossRef]
- Mu, F.; Tang, M.; Guan, Y.; Lin, R.; Zhao, M.; Zhao, J.; Huang, S.; Zhang, H.; Wang, J.; Tang, H. Knowledge Mapping of the Links Between the Gut Microbiota and Heart Failure: A Scientometric Investigation (2006–2021). Front. Cardiovasc. Med. 2022, 9, 882660. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Parissis, J.; Butler, J.; Farmakis, D. Pathogenesis of chronic heart failure: Cardiovascular aging, risk factors, comorbidities, and disease modifiers. Heart Fail. Rev. 2022, 27, 337–344. [Google Scholar] [CrossRef]
- Dias, C.K.; Starke, R.; Pylro, V.S.; Morais, D.K. Database limitations for studying the human gut microbiome. PeerJ Comput. Sci. 2020, 6, e289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).