A Word of Caution—Potential Limitations of Pulmonary Artery Pressure Monitoring in Detecting Congestion Caused by Right-Sided Heart Failure
Abstract
1. Introduction
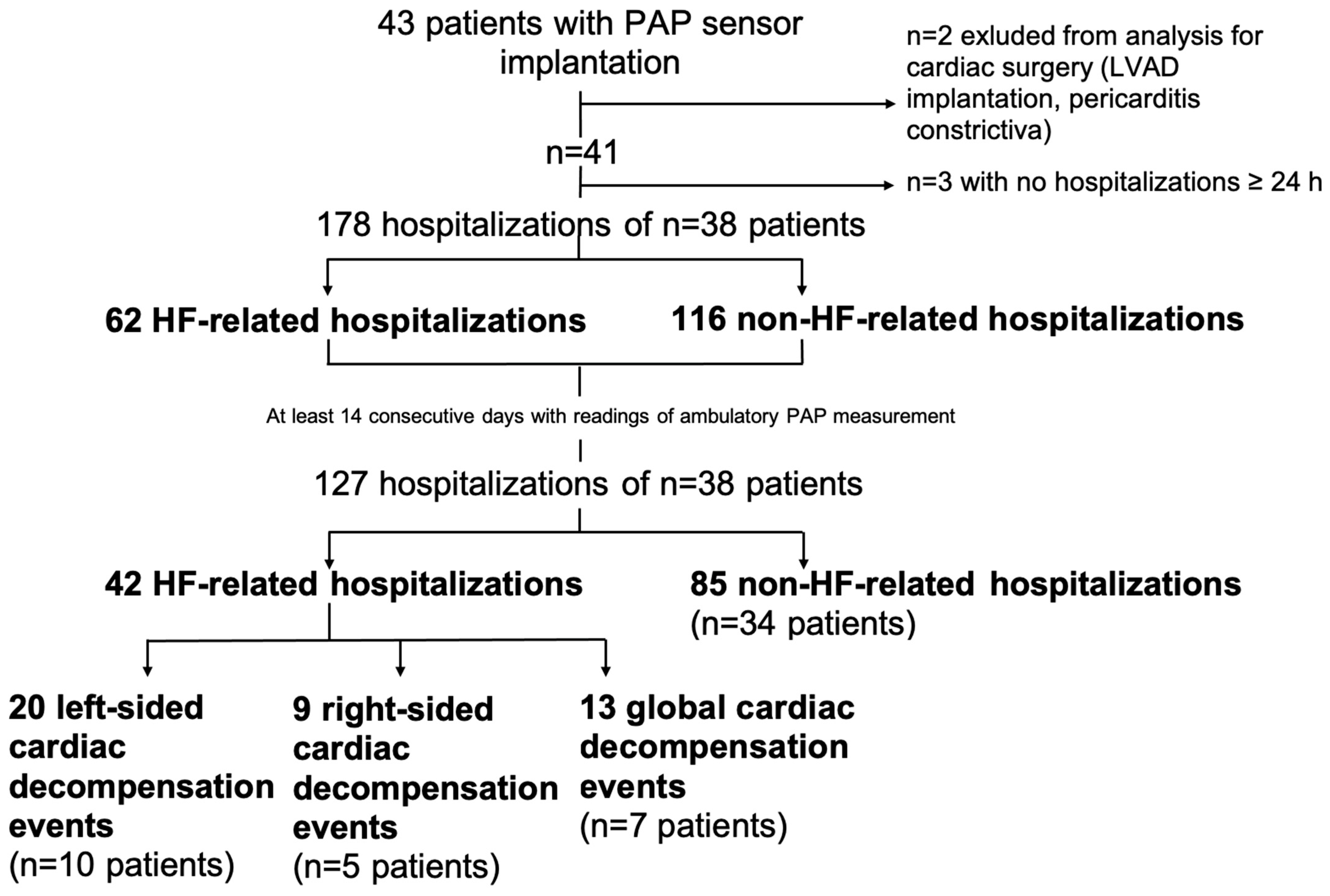
2. Materials and Methods
Statistical Analysis
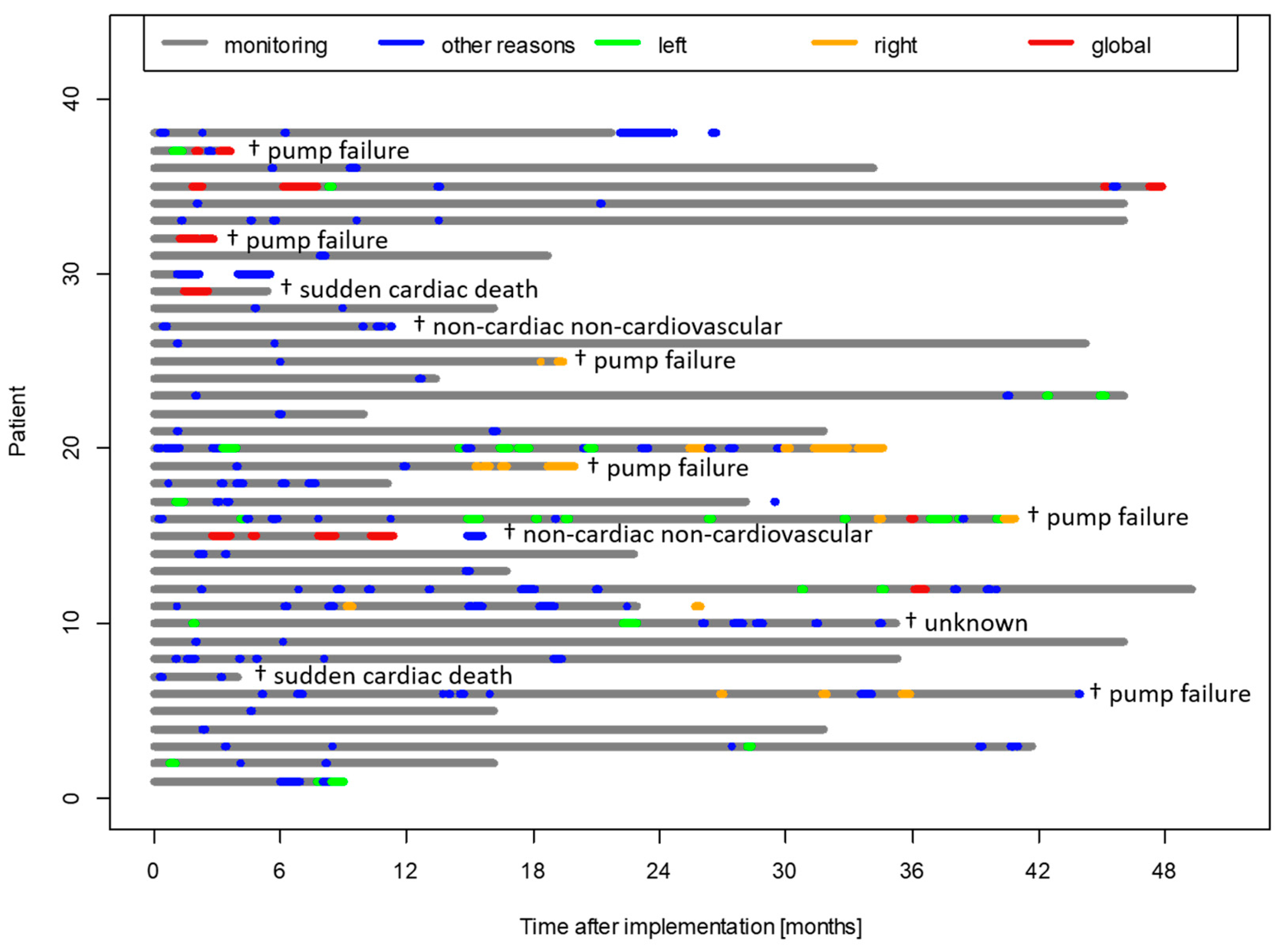
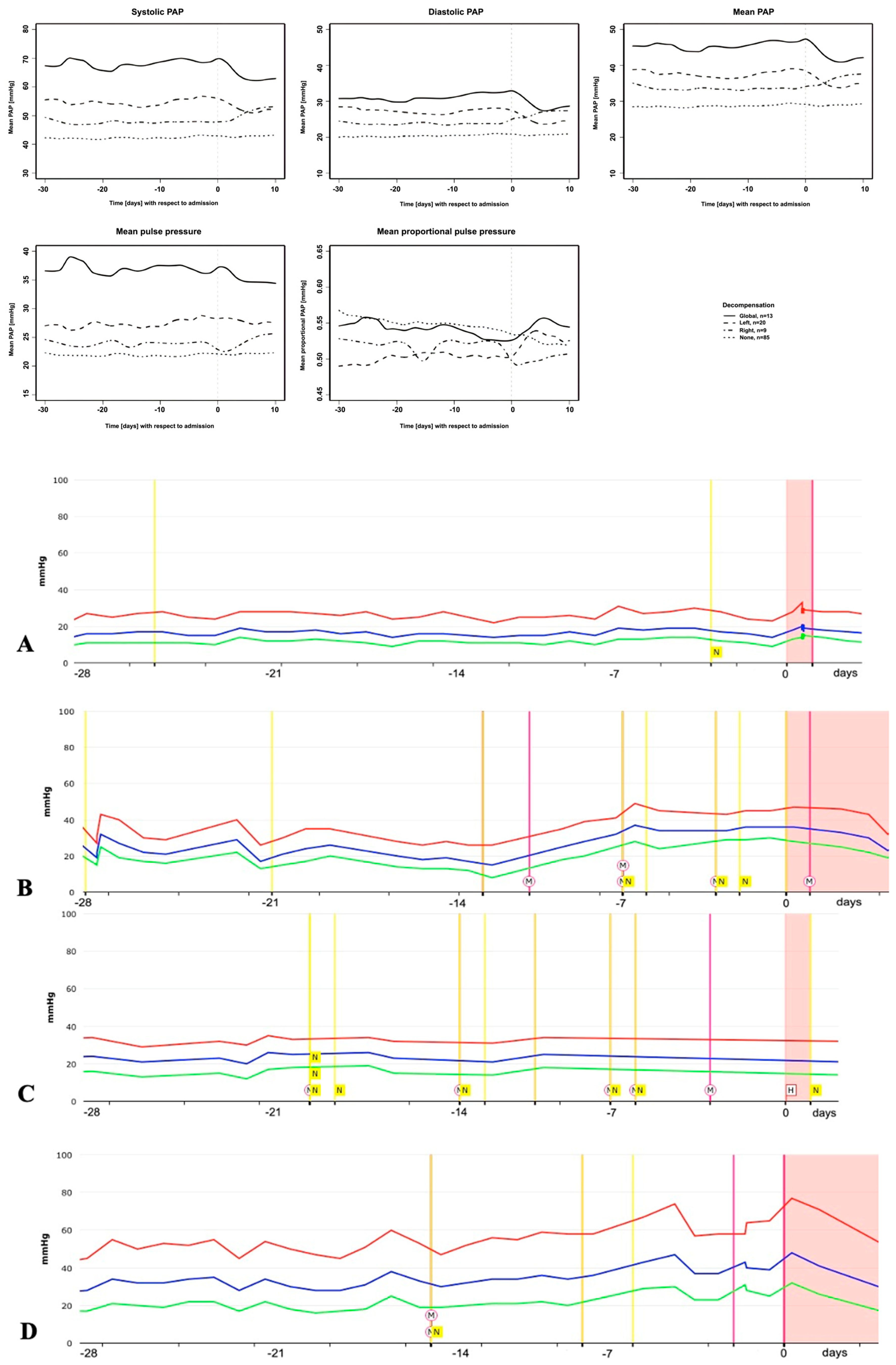
3. Results
3.1. Baseline Characteristics
3.2. Association of Follow-Up Characteristics with Subsequent Decompensation Events
3.3. PAP Course Prior to Different Types of Cardiac Decompensation
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEI-I | angiotensin-converting enzyme inhibitor |
| ARNI | angiotensin receptor blocker neprilysin inhibitor |
| ATRB | angiotensin receptor blocker |
| CHF | chronic heart failure |
| cpcPH | combined post- and precapillary pulmonary hypertension |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| LVEF | left ventricular ejection fraction |
| MRA | mineralocorticoid antagonist |
| NYHA | New York Heart Association |
| NT-proBNP | N-terminal fragment of pro-brain natriuretic protein |
| PASP | systolic pulmonary artery pressure |
| PAP | pulmonary artery pressure |
| PCWP | pulmonary capillary wedge pressure |
| TAPSE | tricuspid annular plane systolic excursion |
| RV | right ventricular |
| PVR | pulmonary vascular resistance |
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Anker, S.D.; AlHabib, K.F.; Cowie, M.R.; Force, T.L.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014, 1, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.S.; Brutsaert, D.; Dickstein, K.; Drexler, H.; Follath, F.; Harjola, V.-P.; Hochadel, M.; Komajda, M.; Lassus, J.; Lopez-Sendon, J.L.; et al. EuroHeart Failure Survey II (EHFS II): A survey on hospitalized acute heart failure patients: Description of population. Eur. Heart J. 2006, 27, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.J.; Gelijns, A.C.; Stevenson, L.W.; Sampat, B.; Aaronson, K.D.; Renlund, D.G.; Ascheim, D.D.; Hong, K.N.; Oz, M.C.; Moskowitz, A.J.; et al. The Cost of Medical Management in Advanced Heart Failure During the Final Two Years of Life. J. Card. Fail. 2008, 14, 651–658. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Vaduganathan, M.; Fonarow, G.C.; Bonow, R.O. Rehospitalization for heart failure: Problems and perspectives. J. Am. Coll. Cardiol. 2013, 61, 391–403. [Google Scholar] [CrossRef]
- Jencks, S.F.; Williams, M.V.; Coleman, E.A. Rehospitalizations among patients in the medicare fee-for-service program. N. Engl. J. Med. 2009, 360, 1418–1428. [Google Scholar] [CrossRef]
- Anker, S.D.; Koehler, F.; Abraham, W.T. Telemedicine and remote management of patients with heart failure. Lancet 2011, 378, 731–739. [Google Scholar] [CrossRef]
- Morgan, J.M.; Kitt, S.; Gill, J.; McComb, J.M.; Ng, G.A.; Raftery, J.; Roderick, P.; Seed, A.; Williams, S.G.; Witte, K.K.; et al. Remote management of heart failure using implantable electronic devices. Eur. Heart J. 2017, 38, 2352–2360. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Abraham, W.T.; Stevenson, L.W.; Bourge, R.C.; Lindenfeld, J.A.; Bauman, J.G.; Adamson, P.B.; CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet 2016, 387, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Angermann, C.E.; Alkhlout, B.; Asselbergs, F.W.; Schnupp, S.; Brugts, J.J.; Nordbeck, P.; Zhou, Q.; Brett, M.; Ginn, G.; et al. Effects of remote haemodynamic-guided heart failure management in patients with different subtypes of pulmonary hypertension: Insights from the MEMS-HF study. Eur. J. Heart Fail. 2022, 24, 2320–2330. [Google Scholar] [CrossRef] [PubMed]
- Brugts, J.J.; Radhoe, S.P.; Clephas, P.R.D.; Aydin, D.; van Gent, M.W.F.; Szymanski, M.K.; Rienstra, M.; Heuvel, M.H.v.D.; da Fonseca, C.A.; Linssen, G.C.M.; et al. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF): A randomised clinical trial. Lancet 2023, 401, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef]
- Mazimba, S.; Ginn, G.; Mwansa, H.; Laja, O.; Jeukeng, C.; Elumogo, C.; Patterson, B.; Kennedy, J.L.; Mehta, N.; Hossack, J.A.; et al. Pulmonary Artery Proportional Pulse Pressure (PAPP) Index Identifies Patients With Improved Survival From the CardioMEMS Implantable Pulmonary Artery Pressure Monitor. Heart Lung Circ. 2021, 30, 1389–1396. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Vachiéry, J.-L.; Adir, Y.; Barberà, J.A.; Champion, H.; Coghlan, J.G.; Cottin, V.; De Marco, T.; Galiè, N.; Ghio, S.; Gibbs, J.S.R.; et al. Pulmonary hypertension due to left heart diseases. J. Am. Coll. Cardiol. 2013, 62 (Suppl. S25), D100–D108. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Hoeper, M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endo. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Gibbs, J.S.R.; Wachter, R.; De Marco, T.; Vonk-Noordegraaf, A.; Vachiéry, J.-L. Left ventricular heart failure and pulmonary hypertension. Eur. Heart J. 2015, 37, 942–954. [Google Scholar] [CrossRef]
- Guazzi, M.; Borlaug, B.A. Pulmonary hypertension due to left heart disease. Circulation 2012, 126, 975–990. [Google Scholar] [CrossRef]
- Ghio, S.; Schirinzi, S.; Pica, S. Pulmonary arterial compliance: How and why should we measure it? Glob. Cardiol. Sci. Pract. 2015, 2015, 58. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Yasumura, Y.; Yoshida, C.; Okumura, T.; Tateishi, J.; Yoshida, J.; Abe, H.; Tamaki, S.; Yano, M.; Hayashi, T.; et al. Prognostic Importance of Pulmonary Arterial Capacitance in Acute Decompensated Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2021, 10, e023043. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Doyle, M.; Lasorda, D.; Parikh, K.S.; Correa-Jaque, P.; Badie, N.; Ginn, G.; Airhart, S.; Franco, V.; Kanwar, M.K.; et al. Monitoring Pulmonary Arterial Hypertension Using an Implantable Hemodynamic Sensor. Chest 2019, 156, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Rechtsventrikuläre Dysfunktion und beeinträchtigtes RV–PA Coupling Bei Paradoxer Low-Flow, Low-Gradient Aortenklappenstenose. Available online: https://herzmedizin.de/fuer-aerzte-und-fachpersonal/kongresse/dgk-jahrestagung-2024/programm/strukturelle_herzerkrankungen/v311.html (accessed on 25 May 2025).
- Boehm, M.; Lawrie, A.; Wilhelm, J.; Ghofrani, H.A.; Grimminger, F.; Weissmann, N.; Seeger, W.; Schermuly, R.T.; Kojonazarov, B. Maintained right ventricular pressure overload induces ventricular-arterial decoupling in mice. Exp. Physiol. 2017, 102, 180–189. [Google Scholar] [CrossRef]
- Gorcsan, J.; Murali, S.; Counihan, P.J.; Mandarino, W.A.; Kormos, R.L. Right Ventricular Performance and Contractile Reserve in Patients With Severe Heart Failure. Circulation 1996, 94, 3190–3197. [Google Scholar] [CrossRef]
- Grünig, E.; Tiede, H.; Enyimayew, E.O.; Ehlken, N.; Seyfarth, H.-J.; Bossone, E.; D’aNdrea, A.; Naeije, R.; Olschewski, H.; Ulrich, S.; et al. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation 2013, 128, 2005–2015. [Google Scholar] [CrossRef]
- Ireland, C.G.; Damico, R.L.; Kolb, T.M.; Mathai, S.C.; Mukherjee, M.; Zimmerman, S.L.; Shah, A.A.; Wigley, F.M.; Houston, B.A.; Hassoun, P.M.; et al. Exercise right ventricular ejection fraction predicts right ventricular contractile reserve. J. Heart Lung Transplant. 2021, 40, 504–512. [Google Scholar] [CrossRef]
- Tedford, R.J.; Mudd, J.O.; Girgis, R.E.; Mathai, S.C.; Zaiman, A.L.; Housten-Harris, T.; Boyce, D.; Kelemen, B.W.; Bacher, A.C.; Shah, A.A.; et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ. Heart Fail. 2013, 6, 953–963. [Google Scholar] [CrossRef]
- Hsu, S.; Kokkonen-Simon, K.M.; Kirk, J.A.; Kolb, T.M.; Damico, R.L.; Mathai, S.C.; Mukherjee, M.; Shah, A.A.; Wigley, F.M.; Margulies, K.B.; et al. Right Ventricular Myofilament Functional Differences in Humans With Systemic Sclerosis–Associated Versus Idiopathic Pulmonary Arterial Hypertension. Circulation 2018, 137, 2360–2370. [Google Scholar] [CrossRef]
- Tello, K.; Dalmer, A.; Axmann, J.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Seeger, W.; Sommer, N.; Wilhelm, J.; et al. Reserve of Right Ventricular-Arterial Coupling in the Setting of Chronic Overload. Circ. Heart Fail. 2019, 12, e005512. [Google Scholar] [CrossRef]
- Trejo-Velasco, B.; Estevez-Loureiro, R.; Carrasco-Chinchilla, F.; Fernández-Vázquez, F.; Arzamendi, D.; Pan, M.; Pascual, I.; Nombela-Franco, L.; Amat-Santos, I.J.; Freixa, X.; et al. Prognostic Role of TAPSE to PASP Ratio in Patients Undergoing MitraClip Procedure. J. Clin. Med. 2021, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, F.; Butcher, S.C.; Dietz, M.F.; van der Bijl, P.; Prihadi, E.A.; De Ferrari, G.M.; Marsan, N.A.; Bax, J.J.; Delgado, V. Right Ventricular–Pulmonary Arterial Coupling in Secondary Tricuspid Regurgitation. Am. J. Cardiol. 2021, 148, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Jobs, A.; Vonthein, R.; König, I.R.; Schäfer, J.; Nauck, M.; Haag, S.; Fichera, C.F.; Stiermaier, T.; Ledwoch, J.; Schneider, A.; et al. Inferior vena cava ultrasound in acute decompensated heart failure: Design rationale of the CAVA-ADHF-DZHK10 trial. ESC Heart Fail. 2020, 7, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Sorimachi, H.; Grill, D.E.; Fischer, K.; Borlaug, B.A. Contributions of cardiac dysfunction and volume status to central haemodynamics in chronic heart failure. Eur. J. Heart Fail. 2021, 23, 1097–1105. [Google Scholar] [CrossRef]
- Haag, S.; Jobs, A.; Stiermaier, T.; Fichera, C.-F.; Paitazoglou, C.; Eitel, I.; Desch, S.; Thiele, H. Lack of correlation between different congestion markers in acute decompensated heart failure. Clin. Res. Cardiol. 2022, 112, 75–86. [Google Scholar] [CrossRef]
| Variables | Hospitalizations Without Cardiac Decompensation (n = 85;%) | Hospitalizations with Cardiac Decompensation (n = 42;%) | p-Value | Left-Sided Decompensation (n = 20;%) | Right-Sided Decompensation (n = 9;%) | Global Cardiac Decompensation (n = 13;%) | p-Values 1,2,3 p-Values a,b,c |
|---|---|---|---|---|---|---|---|
| Clinical characteristics | |||||||
| Age, years | 74 ± 1 | 79 ± 1 | 0.098 | 76 ± 2 | 80 ± 1 | 81 ± 1 | 0.614/0.283/0.738 0.381/0.283/0.940 |
| Etiology of CHF | |||||||
| HFpEF | 2; 8% | 2; 6% | 0.546 | 1; 9% | 0; 0% | 1; 20% | 0.456/0.989/0.589 |
| HFrEF | 83; 92% | 40; 94% | 19; 91% | 9; 100% | 12; 80% | 0.992/0.739/0.984 | |
| NYHA class | |||||||
| IIIA | 83; 94% | 36; 88% | 0.079 | 19; 87% | 8; 80% | 9; 75% | 0.509/0.301/0.670 |
| IIIB | 2; 6% | 6; 12% | 1; 13% | 1; 20% | 4; 25% | 0.595/0.174/0.059 | |
| Body Mass Index (kg/m2) | 26 ± 0.3 | 27 ± 0.4 | 27 ± 0.7 | 26 ± 0.8 | 27 ± 0.7 | ||
| Systolic blood pressure, mmHg | 114 ± 2 | 112 ± 2 | 0.149 | 111 ± 4 | 110 ± 4 | 109 ± 3 | 0.622/<0.001/0.729 0.339/0.252/0.903 |
| Laboratory parameters | |||||||
| Creatinine, mg/dl | 1.44 ± 0.04 | 1.79 ± 0.10 | 0.021 | 1.75 ± 0.12 | 1.40 ± 0.09 | 2.27 ± 0.21 | 0.204/0.698/0.086 0.337/0.030/0.219 |
| Log-NT-proBNP, pg/mL | 3.2 ± 0.07 | 3.5 ± 0.08 | 0.079 | 3.2 ± 0.07 | 3.6 ± 0.13 | 3.5 ± 0.19 | 0.403/0.046/0.551 0.006/0.007/<0.001 |
| Medication at baseline | |||||||
| ACEI/ATRB | 70; 78% | 41; 92% | 0.085 | 20; 100% | 9; 100% | 12; 86% | 0.995/0.997/0.966 1.000/0.983/0.987 |
| ARNI | 15; 20% | 1; 2% | 0.085 | 0; 0% | 0; 0% | 1; 14% | 0.987/0.98/0.965 1.000/0.997/0.999 |
| ACEI/ARB/ARNI | 85; 100% | 42; 100% | 1.000 | 20; 100% | 9; 100% | 13; 100% | 1.000/1.000/1.000 1.000/1.000/1.000 |
| Beta-blockers | 83; 97% | 40; 91% | 0.470 | 18; 92% | 9; 100% | 13; 100% | 0.200/0.998/0.976 0.982/1.000/1.000 |
| MRA | 57; 81% | 30; 69% | 0.468 | 15; 70% | 9; 100% | 6; 40% | 0.922/0.969/0.511 0.991/0.218/0.911 |
| Loop diuretics | 77; 92% | 42; 100% | 0.996 | 20; 100% | 9; 100% | 13; 100% | 0.972/0.997/0.989 1.000/1.000/1.00 |
| Torasemide, dose equivalent in mg | 22 ± 2 | 26 ± 2 | 0.447 | 26 ± 3 | 23 ± 4 | 30 ± 4 | 0.494/0.901/0.680 0.661/0.734/0.550 |
| Furosemide, dose equivalent in mg | 88 ± 8 | 104 ± 8 | 104 ± 12 | 92 ± 16 | 120 ± 16 | ||
| Echocardiography | |||||||
| LVEF, % | 26 ± 1 | 25 ± 2 | 0.598 | 28 ± 3 | 24 ± 3 | 29 ± 3 | 0.688/0.380/0.781 0.324/0.731/0.079 |
| TAPSE, mm | 18 ± 1 | 17 ± 1 | 0.070 | 16± 1 | 21 ± 2 | 13 ± 1 | 0.080/0.154/<0.001 0.161/0.014/0.157 |
| RV fractional area change, % | 39 ± 1.1 | 38 ± 2.4 | 0.131 | 41 ± 3.2 | 48 ± 6.9 | 36.2 ± 9.9 | 0.001/0.277/0.712 0.894/0.201/0.476 |
| TAPSE/PASP ratio, mm/mmHg (Echo) | 0.52 ± 0.04 | 0.42 ± 0.03 | 0.190 | 0.42 ± 0.05 | 0.37 ± 0.08 | 0.28 ± 0.09 | 0.334/0.578/0.099 0.140/0.807/0.454 |
| TAPSE/PASP ratio, mm/mmHg (CardioMEMSTM) | 0.50 ± 0.03 | 0.35 ± 0.03 | 0.007 | 0.36 ± 0.05 | 0.40 ± 0.05 | 0.26 ± 0.04 | 0.060/0.351/0.045 0.626/0.211/0.038 |
| Association TAPSE/PASP ratio (Echo vs. CardioMEMSTM) | p = 0.0012 | p = 0.0014 | - | p = 0.423 | p < 0.001 | p = 0.228 | - |
| Invasive hemodynamics | |||||||
| Cardiac index, l/min/m2 | 2.0 ± 0.1 | 2.0 ± 0.2 | 0.687 | 2.6 ± 0.3 | 2.2 ± 0.5 | 1.8 ± 0.1 | 0.206/0.636/0.768 0.760/0.197/0.062 |
| PAP systolic, mmHg | 44 ± 2 | 53 ± 2 | 0.002 | 53 ± 4 | 54 ± 4 | 54 ± 5 | 0.030/0.169/0.564 0.940/0.422/0.754 |
| PAP mean, mmHg | 27 ±1 | 34 ± 2 | 0.013 | 33 ± 3 | 34 ± 3 | 34 ± 3 | 0.099/0.262/0.667 0.879/0.495/0.804 |
| PAP diastolic, mmHg | 17 ± 1 | 22 ± 1 | 0.031 | 20 ± 2 | 23 ± 2 | 22 ± 2 | 0.261/0.194/0.608 0.561/0.418/0.901 |
| PCWP mean, mmHg | 17 ±1 | 18 ± 1 | 0.524 | 19 ± 2 | 16 ± 3 | 18 ± 2 | 0.870/0.396/0.958 0.396/0.897/0.483 |
| PVR, Wood unit | 3.5 ± 0.3 | 2.7 ± 0.2 | 0.017 | 3.1 ± 0.4 | 3.0 ± 0.7 | 2.4 ± 0.2 | 0.238/0.386/0.625 0.796/0.170/0.074 |
| cpcPH, n, % | 43; 51% | 23; 55% | - | 14; 70% | 6; 67% | 3; 23% | - |
| Variables | Hospitalizations Without Cardiac Decompensation (n = 85;%) | Hospitalizations with Cardiac Decompensation (n = 42;%) | p-Value | Left-Sided Decompensation (n = 20;%) | Right-Sided Decompensation (n = 9;%) | Global Cardiac Decompensation (n = 13;%) | p-Values 1,2,3 p-Values a,b,c p-Values *,§,$ |
|---|---|---|---|---|---|---|---|
| Clinical characteristics | |||||||
| NYHA class | |||||||
| IIA | 18; 12% | 15; 19% | 0.0788 | 9; 18% | 4; 40% | 2; 30% | |
| IIB | 4; 3% | 0; 0% | 0; 0% | 0; 0% | 0; 0% | 0.186 1/0.236 2/0.208 3 | |
| IIIA | 61; 80% | 23; 67% | 10; 72% | 4; 50% | 9; 58% | 0.559 a/0.572 b/0.927 c | |
| IIIB | 2; 6% | 4; 13% | 1; 10% | 1; 10% | 2; 12% | ||
| Echocardiographic parameters | |||||||
| TAPSE, mm | 19.1 ± 0.6 | 17.0 ± 1.1 | 0.010 | 18.1 ± 1.3 | 17.7 ± 0.7 | 14.2 ± 1.5 | 0.272 1/0.357 2/<0.001 3 0.686 a/0.014 b/0.235 c |
| TAPSE/PASP ratio, mm/mmHg (Echo) | 0.53 ± 0.04 | 0.42 ± 0.05 | 0.030 | 0.47 ± 0.06 | 0.41 ±0.04 | 0.28 ± 0.04 | 0.333 1/0.137 2/0.057 3 0.336 a/0.030 b/0.360 c |
| TAPSE/PASP ratio, mm/mmHg (CardioMEMSTM) | 0.53 ± 0.04 | 0.37 ± 0.06 | 0.014 | 0.39 ± 0.08 | 0.37 ± 0.05 | 0.31 ± 0.04 | 0.159 1/0.114 2/0.028 3 0.642 a/0.098 b/0.352 c |
| Association TAPSE/PASP ratio (Echo vs. CardioMEMSTM) | p < 0.001 | p < 0.001 | - | p = 0.894 | p = 0.999 | p = 0.981 | - |
| RV fractional area change, % | 37 ± 1 | 34 ± 2 | 0.066 | 36 ± 2 | 35 ± 2 | 30 ± 2 | 0.491 1/0.502 2/0.219 3 0.620 a/0.034 b/0.463 c |
| Laboratory parameters | |||||||
| Creatinine, mg/dl | 1.61 ± 0.08; # p < 0.001 | 2.40 ± 0.45; & p = 0.025 | 0.021 | 2.51 ± 0.74 | 1.91 ± 0.21 | 3.07 ± 0.90 | 0.345 1/ 0.241 2/0.319 3 0.922 a/ 0.187 b/0.482 c 0.176 */0.142 §/0.231 $ |
| Rel. Creatinine, % | 17% ± 3% | 33% ± 16% | 0.310 | 33% ± 26% | 35% ± 13% | 40% ± 33% | 0.953 1/0.095 2/0.877 3 0.391 a/0.836 b/0.867 c |
| Log-NT-proBNP, pg/ml | 3.2 ± 0.08; # p = 0.172 | 3.6 ± 0.13; & p = 0.228 | 0.015 | 3.2 ± 0.13 | 3.6 ± 0.19 | 3.7 ± 0.25 | 0.870 1/0.067 2/0.310 3 0.024 a/0.012 b/0.861 c 0.305 */0.414 §/0.478 $ |
| Δ log-NT-proBNP, pg/ml | −0.06 ± 0.04 | 0.12 ± 0.10 | 0.098 | 0.16 ± 0.14 | 0.07 ± 0.08 | 0.16 ± 0.21 | 0.318 1/0.404 2/0.016 3 0.623 a/0.945 b/0.487 c |
| Urgent admission, n; % | 60; 78% 23; 22% | 22; 67% 16; 33% | 0.311 | 10; 70% 10; 30% | 5; 60% 4; 40% | 7; 67% 2; 33% | 0.291 1/0.498 2/0.931 3 0.860 a/0.283 b/0.692 c |
| Length of hospitalization, days | 4.5 ± 0.6 | 11.0 ± 1.8 | 0.003 | 8.0 ± 2.7 | 5.3 ± 1.6 | 14.8 ± 3.14 | 0.046 1/ 0.731 2/0.083 3 0.437 a/0.073 b/0.292 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrmann, E.J.; Herrmann, E.; Tello, K.; Mantzsch, K.; Tekeste, M.; Fichtlscherer, S.; Hamm, C.W.; Assmus, B. A Word of Caution—Potential Limitations of Pulmonary Artery Pressure Monitoring in Detecting Congestion Caused by Right-Sided Heart Failure. Biomedicines 2025, 13, 1469. https://doi.org/10.3390/biomedicines13061469
Herrmann EJ, Herrmann E, Tello K, Mantzsch K, Tekeste M, Fichtlscherer S, Hamm CW, Assmus B. A Word of Caution—Potential Limitations of Pulmonary Artery Pressure Monitoring in Detecting Congestion Caused by Right-Sided Heart Failure. Biomedicines. 2025; 13(6):1469. https://doi.org/10.3390/biomedicines13061469
Chicago/Turabian StyleHerrmann, Ester Judith, Eva Herrmann, Khodr Tello, Kathleen Mantzsch, Meaza Tekeste, Stephan Fichtlscherer, Christian W. Hamm, and Birgit Assmus. 2025. "A Word of Caution—Potential Limitations of Pulmonary Artery Pressure Monitoring in Detecting Congestion Caused by Right-Sided Heart Failure" Biomedicines 13, no. 6: 1469. https://doi.org/10.3390/biomedicines13061469
APA StyleHerrmann, E. J., Herrmann, E., Tello, K., Mantzsch, K., Tekeste, M., Fichtlscherer, S., Hamm, C. W., & Assmus, B. (2025). A Word of Caution—Potential Limitations of Pulmonary Artery Pressure Monitoring in Detecting Congestion Caused by Right-Sided Heart Failure. Biomedicines, 13(6), 1469. https://doi.org/10.3390/biomedicines13061469






