Point-of-Care Ultrasound Use in Hemodynamic Assessment
Abstract
1. Introduction
Literature Search Methodology
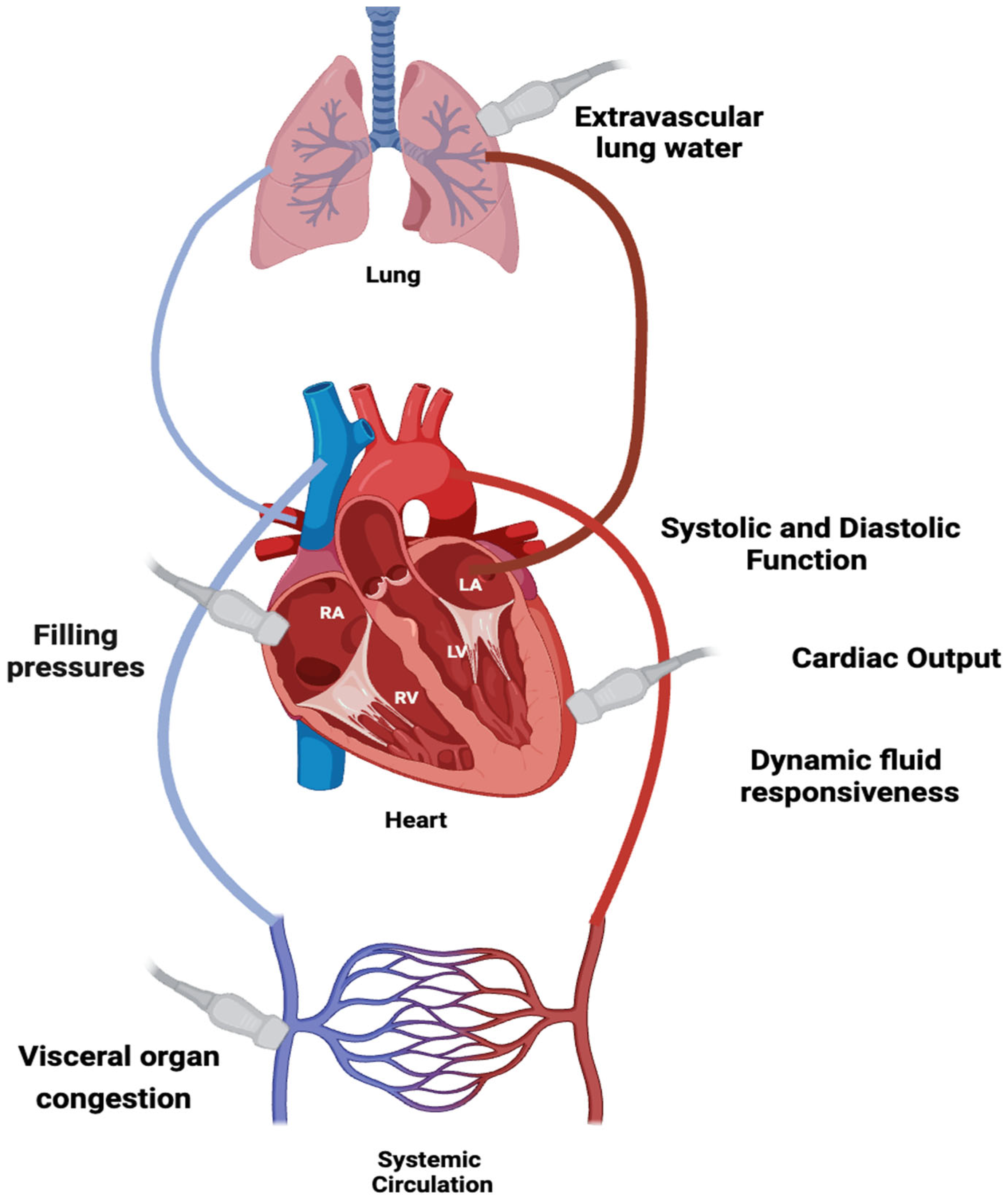
2. Critical Care Ultrasonography
3. Right Ventricular Assessment and Integration of Ultrasound
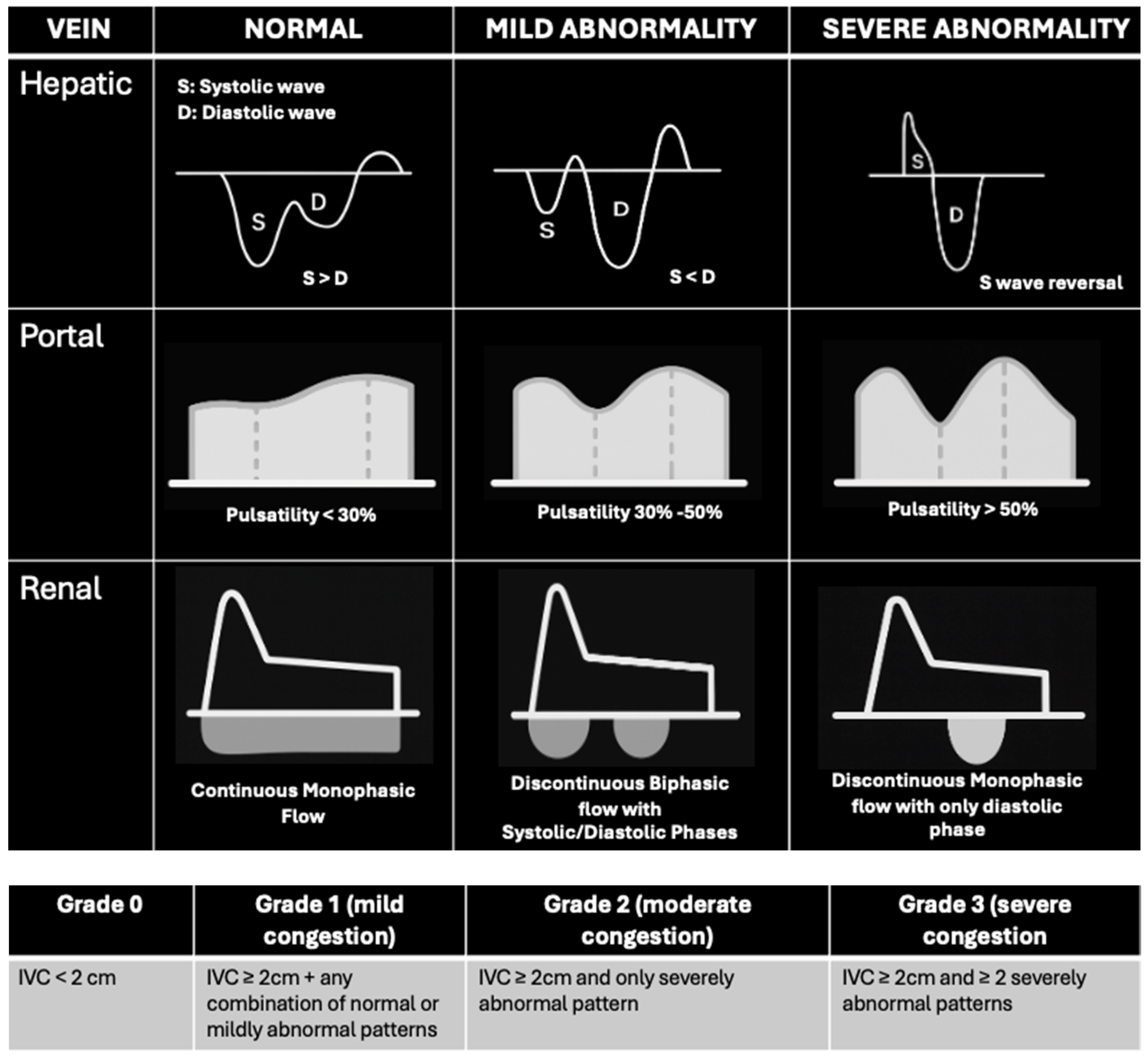
3.1. Right Ventricular Preload
3.2. Right Ventricular Contractility
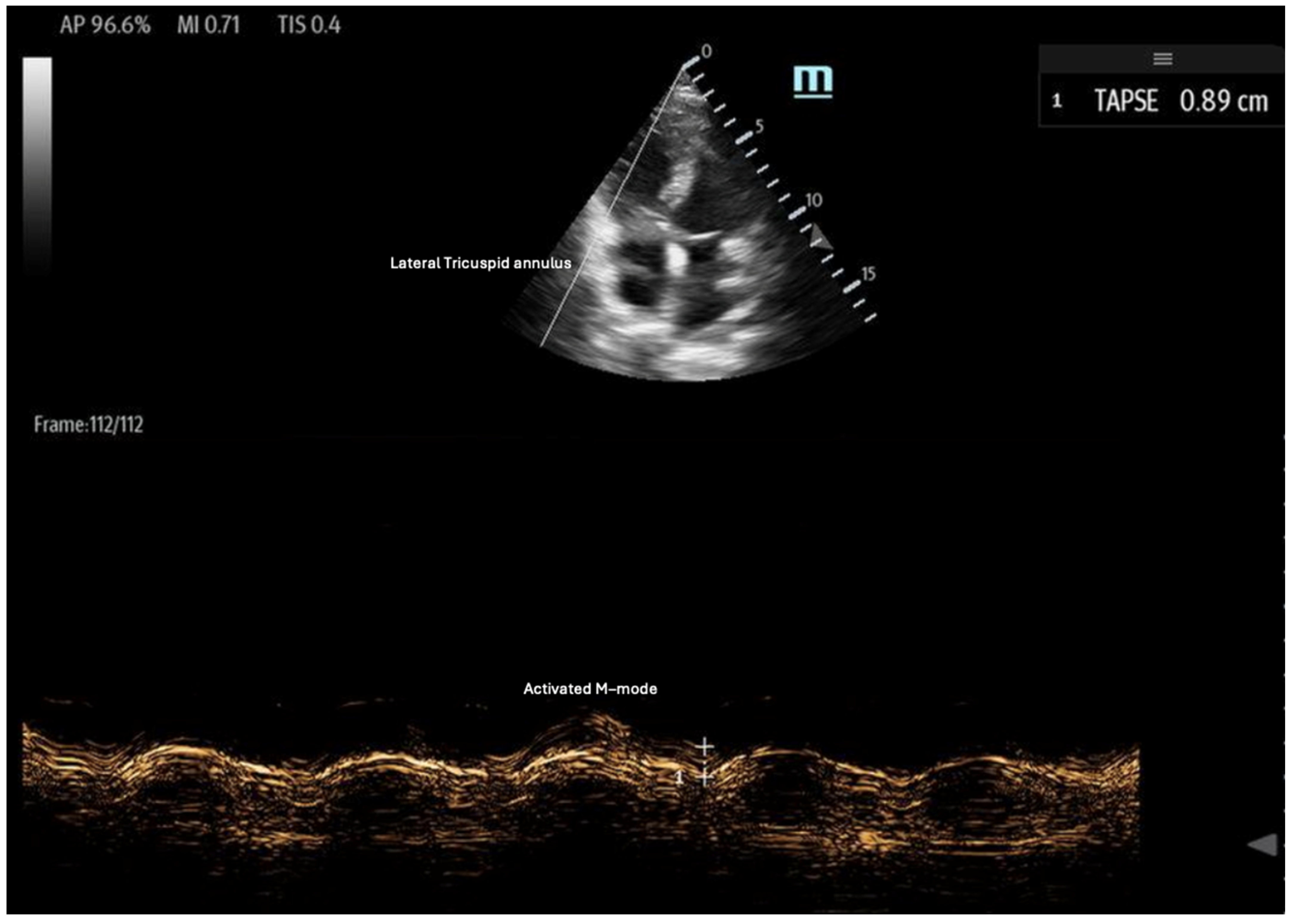
- Tricuspid annular plane systolic excursion (TAPSE): TAPSE is a simple and widely used measure of RV systolic function, assessed using the M-mode of the 2D ultrasound in the apical four-chamber view (Figure 6). It quantifies the displacement of the tricuspid lateral annulus towards the cardiac apex during systole, reflecting the RV’s longitudinal contraction [35]. Greater displacement indicates better RV contractility, while a TAPSE < 17 mm is indicative of RV systolic dysfunction, with high specificity and low sensitivity [35,36,37]. TAPSE is advantageous due to its ease of measurement; however, it is limited by its two-dimensional (2D) interpretation of a 3D structure. Additionally, its accuracy can be affected by ultrasound probe positioning and increased RV afterload [36].
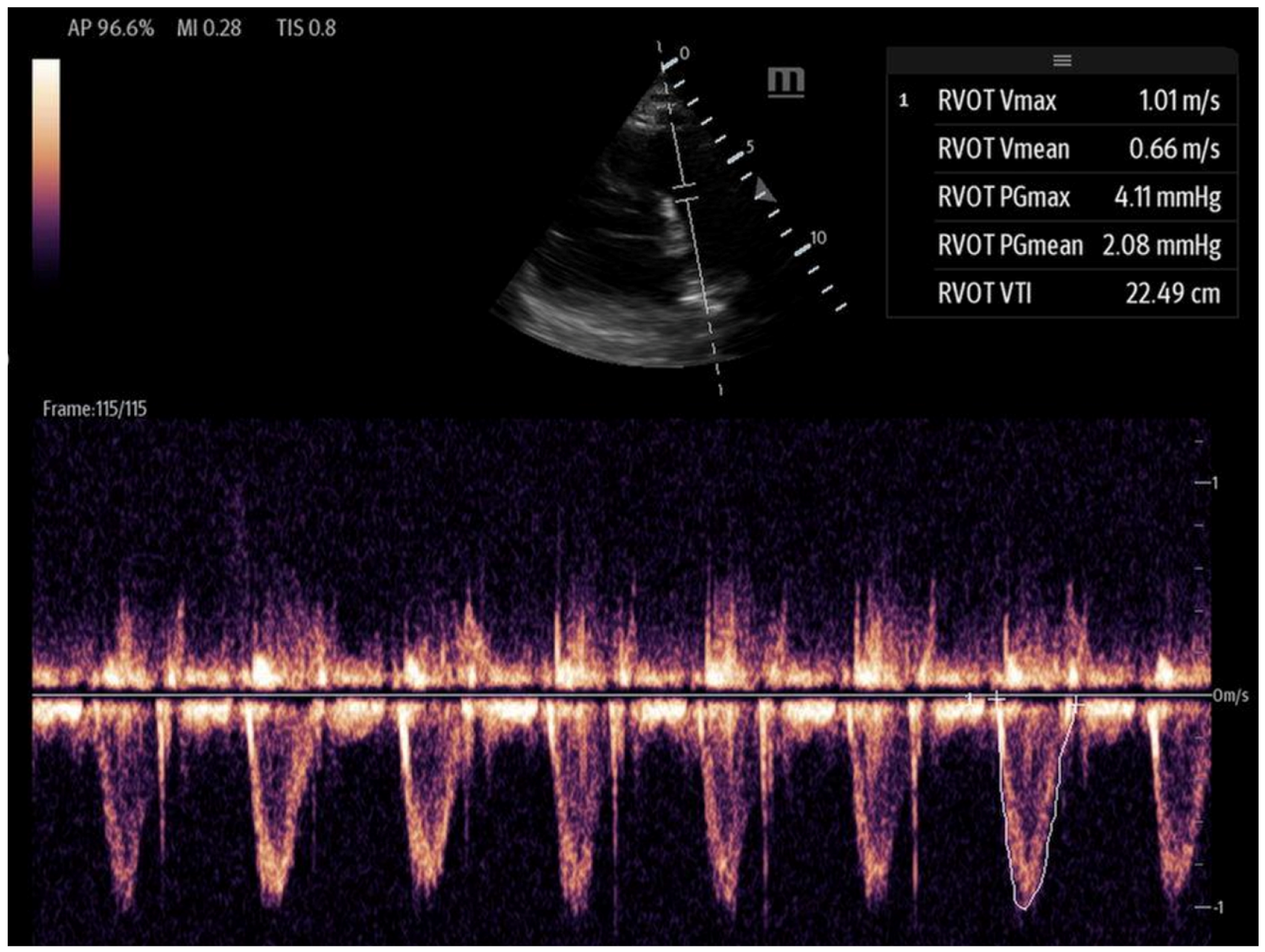
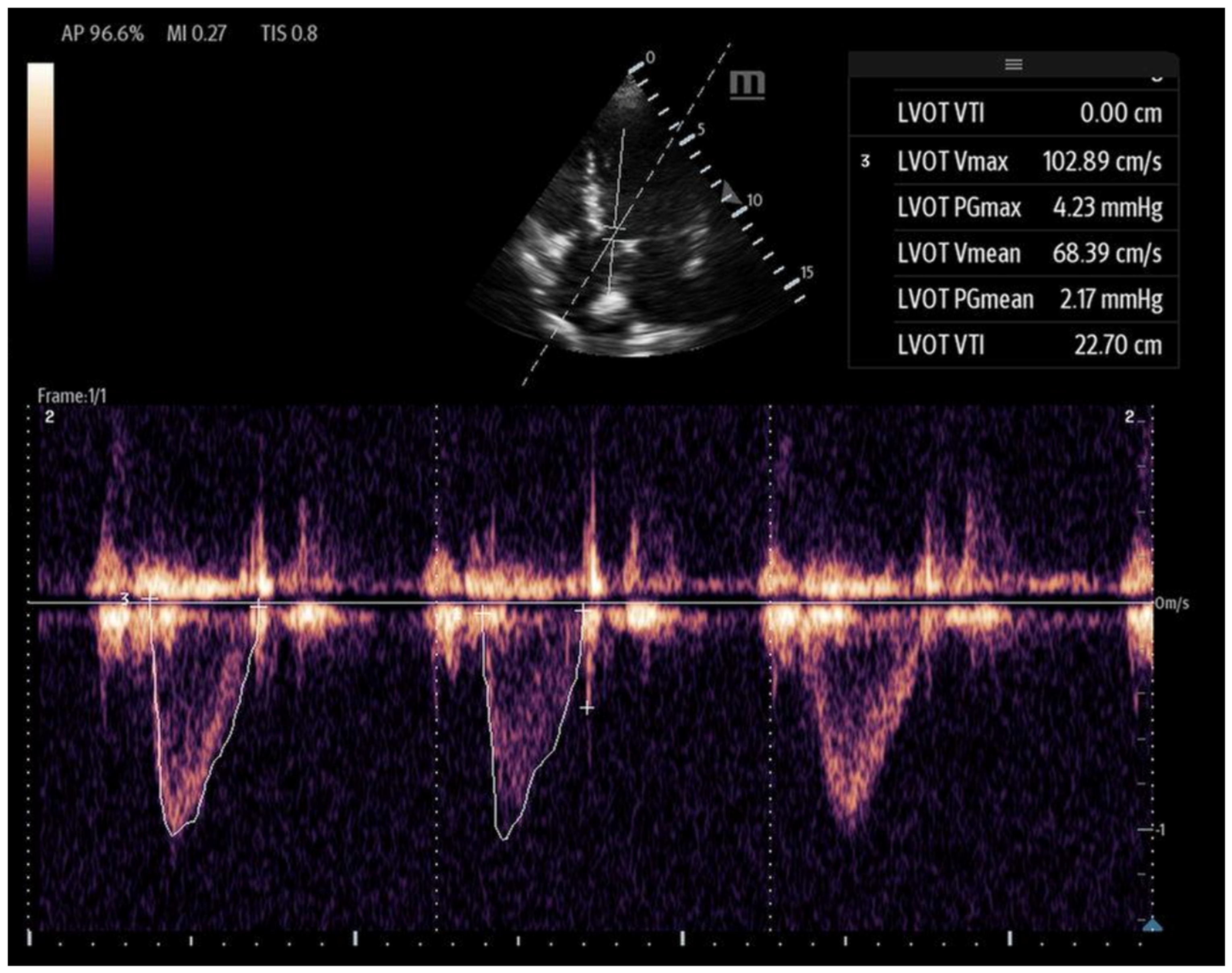
- Right ventricular outflow tract velocity–time integral (RVOT-VTI): RV contractility can be evaluated using the RVOT-VTI, which represents the distance traveled by blood across the RVOT during systole. Stroke volume is calculated by multiplying the RVOT cross-sectional area by the RVOT-VTI. This measurement provides insight into both RV function and pulmonary vascular resistance, with an RVOT-VTI ≥ 12 cm considered normal [38] (Figure 7). The RVOT VTI is a better predictor of RV dysfunction in cardiogenic shock states compared to TAPSE and may also have prognostic value in patients with pulmonary hypertension [39,40].
- Peak lateral tricuspid annular systolic velocity (S′ or systolic wave prime): S′ can be calculated by activating tissue Doppler imaging (TDI) at the lateral tricuspid annulus in the apical four-chamber view. An S′ velocity of less than 10 cm/s correlates strongly with RV systolic dysfunction and may even correlate better than TAPSE for normal RVEF to mild degrees of RVEF reduction. Specifically, an S′ < 11.5 cm/s correlates with an RVEF < 45%, with a sensitivity of 90% and a specificity of 85% [41]. The advantages of S′ include its ease and simplicity; however, similar to TAPSE, it is also limited by its one-dimensionality, as well as its load- and angle-dependence [36,42]. S′ alone is insufficient to capture regional wall motion abnormalities [36].
3.3. Right Ventricular Afterload
- Point-of-care ultrasound markers of RV afterload:
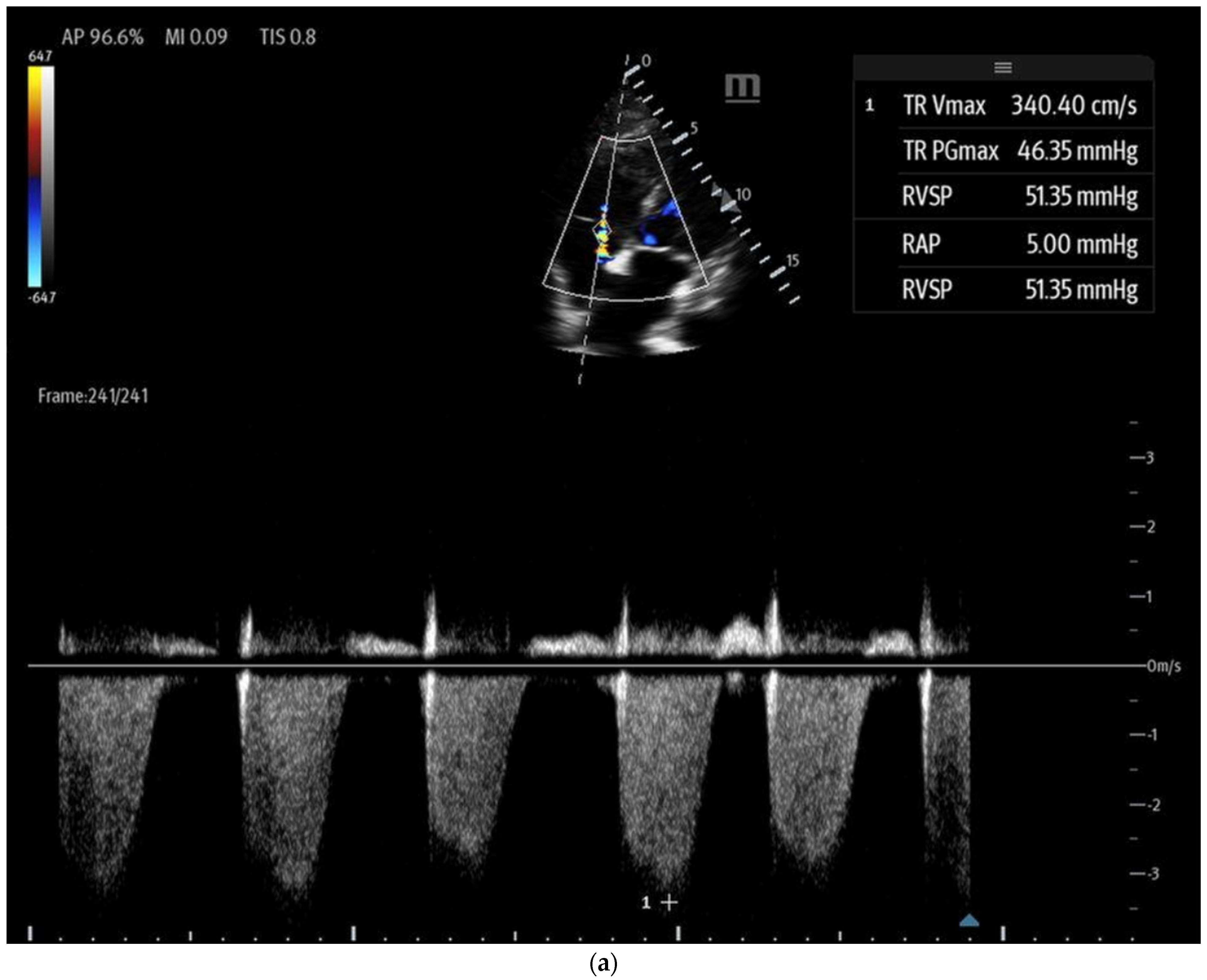
- Tricuspid regurgitation (TR) jet velocity: Pulmonary artery (PA) pressures can be estimated using TR jet peak velocity (Figure 8a). The evidence suggests that in the right clinical context, a TR jet velocity greater than 2.8 m/s is an independent predictor of pulmonary hypertension, while a velocity > 3.4 m/s strongly indicates this condition [35,43,44]. However, this method has limitations as TR jet velocity may underestimate PA pressures in cases of severe TR and is unreliable when TR is insufficient for evaluation [43]. Additionally, this technique cannot differentiate pulmonary hypertension from other causes of RV outflow obstruction, such as pulmonary or RVOT stenosis [44].
- McConnell’s sign: A POCUS finding defined as hypo or akinesis of the mid-RV free wall with preserved apical contraction on ultrasound is a widely recognized marker of right heart strain, particularly in the setting of acute pulmonary embolism (PE) [32,35]. In the original 1996 study describing McConnell’s sign, a sensitivity of 77% and a specificity of 94% were markers for acute PE, even after comparison with patients with other causes of RV dysfunction, such as pulmonary hypertension [45,46]. However, additional studies since then have cast doubt on the high specificity of McConnell’s sign, with one study showing equal prevalence of McConnell’s sign in patients with acute PE or RV myocardial infarction and another study demonstrating that patients diagnosed with acute PE with McConnell’s sign present had similar echocardiographic findings as a comparison group of patients with chronic pulmonary hypertension [47,48]. In contrast, a recent meta-analysis still found a pooled sensitivity of 22% and specificity of 97% for McConnell’s sign in the setting of acute PE, although they noted that high suspicion for acute PE prompted POCUS use in the majority of the studies [49]. Its positive predictive value ranges between 57 and 71% [50]. These findings suggest that while McConnell’s sign may be consistent with right heart strain in acute PE, caution should be utilized regarding its diagnostic capabilities for PE. Accordingly, in 2010, the American Society of Echocardiography and American College of Emergency Physicians recommended against the sole use of POCUS and McConnell’s sign for diagnosing and treating presumed acute PE except in patients too unstable to undergo additional confirmatory testing such as CT imaging [51].
- The “D-Sign”: This refers to the flattening of the interventricular septum with a leftward deviation into the left ventricle, causing the left ventricle to appear as a D-shaped structure, as seen in the parasternal short-axis view (Figure 8b). This finding suggests increased RV afterload and can be associated with pulmonary hypertension, acute PE, and RV failure [35]. Diastolic septal flattening indicates volume overload (e.g., severe tricuspid regurgitation or atrial septal defect), while systolic flattening points to pressure overload (e.g., pulmonary hypertension or acute PE). In volume overload, the D shape is most pronounced at end-diastole and resolves in systole, whereas in pressure overload, flattening persists throughout the cardiac cycle [36].
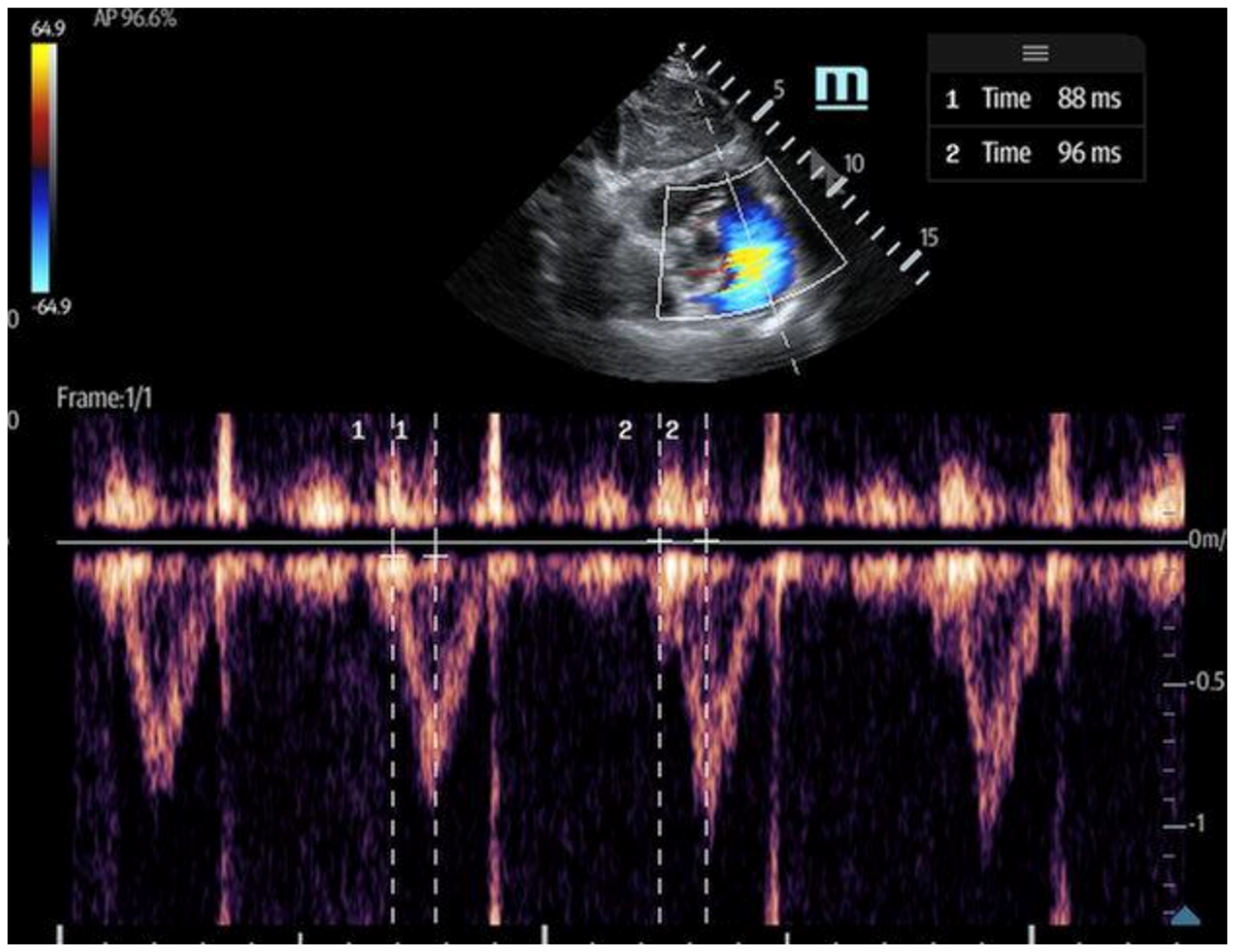
- The 60/60 sign: This is an echocardiography sign that refers to the presence of both pulmonary acceleration time (PAT) ≤60 milliseconds (Figure 9) and tricuspid pressure gradient ≤60 mmHg as a marker for acute RV strain in acute PE, although it has poor sensitivity [52]. It is thought to be more advantageous than McConnell’s sign due to its objectivity and reproducibility [50]. The 60/60 sign has a sensitivity of 36% and a specificity of 94% when seen in combination with McConnell’s sign, emphasizing the utility of various ultrasound findings when assessed in conjunction with each other [50].
4. Left Ventricular Assessment and Integration of Ultrasound
4.1. Left Ventricular Preload
4.2. Left Ventricular Function
4.2.1. Point-of-Care Ultrasound Assessment of Left Ventricular Function
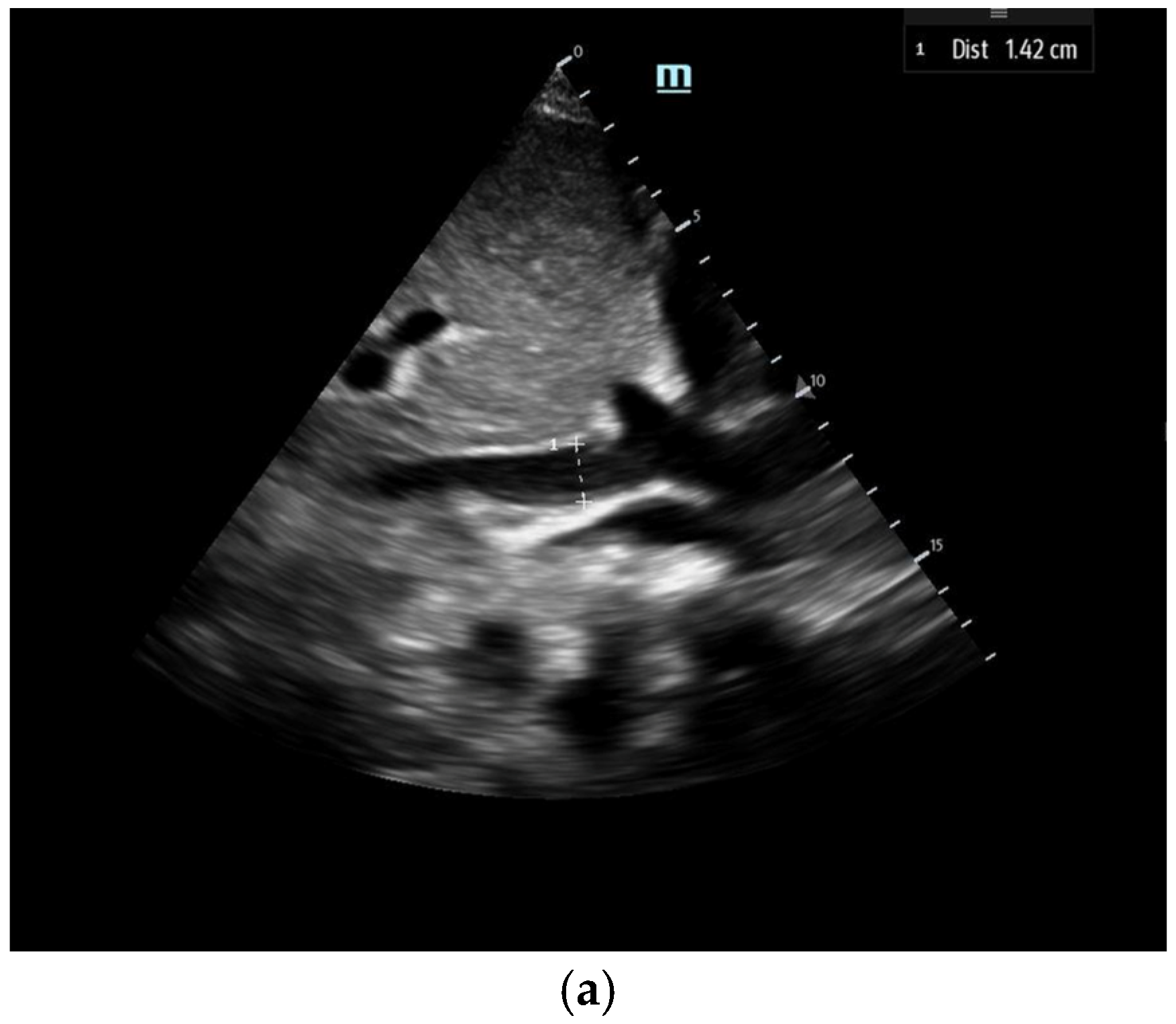
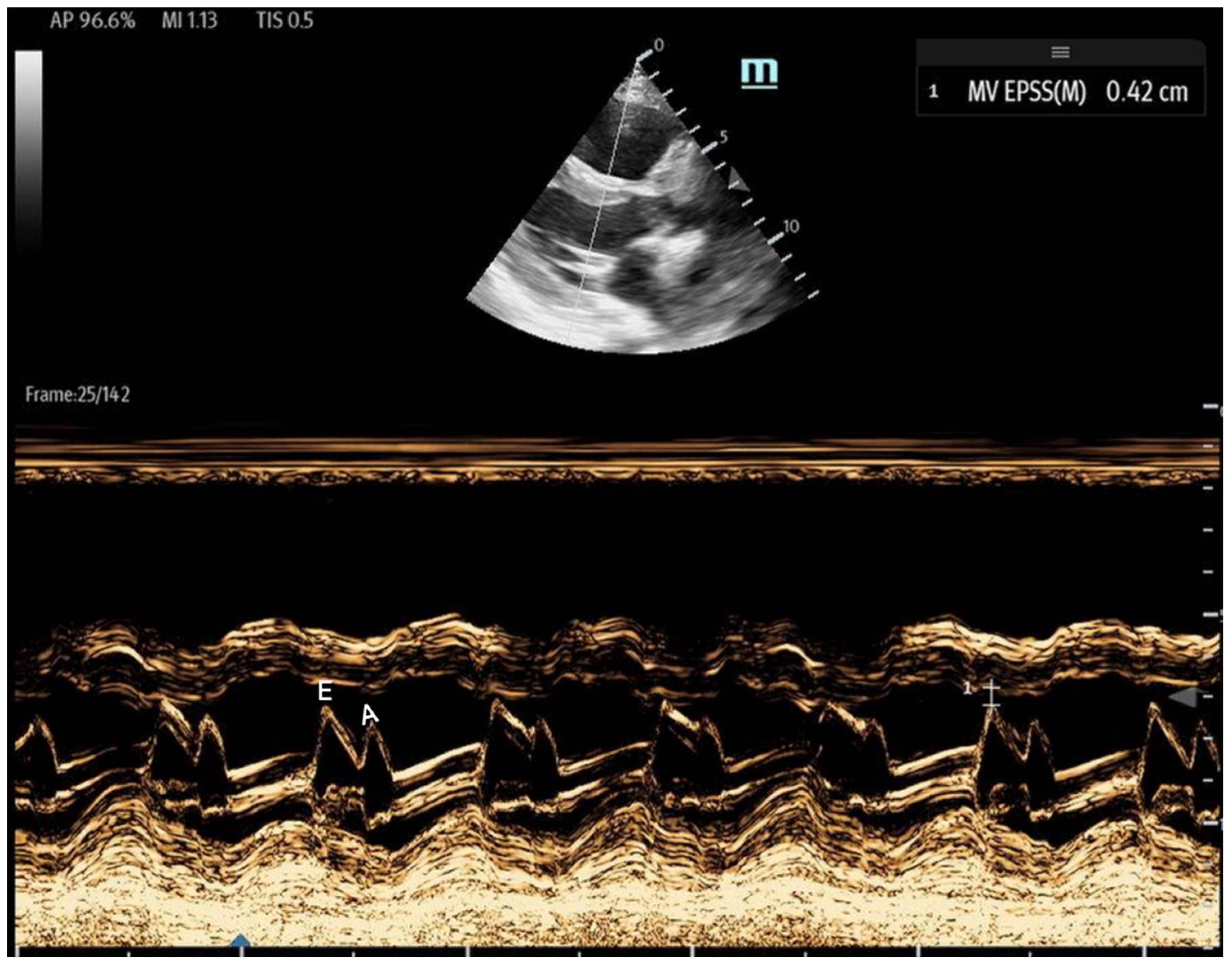
- 1E-point Septal Separation (EPSS): Obtained in the parasternal long-axis (PLAX) view, EPSS quantifies the distance between the anterior mitral valve leaflet tip and the interventricular septum (Figure 11). An EPSS greater than 1 cm may indicate a reduced LVEF of less than 40%, with a sensitivity of 69% and specificity of 91% [54]. This method requires only brief bedside training [55]. However, EPSS may not accurately reflect cardiac function in certain patients. In those with valvular pathologies (e.g., mitral stenosis/prosthesis or aortic regurgitation), abnormal valve or leaflet movement can lead to falsely high EPSS measurements despite normal LVEF. Septal hypertrophy may also underestimate EPSS. Lastly, in atrial fibrillation, the lack of coordinated atrial contractions necessitates multiple measurements for reliable assessments [56].
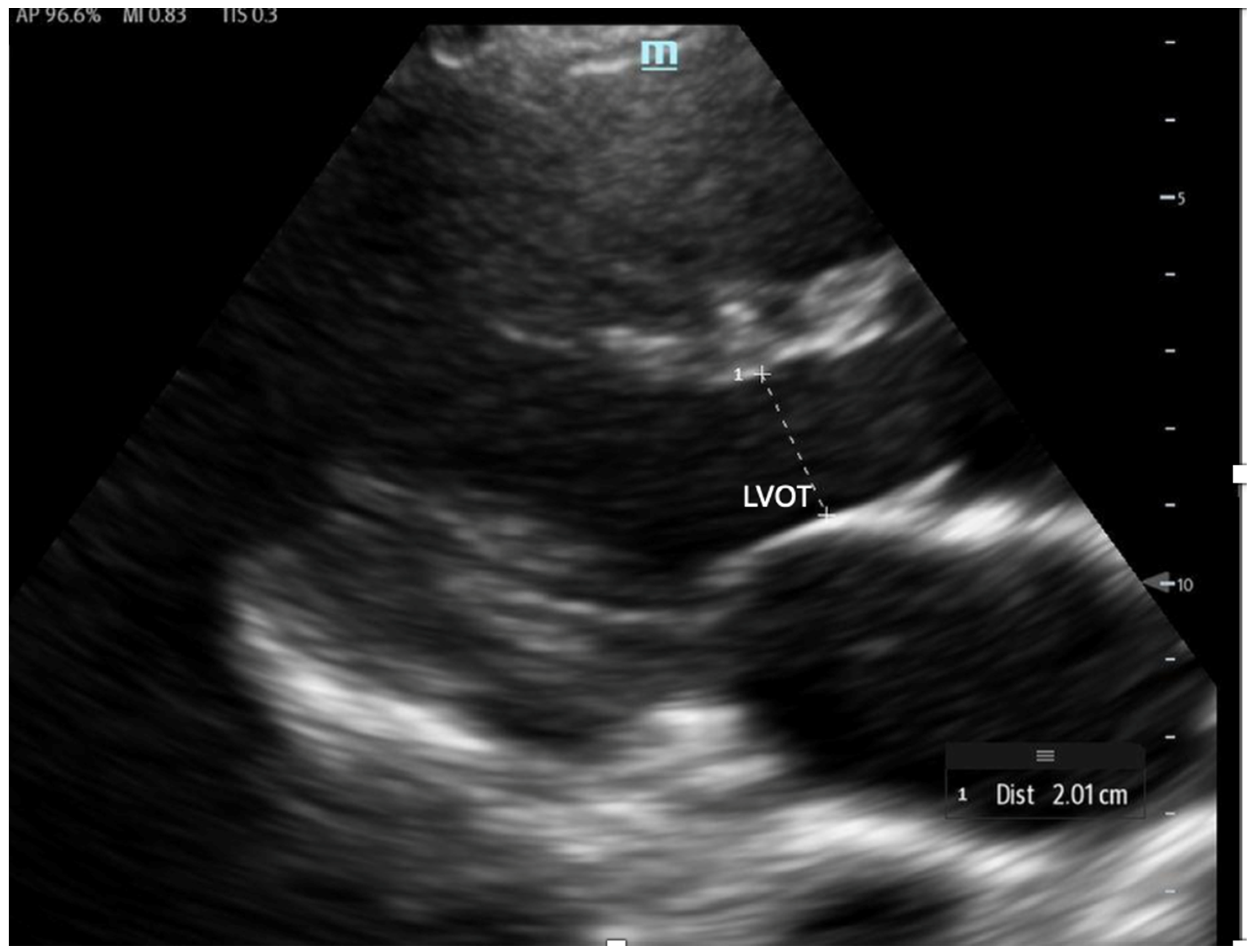
- Fractional Shortening (FS): Obtained in the PLAX view, FS measures the left ventricular (LV) end-systolic and end-diastolic diameters. It evaluates the percentage change in LV diameter between diastole and systole, which is calculated as:FS serves as an estimate of how effectively the LV contracts and can be used as a surrogate for LVEF. However, it may be falsely low in areas of wall motion abnormalities and is highly influenced by preload and afterload. Additionally, FS has been shown to underestimate LVEF in patients with concentric left ventricular hypertrophy [57,58].
4.2.2. Left Ventricular Velocity–Time Integral (LVOT-VTI)
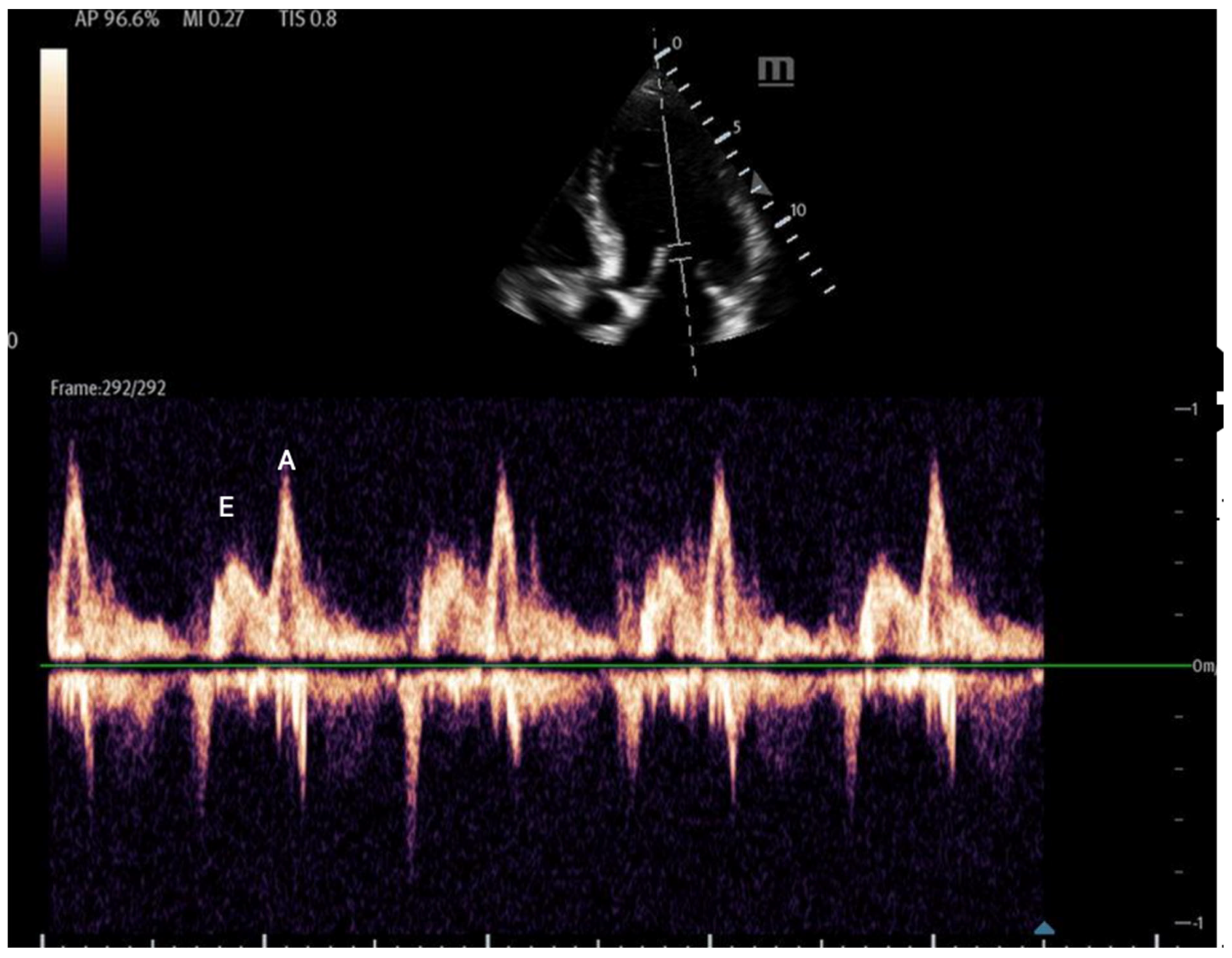
- Mitral inflow: Assessed via pulsed-wave (PW) Doppler at the mitral valve tips in the apical four-chamber view, this measures the blood flow velocities entering the left ventricle as an upward deflection of the PW Doppler signal. The resultant E and A waves are then evaluated for their ratio (Figure 14).
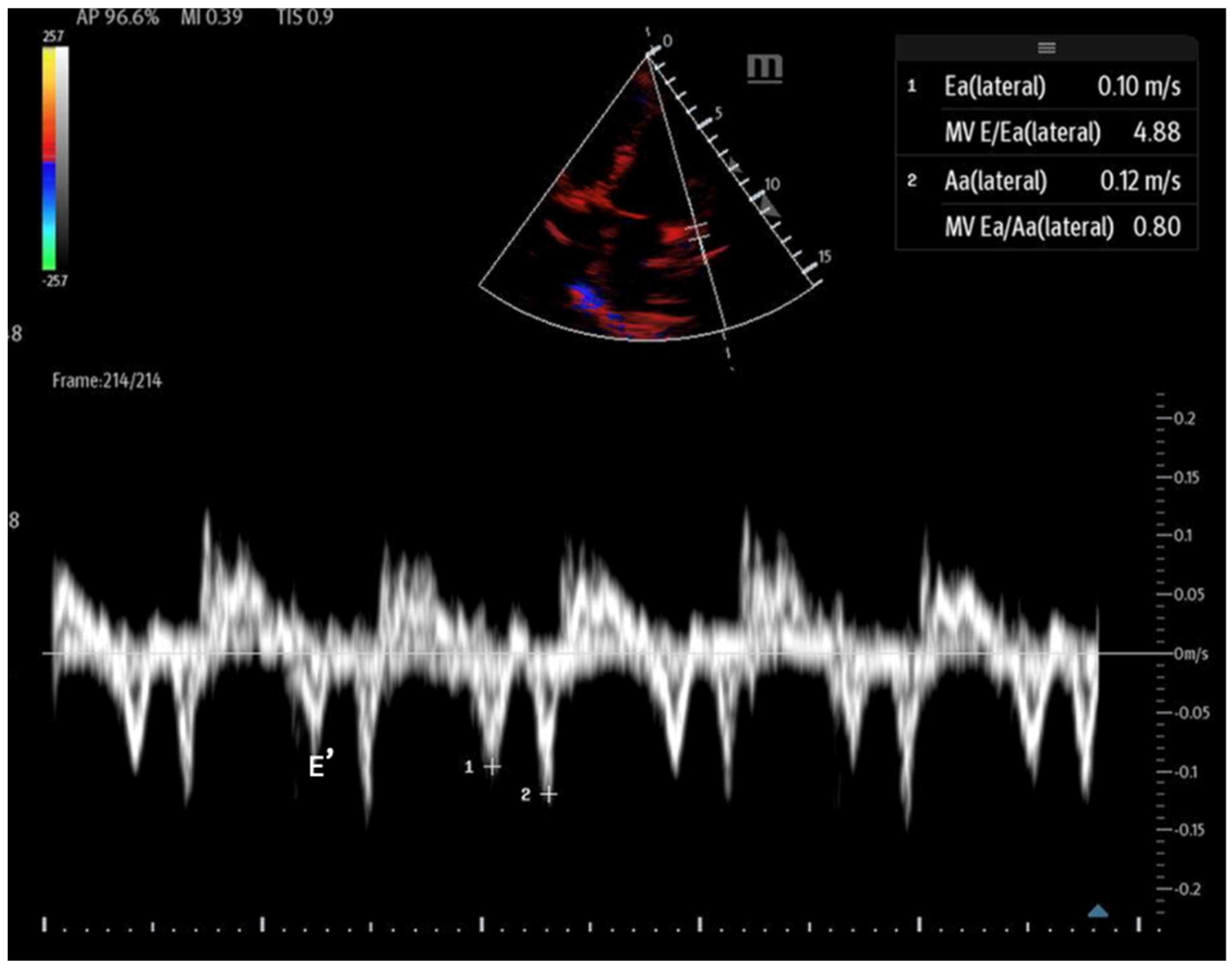
- Tissue Doppler: Measures myocardial movement during diastole using tissue Doppler at the septal annulus. The E/e′ ratio is calculated from the recorded e′ wave (Figure 15).
4.3. Left Ventricular Afterload
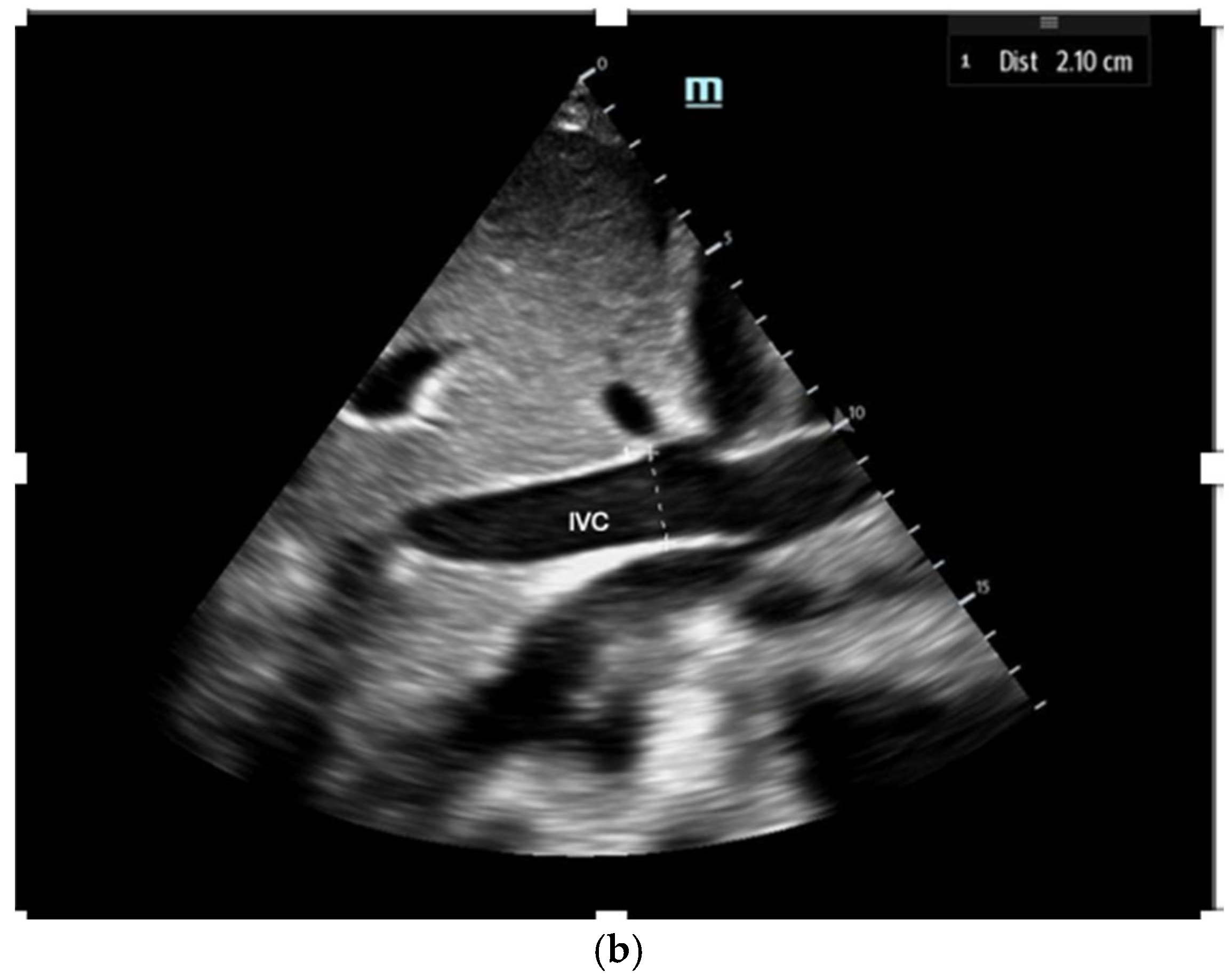
5. Detecting Fluid Responsiveness Using POCUS
6. Transesophageal Echocardiography (TEE)
7. Ultrasound Evaluation of Indwelling Hemodynamic Monitors and Assist Devices
8. Discussion
9. Future Directions
10. Conclusions
Funding
Conflicts of Interest
References
- Kislitsina, O.N.; Rich, J.D.; Wilcox, J.E.; Pham, D.T.; Churyla, A.; Vorovich, E.B.; Ghafourian, K.; Yancy, C.W. Shock—Classification and Pathophysiological Principles of Therapeutics. Curr. Cardiol. Rev. 2019, 15, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Ince, C. Monitoring coherence between the macro and microcirculation in septic shock. Curr. Opin. Crit. Care 2020, 26, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.; Parker, K. Mechanics and Imaging of the Macrocirculation. Magn. Reson. Med. 1990, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Karamchandani, K. Macrocirculation and Microcirculation: The “Batman and Superman” Story of Critical Care Resuscitation. Anesth. Analg. 2021, 132, 280–283. [Google Scholar] [CrossRef]
- Ince, C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit. Care 2015, 19 (Suppl. S3), S8. [Google Scholar] [CrossRef]
- Vincent, J.L.; Rhodes, A.; Perel, A.; Martin, G.S.; Della Rocca, G.; Vallet, B.; Pinsky, M.R.; Hofer, C.K.; Teboul, J.L.; de Boode, W.P.; et al. Clinical review: Update on hemodynamic monitoring—A consensus of 16. Crit. Care 2011, 15, 229. [Google Scholar] [CrossRef]
- Soni, N.; Kory, P.; Arntfield, R. Point-of-Care Ultrasound, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; p. 546. [Google Scholar]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Velissaris, D.; Karamouzos, V.; Kotroni, I.; Pierrakos, C.; Karanikolas, M. The Use of Pulmonary Artery Catheter in Sepsis Patients: A Literature Review. J. Clin. Med. Res. 2016, 8, 769–776. [Google Scholar] [CrossRef]
- Eckel, H. Sonography in emergency diagnosis of the abdomen (author’s transl). Rontgenblatter 1980, 33, 244–248. [Google Scholar]
- Barjaktarevic, I.; Kenny, J.S.; Berlin, D.; Cannesson, M. The Evolution of Ultrasound in Critical Care: From Procedural Guidance to Hemodynamic Monitor. J. Ultrasound Med. 2021, 40, 401–405. [Google Scholar] [CrossRef]
- Mercado, P.; Maizel, J.; Beyls, C.; Titeca-Beauport, D.; Joris, M.; Kontar, L.; Riviere, A.; Bonef, O.; Soupison, T.; Tribouilloy, C.; et al. Transthoracic echocardiography: An accurate and precise method for estimating cardiac output in the critically ill patient. Crit. Care 2017, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Koster, G.; van der Horst, I.C.C. Critical care ultrasonography in circulatory shock. Curr. Opin. Crit. Care 2017, 23, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Ventetuolo, C.E.; Klinger, J.R. Management of acute right ventricular failure in the intensive care unit. Ann. Am. Thorac. Soc. 2014, 11, 811–822. [Google Scholar] [CrossRef]
- Dyke, C.; Brunsting, L.; Salter, D.; Murphy, C.; Abd-Elfattah, A.; Wechsler, A. Preload Dependence of Right Ventricular Blood Flow: I. The Normal Right Ventricle. Ann. Thorac. Surg. 1987, 43, 478–483. [Google Scholar] [CrossRef]
- Galindo, P.; Gasca, C.; Argaiz, E.R.; Koratala, A. Point of care venous Doppler ultrasound: Exploring the missing piece of bedside hemodynamic assessment. World J. Crit. Care Med. 2021, 10, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Ciozda, W.; Kedan, I.; Kehl, D.W.; Zimmer, R.; Khandwalla, R.; Kimchi, A. The efficacy of sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure. Cardiovasc. Ultrasound 2016, 14, 33. [Google Scholar] [CrossRef]
- Orso, D.; Paoli, I.; Piani, T.; Cilenti, F.L.; Cristiani, L.; Guglielmo, N. Accuracy of Ultrasonographic Measurements of Inferior Vena Cava to Determine Fluid Responsiveness: A Systematic Review and Meta-Analysis. J. Intensive Care Med. 2020, 35, 354–363. [Google Scholar] [CrossRef]
- Long, E.; Oakley, E.; Duke, T.; Babl, F.E.; Paediatric Research in Emergency Departments International Collaborative. Does Respiratory Variation in Inferior Vena Cava Diameter Predict Fluid Responsiveness: A Systematic Review and Meta-Analysis. Shock 2017, 47, 550–559. [Google Scholar] [CrossRef]
- Di Nicolo, P.; Tavazzi, G.; Nannoni, L.; Corradi, F. Inferior Vena Cava Ultrasonography for Volume Status Evaluation: An Intriguing Promise Never Fulfilled. J. Clin. Med. 2023, 12, 2217. [Google Scholar] [CrossRef]
- Berikashvili, L.B.; Kuznetsov, I.V.; Yadgarov, M.Y.; Ryzhkov, P.V.; Polyakov, P.A.; Yavorovskiy, A.G.; Yakovlev, A.A.; Grechko, A.V.; Likhvantsev, V.V. Assessment of Fluid Responsiveness via Central Venous Ultrasound Measurement: A Network Meta-Analysis. J. Clin. Med. 2025, 14, 492. [Google Scholar] [CrossRef]
- Guarracino, F.; Ferro, B.; Forfori, F.; Bertini, P.; Magliacano, L.; Pinsky, M.R. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit. Care 2014, 18, 647. [Google Scholar] [CrossRef] [PubMed]
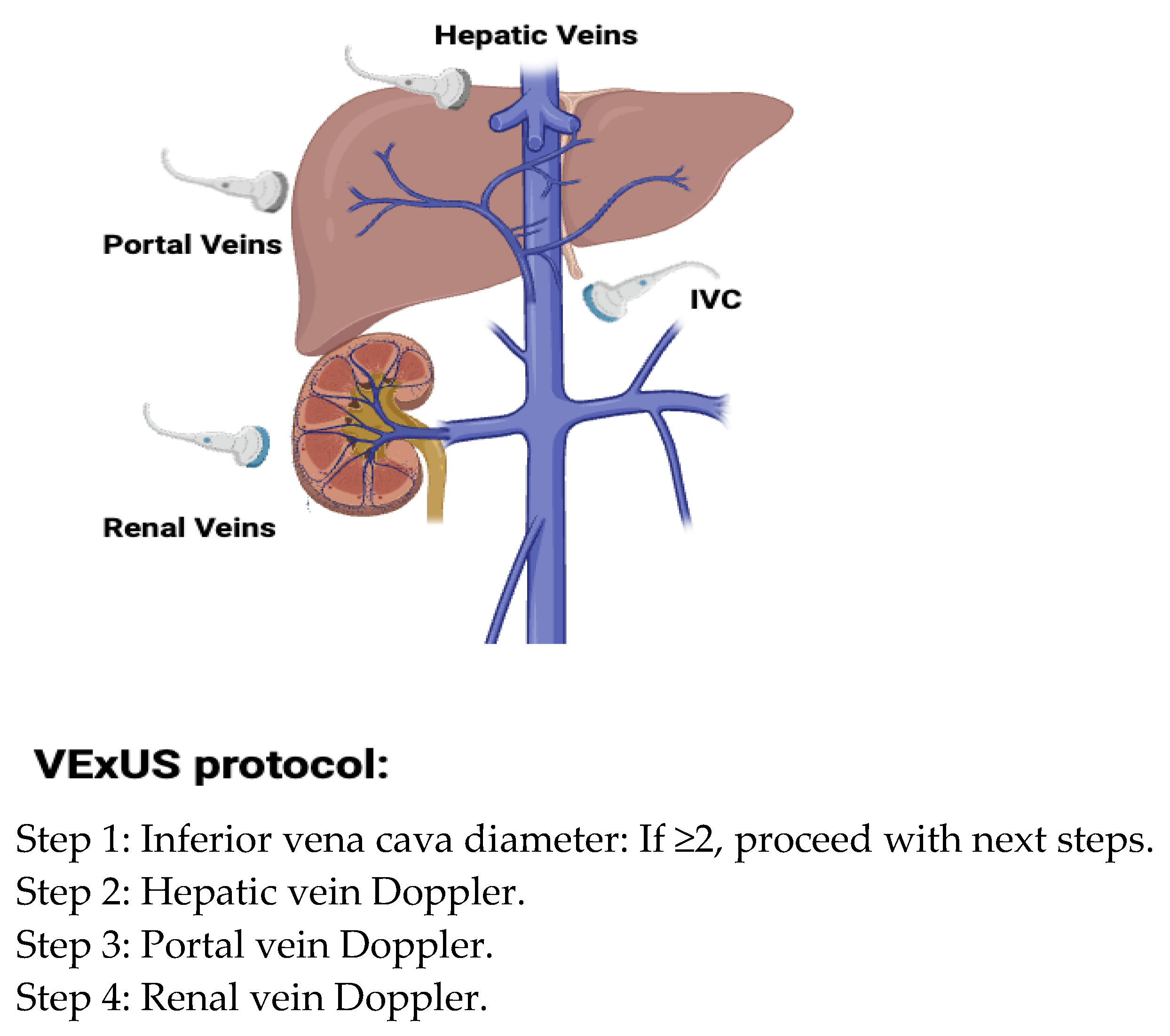
- Khan, A.A.; Saeed, H.; Haque, I.U.; Iqbal, A.; Du, D.; Koratala, A. Point-of-care ultrasonography spotlight: Could venous excess ultrasound serve as a shared language for internists and intensivists? World J. Crit. Care Med. 2024, 13, 93206. [Google Scholar] [CrossRef] [PubMed]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Galarza, L.; Buchannan, B.; Lau, V.I.; Adhikari, N.K.J.; Deschamps, J.; Charbonney, E.; Denault, A.; Wald, R. Prospective Study of Ultrasound Markers of Organ Congestion in Critically Ill Patients with Acute Kidney Injury. Kidney Int. Rep. 2023, 9, 694–702. [Google Scholar]
- Andrei, S.; Bahr, P.-A.; Nguyen, M.; Bouhemad, B.; Guinot, P.-G. Prevalence of systemic venous congestion assessed by Venous Excess Ultrasound Grading System (VExUS) and association with acute kidney injury in a general ICU cohort: A prospective multicentric study. Crit. Care 2023, 27, 224. [Google Scholar] [CrossRef]
- Klompmaker, P.; Mousa, A.; Allard, D.J.; Hagen, B.S.H.; Bánki, T.; Vermeulen, W.; de Waal, M.; van Wolfswinkel, S.; De Grooth, H.J.S.; Veelo, D.P.; et al. The association between venous excess ultrasound grading system (VExUS) and major adverse kidney events after 30 days in critically ill patients: A prospective cohort study. J. Crit. Care 2025, 88, 155097. [Google Scholar] [CrossRef]
- Landi, I.; Guerritore, L.; Iannaccone, A.; Ricotti, A.; Rola, P.; Garrone, M. Assessment of venous congestion with venous excess ultrasound score in the prognosis of acute heart failure in the emergency department: A prospective study. Eur. Heart J. Open 2024, 4, oeae050. [Google Scholar] [CrossRef]
- Rihl, M.F.; Pellegrini, J.A.S.; Boniatti, M.M. VExUS Score in the Management of Patients with Acute Kidney Injury in the Intensive Care Unit: AKIVEX Study. J. Ultrasound Med. 2023, 42, 2547–2556. [Google Scholar] [CrossRef]
- Islas-Rodriguez, J.P.; Miranda-Aquino, T.; Romero-Gonzalez, G.; Hernandez-Del Rio, J.; Camacho-Guerrero, J.R.; Covarrubias-Villa, S.; Ivey-Miranda, J.B.; Chavez-Iniguez, J.S. Effect on Kidney Function Recovery Guiding Decongestion with VExUS in Patients with Cardiorenal Syndrome 1: A Randomized Control Trial. Cardiorenal Med. 2024, 14, 1–11. [Google Scholar] [CrossRef]
- Longino, A.A.; Martin, K.C.; Leyba, K.R.; McCormack, L.; Siegel, G.; Sharma, V.M.; Riscinti, M.; Lopez, C.O.; Douglas, I.S.; Gill, E.A. Reliability and reproducibility of the venous excess ultrasound (VExUS) score, a multi-site prospective study: Validating a novel ultrasound technique for comprehensive assessment of venous congestion. Crit. Care 2024, 28, 197. [Google Scholar] [CrossRef]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
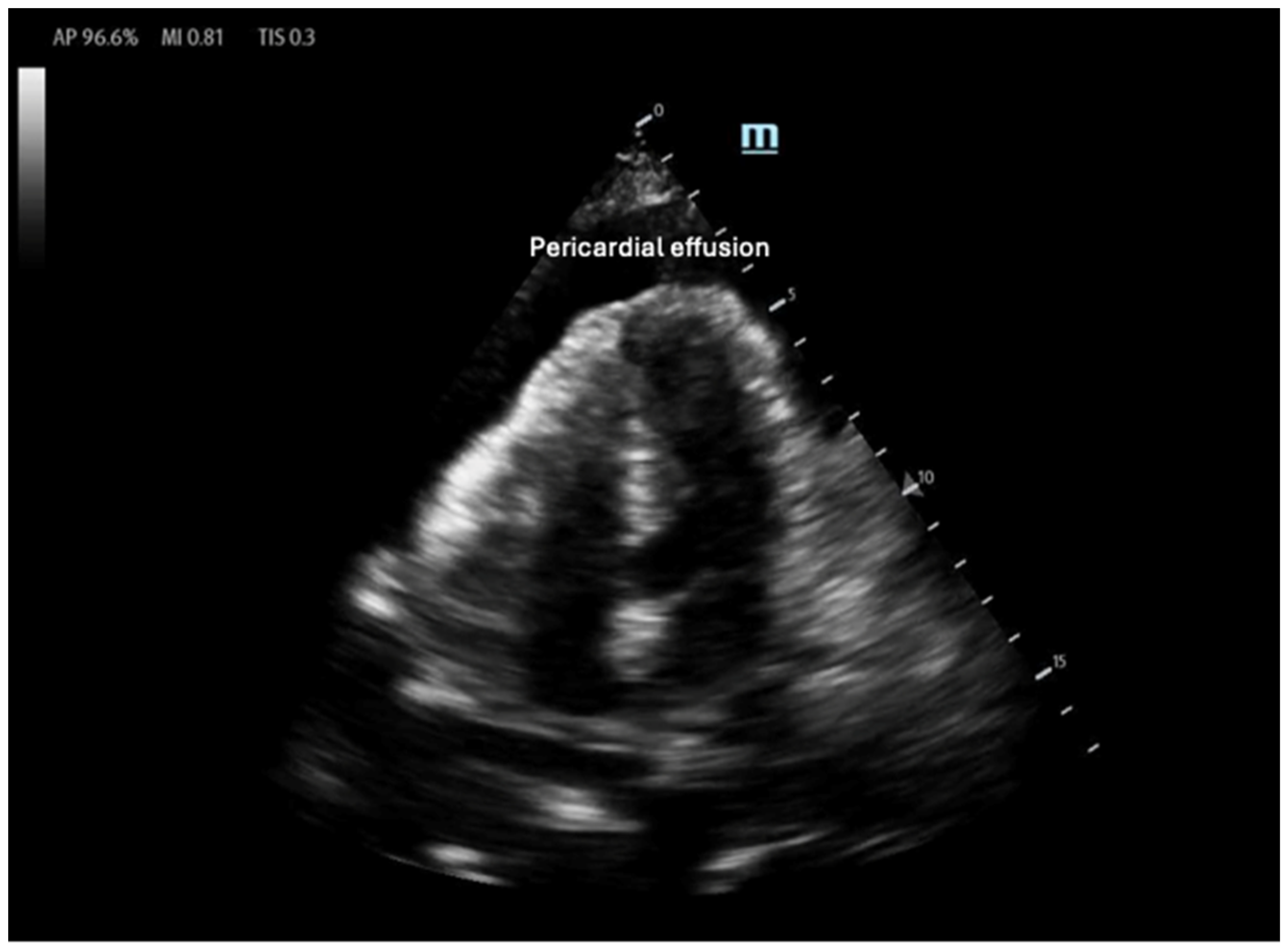
- Stashko, E.; Meer, J.M. Cardiac Tamponade; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Miller, A.; Peck, M.; Clark, T.; Conway, H.; Olusanya, S.; Fletcher, N.; Coleman, N.; Parulekar, P.; Aron, J.; Kirk-Bayley, J.; et al. FUSIC HD. Comprehensive haemodynamic assessment with ultrasound. J. Intensive Care Soc. 2022, 23, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Parfianowicz, D.; Bennett, C. Point of care ultrasound and shock: The value in bedside diagnosis and hemodynamic assessment in undifferentiated shock patients. J. Transl. Crit. Care Med. 2024, 6, e24-00010. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713, quiz 786–788. [Google Scholar]
- Mukherjee, M.; Rudski, L.G.; Addetia, K.; Afilalo, J.; D’Alto, M.; Freed, B.H.; Friend, L.B.; Gargani, L.; Grapsa, J.; Hassoun, P.M.; et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults and Special Considerations in Pulmonary Hypertension: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 141–186. [Google Scholar] [CrossRef]
- Pastore, M.C.; Ilardi, F.; Stefanini, A.; Mandoli, G.E.; Palermi, S.; Bandera, F.; Benfari, G.; Esposito, R.; Lisi, M.; Pasquini, A.; et al. Bedside Ultrasound for Hemodynamic Monitoring in CardiacIntensive Care Unit. J. Clin. Med. 2022, 11, 7538. [Google Scholar] [CrossRef]
- Lashin, H.; Olusanya, O.; Smith, A.; Bhattacharyya, S. Right ventricular echocardiographic parameters and prediction of stroke volume in ischemic cardiogenic shock: A retrospective study. J. Crit. Care 2023, 74, 154219. [Google Scholar] [CrossRef] [PubMed]
- Colak, A.; Kumral, Z.; Sezgin, D.; Simsek, G.O.; Yildirim, T.D.; Kis, M.; Ozpelit, E.; Akdeniz, B. The prognostic value of right ventricular outflow tract velocity time integral in patients with pulmonary hypertension. ESC Heart Fail. 2024, 11, 3332–3340. [Google Scholar] [CrossRef]
- Wu, V.C.; Takeuchi, M. Echocardiographic assessment of right ventricular systolic function. Cardiovasc. Diagn. Ther. 2018, 8, 70–79. [Google Scholar] [CrossRef]
- O’Donnell, C.; Sanchez, P.A.; Celestin, B.; McConnell, M.V.; Haddad, F. The Echocardiographic Evaluation of the Right Heart: Current and Future Advances. Curr. Cardiol. Rep. 2023, 25, 1883–1896. [Google Scholar] [CrossRef]
- Naddaf, N.; Dianati Maleki, N.; Goldschmidt, M.E.; Kalogeropoulos, A.P. Point of Care Ultrasound (POCUS) in the Management of Heart Failure: A Narrative Review. J. Pers. Med. 2024, 14, 766. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S.; Walker, S.; Loudon, B.L.; Gollop, N.D.; Wilson, A.M.; Lowery, C.; Frenneaux, M.P. Assessment of pulmonary artery pressure by echocardiography-A comprehensive review. Int. J. Cardiol. Heart Vasc. 2016, 12, 45–51. [Google Scholar] [CrossRef]
- Sosland, R.P.; Gupta, K. Images in cardiovascular medicine: McConnell’s Sign. Circulation 2008, 118, e517–e518. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.V.; Solomon, S.D.; Rayan, M.E.; Come, P.C.; Goldhaber, S.Z.; Lee, R.T. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am. J. Cardiol. 1996, 78, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Casazza, F.; Bongarzoni, A.; Capozi, A.; Agostoni, O. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur. J. Echocardiogr. 2005, 6, 11–14. [Google Scholar] [CrossRef] [PubMed]
- López-Candales, A.; Edelman, K.; Candales, M.D. Right ventricular apical contractility in acute pulmonary embolism: The McConnell sign revisited. Echocardiography 2010, 27, 614–620. [Google Scholar] [CrossRef]
- Fields, J.M.; Davis, J.; Girson, L.; Au, A.; Potts, J.; Morgan, C.J.; Vetter, I.; Riesenberg, L.A. Transthoracic Echocardiography for Diagnosing Pulmonary Embolism: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 714–723.e4. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.F.; Jabri, A.; Limaye, S.; Sharma, S.; Hamade, H.; Mhanna, M.; Aneja, A.; Gandhi, S. Echocardiographic Evaluation of Pulmonary Embolism: A Review. J. Am. Soc. Echocardiogr. 2023, 36, 906–912. [Google Scholar] [CrossRef]
- Labovitz, A.J.; Noble, V.E.; Bierig, M.; Goldstein, S.A.; Jones, R.; Kort, S.; Porter, T.R.; Spencer, K.T.; Tayal, V.S.; Wei, K. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J. Am. Soc. Echocardiogr. 2010, 23, 1225–1230. [Google Scholar] [CrossRef]
- Shah, B.R.; Velamakanni, S.M.; Patel, A.; Khadkikar, G.; Patel, T.M.; Shah, S.C. Analysis of the 60/60 Sign and Other Right Ventricular Parameters by 2D Transthoracic Echocardiography as Adjuncts to Diagnosis of Acute Pulmonary Embolism. Cureus 2021, 13, e13800. [Google Scholar] [CrossRef]
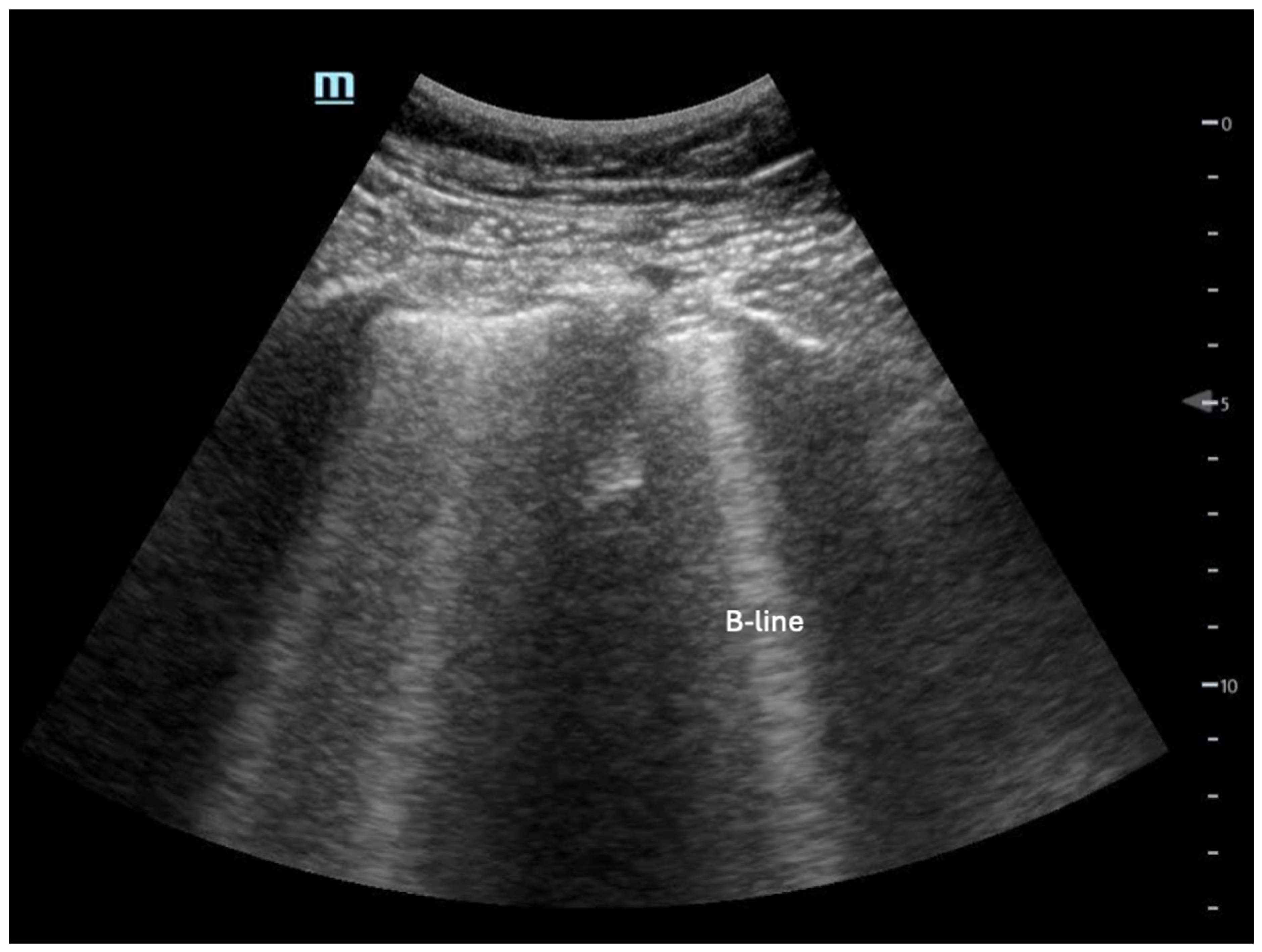
- Mayr, U.; Lukas, M.; Habenicht, L.; Wiessner, J.; Heilmaier, M.; Ulrich, J.; Rasch, S.; Schmid, R.M.; Lahmer, T.; Huber, W.; et al. B-Lines Scores Derived From Lung Ultrasound Provide Accurate Prediction of Extravascular Lung Water Index: An Observational Study in Critically Ill Patients. J. Intensive Care Med. 2022, 37, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kimura, B.J.; Yogo, N.; O’Connell, C.W.; Phan, J.N.; Showalter, B.K.; Wolfson, T. Cardiopulmonary limited ultrasound examination for "quick-look" bedside application. Am. J. Cardiol. 2011, 108, 586–590. [Google Scholar] [CrossRef] [PubMed]
- McKaigney, C.J.; Krantz, M.J.; La Rocque, C.L.; Hurst, N.D.; Buchanan, M.S.; Kendall, J.L. E-point septal separation: A bedside tool for emergency physician assessment of left ventricular ejection fraction. Am. J. Emerg. Med. 2014, 32, 493–497. [Google Scholar] [CrossRef]
- Massie, B.M.; Schiller, N.B.; Ratshin, R.A.; Parmley, W.W. Mitral-septal separation: New echocardiographic index of left ventricular function. Am. J. Cardiol. 1977, 39, 1008–1016. [Google Scholar] [CrossRef]
- Chengode, S. Left ventricular global systolic function assessment by echocardiography. Ann. Card Anaesth. 2016, 19, S26–S34. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Greer, J. Pathophysiology of cardiovascular dysfunction in sepsis. BJA Educ. 2015, 15, 316–321. [Google Scholar] [CrossRef]
- Blanco, P. Rationale for using the velocity-time integral and the minute distance for assessing the stroke volume and cardiac output in point-of-care settings. Ultrasound J. 2020, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.; Leng, A.; Gardiner, S.; Vanderwerff, L.; Jones, S.; Thompson, D.; Leng, P. Using Point-of-Care Bedside Ultrasound for Volume Assessment in Early Shock: An Outcome Study. Chest 2016, 149, A148. [Google Scholar] [CrossRef]
- Lanspa, M.J.; Gutsche, A.R.; Wilson, E.L.; Olsen, T.D.; Hirshberg, E.L.; Knox, D.B.; Brown, S.M.; Grissom, C.K. Application of a simplified definition of diastolic function in severe sepsis and septic shock. Crit. Care 2016, 20, 243. [Google Scholar] [CrossRef]
- Greenstein, Y.Y.; Mayo, P.H. Evaluation of Left Ventricular Diastolic Function by the Intensivist. Chest 2018, 153, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., III; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Slama, M.; Tribouilloy, C.; Maizel, J. Left ventricular outflow tract obstruction in ICU patients. Curr. Opin. Crit. Care 2016, 22, 260–266. [Google Scholar] [CrossRef]
- Caselli, S.; Martino, A.; Genuini, I.; Santini, D.; Carbone, I.; Agati, L.; Fedele, F. Pathophysiology of dynamic left ventricular outflow tract obstruction in a critically ill patient. Echocardiography 2010, 27, E122–E124. [Google Scholar] [CrossRef]
- Marik, P.E. Fluid Responsiveness and the Six Guiding Principles of Fluid Resuscitation. Crit. Care Med. 2016, 44, 1920–1922. [Google Scholar] [CrossRef]
- Kattan, E.; Castro, R.; Miralles-Aguiar, F.; Hernandez, G.; Rola, P. The emerging concept of fluid tolerance: A position paper. J. Crit. Care 2022, 71, 154070. [Google Scholar] [CrossRef]
- Monnet, X.; Shi, R.; Teboul, J.L. Prediction of fluid responsiveness. What’s new? Ann. Intensive Care 2022, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Feissel, M.; Michard, F.; Mangin, I.; Ruyer, O.; Faller, J.P.; Teboul, J.L. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest 2001, 119, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, Y.; Lejeune, V.; Muller, L.; Perbet, S.; Zieleskiewicz, L.; Bart, F.; Veber, B.; Paugam-Burtz, C.; Jaber, S.; Ayham, A.; et al. Evaluation of pulse pressure variation validity criteria in critically ill patients: A prospective observational multicentre point-prevalence study. Br. J. Anaesth. 2014, 112, 681–685. [Google Scholar] [CrossRef]
- Xie, J.; Wan, J.; Xu, L.; Zhang, Y.; Chen, J. The Accuracy of Velocity-Time Integral Variation and Peak Velocity Variation of the Left Ventricular Outflow Tract in Predicting Fluid Responsiveness in Postoperative Patients Mechanically Ventilated at Low Tidal Volumes. J. Cardiothorac. Vasc. Anesth. 2023, 37, 911–918. [Google Scholar] [CrossRef]
- Vignon, P.; Repessé, X.; Bégot, E.; Léger, J.; Jacob, C.; Bouferrache, K.; Slama, M.; Prat, G.; Vieillard-Baron, A. Comparison of Echocardiographic Indices Used to Predict Fluid Responsiveness in Ventilated Patients. Am. J. Respir. Crit. Care Med. 2017, 195, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Barjaktarevic, I.; Toppen, W.E.; Hu, S.; Aquije Montoya, E.; Ong, S.; Buhr, R.; David, I.J.; Wang, T.; Rezayat, T.; Chang, S.Y.; et al. Ultrasound Assessment of the Change in Carotid Corrected Flow Time in Fluid Responsiveness in Undifferentiated Shock. Crit. Care Med. 2018, 46, e1040–e1046. [Google Scholar] [CrossRef] [PubMed]
- Mayo, P.H.; Narasimhan, M.; Koenig, S. Critical Care Transesophageal Echocardiography. Chest 2015, 148, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Colreavy, F.B.; Donovan, K.; Lee, K.Y.; Weekes, J. Transesophageal echocardiography in critically ill patients. Crit. Care Med. 2002, 30, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Bouferrache, K.; Amiel, J.B.; Chimot, L.; Caille, V.; Charron, C.; Vignon, P.; Vieillard-Baron, A. Initial resuscitation guided by the Surviving Sepsis Campaign recommendations and early echocardiographic assessment of hemodynamics in intensive care unit septic patients: A pilot study. Crit. Care Med. 2012, 40, 2821–2827. [Google Scholar] [CrossRef]
- Teran, F.; Prats, M.I.; Nelson, B.P.; Kessler, R.; Blaivas, M.; Peberdy, M.A.; Shillcutt, S.K.; Arntfield, R.T.; Bahner, D. Focused Transesophageal Echocardiography During Cardiac Arrest Resuscitation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 745–754. [Google Scholar] [CrossRef]
- Ho, Y.J.; Sung, C.W.; Chen, Y.C.; Lien, W.C.; Chang, W.T.; Huang, C.H. Performance of Intra-arrest Echocardiography: A Systematic Review. West J. Emerg. Med. 2024, 25, 166–174. [Google Scholar]
- Shaikh, F.; Kenny, J.E.; Awan, O.; Markovic, D.; Friedman, O.; He, T.; Singh, S.; Yan, P.; Qadir, N.; Barjaktarevic, I. Measuring the accuracy of cardiac output using POCUS: The introduction of artificial intelligence into routine care. Ultrasound J. 2022, 14, 47. [Google Scholar] [CrossRef]
| POCUS Exam | Hypovolemic | Cardiogenic | Obstructive | Distributive |
|---|---|---|---|---|
| Heart | Hyperdynamic left ventricle (LV) | Dilated chambers or reduced LV/RV function | Pulmonary embolism (PE): dilated, strained RV, with a D-shaped septum and positive McConell’s sign. Tamponade: pericardial effusion with RA systolic and RV diastolic collapse | Hyperdynamic LV in early sepsis and hypodynamic in late sepsis |
| Lung | A-Line pattern (normal lung) | B-lines and/or pleural effusion | PE: small pleural effusion or pulmonary infarcts (subpleural consolidations) Pneumothorax: absent lung sliding | Pneumonia: consolidation and/or focal B-lines, pleural effusion |
| IVC | Small and collapsible | Distended, non-collapsible | Distended, non-collapsible | Normal or collapsed |
| Others | Abdominal exam may show abdominal aortic aneurysm, aortic dissection, or intra-abdominal hemorrhage. Vascular exam may reveal deep vein thrombosis (DVT) or collapsed vessels | Abdomen exam may show peritoneal fluid | Vascular exam may show DVT | An abdomen exam could show peritoneal fluid. Musculoskeletal ultrasound may detect a focal abscess as a fluid collection |
| Parameter | Normal Value | Sensitivity | Specificity | Reference |
|---|---|---|---|---|
| TAPSE | ≥16 mm | 74% | 81% | [33] |
| RVOT-VTI | ≥12 cm | 84% | 78% | [34,35,36] |
| S′ | ≥10 cm/s | 90% | 85% | [37] |
| Normal | Grade 1 (Impaired Relaxation) | Grade 2 (Pseudo Normal) | Grade 3 (Restrictive) |
|---|---|---|---|
| E/A ≥ 0.8 e′ ≥ 8 cm/s E/e′ < 8 | E/A < 0.8 e′ < 8 cm/s E/e′ <8 | E/A ≥ 0.8 e′ < 8 cm/s E/e′ 8–15 | E/A ≥ 2 e′ < 8 cm/s E/e′ > 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, A.; Liu, M.; Jarman, A.; Yamanaka, T.; Kaul, M. Point-of-Care Ultrasound Use in Hemodynamic Assessment. Biomedicines 2025, 13, 1426. https://doi.org/10.3390/biomedicines13061426
Noor A, Liu M, Jarman A, Yamanaka T, Kaul M. Point-of-Care Ultrasound Use in Hemodynamic Assessment. Biomedicines. 2025; 13(6):1426. https://doi.org/10.3390/biomedicines13061426
Chicago/Turabian StyleNoor, Ahmed, Margaret Liu, Alan Jarman, Travis Yamanaka, and Malvika Kaul. 2025. "Point-of-Care Ultrasound Use in Hemodynamic Assessment" Biomedicines 13, no. 6: 1426. https://doi.org/10.3390/biomedicines13061426
APA StyleNoor, A., Liu, M., Jarman, A., Yamanaka, T., & Kaul, M. (2025). Point-of-Care Ultrasound Use in Hemodynamic Assessment. Biomedicines, 13(6), 1426. https://doi.org/10.3390/biomedicines13061426






