Altered Expression of Cell Cycle Regulators and Factors Released by Aged Cells in Skeletal Muscle of Patients with Bone Fragility: A Pilot Study on the Potential Role of SIRT1 in Muscle Atrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Clinical Parameters
2.3. Sample Collection
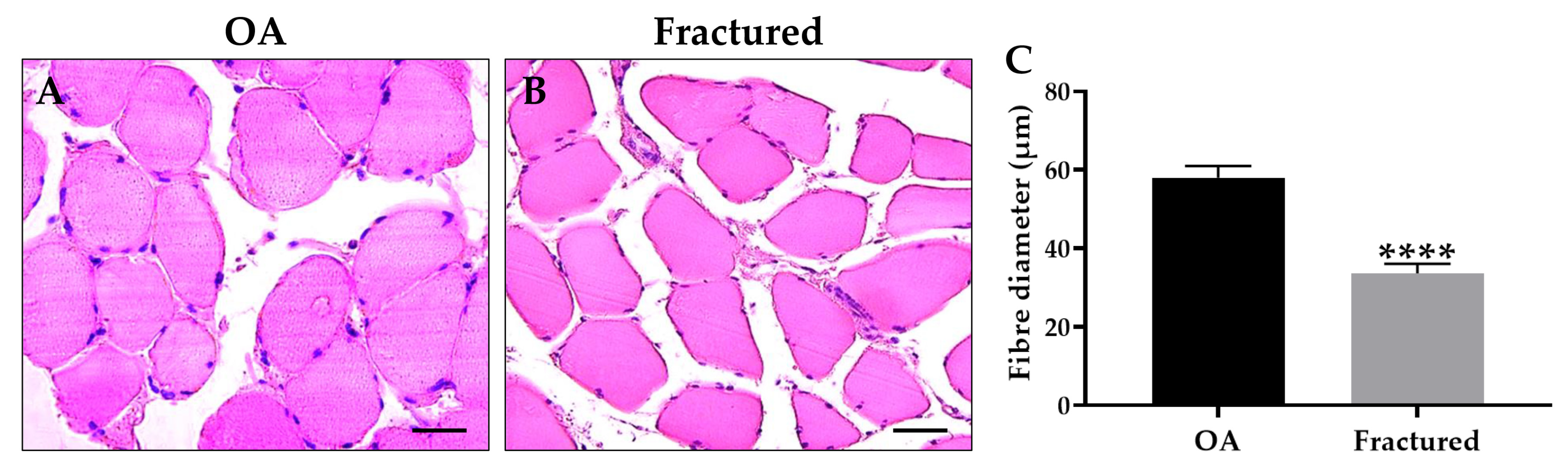
2.4. Morphometric Analysis
2.5. Immunohistochemical Analysis
2.6. Western Blotting Analysis
2.7. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR) Analysis
2.8. Statistical Analysis
3. Results
3.1. Clinical Profile and Muscle Histology in OA and Fractured Patients
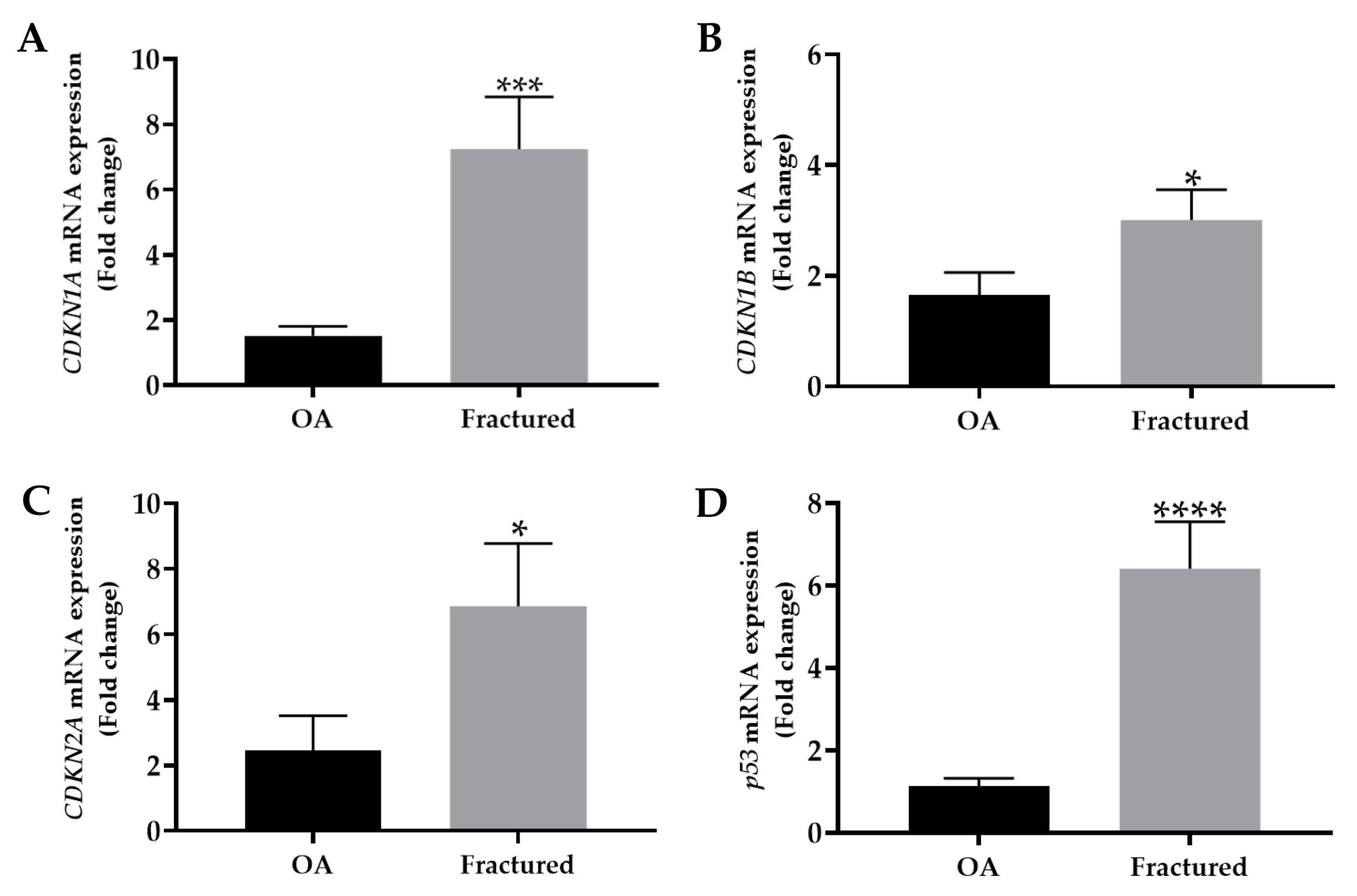
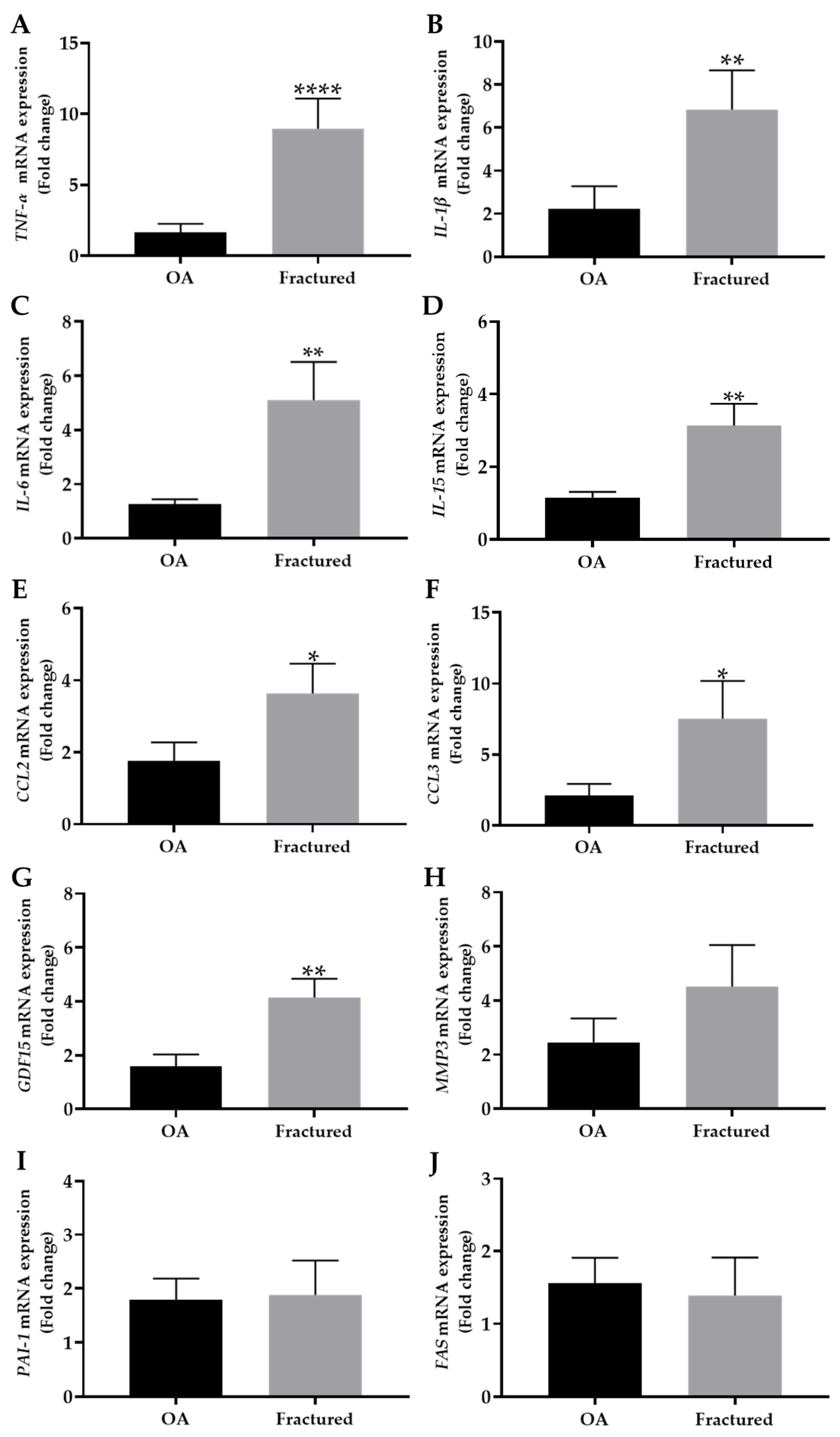
3.2. mRNA Expression of Cell Cycle Regulators and Factors Released by Aged Cells in Muscle Tissue of OA and Fractured Patients
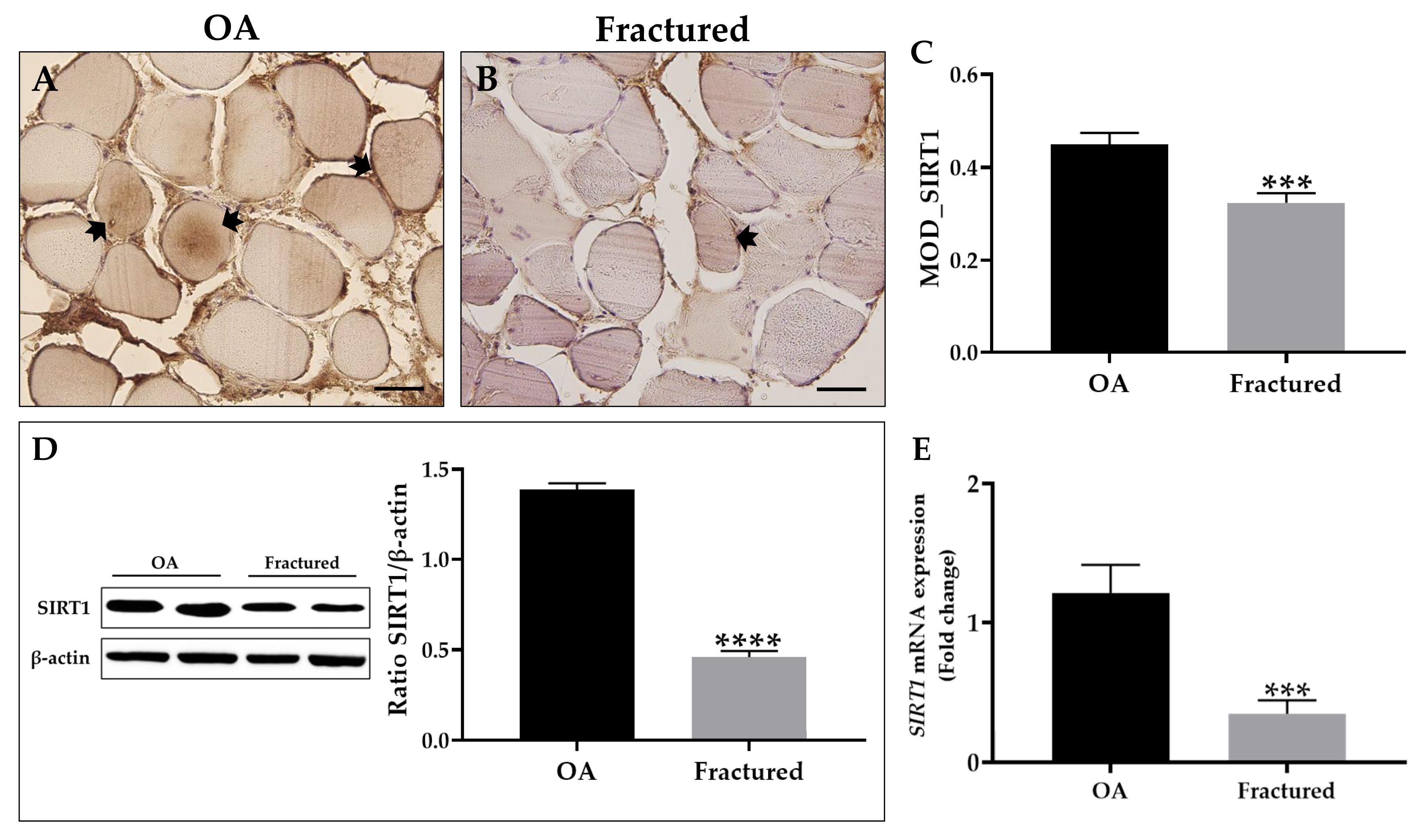
3.3. SIRT1 Expression in Muscle Tissue of OA and Fractured Patients
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SIRT1 | Sirtuin 1 | |
| OA | Patients undergoing hip arthroplasty for coxarthrosis | |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A | |
| CDKN1B | Cyclin-dependent kinase inhibitor 1B | |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A | |
| TNF-α | Tumor necrosis factor alpha | |
| IL-1β | Interleukin-1 beta | |
| IL-6 | Interleukin-6 | |
| IL-15 | Interleukin-15 | |
| CCL2 | Chemokine (C-C motif) ligand 2 | |
| CCL3 | Chemokine (C-C motif) ligand 3 | |
| GDF15 | Growth differentiation factor 15 | |
| MMP3 | Matrix metallopeptidase 3 | |
| PAI-1 | Plasminogen activator inhibitor 1 | |
| FAS | Fas cell surface death receptor | |
| SASP | Senescence-associated secretory phenotype | |
| NAD | Nicotinamide adenine dinucleotide | |
| BMD | Bone mineral density | |
| DXA | Dual-energy X-ray absorptiometry | |
| CET | Territorial ethics committee | |
| H&E | Hematoxylin and eosin | |
| EDTA | Ethylenediaminetetraacetic acid | |
| HRP | Horseradish peroxidase | |
| DAB | 3,3’-diaminobenzidine | |
| MOD | Mean optical density | |
| qRT-PCR | Real-time quantitative polymerase chain reaction | |
| BMI | Body mass index | |
| PTH | Parathyroid hormone | |
| NF-κB | Kappa-light-chain-enhancer of activated B cells | |
References
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular Mechanisms of Aging and Anti-Aging Strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Cariati, I.; Greggi, C.; Iundusi, R.; Gasbarra, E.; Iolascon, G.; Kurth, A.; Akesson, K.E.; Bouxsein, M.; Tranquilli Leali, P.; et al. Gaps and Alternative Surgical and Non-Surgical Approaches in the Bone Fragility Management: An Updated Review. Osteoporos. Int. 2022, 33, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular Senescence: A Key Therapeutic Target in Aging and Diseases. J. Clin. Invest. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Carlos Acosta, J.; Adams, P.D.; d’Adda di Fagagna, F.; Baker, D.J.; Bishop, C.L.; Chandra, T.; Collado, M.; Gil, J.; Gorgoulis, V.; et al. Guidelines for Minimal Information on Cellular Senescence Experimentation in Vivo. Cell 2024, 187, 4150–4175. [Google Scholar] [CrossRef]
- Lucas, V.; Cavadas, C.; Aveleira, C.A. Cellular Senescence: From Mechanisms to Current Biomarkers and Senotherapies. Pharmacol. Rev. 2023, 75, 675–713. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular Senescence in Aging and Age-Related Disease: From Mechanisms to Therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Murakami, T.; Inagaki, N.; Kondoh, H. Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells. Front. Endocrinol. 2022, 13, 869414. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Liu, T.; Zhu, X.; Pan, X. The Multifaceted Role of the SASP in Atherosclerosis: From Mechanisms to Therapeutic Opportunities. Cell Biosci. 2022, 12, 74. [Google Scholar] [CrossRef]
- Herdy, J.R.; Traxler, L.; Agarwal, R.K.; Karbacher, L.; Schlachetzki, J.C.M.; Boehnke, L.; Zangwill, D.; Galasko, D.; Glass, C.K.; Mertens, J.; et al. Increased Post-Mitotic Senescence in Aged Human Neurons Is a Pathological Feature of Alzheimer’s Disease. Cell Stem Cell 2022, 29, 1637–1652.e6. [Google Scholar] [CrossRef]
- Falvino, A.; Gasperini, B.; Cariati, I.; Bonanni, R.; Chiavoghilefu, A.; Gasbarra, E.; Botta, A.; Tancredi, V.; Tarantino, U. Cellular Senescence: The Driving Force of Musculoskeletal Diseases. Biomedicines 2024, 12, 1948. [Google Scholar] [CrossRef]
- He, Y.; Xie, W.; Li, H.; Jin, H.; Zhang, Y.; Li, Y. Cellular Senescence in Sarcopenia: Possible Mechanisms and Therapeutic Potential. Front. Cell Dev. Biol. 2021, 9, 793088. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Visconti, V.V.; Bonanni, R.; Gatti, A.; Marcozzi, M.; Calabrò, D.; Cariati, I. Osteosarcopenia and Long-COVID: A Dangerous Combination. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221130485. [Google Scholar] [CrossRef]
- Kirk, B.; Lombardi, G.; Duque, G. Bone and Muscle Crosstalk in Ageing and Disease. Nat. Rev. Endocrinol. 2025, 21, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal Muscle as Potential Central Link between Sarcopenia and Immune Senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef]
- Suetta, C.; Frandsen, U.; Mackey, A.L.; Jensen, L.; Hvid, L.G.; Bayer, M.L.; Petersson, S.J.; Schrøder, H.D.; Andersen, J.L.; Aagaard, P.; et al. Ageing Is Associated with Diminished Muscle Re-Growth and Myogenic Precursor Cell Expansion Early after Immobility-Induced Atrophy in Human Skeletal Muscle. J. Physiol. 2013, 591, 3789–3804. [Google Scholar] [CrossRef]
- Jung, W.; Juang, U.; Gwon, S.; Nguyen, H.; Huang, Q.; Lee, S.; Lee, B.; Kim, S.-H.; Ryu, S.; Park, J.; et al. Identifying the Potential Therapeutic Effects of MiR-6516 on Muscle Disuse Atrophy. Mol. Med. Rep. 2024, 30, 119. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Greenhaff, P.L.; Phillips, S.M.; Bodine, S.C.; Adams, C.M.; Lang, C.H. Control of Skeletal Muscle Atrophy in Response to Disuse: Clinical/Preclinical Contentions and Fallacies of Evidence. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E594–E604. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 Myokine Signaling in Skeletal Muscle: A Double-Edged Sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Pistilli, E.E.; Siu, P.M.; Alway, S.E. Interleukin-15 Responses to Aging and Unloading-Induced Skeletal Muscle Atrophy. Am. J. Physiol. Cell Physiol. 2007, 292, C1298–C1304. [Google Scholar] [CrossRef]
- Kamal, M.; Shanmuganathan, M.; Kroezen, Z.; Joanisse, S.; Britz-McKibbin, P.; Parise, G. Senescent Myoblasts Exhibit an Altered Exometabolome That Is Linked to Senescence-Associated Secretory Phenotype Signaling. Am. J. Physiol. Cell Physiol. 2025, 328, C440–C451. [Google Scholar] [CrossRef]
- Yu, S.; Ren, B.; Chen, H.; Goltzman, D.; Yan, J.; Miao, D. 1,25-Dihydroxyvitamin D Deficiency Induces Sarcopenia by Inducing Skeletal Muscle Cell Senescence. Am. J. Transl. Res. 2021, 13, 12638–12649. [Google Scholar] [PubMed]
- Schafer, M.J.; Zhang, X.; Kumar, A.; Atkinson, E.J.; Zhu, Y.; Jachim, S.; Mazula, D.L.; Brown, A.K.; Berning, M.; Aversa, Z.; et al. The Senescence-Associated Secretome as an Indicator of Age and Medical Risk. JCI Insight 2020, 5, e133668. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and Aging Related Signaling Pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Tonkin, J.; Villarroya, F.; Puri, P.L.; Vinciguerra, M. SIRT1 Signaling as Potential Modulator of Skeletal Muscle Diseases. Curr. Opin. Pharmacol. 2012, 12, 372–376. [Google Scholar] [CrossRef]
- Myers, M.J.; Shepherd, D.L.; Durr, A.J.; Stanton, D.S.; Mohamed, J.S.; Hollander, J.M.; Alway, S.E. The Role of SIRT1 in Skeletal Muscle Function and Repair of Older Mice. J. Cachexia. Sarcopenia Muscle 2019, 10, 929–949. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Rinaldi, A.M.; Marini, M.; Iundusi, R.; Gasbarra, E.; Tancredi, V.; Tarantino, U. Recombinant Irisin Prevents Cell Death and Mineralization Defects Induced by Random Positioning Machine Exposure in Primary Cultures of Human Osteoblasts: A Promising Strategy for the Osteoporosis Treatment. Front. Physiol. 2023, 14, 1107933. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Romagnoli, C.; Caprioli, L.; D’Arcangelo, G.; Tancredi, V.; Annino, G. Bone Adaptations to a Whole Body Vibration Protocol in Murine Models of Different Ages: A Preliminary Study on Structural Changes and Biomarker Evaluation. J. Funct. Morphol. Kinesiol. 2025, 10, 26. [Google Scholar] [CrossRef]
- Yin, M.; Xu, Y. The Protective Effects of Etomidate against Interleukin-1β (IL-1β)-Induced Oxidative Stress, Extracellular Matrix Alteration and Cellular Senescence in Chondrocytes. Bioengineered 2022, 13, 985–994. [Google Scholar] [CrossRef]
- Shaikh, S.B.; Balaya, R.D.A.; Dagamajalu, S.; Bhandary, Y.P.; Unwalla, H.; Prasad, T.S.K.; Rahman, I. A Signaling Pathway Map of Plasminogen Activator Inhibitor-1 (PAI-1/SERPINE-1): A Review of an Innovative Frontier in Molecular Aging and Cellular Senescence. Cell Commun. Signal. 2024, 22, 544. [Google Scholar] [CrossRef]
- White, J.P.; Billin, A.N.; Campbell, M.E.; Russell, A.J.; Huffman, K.M.; Kraus, W.E. The AMPK/P27(Kip1) Axis Regulates Autophagy/Apoptosis Decisions in Aged Skeletal Muscle Stem Cells. Stem Cell Rep. 2018, 11, 425–439. [Google Scholar] [CrossRef]
- Bonanni, R.; Gino Grillo, S.; Cariati, I.; Tranquillo, L.; Iundusi, R.; Gasbarra, E.; Tancredi, V.; Tarantino, U. Osteosarcopenia and Pain: Do We Have a Way Out? Biomedicines 2023, 11, 1285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Habiballa, L.; Aversa, Z.; Ng, Y.E.; Sakamoto, A.E.; Englund, D.A.; Pearsall, V.M.; White, T.A.; Robinson, M.M.; Rivas, D.A.; et al. Characterization of Cellular Senescence in Aging Skeletal Muscle. Nat. Aging 2022, 2, 601–615. [Google Scholar] [CrossRef]
- Perez, K.; Ciotlos, S.; McGirr, J.; Limbad, C.; Doi, R.; Nederveen, J.P.; Nilsson, M.I.; Winer, D.A.; Evans, W.; Tarnopolsky, M.; et al. Single Nuclei Profiling Identifies Cell Specific Markers of Skeletal Muscle Aging, Frailty, and Senescence. Aging 2022, 14, 9393–9422. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.F.L.; Ogrodnik, M.; Kucheryavenko, O.; Glibert, J.; Miwa, S.; Cameron, K.; Ishaq, A.; Saretzki, G.; Nagaraja-Grellscheid, S.; Nelson, G.; et al. The Bystander Effect Contributes to the Accumulation of Senescent Cells in Vivo. Aging Cell 2019, 18, e12848. [Google Scholar] [CrossRef]
- Dong, Y.; Yuan, H.; Ma, G.; Cao, H. Bone-Muscle Crosstalk under Physiological and Pathological Conditions. Cell. Mol. Life Sci. 2024, 81, 310. [Google Scholar] [CrossRef]
- Englund, D.A.; Jolliffe, A.; Aversa, Z.; Zhang, X.; Sturmlechner, I.; Sakamoto, A.E.; Zeidler, J.D.; Warner, G.M.; McNinch, C.; White, T.A.; et al. P21 Induces a Senescence Program and Skeletal Muscle Dysfunction. Mol. Metab. 2023, 67, 101652. [Google Scholar] [CrossRef]
- Wan, M.; Gray-Gaillard, E.F.; Elisseeff, J.H. Cellular Senescence in Musculoskeletal Homeostasis, Diseases, and Regeneration. Bone Res. 2021, 9, 41. [Google Scholar] [CrossRef]
- He, X.; Hu, W.; Zhang, Y.; Chen, M.; Ding, Y.; Yang, H.; He, F.; Gu, Q.; Shi, Q. Cellular Senescence in Skeletal Disease: Mechanisms and Treatment. Cell. Mol. Biol. Lett. 2023, 28, 88. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.K.; Ebert, S.M.; Bongers, K.S.; Dyle, M.C.; Bullard, S.A.; Dierdorff, J.M.; Kunkel, S.D.; Adams, C.M. P53 and ATF4 Mediate Distinct and Additive Pathways to Skeletal Muscle Atrophy during Limb Immobilization. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E245–E261. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Di, D.; Zhou, H.; Cui, Z.; Zhang, J.; Liu, Q.; Yuan, T.; Zhou, T.; Luo, X.; Ling, D.; Wang, Q. Frailty Phenotype as Mediator between Systemic Inflammation and Osteoporosis and Fracture Risks: A Prospective Study. J. Cachexia. Sarcopenia Muscle 2024, 15, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-W.; Yu, K.; Shyh-Chang, N.; Li, G.-X.; Jiang, L.-J.; Yu, S.-L.; Xu, L.-Y.; Liu, R.-J.; Guo, Z.-J.; Xie, H.-Y.; et al. Circulating Factors Associated with Sarcopenia during Ageing and after Intensive Lifestyle Intervention. J. Cachexia Sarcopenia Muscle 2019, 10, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-V.; Wu, W.-T.; Chen, Y.-H.; Chen, L.-R.; Hsu, W.-H.; Lin, Y.-L.; Han, D.-S. Enhanced Serum Levels of Tumor Necrosis Factor-α, Interleukin-1β, and -6 in Sarcopenia: Alleviation through Exercise and Nutrition Intervention. Aging 2023, 15, 13471–13485. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.; Khayrullin, A.; Kent, A.; Mendhe, B.; Youssef El Baradie, K.B.; Yu, K.; Pihkala, J.; Liu, Y.; McGee-Lawrence, M.; Johnson, M.; et al. Hindlimb Immobilization Increases IL-1β and Cdkn2a Expression in Skeletal Muscle Fibro-Adipogenic Progenitor Cells: A Link Between Senescence and Muscle Disuse Atrophy. Front. Cell Dev. Biol. 2021, 9, 790437. [Google Scholar] [CrossRef]
- Xiong, L.; Zhao, K.; Cao, Y.; Guo, H.-H.; Pan, J.-X.; Yang, X.; Ren, X.; Mei, L.; Xiong, W.-C. Linking Skeletal Muscle Aging with Osteoporosis by Lamin A/C Deficiency. PLoS Biol. 2020, 18, e3000731. [Google Scholar] [CrossRef]
- Ding, J.; Yang, G.; Sun, W.; Li, Y.; Wang, N.; Wang, J.; Zhao, Y. Association of Interleukin-6 with Sarcopenia and Its Components in Older Adults: A Systematic Review and Meta-Analysis of Cross-Sectional Studies. Ann. Med. 2024, 56, 2384664. [Google Scholar] [CrossRef]
- Iwasaki, S.; Miyake, M.; Hayashi, S.; Watanabe, H.; Nagasawa, Y.; Terada, S.; Watanabe, K.; Ohwada, S.; Kitazawa, H.; Rose, M.T.; et al. Effect of Myostatin on Chemokine Expression in Regenerating Skeletal Muscle Cells. Cells. Tissues. Organs 2013, 198, 66–74. [Google Scholar] [CrossRef]
- Kedlian, V.R.; Wang, Y.; Liu, T.; Chen, X.; Bolt, L.; Tudor, C.; Shen, Z.; Fasouli, E.S.; Prigmore, E.; Kleshchevnikov, V.; et al. Human Skeletal Muscle Aging Atlas. Nat. Aging 2024, 4, 727–744. [Google Scholar] [CrossRef]
- Hong, S.W.; Kang, J.-H. Growth Differentiation Factor-15 as a Modulator of Bone and Muscle Metabolism. Front. Endocrinol. 2022, 13, 948176. [Google Scholar] [CrossRef]
- Francis, T.G.; Jaka, O.; Ellison-Hughes, G.M.; Lazarus, N.R.; Harridge, S.D.R. Human Primary Skeletal Muscle-derived Myoblasts and Fibroblasts Reveal Different Senescent Phenotypes. JCSM Rapid Commun. 2022, 5, 226–238. [Google Scholar] [CrossRef]
- Aihemaiti, A.; Yamamoto, N.; Piao, J.; Oyaizu, T.; Ochi, H.; Sato, S.; Okawa, A.; Miyata, T.; Tsuji, K.; Ezura, Y.; et al. A Novel PAI-1 Inhibitor Prevents Ageing-Related Muscle Fiber Atrophy. Biochem. Biophys. Res. Commun. 2021, 534, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Pistilli, E.E.; Jackson, J.R.; Alway, S.E. Death Receptor-Associated pro-Apoptotic Signaling in Aged Skeletal Muscle. Apoptosis 2006, 11, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Rogina, B.; Tissenbaum, H.A. SIRT1, Resveratrol and Aging. Front. Genet. 2024, 15, 1393181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, F.; He, Z.; Che, L.; Chen, X.; Yuan, Y.; Liu, C. Senescence-Targeted and NAD(+)-Dependent SIRT1-Activated Nanoplatform to Counteract Stem Cell Senescence for Promoting Aged Bone Regeneration. Small 2024, 20, e2304433. [Google Scholar] [CrossRef]
- You, Y.; Liang, W. SIRT1 and SIRT6: The Role in Aging-Related Diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166815. [Google Scholar] [CrossRef]
- Lakhdar, R.; McGuinness, D.; Drost, E.M.; Shiels, P.G.; Bastos, R.; MacNee, W.; Rabinovich, R.A. Role of Accelerated Aging in Limb Muscle Wasting of Patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 1987–1998. [Google Scholar] [CrossRef]
- Machida, S.; Booth, F.W. Increased Nuclear Proteins in Muscle Satellite Cells in Aged Animals as Compared to Young Growing Animals. Exp. Gerontol. 2004, 39, 1521–1525. [Google Scholar] [CrossRef]
- Mañas-García, L.; Guitart, M.; Duran, X.; Barreiro, E. Satellite Cells and Markers of Muscle Regeneration during Unloading and Reloading: Effects of Treatment with Resveratrol and Curcumin. Nutrients 2020, 12, 1870. [Google Scholar] [CrossRef]
- Rathbone, C.R.; Booth, F.W.; Lees, S.J. Sirt1 Increases Skeletal Muscle Precursor Cell Proliferation. Eur. J. Cell Biol. 2009, 88, 35–44. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Liu, J.; Pan, R.; Lee, S.M.-Y.; Lin, L. Myricanol Rescues Dexamethasone-Induced Muscle Dysfunction via a Sirtuin 1-Dependent Mechanism. J. Cachexia. Sarcopenia Muscle 2019, 10, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.J.; Shaik, F.; Shaik, F.; Alway, S.E.; Mohamed, J.S. Skeletal Muscle Gene Expression Profile in Response to Caloric Restriction and Aging: A Role for SirT1. Genes 2021, 12, 691. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, D.; Jiang, S.; Li, H.; Chen, L.; Wu, Y.; Essien, A.E.; Opoku, M.; Naranmandakh, S.; Liu, S.; et al. SIRT1 Signaling Pathways in Sarcopenia: Novel Mechanisms and Potential Therapeutic Targets. Biomed. Pharmacother. 2024, 177, 116917. [Google Scholar] [CrossRef] [PubMed]
- Cariati, I.; Bonanni, R.; Annino, G.; Scimeca, M.; Bonanno, E.; D’Arcangelo, G.; Tancredi, V. Dose-Response Effect of Vibratory Stimulus on Synaptic and Muscle Plasticity in a Middle-Aged Murine Model. Front. Physiol. 2021, 12, 678449. [Google Scholar] [CrossRef]
| Gene | Sequence (5′–3′) | |
|---|---|---|
| CDKN1A | Forward | GACACCACTGGAGGGTGACT |
| Reverse | CAGGTCCACATGGTCTTCCT | |
| CDKN1B | Forward | ATAAGGAAGCGACCTGCAACCG |
| Reverse | TTCTTGGGCGTCTGCTCCACAG | |
| CDKN2A | Forward | CTTCCTGGACAGGCTGGTG |
| Reverse | ATGGTTACTGCCTCTGGTGC | |
| p53 | Forward | TAAGCGAGCACTGCCCAACA |
| Reverse | TCCTTGAGTTCCAAGGCCTC | |
| TNF-α | Forward | CCTCTCTCTAATCAGCCCTCTG |
| Reverse | GAGGACCTGGGAGTAGATGAG | |
| IL-1β | Forward | ATGATGGCTTATTACAGTGGCAA |
| Reverse | GTCGGAGATTCGTAGCTGGA | |
| IL-6 | Forward | GGTACATCCTCGACGGCATCT |
| Reverse | GTGCCTCTTTGCTGCTTTCAC | |
| IL-15 | Forward | CATGGTATTGGGAACCATAGATTTG |
| Reverse | CATTCACCCAGTTGGCTTCTG | |
| CCL2 | Forward | AGAATCACCAGCAGCAAGTGTC |
| Reverse | TCCTGAACCCACTTCTGCTTGG | |
| CCL3 | Forward | AGCTGACTACTTTGAGACGAGCA |
| Reverse | CGGCTTCGCTTGGTTAGGA | |
| GDF15 | Forward | CACCCTCAGAGTTGCACTCC |
| Reverse | GCCTGGTTAGCAGGTCCTC | |
| MMP3 | Forward | CACTCACAGACCTGACTCGGTT |
| Reverse | AAGCAGGATCACAGTTGGCTGG | |
| PAI-1 | Forward | CTCATCAGCCACTGGAAAGGCA |
| Reverse | GACTCGTGAAGTCAGCCTGAAAC | |
| FAS | Forward | TGAAGGACATGGCTTAGAAGTG |
| Reverse | GGTGCAAGGGTCACAGTGTT | |
| SIRT1 | Forward | TAGACACGCTGGAACAGGTTGC |
| Reverse | CTCCTCGTACAGCTTCACAGTC | |
| β-ACTIN | Forward | ACTCCATGCCCAGGAAGGAA |
| Reverse | GAGATGGCCACGGCTGCTT |
| Parameters | OA (n = 13) | Fractured (n = 13) | p-Value | Variance (s2) |
|---|---|---|---|---|
| Age (years) | 75.1 ± 7.0 | 74.3 ± 7.9 | p = 0.7949 | OA: 49.1 Fractured: 62.2 |
| BMI (Kg/m2) | 27.2 ± 5.1 | 26.8 ± 3.4 | p = 0.7954 | OA: 26.0 Fractured: 11.6 |
| T-score (L1–L4) | 1.0 ± 0.8 | -2.6 ± 0.6 | **** p < 0.0001 | OA: 0.7 Fractured: 0.4 |
| T-score (femoral neck) | 0.4 ± 1.2 | -2.5 ± 0.6 | **** p < 0.0001 | OA: 1.5 Fractured: 0.3 |
| T-score (total femur) | 0.7 ± 1.5 | -2.8± 0.6 | **** p < 0.0001 | OA: 2.3 Fractured: 0.4 |
| 25-(OH)-Vit D (ng/mL) | 20.1 ± 9.5 | 13.4 ± 6.0 | * p < 0.05 | OA: 89.5 Fractured: 36.6 |
| PTH (pg/mL) | 72.2 ± 21.6 | 111.8 ± 11.2 | **** p < 0.0001 | OA: 465.1 Fractured: 125.7 |
| Gene | OA (n = 13) | Fractured (n = 13) | p-Value | Variance (s2) |
|---|---|---|---|---|
| CDKN1A | 1.5 ± 0.3 | 7.2 ± 1.6 | *** p < 0.001 | OA: 1.1 Fractured: 33.6 |
| CDKN1B | 1.6 ± 0.4 | 3.0 ± 0.5 | * p < 0.05 | OA: 2.2 Fractured: 3.9 |
| CDKN2A | 2.4 ± 1.1 | 6.9 ± 1.9 | * p < 0.05 | OA: 15.0 Fractured: 47.6 |
| p53 | 1.1 ± 0.2 | 6.4 ± 1.1 | **** p < 0.0001 | OA: 0.5 Fractured: 17.0 |
| Gene | OA (n = 13) | Fractured (n = 13) | p-Value | Variance (s2) |
|---|---|---|---|---|
| TNF-α | 1.7 ± 0.6 | 9.0 ± 2.1 | **** p < 0.0001 | OA: 5.2 Fractured: 59.0 |
| IL-1β | 2.2 ± 1.0 | 6.8 ± 1.8 | ** p < 0.01 | OA: 13.1 Fractured: 40.1 |
| IL-6 | 1.2 ± 0.2 | 5.1 ± 1.4 | ** p < 0.01 | OA: 0.5 Fractured: 25.6 |
| IL-15 | 1.1 ± 0.1 | 3.1 ± 0.6 | ** p < 0.01 | OA: 0.3 Fractured: 4.6 |
| CCL2 | 1.8 ± 0.5 | 3.6 ± 0.8 | * p < 0.05 | OA: 3.1 Fractured: 8.0 |
| CCL3 | 2.1 ± 0.8 | 7.5 ± 2.6 | * p < 0.05 | OA: 7.0 Fractured: 70.3 |
| GDF15 | 1.6 ± 0.4 | 4.1 ± 0.7 | ** p < 0.01 | OA: 2.5 Fractured: 6.3 |
| MMP3 | 2.4 ± 0.9 | 4.5 ± 1.5 | p = 0.6139 | OA: 10.1 Fractured: 30.5 |
| PAI-1 | 1.8 ± 0.4 | 1.9 ± 0.6 | p = 0.8010 | OA: 2.0 Fractured: 5.3 |
| FAS | 1.5 ± 0.3 | 1.4 ± 0.5 | p = 0.3358 | OA: 1.6 Fractured: 3.5 |
| SIRT1 Expression | OA (n = 13) | Fractured (n = 13) | p-Value | Variance (s2) |
|---|---|---|---|---|
| Immunohistochemistry (MOD value) | 0.45 ± 0.02 | 0.32 ± 0.02 | *** p < 0.001 | OA: 0.01 Fractured: 0.01 |
| Western Blotting (protein levels) | 1.39 ± 0.04 | 0.46 ± 0.05 | **** p < 0.0001 | OA: 0.01 Fractured: 0.01 |
| qRT-PCR (mRNA levels) | 1.2 ± 0.2 | 0.3 ± 0.1 | *** p < 0.001 | OA: 0.5 Fractured: 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falvino, A.; Bonanni, R.; Gasperini, B.; Cariati, I.; Chiavoghilefu, A.; Smakaj, A.; Visconti, V.V.; Botta, A.; Iundusi, R.; Gasbarra, E.; et al. Altered Expression of Cell Cycle Regulators and Factors Released by Aged Cells in Skeletal Muscle of Patients with Bone Fragility: A Pilot Study on the Potential Role of SIRT1 in Muscle Atrophy. Biomedicines 2025, 13, 1350. https://doi.org/10.3390/biomedicines13061350
Falvino A, Bonanni R, Gasperini B, Cariati I, Chiavoghilefu A, Smakaj A, Visconti VV, Botta A, Iundusi R, Gasbarra E, et al. Altered Expression of Cell Cycle Regulators and Factors Released by Aged Cells in Skeletal Muscle of Patients with Bone Fragility: A Pilot Study on the Potential Role of SIRT1 in Muscle Atrophy. Biomedicines. 2025; 13(6):1350. https://doi.org/10.3390/biomedicines13061350
Chicago/Turabian StyleFalvino, Angela, Roberto Bonanni, Beatrice Gasperini, Ida Cariati, Angela Chiavoghilefu, Amarildo Smakaj, Virginia Veronica Visconti, Annalisa Botta, Riccardo Iundusi, Elena Gasbarra, and et al. 2025. "Altered Expression of Cell Cycle Regulators and Factors Released by Aged Cells in Skeletal Muscle of Patients with Bone Fragility: A Pilot Study on the Potential Role of SIRT1 in Muscle Atrophy" Biomedicines 13, no. 6: 1350. https://doi.org/10.3390/biomedicines13061350
APA StyleFalvino, A., Bonanni, R., Gasperini, B., Cariati, I., Chiavoghilefu, A., Smakaj, A., Visconti, V. V., Botta, A., Iundusi, R., Gasbarra, E., Tancredi, V., & Tarantino, U. (2025). Altered Expression of Cell Cycle Regulators and Factors Released by Aged Cells in Skeletal Muscle of Patients with Bone Fragility: A Pilot Study on the Potential Role of SIRT1 in Muscle Atrophy. Biomedicines, 13(6), 1350. https://doi.org/10.3390/biomedicines13061350







