Interaction Between Periodontitis and MASLD: Pathophysiological Associations and Possibilities of Prevention and Therapy
Abstract
1. Introduction
2. Data Collection
3. Periodontitis
3.1. Etiology, Pathogenesis, and Immune Response
3.2. Clinical Features, Progression, and Therapeutic Interventions in Periodontitis
4. MASLD
4.1. Pathophysiological Mechanisms and Risk Factors
4.2. Clinical Features, Progression, and Therapeutic Interventions in MASLD
5. Bidirectional Relationship Between Periodontitis and MASLD
5.1. Shared Inflammatory Mechanisms
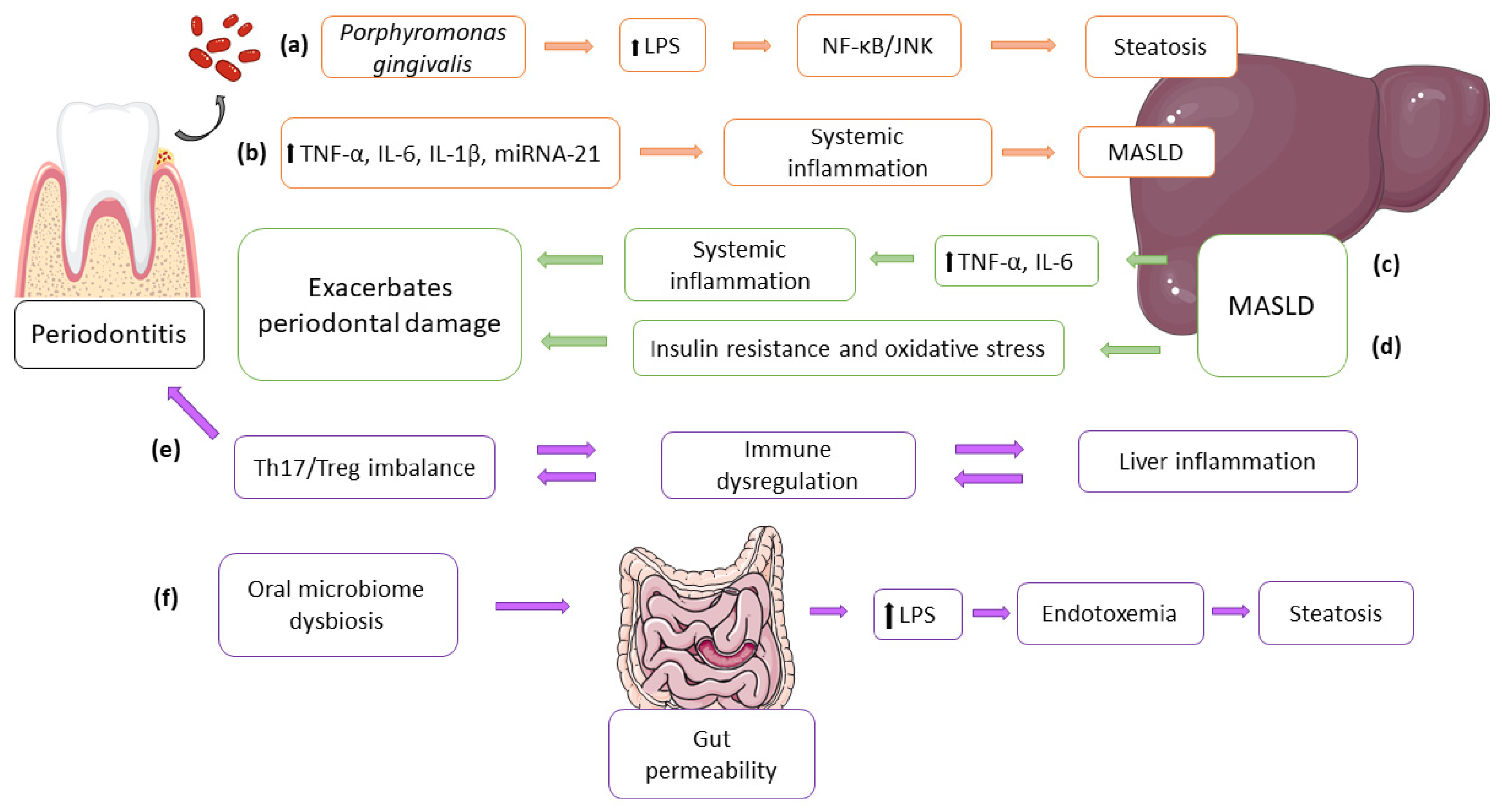
5.2. Impact of Porphyromonas Gingivalis on Liver Function: Direct and Indirect Pathways
6. Prevention Strategies, Lifestyle Modifications, and Oral Health
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kane, S.F. The Effects of Oral Health on Systemic Health. Gen. Dent. 2017, 65, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.M.; Reis, C.; Manzanares-Céspedes, M.C. Chronic Periodontitis, Inflammatory Cytokines, and Interrelationship with Other Chronic Diseases. Postgrad. Med. 2018, 130, 98–104. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and Systemic Mechanisms Linking Periodontal Disease and Inflammatory Comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Winning, L.; Linden, G.J. Periodontitis and Systemic Disease. BDJ Team 2015, 2, 15163. [Google Scholar] [CrossRef]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of Periodontitis in Dentate People between 2011 and 2020: A Systematic Review and Meta-Analysis of Epidemiological Studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.Y.; Li, B.H.; Wu, L.; Xie, W.Z.; Zhou, X.; Ma, B. Association between Periodontal Disease and Systemic Diseases: A Cross-Sectional Analysis of Current Evidence. Mil. Med. Res. 2024, 11, 74. [Google Scholar] [CrossRef]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between Periodontal Pathogens and Systemic Disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Kumar, P.S. From Focal Sepsis to Periodontal Medicine: A Century of Exploring the Role of the Oral Microbiome in Systemic Disease. J. Physiol. 2017, 595, 465–476. [Google Scholar] [CrossRef]
- Loos, B.G.; Van Dyke, T.E. The Role of Inflammation and Genetics in Periodontal Disease. Periodontol. 2000 2020, 83, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated Naming and Diagnosis Criteria for Fatty Liver Disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef] [PubMed]
- Shine, B.K.; Son, M.; Moon, S.Y.; Han, S.H. Metabolic Dysfunction-Associated Steatotic Liver Disease and the Risk of Chronic Periodontitis: A Nationwide Cohort Study. Nutrients 2025, 17, 125. [Google Scholar] [CrossRef]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Belluz, M.; Longhi, E.V. Periodontal Disease. In Managing Psychosexual Consequences in Chronic Diseases; Springer: Cham, Switzerland, 2023; pp. 329–336. [Google Scholar] [CrossRef]
- Slots, J. Primer on Etiology and Treatment of Progressive/Severe Periodontitis: A Systemic Health Perspective. Periodontol. 2000 2020, 83, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S162–S170. [Google Scholar] [CrossRef]
- Kwon, T.H.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Liu, S.; Zhang, S.; Pan, Y. The Role of Porphyromonas Gingivalis Outer Membrane Vesicles in Periodontal Disease and Related Systemic Diseases. Front. Cell. Infect. Microbiol. 2021, 10, 585917. [Google Scholar] [CrossRef]
- Czerniuk, M.R.; Surma, S.; Romańczyk, M.; Nowak, J.M.; Wojtowicz, A.; Filipiak, K.J. Unexpected Relationships: Periodontal Diseases: Atherosclerosis–Plaque Destabilization? From the Teeth to a Coronary Event. Biology 2022, 11, 272. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current Understanding of Periodontal Disease Pathogenesis and Targets for Host-Modulation Therapy. Periodontol. 2000 2020, 84, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.A.; Dou, Y.; Fletcher, H.M.; Boskovic, D.S. Local and Systemic Effects of Porphyromonas Gingivalis Infection. Microorganisms 2023, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Abu, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.R. Periodontitis: An Oral Disease with Severe Consequences. Appl. Biochem. Biotechnol. 2023, 195, 17–32. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Leira, Y.; Seoane, J.; Blanco, M.; Rodríguez-Yáñez, M.; Takkouche, B.; Blanco, J.; Castillo, J. Association between Periodontitis and Ischemic Stroke: A Systematic Review and Meta-Analysis. Eur. J. Epidemiol. 2017, 32, 43–53. [Google Scholar] [CrossRef]
- Gomes-Filho, I.S.; Coelho, J.M.F.; Miranda, S.S.; Cruz, S.S.; Trindade, S.C.; Cerqueira, E.M.M.; Passos-Soares, J.S.; Costa, M.d.C.N.; Vianna, M.I.P.; Figueiredo, A.C.M.G.; et al. Severe and Moderate Periodontitis Are Associated with Acute Myocardial Infarction. J. Periodontol. 2020, 91, 1444–1452. [Google Scholar] [CrossRef]
- Zardawi, F.; Gul, S.; Abdulkareem, A.; Sha, A.; Yates, J. Association Between Periodontal Disease and Atherosclerotic Cardiovascular Diseases: Revisited. Front. Cardiovasc. Med. 2021, 7, 625579. [Google Scholar] [CrossRef]
- Freiherr Von Seckendorff, A.; Nomenjanahary, M.S.; Labreuche, J.; Ollivier, V.; Di Meglio, L.; Dupont, S.; Hamdani, M.; Brikci-Nigassa, N.; Brun, A.; Boursin, P.; et al. Periodontitis in Ischemic Stroke: Impact of Porphyromonas Gingivalis on Thrombus Composition and Ischemic Stroke Outcomes. Res. Pract. Thromb. Haemost. 2024, 8, 102313. [Google Scholar] [CrossRef]
- Imai, K.; Iinuma, T.; Sato, S. Relationship between the Oral Cavity and Respiratory Diseases: Aspiration of Oral Bacteria Possibly Contributes to the Progression of Lower Airway Inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 224–230. [Google Scholar] [CrossRef]
- Molina, A.; Huck, O.; Herrera, D.; Montero, E. The Association between Respiratory Diseases and Periodontitis: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2023, 50, 842–887. [Google Scholar] [CrossRef]
- Nemesh, O.M.; Honta, Z.M.; Slaba, O.M.; Shylivskyi, I.V. Pathogenetic Mechanisms of Comorbidity of Systemic Diseases and Periodontal Pathology. Wiad. Lek. 2021, 74, 1262–1267. [Google Scholar] [CrossRef]
- Qasim, S.S.B.; Al-Otaibi, D.; Al-Jasser, R.; Gul, S.S.; Zafar, M.S. An Evidence-Based Update on the Molecular Mechanisms Underlying Periodontal Diseases. Int. J. Mol. Sci. 2020, 21, 3829. [Google Scholar] [CrossRef] [PubMed]
- Hashioka, S.; Inoue, K.; Hayashida, M.; Wake, R.; Oh-Nishi, A.; Miyaoka, T. Implications of Systemic Inflammation and Periodontitis for Major Depression. Front. Neurosci. 2018, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Al-Nasser, L.; Lamster, I.B. Prevention and Management of Periodontal Diseases and Dental Caries in the Older Adults. Periodontol. 2000 2020, 84, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute Periodontal Lesions (Periodontal Abscesses and Necrotizing Periodontal Diseases) and Endo-Periodontal Lesions. J. Periodontol. 2018, 89 (Suppl. S1), S85–S102. [Google Scholar] [CrossRef]
- Jervøe-Storm, P.M.; Eberhard, J.; Needleman, I.; Worthington, H.V.; Jepsen, S. Full-Mouth Treatment Modalities (within 24 Hours) for Periodontitis in Adults. Cochrane Database Syst. Rev. 2022, 6, CD004622. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions—Introduction and Key Changes from the 1999 Classification. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S1–S8. [Google Scholar] [CrossRef]
- Falcao, A.; Bullón, P. A Review of the Influence of Periodontal Treatment in Systemic Diseases. Periodontol. 2000 2019, 79, 117–128. [Google Scholar] [CrossRef]
- Dioguardi, M.; Crincoli, V.; Laino, L.; Alovisi, M.; Sovereto, D.; Mastrangelo, F.; Lo Russo, L.; Lo Muzio, L. The Role of Periodontitis and Periodontal Bacteria in the Onset and Progression of Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; Papapanou, P.N. Clinical Application of the New Classification of Periodontal Diseases: Ground Rules, Clarifications and ‘Gray Zones’. J. Periodontol. 2020, 91, 352–360. [Google Scholar] [CrossRef]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Kebschull, M.; Jepsen, S.; Sculean, A.; Berglundh, T.; Papapanou, P.N.; Chapple, I.; Tonetti, M.S. Treatment of Stage IV Periodontitis: The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2022, 49 (Suppl. S24), 4–71. [Google Scholar] [CrossRef]
- Caffesse, R.G.; Echeverría, J.J. Treatment Trends in Periodontics. Periodontol. 2000 2019, 79, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, M.; Muñoz Aguilera, E.; Marletta, D.; Petrie, A.; Suvan, J.; D’Aiuto, F. Impact of the Treatment of Periodontitis on Systemic Health and Quality of Life: A Systematic Review. J. Clin. Periodontol. 2022, 49 (Suppl. S24), 314–327. [Google Scholar] [CrossRef]
- Cope, A.L.; Francis, N.; Wood, F.; Thompson, W.; Chestnutt, I.G. Systemic Antibiotics for Symptomatic Apical Periodontitis and Acute Apical Abscess in Adults. Cochrane Database Syst. Rev. 2024, 5, CD010136. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Merete Aass, A.; Aimetti, M.; et al. Treatment of Stage I-III Periodontitis-The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef]
- Graetz, C.; Mann, L.; Krois, J.; Sälzer, S.; Kahl, M.; Springer, C.; Schwendicke, F. Comparison of Periodontitis Patients’ Classification in the 2018 versus 1999 Classification. J. Clin. Periodontol. 2019, 46, 908–917. [Google Scholar] [CrossRef]
- Newman, M.G.; Klokkevold, P.R.; Elangovan, S.; Kapila, Y. Newman and Carranza’s Clinical Periodontology and Implantology, 14th ed.; Carranza, F.A., Takei, H., Eds.; Elsevier: St. Louis, MO, USA, 2023; p. 1048. [Google Scholar]
- Scannapieco, F.A.; Gershovich, E. The Prevention of Periodontal Disease-An Overview. Periodontol. 2000 2020, 84, 9–13. [Google Scholar] [CrossRef]
- Salvi, G.E.; Roccuzzo, A.; Imber, J.C.; Stähli, A.; Klinge, B.; Lang, N.P. Clinical Periodontal Diagnosis. Periodontol. 2000 2023. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-Regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef]
- Nakahara, T.; Hyogo, H.; Ono, A.; Nagaoki, Y.; Kawaoka, T.; Miki, D.; Tsuge, M.; Hiraga, N.; Hayes, C.N.; Hiramatsu, A.; et al. Involvement of Porphyromonas Gingivalis in the Progression of Non-Alcoholic Fatty Liver Disease. J. Gastroenterol. 2018, 53, 269–280. [Google Scholar] [CrossRef]
- Portincasa, P.; Khalil, M.; Mahdi, L.; Perniola, V.; Idone, V.; Graziani, A.; Baffy, G.; Di Ciaula, A. Metabolic Dysfunction–Associated Steatotic Liver Disease: From Pathogenesis to Current Therapeutic Options. Int. J. Mol. Sci. 2024, 25, 5640. [Google Scholar] [CrossRef]
- Tauil, R.B.; Golono, P.T.; de Lima, E.P.; de Alvares Goulart, R.; Guiguer, E.L.; Bechara, M.D.; Nicolau, C.C.T.; Yanaguizawa Junior, J.L.; Fiorini, A.M.R.; Méndez-Sánchez, N.; et al. Metabolic-Associated Fatty Liver Disease: The Influence of Oxidative Stress, Inflammation, Mitochondrial Dysfunctions, and the Role of Polyphenols. Pharmaceuticals 2024, 17, 1354. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.K.; Bansal, M.B. Pathogenesis of MASLD and MASH—Role of Insulin Resistance and Lipotoxicity. Aliment. Pharmacol. Ther. 2024, 59 (Suppl. S1), S10–S22. [Google Scholar] [CrossRef]
- Schwärzler, J.; Grabherr, F.; Grander, C.; Adolph, T.E.; Tilg, H. The Pathophysiology of MASLD: An Immunometabolic Perspective. Expert Rev. Clin. Immunol. 2024, 20, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.K.; Tripathi, M.; Singh, B.K.; Verma, M.K.; Tripathi, M.; Singh, B.K. Dietary Determinants of Metabolic Syndrome: Focus on the Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). In Metabolic Syndrome—Lifestyle and Biological Risk; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Habib, S. Team Players in the Pathogenesis of Metabolic Dysfunctions-Associated Steatotic Liver Disease: The Basis of Development of Pharmacotherapy. World J. Gastrointest. Pathophysiol. 2024, 15, 93606. [Google Scholar] [CrossRef]
- Ha, S.; Wong, V.W.S.; Zhang, X.; Yu, J. Interplay between Gut Microbiome, Host Genetic and Epigenetic Modifications in MASLD and MASLD-Related Hepatocellular Carcinoma. Gut 2024, 74, 141–152. [Google Scholar] [CrossRef]
- Verdelho Machado, M. Circadian Deregulation: Back Facing the Sun Toward Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Development. Nutrients 2024, 16, 4294. [Google Scholar] [CrossRef]
- Sookoian, S.; Rotman, Y.; Valenti, L. Genetics of Metabolic Dysfunction-Associated Steatotic Liver Disease: The State of the Art Update. Clin. Gastroenterol. Hepatol. 2024, 22, 2177–2187.e3. [Google Scholar] [CrossRef] [PubMed]
- Theys, C.; Lauwers, D.; Perez-Novo, C.; Vanden Berghe, W. PPARα in the Epigenetic Driver Seat of NAFLD: New Therapeutic Opportunities for Epigenetic Drugs? Biomedicines 2022, 10, 3041. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Johnson, A. An Overview of Pathogenesis of Metabolic Dysfunction-Associated Steatotic Liver Disease. Explor. Dig. Dis. 2024, 3, 459–473. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N. There and Back Again: Leptin Actions in White Adipose Tissue. Int. J. Mol. Sci. 2020, 21, 6039. [Google Scholar] [CrossRef]
- Bourganou, M.V.; Chondrogianni, M.E.; Kyrou, I.; Flessa, C.-M.; Chatzigeorgiou, A.; Oikonomou, E.; Lambadiari, V.; Randeva, H.S.; Kassi, E. Unraveling Metabolic Dysfunction-Associated Steatotic Liver Disease Through the Use of Omics Technologies. Int. J. Mol. Sci. 2025, 26, 1589. [Google Scholar] [CrossRef]
- Vidal-Cevallos, P.; Sorroza-Martínez, A.P.; Chávez-Tapia, N.C.; Uribe, M.; Montalvo-Javé, E.E.; Nuño-Lámbarri, N. The Relationship between Pathogenesis and Possible Treatments for the MASLD-Cirrhosis Spectrum. Int. J. Mol. Sci. 2024, 25, 4397. [Google Scholar] [CrossRef]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Njei, B.; Ameyaw, P.; Al-Ajlouni, Y.; Njei, L.-P.; Boateng, S. Diagnosis and Management of Lean Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Systematic Review. Cureus 2024, 16, e71451. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Jin, Y.; Li, R.; Yin, Q.; Chen, J.; Li, T.; Zhang, M.; Zhuge, Y. Clinical Characteristics of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)-Related Decompensated Cirrhosis: Comparison with Hepatitis B Virus (HBV)-Associated Decompensated Cirrhosis. SSRN 2023. [Google Scholar] [CrossRef]
- Ali, H.; Shahzil, M.; Moond, V.; Shahzad, M.; Thandavaram, A.; Sehar, A.; Waseem, H.; Siddiqui, T.; Dahiya, D.S.; Patel, P.; et al. Non-Pharmacological Approach to Diet and Exercise in Metabolic-Associated Fatty Liver Disease: Bridging the Gap between Research and Clinical Practice. J. Pers. Med. 2024, 14, 61. [Google Scholar] [CrossRef]
- Armandi, A.; Bugianesi, E. Dietary and Pharmacological Treatment in Patients with Metabolic-Dysfunction Associated Steatotic Liver Disease. Eur. J. Intern. Med. 2024, 122, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Beygi, M.; Ahi, S.; Zolghadri, S.; Stanek, A. Management of Metabolic-Associated Fatty Liver Disease/Metabolic Dysfunction-Associated Steatotic Liver Disease: From Medication Therapy to Nutritional Interventions. Nutrients 2024, 16, 2220. [Google Scholar] [CrossRef] [PubMed]
- Gries, J.J.; Lazarus, J.V.; Brennan, P.N.; Siddiqui, M.S.; Targher, G.; Lang, C.C.; Virani, S.S.; Lavie, C.J.; Isaacs, S.; Arab, J.P.; et al. Interdisciplinary Perspectives on the Co-Management of Metabolic Dysfunction-Associated Steatotic Liver Disease and Coronary Artery Disease. Lancet Gastroenterol. Hepatol. 2025, 10, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Wajcman, D.I.; Byrne, C.J.; Dillon, J.F.; Brennan, P.N.; Villota-Rivas, M.; Younossi, Z.M.; Allen, A.M.; Crespo, J.; Gerber, L.H.; Lazarus, J.V. A Narrative Review of Lifestyle Management Guidelines for Metabolic Dysfunction-Associated Steatotic Liver Disease. Hepatology 2024. [Google Scholar] [CrossRef]
- Zeng, J.; Fan, J.G.; Francque, S.M. Therapeutic Management of Metabolic Dysfunction Associated Steatotic Liver Disease. United Eur. Gastroenterol. J. 2024, 12, 177–186. [Google Scholar] [CrossRef]
- Stefan, N.; Yki-Järvinen, H.; Neuschwander-Tetri, B.A. Metabolic Dysfunction-Associated Steatotic Liver Disease: Heterogeneous Pathomechanisms and Effectiveness of Metabolism-Based Treatment. Lancet Diabetes Endocrinol. 2025, 13, 134–148. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Mehta, A.; Goh, R.S.J.; Zhang, A.; Chen, Y.; Chong, B.; Chew, H.S.J.; Shabbir, A.; Brown, A.; Dimitriadis, G.K.; et al. Cardiovascular-Liver-Metabolic Health: Recommendations in Screening, Diagnosis, and Management of Metabolic Dysfunction-Associated Steatotic Liver Disease in Cardiovascular Disease via Modified Delphi Approach. Circulation 2025, 151, 98–119. [Google Scholar] [CrossRef]
- Kang, M.K.; Song, J.; Loomba, R.; Park, S.; Tak, W.; Kweon, Y.; Lee, Y.; Park, J.G. Comparative Associations of MASLD and MAFLD with the Presence and Severity of Coronary Artery Calcification. Res. Rep. 2024, 14, 22917. [Google Scholar] [CrossRef]
- De Filippo, O.; Di Pietro, G.; Nebiolo, M.; Ribaldone, D.G.; Gatti, M.; Bruno, F.; Gallone, G.; Armandi, A.; Birtolo, L.I.; Zullino, V.; et al. Increased Prevalence of High-Risk Coronary Plaques in Metabolic Dysfunction Associated Steatotic Liver Disease Patients: A Meta-Analysis. Eur. J. Clin. Investig. 2024, 54, e14188. [Google Scholar] [CrossRef]
- Tacke, F.; Horn, P.; Wai-Sun Wong, V.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Mellemkjær, A.; Kjær, M.B.; Haldrup, D.; Grønbæk, H.; Thomsen, K.L. Management of Cardiovascular Risk in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease. Eur. J. Intern. Med. 2024, 122, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Lionis, C.; Papadakis, S.; Anastasaki, M.; Aligizakis, E.; Anastasiou, F.; Francque, S.; Gergianaki, I.; Mendive, J.M.; Marketou, M.; Muris, J.; et al. Practice Recommendations for the Management of MASLD in Primary Care: Consensus Results. Diseases 2024, 12, 180. [Google Scholar] [CrossRef]
- Lara-Romero, C.; Romero-Gómez, M. Treatment Options and Continuity of Care in Metabolic-Associated Fatty Liver Disease: A Multidisciplinary Approach. Eur. Cardiol. 2024, 19, e06. [Google Scholar] [CrossRef]
- Elshaer, A.; Chascsa, D.M.H.; Lizaola-Mayo, B.C. Exploring Varied Treatment Strategies for Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Life 2024, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Alakhali, M.S.; Al-Maweri, S.A.; Al-Shamiri, H.M.; Al-haddad, K.; Halboub, E. The Potential Association between Periodontitis and Non-Alcoholic Fatty Liver Disease: A Systematic Review. Clin. Oral Investig. 2018, 22, 2965–2974. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chen, J.; Liu, S.; Bu, S. Assessing Causal Relationships Between Periodontitis and Non-Alcoholic Fatty Liver Disease: A Two-Sample Bidirectional Mendelian Randomisation Study. Oral Health Prev. Dent. 2024, 22, 189–202. [Google Scholar] [CrossRef]
- Hajishengallis, G. Interconnection of Periodontal Disease and Comorbidities: Evidence, Mechanisms, and Implications. Periodontol. 2000 2022, 89, 9–18. [Google Scholar] [CrossRef]
- Xu, X.W.; Liu, X.; Shi, C.; Sun, H.C. Roles of Immune Cells and Mechanisms of Immune Responses in Periodontitis. Chin. J. Dent. Res. 2021, 24, 219–230. [Google Scholar] [CrossRef]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and Chemokines in Periodontitis. Eur. J. Dent. 2020, 14, 483–495. [Google Scholar] [CrossRef]
- Becerra-Ruiz, J.S.; Guerrero-Velázquez, C.; Martínez-Esquivias, F.; Martínez-Pérez, L.A.; Guzmán-Flores, J.M. Innate and Adaptive Immunity of Periodontal Disease. From Etiology to Alveolar Bone Loss. Oral Dis. 2022, 28, 1441–1447. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The Cytokine Network Involved in the Host Immune Response to Periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Carrillo, J.L.; Hernández-Reyes, V.E.; García-Huerta, O.E.; Chávez-Ruvalcaba, F.; Chávez-Ruvalcaba, M.I.; Chávez-Ruvalcaba, K.M.; Díaz-Alfaro, L.; Muñoz-Carrillo, J.L.; Hernández-Reyes, V.E.; García-Huerta, O.E.; et al. Pathogenesis of Periodontal Disease. In Periodontal Disease—Diagnostic and Adjunctive Non-Surgical Considerations; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Degasperi, G.R.; Etchegaray, A.; Marcelino, L.; Sicard, A.; Villalpando, K.; Pinheiro, S.L.; Degasperi, G.R.; Etchegaray, A.; Marcelino, L.; Sicard, A.; et al. Periodontal Disease: General Aspects from Biofilm to the Immune Response Driven by Periodontal Pathogens. Adv. Microbiol. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef]
- Han, N.; Liu, Y.; Du, J.; Xu, J.; Guo, L.; Liu, Y. Regulation of the Host Immune Microenvironment in Periodontitis and Periodontal Bone Remodeling. Int. J. Mol. Sci. 2023, 24, 3158. [Google Scholar] [CrossRef]
- Alazawi, W.; Bernabe, E.; Tai, D.; Janicki, T.; Kemos, P.; Samsuddin, S.; Syn, W.K.; Gillam, D.; Turner, W. Periodontitis Is Associated with Significant Hepatic Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2017, 12, e0185902. [Google Scholar] [CrossRef] [PubMed]
- Kuraji, R.; Sekino, S.; Kapila, Y.; Numabe, Y. Periodontal Disease-Related Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Emerging Concept of Oral-Liver Axis. Periodontol. 2000 2021, 87, 204–240. [Google Scholar] [CrossRef] [PubMed]
- Rinčić, G.; Gaćina, P.; Jukić, L.V.; Rinčić, N.; Božić, D.; Badovinac, A. ASSOCIATION BETWEEN PERIODONTITIS AND LIVER DISEASE. Acta Clin. Croat. 2022, 60, 510–518. [Google Scholar] [CrossRef]
- Vasconcelos, A.C.C.G.; Vasconcelos, D.F.P.; Pereira da Silva, F.R.; de Carvalho França, L.F.; Alves, E.H.P.; Di Lenardo, D.; dos Santos Pessoa, L.; Novaes, P.D.; Luiz dos Reis Barbosa, A.; Mani, A.; et al. Periodontitis Causes Abnormalities in the Liver of Rats. J. Periodontol. 2018, 90, 295. [Google Scholar] [CrossRef]
- Gheorghe, D.N.; Camen, A.; Popescu, D.M.; Sincar, C.; Pitru, A.; Ionele, C.M.; Nicolae, F.M.; Danilescu, C.M.; Roman, A.; Florescu, C. Periodontitis, Metabolic and Gastrointestinal Tract Diseases: Current Perspectives on Possible Pathogenic Connections. J. Pers. Med. 2022, 12, 341. [Google Scholar] [CrossRef]
- Hatipoglu, H.; Kartal, A.; Kartal, I.; Yaylak, F. Non-Alcoholic Fatty Liver and Periodontal Disease: Is There a Relationship? A Contemporary Review. J. Inonu Liver Transplant. Inst. 2023, 1, 81–89. [Google Scholar] [CrossRef]
- Grønkjær, L.L.; Vilstrup, H. Oral Health in Patients with Liver Cirrhosis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 834–839. [Google Scholar] [CrossRef]
- Qiao, F.; Li, X.; Liu, Y.; Zhang, S.; Liu, D.; Li, C. Periodontitis and NAFLD-Related Diseases: A Bidirectional Two-Sample Mendelian Randomization Study. Oral Dis. 2024, 30, 3452–3461. [Google Scholar] [CrossRef]
- Tan, L.; He, Y.; Wang, T.; Gao, X.; Fan, W.; Fan, B. A Mendelian Randomization Study between Chronic Periodontitis and Non-Alcoholic Fatty Liver Disease. J. Periodontal Res. 2024, 59, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Vegda, H.S.; Patel, B.; Girdhar, G.A.; Pathan, M.S.H.; Ahmad, R.; Haque, M.; Sinha, S.; Kumar, S. Role of Nonalcoholic Fatty Liver Disease in Periodontitis: A Bidirectional Relationship. Cureus 2024, 16, e63775. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iwaki, M.; Nogami, A.; Honda, Y.; Ogawa, Y.; Imajo, K.; Saito, S.; Nakajima, A.; Yoneda, M. Involvement of Periodontal Disease in the Pathogenesis and Exacerbation of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis: A Review. Nutrients 2023, 15, 1269. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Li, J.; Liu, Y.; Zhou, S.; Wei, X.; Hua, H.; Tang, K.; Zhang, X.; Wang, Y.; Wu, Z.; et al. Roles of Immune Dysregulation in MASLD. Biomed. Pharmacother. 2024, 170, 116069. [Google Scholar] [CrossRef]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic Impacts of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Metabolic Dysfunction-Associated Steatohepatitis (MASH) on Heart, Muscle, and Kidney Related Diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Dai, K.; Liu, N.; Wang, J.; Lu, X.; Ma, J.; Zhang, M.; Xu, M.; Long, X.; et al. Inflammatory Response of Gut, Spleen, and Liver in Mice Induced by Orally Administered Porphyromonas Gingivalis. J. Oral Microbiol. 2022, 14, 2088936. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Huang, Z.; Mo, K.; Li, Z. Salivary Lipid Metabolism in Periodontitis Patients with Spleen-Stomach Dampness-Heat Syndrome. BMC Oral Health 2025, 25, 476. [Google Scholar] [CrossRef]
- Zhu, Y.-N.; Gu, X.-L.; Wang, L.-Y.; Guan, N.; Li, C.-G. All-Trans Retinoic Acid Promotes M2 Macrophage Polarization in Vitro by Activating the P38MAPK/STAT6 Signaling Pathway. Immunol. Investig. 2023, 52, 298–318. [Google Scholar] [CrossRef]
- Tarantino, G.; Citro, V.; Balsano, C. Liver-Spleen Axis in Nonalcoholic Fatty Liver Disease. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Sun, D.; Yang, J. Interaction between Periodontitis and Liver Diseases. Biomed. Rep. 2016, 5, 267–276. [Google Scholar] [CrossRef]
- Albuquerque-Souza, E.; Sahingur, S.E. Periodontitis, Chronic Liver Diseases, and the Emerging Oral-Gut-Liver Axis. Periodontol. 2000 2022, 89, 125–141. [Google Scholar] [CrossRef]
- Kuraji, R.; Shiba, T.; Dong, T.S.; Numabe, Y.; Kapila, Y.L. Periodontal Treatment and Microbiome-Targeted Therapy in Management of Periodontitis-Related Nonalcoholic Fatty Liver Disease with Oral and Gut Dysbiosis. World J. Gastroenterol. 2023, 29, 967–996. [Google Scholar] [CrossRef] [PubMed]
- Zenobia, C.; Darveau, R.P. Does Oral Endotoxin Contribute to Systemic Inflammation? Front. Oral Health 2022, 3, 911420. [Google Scholar] [CrossRef]
- Marroncini, G.; Naldi, L.; Martinelli, S.; Amedei, A. Gut–Liver–Pancreas Axis Crosstalk in Health and Disease: From the Role of Microbial Metabolites to Innovative Microbiota Manipulating Strategies. Biomedicines 2024, 12, 1398. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Katagiri, S.; Komazaki, R.; Watanabe, K.; Maekawa, S.; Shiba, T.; Udagawa, S.; Takeuchi, Y.; Ohtsu, A.; Kohda, T.; et al. Endotoxemia by Porphyromonas Gingivalis Injection Aggravates Non-Alcoholic Fatty Liver Disease, Disrupts Glucose/Lipid Metabolism, and Alters Gut Microbiota in Mice. Front. Microbiol. 2018, 9, 2470. [Google Scholar] [CrossRef]
- Kuraji, R.; Ye, C.; Zhao, C.; Gao, L.; Martinez, A.; Miyashita, Y.; Radaic, A.; Kamarajan, P.; Le, C.; Zhan, L.; et al. Nisin Lantibiotic Prevents NAFLD Liver Steatosis and Mitochondrial Oxidative Stress Following Periodontal Disease by Abrogating Oral, Gut and Liver Dysbiosis. NPJ Biofilms Microbiomes 2024, 10, 3. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; Bilodeau, S.; Greten, T.F.; Wang, X.W.; Trinchieri, G. The Gut-Liver Axis: Host Microbiota Interactions Shape Hepatocarcinogenesis. Trends Cancer 2022, 8, 583–597. [Google Scholar] [CrossRef]
- Ding, L.; Liang, L.; Zhao, Y.; Yang, Y.; Liu, F.; Ding, Q.; Luo, L. Porphyromonas Gingivalis-Derived Lipopolysaccharide Causes Excessive Hepatic Lipid Accumulation via Activating NF-ΚB and JNK Signaling Pathways. Oral Dis. 2019, 25, 1789–1797. [Google Scholar] [CrossRef]
- Nagasaki, A.; Sakamoto, S.; Chea, C.; Ishida, E.; Furusho, H.; Fujii, M.; Takata, T.; Miyauchi, M. Odontogenic Infection by Porphyromonas Gingivalis Exacerbates Fibrosis in NASH via Hepatic Stellate Cell Activation. Sci. Rep. 2020, 10, 4134. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tsung, A.; Mishra, L.; Huang, H. Regulatory T Cell: A Double-Edged Sword from Metabolic-Dysfunction-Associated Steatohepatitis to Hepatocellular Carcinoma. EBioMedicine 2024, 101, 105031. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Lan, D.; Li, X.; Wang, Y.; Qi, S.; Liu, Y. Porphyromonas Gingivalis Is a Risk Factor for the Development of Nonalcoholic Fatty Liver Disease via Ferroptosis. Microbes Infect. 2023, 25, 105040. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, P.; Wei, Y.; Ye, C.; Mao, D.; Xia, D.; Luo, Y. Porphyromonas Gingivalis Exacerbates Alcoholic Liver Disease by Altering Gut Microbiota Composition and Host Immune Response in Mice. J. Clin. Periodontol. 2023, 50, 1253–1263. [Google Scholar] [CrossRef]
- Ahn, J.S.; Yang, J.W.; Oh, S.J.; Shin, Y.Y.; Kang, M.J.; Park, H.R.; Seo, Y.; Kim, H.S. Porphyromonas Gingivalis Exacerbates the Progression of Fatty Liver Disease via CD36-PPARγ Pathway. BMB Rep. 2021, 54, 323–328. [Google Scholar] [CrossRef]
- Elsayed, R.; Elashiry, M.; Liu, Y.; El-Awady, A.; Hamrick, M.; Cutler, C.W. Porphyromonas Gingivalis Provokes Exosome Secretion and Paracrine Immune Senescence in Bystander Dendritic Cells. Front. Cell. Infect. Microbiol. 2021, 11, 669989. [Google Scholar] [CrossRef]
- Wu, L.; Shi, R.; Bai, H.; Wang, X.; Wei, J.; Liu, C.; Wu, Y. Porphyromonas Gingivalis Induces Increases in Branched-Chain Amino Acid Levels and Exacerbates Liver Injury Through Livh/Livk. Front. Cell. Infect. Microbiol. 2022, 12, 776996. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Xiao, J.; Yang, Q.; Huang, X.; Yang, Z.; Liu, H.; Liu, Y.; Wang, H.; Huang, Z.; et al. Autophagy Mediates the Impact of Porphyromonas Gingivalis on Short-Chain Fatty Acids Metabolism in Periodontitis-Induced Gut Dysbiosis. Sci. Rep. 2024, 14, 26291. [Google Scholar] [CrossRef]
- Lee, Y.H.; Hong, J.Y. Oral Microbiome as a Co-Mediator of Halitosis and Periodontitis: A Narrative Review. Front. Oral Health 2023, 4, 1229145. [Google Scholar] [CrossRef]
- Rabiu, L.; Zhang, P.; Afolabi, L.O.; Saliu, M.A.; Dabai, S.M.; Suleiman, R.B.; Gidado, K.I.; Ige, M.A.; Ibrahim, A.; Zhang, G.; et al. Immunological Dynamics in MASH: From Landscape Analysis to Therapeutic Intervention. J. Gastroenterol. 2024, 59, 1053–1078. [Google Scholar] [CrossRef]
- Bose, S.; Yashoda, R.; Puranik, M. Association between Oral Health and Alcoholic Liver Disease—A Cross-Sectional Analytical Study. J. Dent. Def. Sect. 2021, 15, 5. [Google Scholar] [CrossRef]
- Foo, L.H.; Balan, P.; Pang, L.M.; Laine, M.L.; Seneviratne, C.J. Role of the Oral Microbiome, Metabolic Pathways, and Novel Diagnostic Tools in Intra-Oral Halitosis: A Comprehensive Update. Crit. Rev. Microbiol. 2021, 47, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Durey, A.; Naoum, S.; Kruger, E.; Slack-Smith, L. Oral Health Education and Prevention Strategies among Remote Aboriginal Communities: A Qualitative Study. Aust. Dent. J. 2022, 67, 83–93. [Google Scholar] [CrossRef]
- Jonesn, G.; Wilson, H.; Smith, S.; Brown, T. Periodontitis: Causes, Symptoms, and Steps to Treatment. Fusion Multidiscip. Res. Int. J. 2023, 4, 445–457. [Google Scholar]
- Saengtipbovorn, S.; Taneepanichskul, S. Effectiveness of Lifestyle Change plus Dental Care Program in Improving Glycemic and Periodontal Status in Aging Patients with Diabetes: A Cluster, Randomized, Controlled Trial. J. Periodontol. 2015, 86, 507–515. [Google Scholar] [CrossRef]
- Yazdanian, M.; Armoon, B.; Noroozi, A.; Mohammadi, R.; Bayat, A.H.; Ahounbar, E.; Higgs, P.; Nasab, H.S.; Bayani, A.; Hemmat, M. Dental Caries and Periodontal Disease among People Who Use Drugs: A Systematic Review and Meta-Analysis. BMC Oral Health 2020, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S74–S84. [Google Scholar] [CrossRef]
- Bolukbasi, G.; Dundar, N. Oral Health in Older Adults: Current Insights and Tips. J. Gerontol. Geriatr. 2024, 72, 96–107. [Google Scholar] [CrossRef]
- Åberg, F.; Helenius-Hietala, J. Oral Health and Liver Disease: Bidirectional Associations—A Narrative Review. Dent. J. 2022, 10, 16. [Google Scholar] [CrossRef]
- Fujii, T.; Aoyama, N.; Kida, S.; Taniguchi, K.; Yata, T.; Minabe, M.; Komaki, M. Associations between Periodontal Status and Liver Function in the Japanese Population: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 4759. [Google Scholar] [CrossRef]
- Di Spirito, F. Oral and Systemic Health in the Elderly. Appl. Sci. 2022, 12, 11718. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A Comprehensive Review of the Application of Probiotics and Postbiotics in Oral Health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, S.; Maiorani, C.; Scribante, A.; Butera, A. The Home Use of Probiotics and Paraprobiotics for the Maintenance of Tongue Eubiosis: A Case Report. Case Rep. Dent. 2025, 2025, 5496240. [Google Scholar] [CrossRef] [PubMed]
- Gheisary, Z.; Mahmood, R.; Harri Shivanantham, A.; Liu, J.; Lieffers, J.R.L.; Papagerakis, P.; Papagerakis, S. The Clinical, Microbiological, and Immunological Effects of Probiotic Supplementation on Prevention and Treatment of Periodontal Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1036. [Google Scholar] [CrossRef]
- Carpi, R.Z.; Barbalho, S.M.; Sloan, K.P.; Laurindo, L.F.; Gonzaga, H.F.; Grippa, P.C.; Zutin, T.L.M.; Girio, R.J.S.; Repetti, C.S.F.; Detregiachi, C.R.P.; et al. The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8805. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juzbašić, M.; Tomas, M.; Petrović, A.; Hefer, M.; Sikora, R.; Mačković, A.; Siber, S.; Smolić, M. Interaction Between Periodontitis and MASLD: Pathophysiological Associations and Possibilities of Prevention and Therapy. Biomedicines 2025, 13, 1346. https://doi.org/10.3390/biomedicines13061346
Juzbašić M, Tomas M, Petrović A, Hefer M, Sikora R, Mačković A, Siber S, Smolić M. Interaction Between Periodontitis and MASLD: Pathophysiological Associations and Possibilities of Prevention and Therapy. Biomedicines. 2025; 13(6):1346. https://doi.org/10.3390/biomedicines13061346
Chicago/Turabian StyleJuzbašić, Martina, Matej Tomas, Ana Petrović, Marija Hefer, Renata Sikora, Ana Mačković, Stjepan Siber, and Martina Smolić. 2025. "Interaction Between Periodontitis and MASLD: Pathophysiological Associations and Possibilities of Prevention and Therapy" Biomedicines 13, no. 6: 1346. https://doi.org/10.3390/biomedicines13061346
APA StyleJuzbašić, M., Tomas, M., Petrović, A., Hefer, M., Sikora, R., Mačković, A., Siber, S., & Smolić, M. (2025). Interaction Between Periodontitis and MASLD: Pathophysiological Associations and Possibilities of Prevention and Therapy. Biomedicines, 13(6), 1346. https://doi.org/10.3390/biomedicines13061346






