Multidrug-Resistant Infections and Metabolic Syndrome: An Overlooked Bidirectional Relationship
Abstract
1. Introduction
1.1. Framing the Problem
1.2. Aim and Rationale of the Review
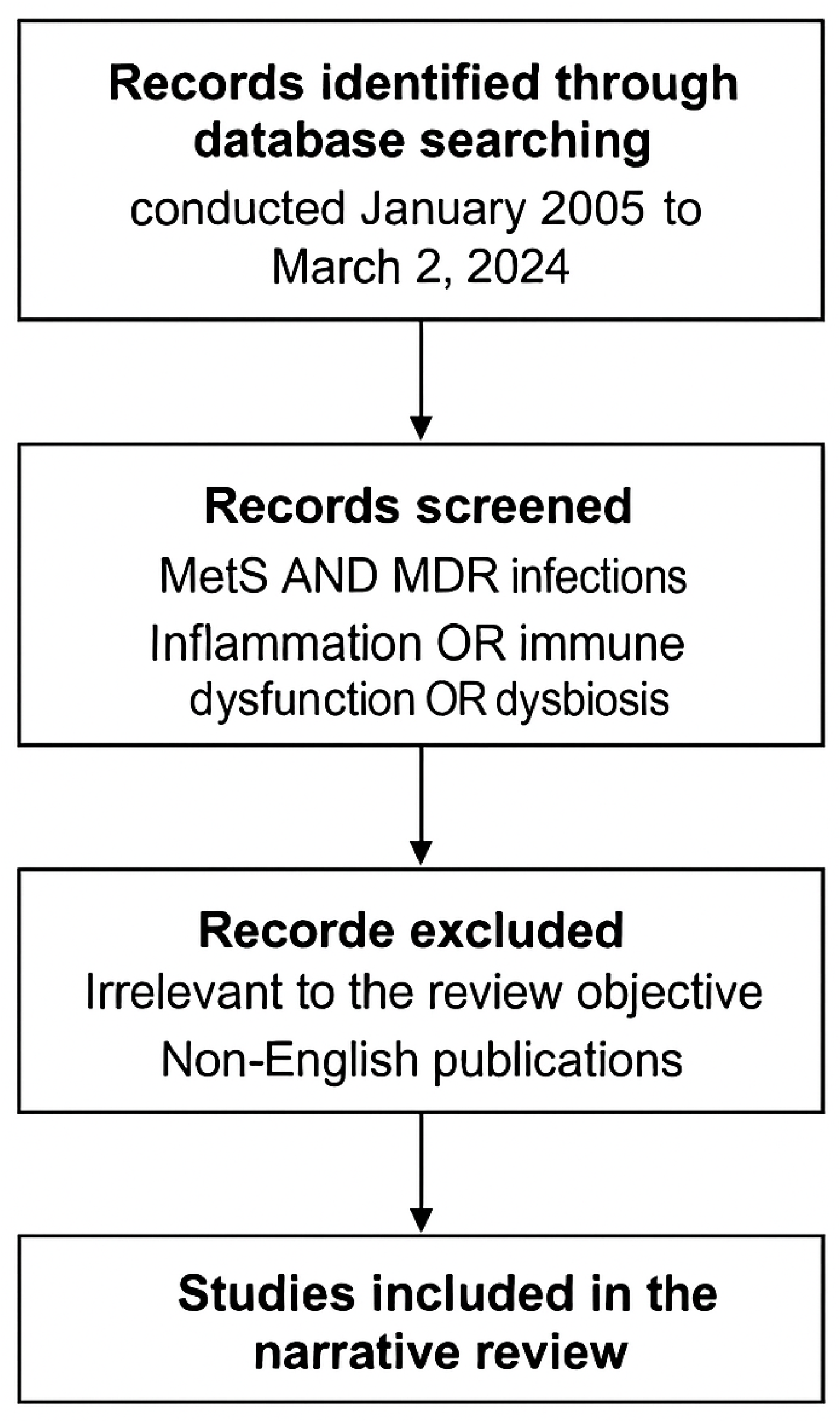
1.3. Materials and Methods
1.4. Data Sources and Search Strategy
- “metabolic syndrome” or “insulin resistance” or “obesity” or “visceral adiposity”
- and
- “multidrug-resistant bacteria” or “MDR pathogens” or “antimicrobial resistance” or “AMR”
- and
- “inflammation” or “immune dysfunction” or “gut microbiota” or “dysbiosis” or “resistome”
1.5. Inclusion and Exclusion Criteria
1.5.1. Inclusion Criteria
- Original research articles, meta-analyses, and systematic or narrative reviews;
- Studies exploring the intersection between metabolic dysfunction and antimicrobial resistance;
- English-language articles published in peer-reviewed journals;
- Human studies, animal models, or in vitro experiments with mechanistic relevance.
1.5.2. Exclusion Criteria
- Case reports, editorials, letters, and conference abstracts;
- Studies unrelated to MetS or MDR infections;
- Non-English articles without accessible translations.
1.6. Data Extraction and Synthesis
- Shared immunometabolic mechanisms (e.g., chronic inflammation, impaired insulin signaling, gut microbiota disruption);
- Impact of MetS on susceptibility to and outcomes of MDR infections;
- Effects of MDR infections and antimicrobial exposure on metabolic regulation;
- Key molecular pathways (e.g., IRS–PI3K–AKT–mTOR, TLR-mediated signaling);
- Therapeutic implications and candidate targets for intervention.
1.7. Limitations of the Methodology
1.8. Methodological Flowchart
2. Metabolic Syndrome: Epidemiology, Clinical Burden, and Molecular Basis
2.1. Definition and Diagnostic Criteria
2.2. Global Epidemiology and Emerging Trends
2.3. Pathophysiological Basis: Chronic Inflammation, Insulin Resistance, and Immune Dysfunction
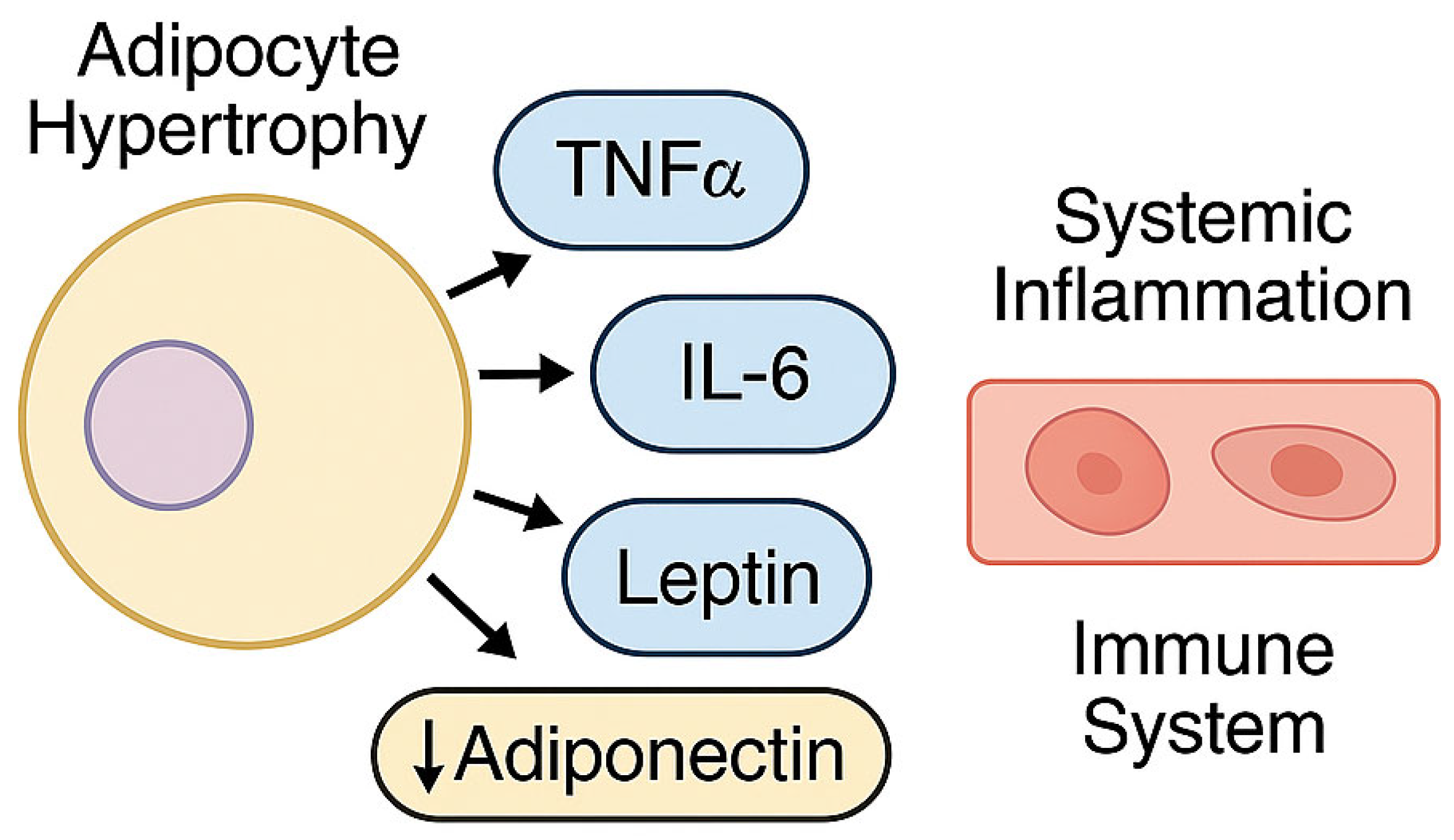
2.3.1. Visceral Adipose Tissue as a Pro-Inflammatory Organ
2.3.2. Low-Grade Chronic Inflammation and Innate Immune Activation
2.3.3. Insulin Resistance: A Central Pathophysiological Node in MetS
2.3.4. Adaptive Immune Dysfunction
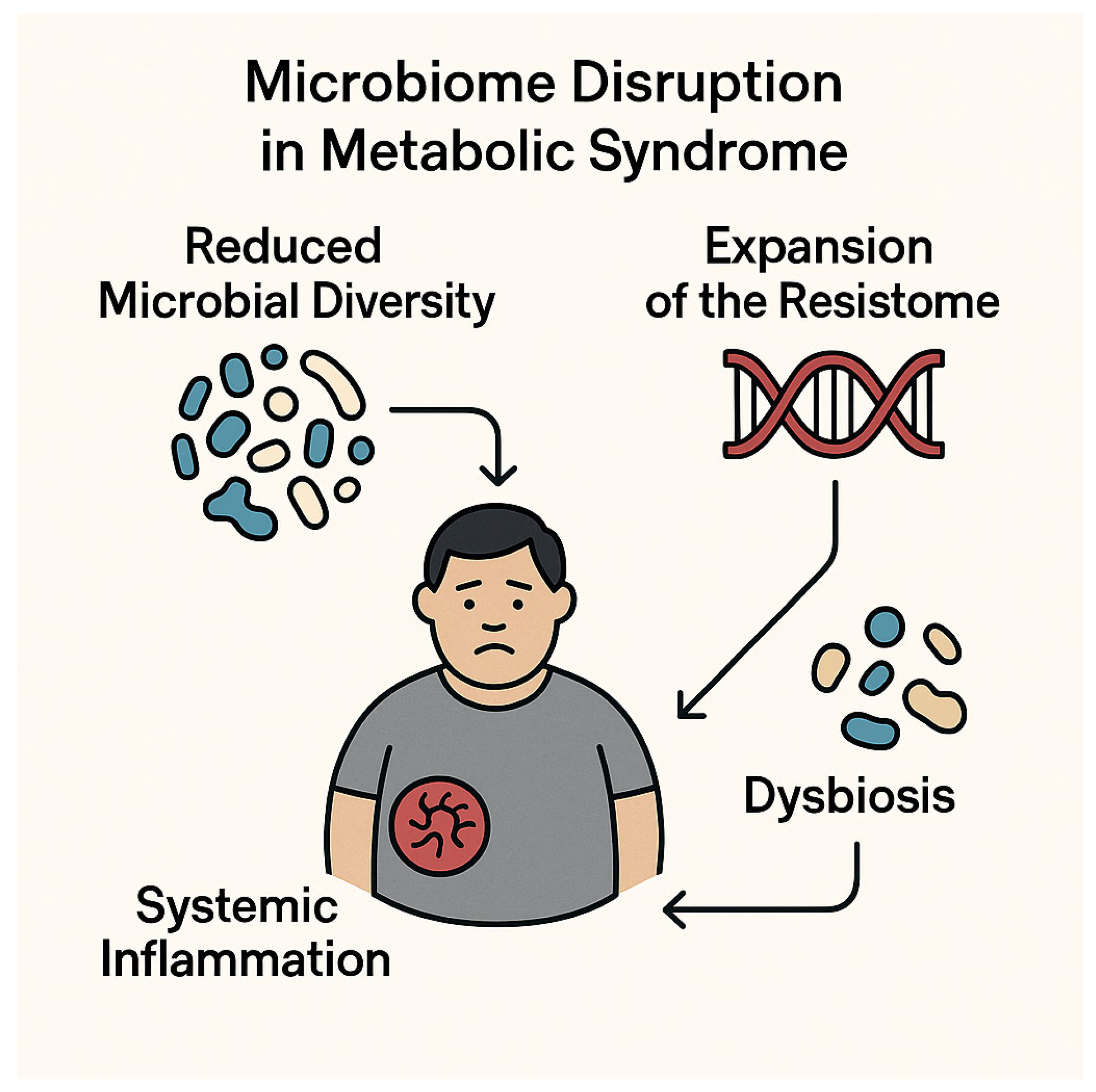
2.3.5. Gut Dysbiosis and Metabolic Endotoxemia
3. Multidrug-Resistant Infections: Definitions, Molecular Basis, Epidemiology, and Clinical Impact
3.1. Definitions and Classification of Multidrug Resistance
3.2. Molecular Mechanisms of Resistance
3.3. Global Epidemiology and Burden of Disease
3.4. Clinical Manifestations and Healthcare Impact
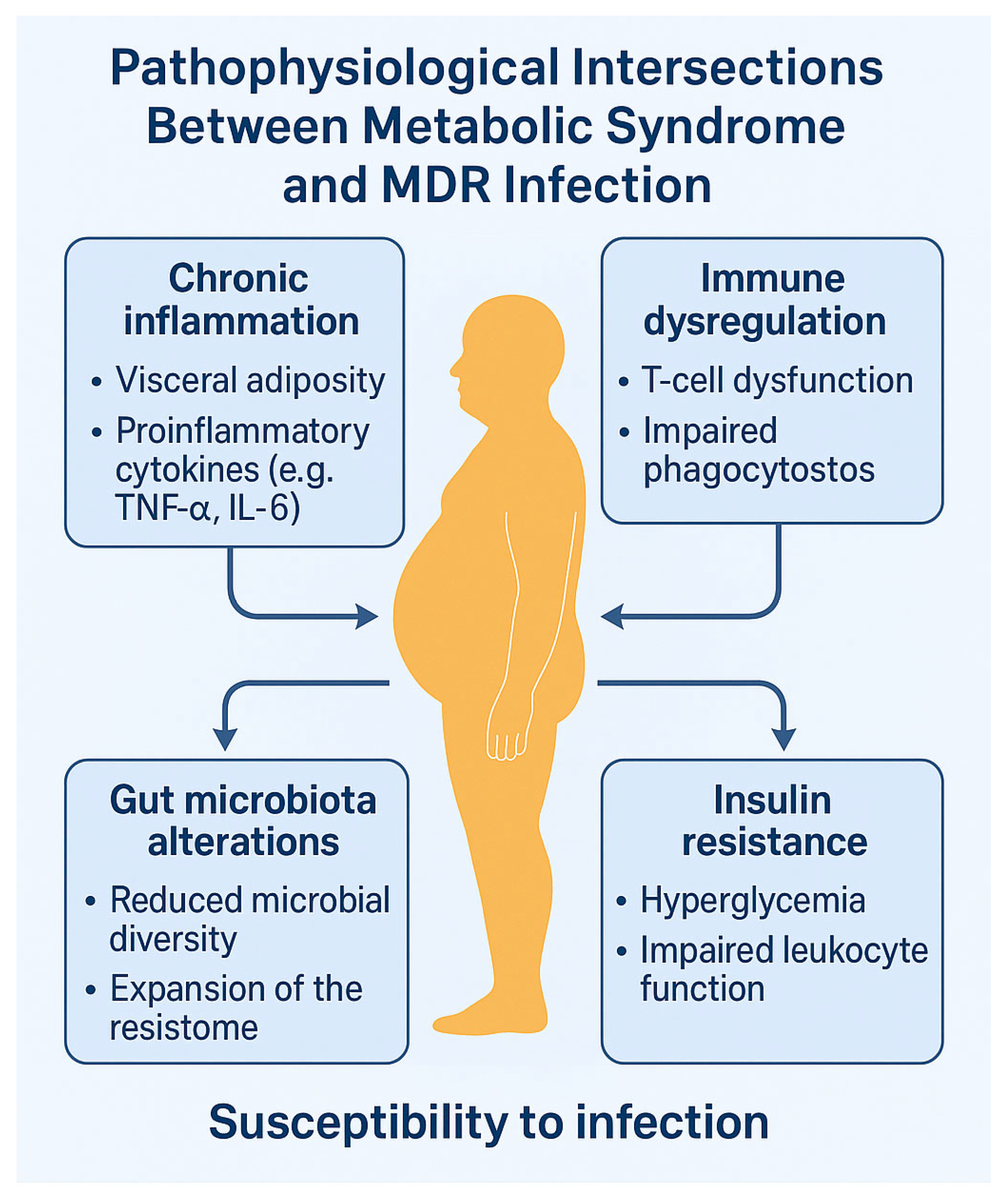
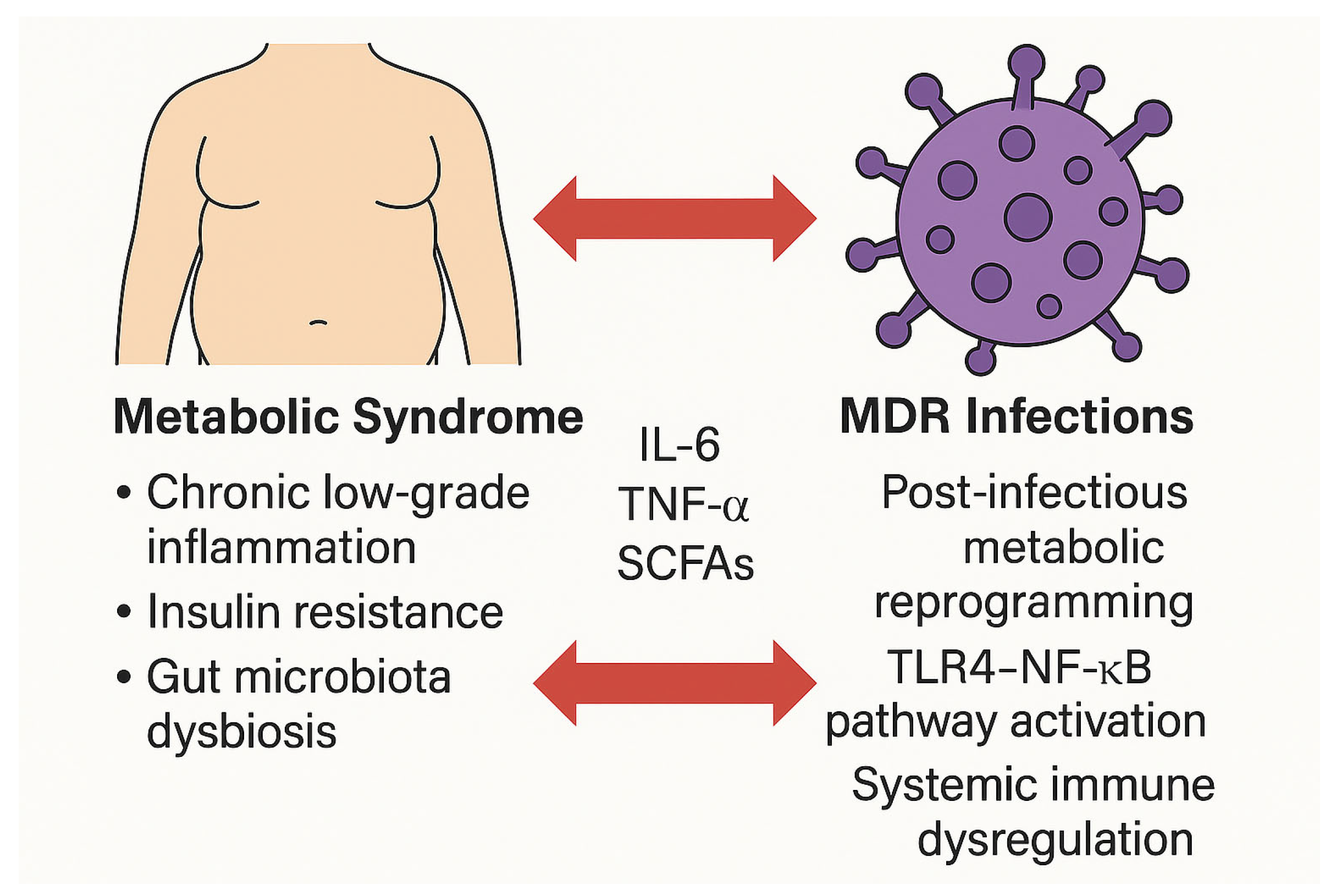
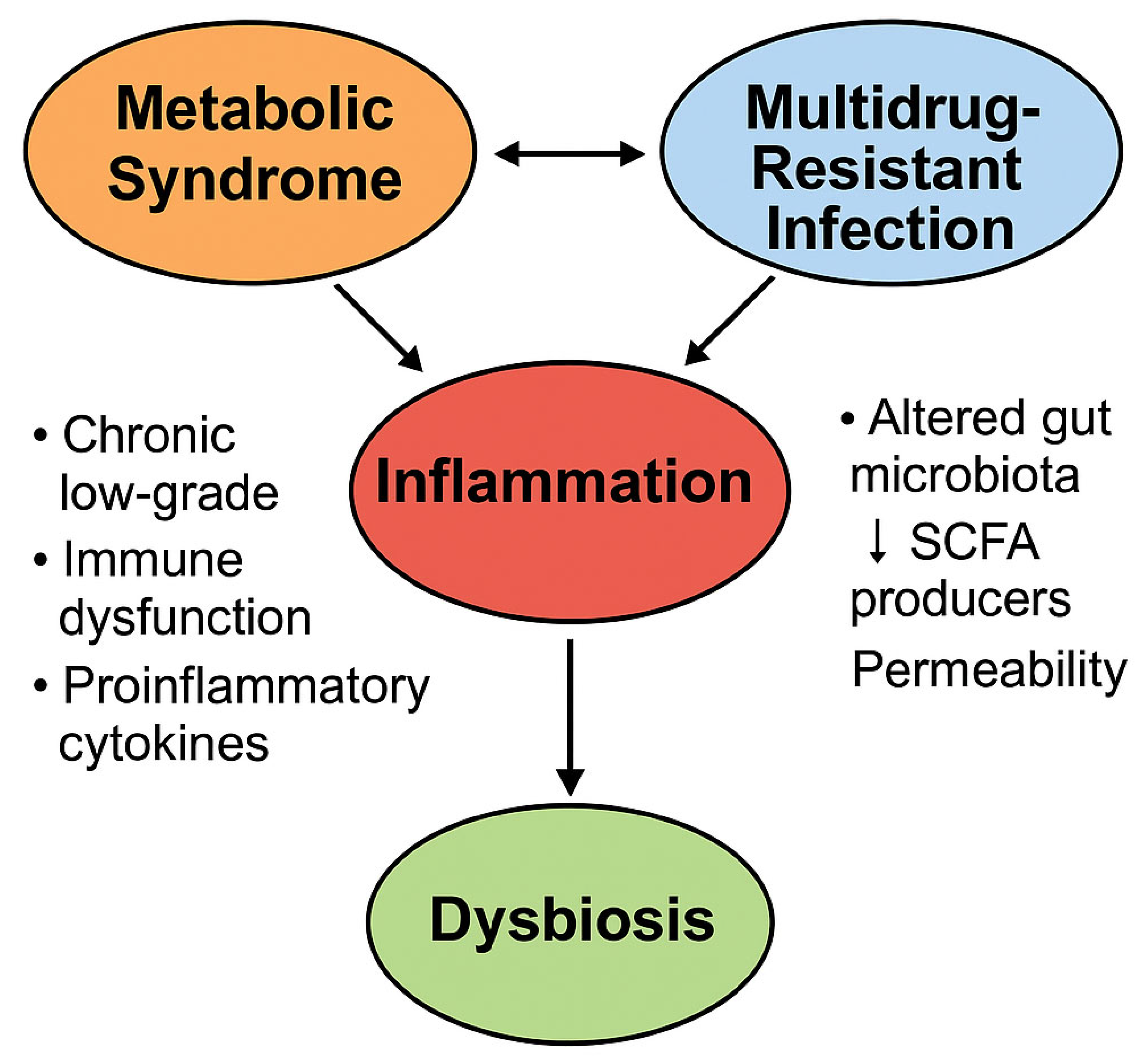
4. Pathophysiological Intersections Between Metabolic Syndrome and Antimicrobial Resistance
4.1. Chronic Inflammation and Immune Dysregulation
4.2. Gut Dysbiosis and Expansion of the Resistome
4.3. Insulin Resistance and Host Defense Impairment
4.4. Microbiota–Immune Axis and Resistance Transmission
5. Impact of Metabolic Syndrome on MDR Infection Risk and Outcomes
5.1. Increased Susceptibility and Delayed Infection Resolution
5.2. Influence on Hospitalization, ICU Stay, and Mortality
5.3. Evidence from Clinical Cohorts and Real-World Studies
6. Can Multidrug-Resistant Infections Contribute to Metabolic Dysfunction?
6.1. Post-Infectious Inflammation and Metabolic Reprogramming
6.2. Antibiotic-Induced Dysbiosis and the Concept of “Infection-Induced MetS”
7. Therapeutic Challenges and Clinical Implications
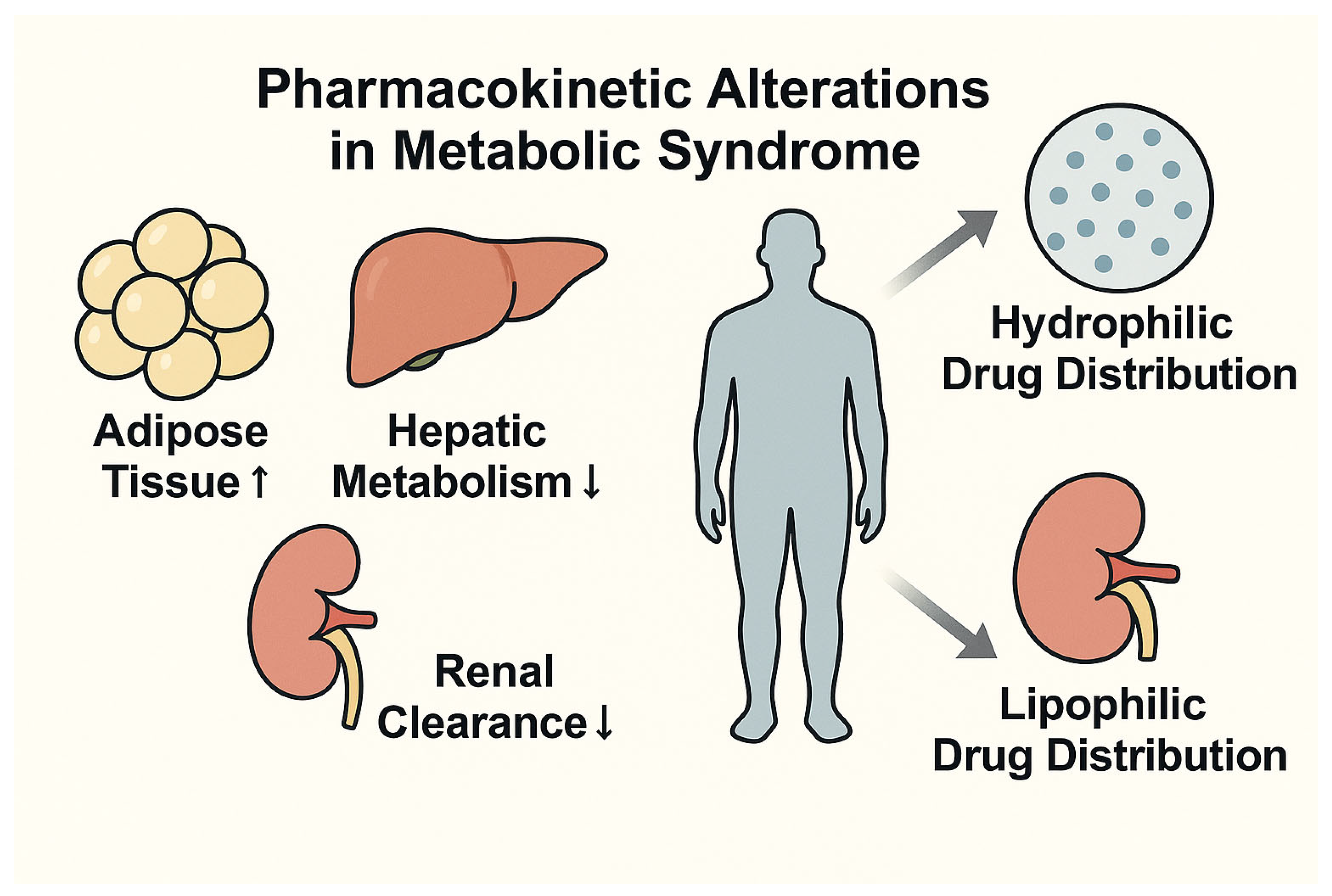
7.1. Pharmacokinetics and Pharmacodynamics in Metabolic Syndrome
7.2. Antimicrobial Stewardship and Optimization of Therapy
7.3. Microbiome-Targeted and Immunometabolic Approaches
8. Health Systems, Surveillance, and Prevention Strategies
8.1. Risk Stratification and Screening Protocols in Patients with MetS
8.2. Metabolism-Adapted Antimicrobial Stewardship
8.3. Public Health Implications and Preventive Policies
8.4. Community-Level Strategies for Managing the MetS–MDR Syndemic
- Systematic documentation of antibiotic histories in patients with MetS;
- Implementation of shared or delayed prescription models;
- Development of “metabolic decolonization” protocols involving dietary modulation, probiotics, and anti-inflammatory strategies;
9. Future Directions and Research Gaps
9.1. Knowledge Gaps in Immuno-Metabolic–Microbial Interactions
9.2. Translational and Model-Based Studies
9.3. Innovation in Microbiome-Driven and Personalized Approaches
10. Conclusions
- (1)
- The absence of prospective, longitudinal studies to define the directionality and magnitude of the MetS–MDR relationship;
- (2)
- The lack of validated biomarkers to predict infection risk or metabolic deterioration in this context;
- (3)
- The limited translational utility of existing animal models, which fail to recapitulate the complexity of the human immune–metabolic–microbial interface;
- (4)
- The need for clinically actionable, microbiota-based interventions capable of modulating both infectious and metabolic outcomes.
- Cohort studies stratified by metabolic phenotype to evaluate incidence, clinical outcomes, and longitudinal trajectories of MDR infections;
- Cross-omic analyses integrating genomics, epigenomics, transcriptomics, and metagenomics to identify susceptibility markers and immunometabolic signatures;
- The development of human-relevant experimental models—such as gut-on-chip systems, intestinal and hepatic organoids, and humanized mouse models—to simulate host–microbiota–pathogen interactions under metabolic stress;
- Clinical trials assessing the efficacy of microbiota-directed interventions, including targeted probiotics and standardized fecal microbiota transplantation, in high-risk metabolic populations;
- The integration of pharmacokinetic and pharmacodynamic data into antimicrobial stewardship protocols for patients with obesity, diabetes, or MASLD, to reduce therapeutic failure and resistance selection.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Aggarwal, R.; Mahajan, P.; Pandiya, S.; Bajaj, A.; Verma, S.K.; Yadav, P.; Kharat, A.S.; Khan, A.U.; Dua, M.; Johri, A.K. Antibiotic resistance: A global crisis, problems and solutions. Crit. Rev. Microbiol. 2024, 50, 896–921. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Makowski, L.; Chaib, M.; Rathmell, J.C. Immunometabolism: From basic mechanisms to translation. Immunol. Rev. 2020, 295, 5–14. [Google Scholar] [CrossRef]
- Geerling, E.; Hameed, M.; Weger-Lucarelli, J.; Pinto, A.K. Metabolic syndrome and aberrant immune responses to viral infection and vaccination: Insights from small animal models. Front. Immunol. 2022, 13, 1015563. [Google Scholar] [CrossRef]
- Brown, T.T.; Glesby, M.J. Management of the metabolic effects of HIV and HIV drugs. Nat. Rev. Endocrinol. 2011, 8, 11–21. [Google Scholar] [CrossRef]
- Shi, Y.-W.; Yang, R.-X.; Fan, J.-G. Chronic hepatitis B infection with concomitant hepatic steatosis: Current evidence and opinion. World J. Gastroenterol. 2021, 27, 3971–3983. [Google Scholar] [CrossRef]
- Bertagnolio, S.; Dobreva, Z.; Centner, C.M.; Olaru, I.D.; Donà, D.; Burzo, S.; Huttner, B.D.; Chaillon, A.; Gebreselassie, N.; Wi, T.; et al. WHO global research priorities for antimicrobial resistance in human health. Lancet Microbe 2024, 5, 100902. [Google Scholar] [CrossRef]
- Mukhopadhyay, H.; Bairagi, A.; Mukherjee, A.; Prasad, A.K.; Roy, A.D.; Nayak, A. Multidrug resistant Acinetobacter baumannii: A study on its pathogenesis and therapeutics. Curr. Res. Microb. Sci. 2025, 8, 100331. [Google Scholar] [CrossRef]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Almutairi, S.M. Metabolic Impairment of Natural Killer Cells in Type 2 Diabetes (T2D) Individuals: A Double-Edged Sword Elevating Susceptibility to Infections and Cancer. Biosci. Biotechnol. Res. Asia 2024, 21, 633–644. [Google Scholar] [CrossRef]
- Richards, A.M.; Abu Kwaik, Y.; Lamont, R.J. Code blue: Acinetobacter baumannii, a nosocomial pathogen with a role in the oral cavity. Mol. Oral Microbiol. 2015, 30, 2–15. [Google Scholar] [CrossRef]
- Guo, Q.; Gao, Z.; Zhao, L.; Wang, H.; Luo, Z.; Vandeputte, D.; He, L.; Li, M.; Di, S.; Liu, Y.; et al. Multiomics Analyses With Stool-Type Stratification in Patient Cohorts and Blautia Identification as a Potential Bacterial Modulator in Type 2 Diabetes Mellitus. Diabetes 2024, 73, 511–527. [Google Scholar] [CrossRef]
- Tang, L.; Wang, H.; Cao, K.; Li, Y.; Li, T.; Huang, Y.; Xu, Y. Epidemiological Features and Impact of High Glucose Level on Virulence Gene Expression and Serum Resistance of Klebsiella pneumoniae Causing Liver Abscess in Diabetic Patients. Infect. Drug Resist. 2023, 16, 1221–1230. [Google Scholar] [CrossRef]
- Huang, J.; Yi, M.; Yuan, Y.; Xia, P.; Yang, B.; Liao, J.; Dang, Z.; Luo, S.; Xia, Y. Emergence of a Fatal ST11-KL64 Tigecycline-Resistant Hypervirulent Klebsiella pneumoniae Clone Cocarrying blaNDM and blaKPC in Plasmids. Garcia-Solache MA, curatore. Microbiol. Spectr. 2022, 10, e0253922. [Google Scholar] [CrossRef]
- Mehdi, S.F.; Qureshi, M.H.; Pervaiz, S.; Kumari, K.; Saji, E.; Shah, M.; Abdullah, A.; Zahoor, K.; Qadeer, H.A.; Katari, D.K.; et al. Endocrine and metabolic alterations in response to systemic inflammation and sepsis: A review article. Mol. Med. 2025, 31, 16. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Moro, M.; Vigezzi, G.P.; Callari, E.; Biancardi, A.; Nizzero, P.; Cichero, P.; Signorelli, C.; Odone, A. Multidrug-resistant organism infections and mortality: Estimates from a large Italian hospital. Eur. J. Public Health 2020, 30 (Suppl. 5), ckaa165.315. [Google Scholar] [CrossRef]
- Al-Dorzi, H.M.; Al Harbi, S.A.; Arabi, Y.M. Antibiotic therapy of pneumonia in the obese patient: Dosing and delivery. Curr. Opin. Infect. Dis. 2014, 27, 165–173. [Google Scholar] [CrossRef]
- Neeland, I.J.; Lim, S.; Tchernof, A.; Gastaldelli, A.; Rangaswami, J.; Ndumele, C.E.; Powell-Wiley, T.M.; Després, J.P. Metabolic syndrome. Nat. Rev. Dis. Primer. 2024, 10, 77. [Google Scholar] [CrossRef]
- Acierno, C.; Caturano, A.; Pafundi, P.C.; Nevola, R.; Adinolfi, L.E.; Sasso, F.C. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes: Pathophysiological Mechanisms Shared Between the Two Faces of the Same Coin. Explor. Med. 2020, 1, 287–306. Available online: https://www.explorationpub.com/Journals/em/Article/100119 (accessed on 17 April 2025). [CrossRef]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Pigeot, I.; Ahrens, W. Epidemiology of metabolic syndrome. Pflüg. Arch.-Eur. J. Physiol. 2025, 477, 669–680. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- de Siqueira Valadares, L.T.; de Souza, L.S.B.; Salgado Júnior, V.A.; de Freitas Bonomo, L.; de Macedo, L.R.; Silva, M. Prevalence of metabolic syndrome in Brazilian adults in the last 10 years: A systematic review and meta-analysis. BMC Public Health 2022, 22, 327. [Google Scholar] [CrossRef]
- Gutierrez-Montiel, D.; Guerrero-Barrera, A.L.; Ramírez-Castillo, F.Y.; Galindo-Guerrero, F.; Ornelas-García, I.G.; Chávez-Vela, N.A.; Costa, M.d.O.; Avelar-Gonzalez, F.J.; Moreno-Flores, A.C.; Vazquez-Pedroza, E.; et al. Guava Leaf Extract Exhibits Antimicrobial Activity in Extensively Drug-Resistant (XDR) Acinetobacter baumannii. Molecules 2024, 30, 70. [Google Scholar] [CrossRef]
- Wang, Y.; Mi, J.; Shan, X.-Y.; Wang, Q.J.; Ge, K.-Y. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int. J. Obes. 2007, 31, 177–188. [Google Scholar] [CrossRef]
- Scuteri, A.; Laurent, S.; Cucca, F.; Cockcroft, J.; Cunha, P.G.; Mañas, L.R.; Raso, F.U.M.; Muiesan, M.L.; Ryliškytė, L.; Rietzschel, E.; et al. Metabolic syndrome across Europe: Different clusters of risk factors. Eur. J. Prev. Cardiol. 2015, 22, 486–491. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Friend, A.; Craig, L.; Turner, S. The Prevalence of Metabolic Syndrome in Children: A Systematic Review of the Literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80. [Google Scholar] [CrossRef]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the Metabolic Syndrome in Children and Adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Winer, S.; Winer, D.A. The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunol. Cell Biol. 2012, 90, 755–762. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Caruso, C.; Candore, G. The Role of Adipose Tissue and Adipokines in Obesity-Related Inflammatory Diseases. Mediat. Inflamm. 2010, 2010, 802078. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Shklyaev, S.S.; Melnichenko, G.A.; Volevodz, N.N.; Falaleeva, N.A.; Ivanov, S.A.; Kaprin, A.D.; Mokrysheva, N.G. Adiponectin: A pleiotropic hormone with multifaceted roles. Probl. Endocrinol. 2021, 67, 98–112. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, W.; Zhou, R. DAMPs and DAMP-sensing receptors in inflammation and diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef]
- Caturano, A.; Rocco, M.; Tagliaferri, G.; Piacevole, A.; Nilo, D.; Di Lorenzo, G.; Iadicicco, I.; Donnarumma, M.; Galiero, R.; Acierno, C.; et al. Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications. Antioxidants 2025, 14, 72. [Google Scholar] [CrossRef]
- Caturano, A.; Acierno, C.; Nevola, R.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Salvatore, T.; Adinolfi, L.E.; Sasso, F.C. Non-Alcoholic Fatty Liver Disease: From Pathogenesis to Clinical Impact. Processes 2021, 9, 135. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef]
- Acierno, C.; Nevola, R.; Rinaldi, L.; Sasso, F.C.; Adinolfi, L.E.; Caturano, A. The Intestinal Thread of Fate: How the Microbiota Shapes the Story of Liver Disease. Livers 2025, 5, 17. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; 28p, Available online: https://iris.who.int/handle/10665/193736 (accessed on 16 May 2025).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, T.; Tsergouli, K.; Behzadi, P. Carbapenem-Resistant Klebsiella pneumoniae: Virulence Factors, Molecular Epidemiology and Latest Updates in Treatment Options. Antibiotics 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Kazi, R.N.A.; Aatif, M.; Azhar, A.; El Oirdi, M.; Farhan, M. Antimicrobial Resistance: Linking Molecular Mechanisms to Public Health Impact. SLAS Discov. Adv. Sci. Drug Discov. 2025, 33, 100232. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Macesic, N.; Uhlemann, A.-C.; Peleg, A.Y. Multidrug-resistant Gram-negative bacterial infections. Lancet 2025, 405, 257–272. [Google Scholar] [CrossRef]
- Ayobami, O.; Brinkwirth, S.; Eckmanns, T.; Markwart, R. Antibiotic resistance in hospital-acquired ESKAPE-E infections in low- and lower-middle-income countries: A systematic review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 443–451. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Surveillance in Europe 2023–2021 Data. 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2023-2021-data (accessed on 16 May 2025).
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Qian, Y.; Bi, Y.; Liu, S.; Li, X.; Dong, S.; Ju, M. Predictors of mortality in patients with carbapenem-resistant Klebsiella pneumoniae infection: A meta-analysis and a systematic review. Ann. Palliat. Med. 2021, 10, 7340–7350. [Google Scholar] [CrossRef] [PubMed]
- Sommer, L.M.; Johansen, H.K.; Molin, S. Antibiotic resistance in Pseudomonas aeruginosa and adaptation to complex dynamic environments. Microb. Genom. 2020, 6, e000370. [Google Scholar] [CrossRef]
- Zhao, L.; Pu, J.; Liu, Y.; Cai, H.; Han, M.; Yu, Y.; Tang, J. High prevalence of carbapenem-resistant Pseudomonas aeruginosa and identification of a novel VIM-type metallo-β-lactamase, VIM-92, in clinical isolates from northern China. Front. Microbiol. 2025, 16, 1543509. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Tabah, A.; Laupland, K.B. Update on Staphylococcus aureus bacteraemia. Curr. Opin. Crit. Care 2022, 28, 495–504. [Google Scholar] [CrossRef] [PubMed]
- David, M.Z.; Daum, R.S. Community-Associated Methicillin-Resistant Staphylococcus aureus: Epidemiology and Clinical Consequences of an Emerging Epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef]
- Wei, Y.; Palacios Araya, D.; Palmer, K.L. Enterococcus faecium: Evolution, adaptation, pathogenesis and emerging therapeutics. Nat. Rev. Microbiol. 2024, 22, 705–721. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, R.F.; Leong, K.W.; Cumming, V.; Van Hal, S.J. Vancomycin-resistant Enterococcus faecium and the emergence of new sequence types associated with hospital infection. Res. Microbiol. 2023, 174, 104046. [Google Scholar] [CrossRef]
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef]
- Mollers, M.; Lutgens, S.P.; Schoffelen, A.F.; Schneeberger, P.M.; Suijkerbuijk, A.W.M. Cost of Nosocomial Outbreak Caused by NDM-1-Containing Klebsiella pneumoniae in The Netherlands, October 2015–January 2016. Emerg. Infect. Dis. 2017, 3, 1574–1576. [Google Scholar] [CrossRef]
- Shimi, G.; Sohouli, M.H.; Ghorbani, A.; Shakery, A.; Zand, H. The interplay between obesity, immunosenescence, and insulin resistance. Immun. Ageing 2024, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, S.; de Warle, S.; van Baarlen, P.; Boekhorst, J.; Wells, J.M. Resistome expansion in disease-associated human gut microbiomes. Microbiome 2023, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Chazal, N.; Gerlier, D. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. MMBR 2003, 67, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.Z.; He, P.; He, P.; Wu, Y.; Munir, S.; He, Y. The Response of Murine Gut Microbiome in the Presence of Altered rpoS Gene of Klebsiella pneumoniae. Int. J. Mol. Sci. 2024, 25, 9222. [Google Scholar] [CrossRef]
- Fessler, M.B.; Rudel, L.L.; Brown, J.M. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 2009, 20, 379–385. [Google Scholar] [CrossRef]
- Todd, K.; Gunter, K.; Bowen, J.M.; Holmes, C.L.; Tilston-Lunel, N.L.; Vornhagen, J. Type-2 diabetes mellitus enhances Klebsiella pneumoniae pathogenesis. BioRxiv 2024. BioRxiv:2024.05.31.596766. [Google Scholar]
- Mantovani, A.; Morandin, R.; Fiorio, V.; Lando, M.G.; Gaviraghi, A.; Motta, L.; Gobbi, F.; Tilg, H.; Byrne, C.D.; Targher, G. Association between MASLD and increased risk of serious bacterial infections requiring hospital admission: A meta-analysis. Liver Int. 2024, 45, e16101. [Google Scholar] [CrossRef]
- Allard, R.; Leclerc, P.; Tremblay, C.; Tannenbaum, T.-N. Diabetes and the Severity of Pandemic Influenza A (H1N1) Infection. Diabetes Care 2010, 33, 1491–1493. [Google Scholar] [CrossRef]
- Sechterberger, M.K.; Bosman, R.J.; Oudemans-van Straaten, H.M.; Siegelaar, S.E.; Hermanides, J.; Hoekstra, J.B.; De Vries, J.H. The effect of diabetes mellitus on the association between measures of glycaemiccontrol and ICU mortality: A retrospective cohort study. Crit. Care 2013, 17, R52. [Google Scholar] [CrossRef]
- Chen, J.; Ma, H.; Huang, X.; Cui, Y.; Peng, W.; Zhu, F.; Ma, S.; Rao, M.; Zhang, P.; Yang, H.; et al. Risk factors and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary-care hospital in China: An eight-year retrospective study. Antimicrob. Resist. Infect. Control 2022, 11, 161. [Google Scholar] [CrossRef]
- Huang, C.; Gao, Y.; Lin, H.; Fan, Q.; Chen, L.; Feng, Y. Prognostic Factors That Affect Mortality Patients with Acinetobacter baumannii Bloodstream Infection. Infect. Drug Resist. 2024, 17, 3825–3837. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, T.A.; Bin Shaman, M.S.; Suliman, D.M.; Alanazi, T.A.; Altawalbeh, S.M.; Alshareef, H.; Lahreche, D.I.; Al-Azzam, S.; Araydah, M.; Karasneh, R.; et al. The Impact of Multidrug-Resistant Acinetobacter baumannii Infection in Critically Ill Patients with or without COVID-19 Infection. Healthcare 2023, 11, 487. [Google Scholar] [CrossRef]
- Holt, R.I.G.; Cockram, C.S.; Ma, R.C.W.; Luk, A.O.Y. Diabetes and infection: Review of the epidemiology, mechanisms and principles of treatment. Diabetologia 2024, 67, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Ata, A.; Lee, J.; Bestle, S.L.; Desemone, J.; Stain, S.C. Postoperative Hyperglycemia and Surgical Site Infection in General Surgery Patients. Arch. Surg. 2010, 145, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Santibañez, M.; Bunnell, K.; Harrington, A.; Bleasdale, S.; Wenzler, E. Association Between Estimated Pharmacokinetic/Pharmacodynamic Predictions of Efficacy and Observed Clinical Outcomes in Obese and Nonobese Patients With Enterobacteriaceae Bloodstream Infections. Open Forum Infect. Dis. 2019, 6, ofz400. [Google Scholar] [CrossRef]
- Peñalva, G.; Cantón, R.; Pérez-Rodríguez, M.T.; González-López, J.J.; Rodríguez-Baño, J.; del Barrio-Tofiño, E.; Kirkegaard-Biosca, C.; Sánchez-Romero, I.; Gutiérrez-Villanueva, A.; Marrodán-Ciordia, T.; et al. Burden of bacterial antimicrobial resistance among hospitalised patients in Spain: Findings from three nationwide prospective studies. Lancet Reg. Health-Eur. 2025, 51, 101220. [Google Scholar] [CrossRef]
- Pepper, D.J.; Sun, J.; Welsh, J.; Cui, X.; Suffredini, A.F.; Eichacker, P.Q. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: A systematic review and meta-analysis. Crit. Care 2016, 20, 181. [Google Scholar] [CrossRef]
- Märtson, A.-G.; Barber, K.E.; Crass, R.L.; Hites, M.; Kloft, C.; Kuti, J.L.; Nielsen, E.I.; Pai, M.P.; Zeitlinger, M.; Roberts, J.A.; et al. The pharmacokinetics of antibiotics in patients with obesity: A systematic review and consensus guidelines for dose adjustments. Lancet Infect. Dis. 2025, in press. [Google Scholar] [CrossRef]
- Gorham, J.; Taccone, F.S.; Hites, M. Therapeutic Drug Monitoring of Antimicrobials in Critically Ill Obese Patients. Antibiotics 2023, 12, 1099. [Google Scholar] [CrossRef]
- Davido, B.; Merrick, B.; Kuijper, E.; Benech, N.; Biehl, L.M.; Corcione, S.; European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Host and Microbiota Interactions (ESGHAMI). How can the gut microbiome be targeted to fight multidrug-resistant organisms? Lancet Microbe. 2025, 101063. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef] [PubMed]
- Darwitz, B.P.; Genito, C.J.; Thurlow, L.R. Triple threat: How diabetes results in worsened bacterial infections. Richardson AR, curatore. Infect. Immun. 2024, 92, e0050923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, J.; Wang, J.; Li, B.; Huang, W. Inhibition of Hyperglycemia and Hyperlipidemia by Blocking Toll-like Receptor 4: Comparison of Wild-Type and Toll-like Receptor 4 Gene Knockout Mice on Obesity and Diabetes Modeling. Biology 2024, 13, 63. [Google Scholar] [CrossRef]
- Jiang, B.; Hebert, V.; Li, Y.; Mathis, J.; Alexander, J.; Dugas, T. HIV antiretroviral drug combination induces endothelial mitochondrial dysfunction and reactive oxygen species production, but not apoptosis. Toxicol. Appl. Pharmacol. 2007, 224, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, S.P.; Khan, A.; Tay, E.S.E.; Garvey, M.; Ajoyan, H.; Diefenbach, E.; Gloss, B.S.; Tu, T.; George, J.; Douglas, M.W. Hepatitis B Virus and Hepatitis C Virus Affect Mitochondrial Function Through Different Metabolic Pathways, Explaining Virus-Specific Clinical Features of Chronic Hepatitis. J. Infect. Dis. 2024, 230, e1012–e1022. [Google Scholar] [CrossRef]
- Dongre, D.S.; Saha, U.B.; Saroj, S.D. Exploring the role of gut microbiota in antibiotic resistance and prevention. Ann. Med. 2025, 57, 2478317. [Google Scholar] [CrossRef]
- Leshem, A.; Liwinski, T.; Elinav, E. Immune-Microbiota Interplay and Colonization Resistance in Infection. Mol. Cell 2020, 78, 597–613. [Google Scholar] [CrossRef]
- Li, W.; Lin, X.; Liang, H.; Wu, Z.; Wang, M.; Sun, J.; Li, X.; He, W.; Gao, X.; Hu, T.; et al. Genomic and functional diversity of the human-derived isolates of Faecalibacterium. Front. Microbiol. 2024, 15, 1379500. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.; Ng, S.C. Gut microbiota in patients with obesity and metabolic disorders—A systematic review. Genes Nutr. 2022, 17, 2. [Google Scholar] [CrossRef]
- Woodworth, M.H.; Conrad, R.E.; Haldopoulos, M.; Pouch, S.M.; Babiker, A.; Mehta, A.K.; Sitchenko, K.L.; Wang, C.H.; Strudwick, A.; Ingersoll, J.M.; et al. Fecal microbiota transplantation promotes reduction of antimicrobial resistance by strain replacement. Sci. Transl. Med. 2023, 15, eabo2750. [Google Scholar] [CrossRef]
- Jones, K.; de Brito, C.B.; Byndloss, M.X. Metabolic tug-of-war: Microbial metabolism shapes colonization resistance against enteric pathogens. Cell Chem. Biol. 2025, 32, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Mui, E.; Ha, D.R.; Stave, C.; Deresinski, S.C.; Holubar, M. Comprehensive guidance for antibiotic dosing in obese adults: 2022 update. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2023, 43, 226–246. [Google Scholar] [CrossRef]
- Merrick, B.; Sergaki, C.; Edwards, L.; Moyes, D.L.; Kertanegara, M.; Prossomariti, D.; Shawcross, D.L.; Goldenberg, S.D. Modulation of the Gut Microbiota to Control Antimicrobial Resistance (AMR)—A Narrative Review with a Focus on Faecal Microbiota Transplantation (FMT). Infect. Dis. Rep. 2023, 15, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Knežević, S.; Filippi-Arriaga, F.; Belančić, A.; Božina, T.; Mršić-Pelčić, J.; Vitezić, D. Metabolic Syndrome Drug Therapy: The Potential Interplay of Pharmacogenetics and Pharmacokinetic Interactions in Clinical Practice: A Narrative Review. Diabetology 2024, 5, 406–429. [Google Scholar] [CrossRef]
- Castro-Balado, A.; Varela-Rey, I.; Mejuto, B.; Mondelo-García, C.; Zarra-Ferro, I.; Rodríguez-Jato, T.; Fernández-Ferreiro, A. Updated antimicrobial dosing recommendations for obese patients. Antimicrob. Agents Chemother. 2024, 68, e0171923. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; Cao, Y.-M.; Cao, Y.-B.; Yan, T.-H.; Jia, C.-L.; He, P. A Review: Cytochrome P450 in Alcoholic and Non-Alcoholic Fatty Liver Disease. Diabetes Metab. Syndr. Obes. Targets Ther. 2024, 17, 1511–1521. [Google Scholar] [CrossRef]
- Tran, T.B.; Velkov, T.; Nation, R.L.; Forrest, A.; Tsuji, B.T.; Bergen, P.J.; Li, J. Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: Are we there yet? Int. J. Antimicrob. Agents 2016, 48, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, T.P.; Lipman, J.; Roberts, J.A. Antibiotic dosing in obesity: A BIG challenge. Crit. Care 2016, 20, 240. [Google Scholar] [CrossRef][Green Version]
- Takahashi, N.; Kondo, Y.; Kubo, K.; Egi, M.; Kano, K.-I.; Ohshima, Y.; Nakada, T.-A. Efficacy of therapeutic drug monitoring-based antibiotic regimen in critically ill patients: A systematic review and meta-analysis of randomized controlled trials. J. Intensiv. Care 2023, 11, 48. [Google Scholar] [CrossRef]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C.; ESGAP (ESCMID Study Group for Antimicrobial stewardshiP). What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef]
- BALANCE Investigators, for the Canadian Critical Care Trials Group, the Association of Medical Microbiology and Infectious Disease Canada Clinical Research Network, the Australian and New Zealand Intensive Care Society Clinical Trials Group, and the Australasian Society for Infectious Diseases Clinical Research Network; Daneman, N.; Rishu, A.; Pinto, R.; Rogers, B.A.; Shehabi, Y.; Parke, R.; Cook, D.; Arabi, Y.; Muscedere, J.; et al. Antibiotic Treatment for 7 versus 14 Days in Patients with Bloodstream Infections. N. Engl. J. Med. 2025, 392, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Vieira, P.; Sá, H.; Malva, J.; Castelo-Branco, M.; Reis, F.; Viana, S. Polyphenols: Immunonutrients tipping the balance of immunometabolism in chronic diseases. Front. Immunol. 2024, 15, 1360065. [Google Scholar] [CrossRef] [PubMed]
- Tummolo, A.; Melpignano, L. The Reciprocal Interplay between Infections and Inherited Metabolic Disorders. Microorganisms 2023, 11, 2545. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Wang, M.; Wang, L.; Wu, Y.; Fang, Y.; Zhao, Y.; Fan, Y.; Liu, X.; Liang, H.; et al. Development and validation of machine learning models to predict MDRO colonization or infection on ICU admission by using electronic health record data. Antimicrob. Resist. Infect. Control 2024, 13, 74. [Google Scholar] [CrossRef]
- Wp, S.E.; Norhidayah, M.; Ar, M.N.A. Factors associated with multidrug-resistant organism (MDRO) mortality: An analysis from the national surveillance of multidrug-resistant organism, 2018–2022. BMC Infect. Dis. 2025, 25, 60. [Google Scholar] [CrossRef]
- Raman, G.; Avendano, E.E.; Chan, J.; Merchant, S.; Puzniak, L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Citro, V. Could Adverse Effects of Antibiotics Due to Their Use/Misuse Be Linked to Some Mechanisms Related to Nonalcoholic Fatty Liver Disease? Int. J. Mol. Sci. 2024, 25, 1993. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Cangini, A.; Fortinguerra, F.; Di Filippo, A.; Pierantozzi, A.; Da Cas, R.; Villa, F.; Trotta, F.; Moro, M.L.; Gagliotti, C. Monitoring the community use of antibiotics in Italy within the National Action Plan on antimicrobial resistance. Br. J. Clin. Pharmacol. 2021, 87, 1033–1042. [Google Scholar] [CrossRef]
- Nokleby, H.; Nicoll, A. Risk groups and other target groups-preliminary ECDC guidance for developing influenza vaccination recommendations for the season 2010-11. Euro Surveill. 2010, 15, 19525. [Google Scholar] [CrossRef]
- Buchy, P.; Badur, S. Who and when to vaccinate against influenza. Int. J. Infect. Dis. 2020, 93, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Amaro, R.; Torres, A. Pneumococcal vaccination. Curr. Opin. Infect. Dis. 2016, 29, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.-J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Floor, E.; Su, J.; Chatterjee, M.; Kuipers, E.S.; Ijssennagger, N.; Heidari, F.; Giordano, L.; Wubbolts, R.W.; Mihăilă, S.M.; Stapels, D.A.C.; et al. Development of a Caco-2-based intestinal mucosal model to study intestinal barrier properties and bacteria–mucus interactions. Gut Microbes 2025, 17, 2434685. [Google Scholar] [CrossRef] [PubMed]
- Schuh, M.G.; Hesse, J.; Sieber, S.A. AI-guided Antibiotic Discovery Pipeline from Target Selection to Compound Identification. arXiv 2025, arXiv:2504.11091. Available online: http://arxiv.org/abs/2504.11091 (accessed on 16 May 2025).
- Yang, S.Y.; Han, S.M.; Lee, J.Y.; Kim, K.S.; Lee, J.E.; Lee, D.W. Advancing Gut Microbiome Research: The Shift from Metagenomics to Multi-Omics and Future Perspectives. J. Microbiol. Biotechnol. 2025, 35, e2412001. [Google Scholar] [CrossRef]
- Yadegar, A.; Bar-Yoseph, H.; Monaghan, T.M.; Pakpour, S.; Severino, A.; Kuijper, E.J.; Smits, W.K.; Terveer, E.M.; Neupane, S.; Nabavi-Rad, A.; et al. Fecal microbiota transplantation: Current challenges and future landscapes. Clin. Microbiol. Rev. 2024, 37, e0006022. [Google Scholar] [CrossRef]

| Diagnostic Criterion | Threshold/Definition | References |
|---|---|---|
| Abdominal Obesity | Increased waist circumference (sex- and ethnicity-specific cut-offs) | [25] |
| Hypertriglyceridemia | Serum triglycerides ≥150 mg/dL | [25] |
| Low HDL Cholesterol | <40 mg/dL in men; <50 mg/dL in women | [25] |
| Elevated Blood Pressure | ≥130/85 mmHg or current use of antihypertensive medications | [25] |
| Mechanism | Description | Common Pathogens | References |
|---|---|---|---|
| ESBL Production | Hydrolyzes extended-spectrum β-lactams | E. coli, K. pneumoniae | [55,56] |
| Carbapenemases (KPC, NDM, VIM, OXA) | Enzymatic degradation of carbapenems | K. pneumoniae, P. aeruginosa, A. baumannii | [55,56] |
| Ribosomal Methylation | Alters antibiotic binding sites | Staphylococcus spp., Enterococcus spp. | [55] |
| Efflux Pump Upregulation | Actively extrudes antibiotics | P. aeruginosa, A. baumannii | [55] |
| Porin Loss/Mutation | Reduces drug uptake | K. pneumoniae, P. aeruginosa | [55] |
| Target Modification | Alters PBPs or other drug targets | S. aureus, E. faecium | [55,56] |
| Horizontal Gene Transfer | Plasmid-mediated gene acquisition | Multiple species | [57] |
| Pathogen | Clinical Manifestations | Mortality/Economic Impact | References |
|---|---|---|---|
| K. pneumoniae (KPC-Kp) | Nosocomial infections: BSI, VAP | Hospital mortality >40% | [55,63] |
| A. baumannii (XDR) | ICU infections, biofilm formation | Mortality >50% in ventilated patients | [9,29] |
| P. aeruginosa (CRPA) | Infections in immunocompromised hosts | Increased length of stay, therapeutic failure | [10,64,65] |
| MRSA | Bacteremia, pneumonia, endocarditis | 20–30% case-fatality in high-risk groups | [66,67] |
| CA-MRSA | Community-acquired infections | Enhanced virulence and spread | [68] |
| VRE (E. faecium) | Infections in immunocompromised patients | Requires expensive, toxic drugs | [69,70,71] |
| K. pneumoniae (NDM-1) | Nosocomial outbreak in NL | Cost USD ~804,263 | [72] |
| Mechanism | Description | Pathogens | References |
|---|---|---|---|
| Visceral Adiposity | Acts as a pro-inflammatory endocrine organ, producing TNF-α, IL-6, and leptin, with reduced adiponectin | K. pneumoniae, A. baumannii | [36,37,38,39] |
| Insulin Resistance | Impairs PI3K–AKT–mTOR pathway, reducing immune cell function (T cells, NK cells, macrophages) | Multiple MDR bacteria | [4,46] |
| Chronic Low-Grade Inflammation | Drives immune exhaustion via TLR-mediated activation and systemic cytokine release | K. pneumoniae, P. aeruginosa | [17,35,50] |
| Adaptive Immune Skewing | Increased Th1/Th17 polarization, reduced Treg function, and enhanced inflammation | K. pneumoniae, A. baumannii | [36,47] |
| Gut Dysbiosis | Promotes resistome expansion and epithelial barrier dysfunction | Enterobacteriaceae, K. pneumoniae | [50,74] |
| Gene/Protein | Function in Immunometabolism | Associated Pathway | Role in MDR Susceptibility | References |
|---|---|---|---|---|
| IRS-1 | Transduces insulin signals to downstream effectors | IRS–PI3K–AKT–mTOR | Serine phosphorylation inhibits PI3K–AKT signaling, reducing immune cell function | [46] |
| AKT | Regulates cell survival, proliferation, and metabolism | PI3K–AKT–mTOR | Impaired activation compromises T-cell and macrophage responses | [46] |
| mTOR | Controls immune cell metabolism and growth | PI3K–AKT–mTOR | Dysregulation limits immune cell activation and proliferation | [46] |
| TLR4 | Recognizes microbial PAMPs and endogenous ligands | TLR–NF-κB | Chronic activation promotes metaflammation, immune exhaustion | [17] |
| IL-6 | Mediator of systemic inflammation | NF-κB pathway | Elevates systemic inflammation, impairs pathogen clearance | [17,39] |
| TNF-α | Pro-inflammatory cytokine | NF-κB pathway | Contributes to chronic inflammation and immune dysregulation | [39] |
| Adiponectin | Anti-inflammatory adipokine | Metabolic regulation | Low levels worsen insulin resistance and inflammatory status | [41] |
| Study Context | MetS Component(s) | Adverse Outcome(s) | References |
|---|---|---|---|
| ICU patients with MDR A. baumannii | Obesity, T2DM | Increased mortality, longer ICU stay, mechanical ventilation | [83,84] |
| Patients with KPC-Kp bacteremia | Obesity (BMI > 30) | Higher in-hospital mortality, treatment failure | [82] |
| Spanish multicenter cohort | Diabetes | Higher 30-day mortality, recurrence | [85] |
| General ICU population with sepsis | Obesity | Increased mortality, worse infection control | [86] |
| Factor | MetS Impact on Infection | Impact of Infection on MetS | References |
|---|---|---|---|
| Insulin Resistance | Impaired phagocytic activity and pathogen clearance | Aggravated via TLR4-mediated inflammation | [27,42,98] |
| Intestinal Dysbiosis | Promotes MDR colonization and translocation | Worsened by antibiotic-induced microbiota disruption | [54,55,102] |
| Chronic Inflammation | Enhances MDR pathogen virulence and persistence | Amplified by recurrent or unresolved infections | [6,45,95] |
| Immune Alterations | Skews toward Th1/Th17 responses, impacting host defense | Triggered and sustained by chronic infectious stimuli | [23,52,53] |
| Factor in MetS | Affected PK/PD Parameter | Antimicrobial Class | Clinical Implication | References |
|---|---|---|---|---|
| Visceral Adiposity | Increased volume of distribution | β-lactams, aminoglycosides (hydrophilic) | Risk of subtherapeutic levels | [104,107] |
| Insulin Resistance and MASLD | Altered hepatic metabolism | Fluoroquinolones, macrolides (lipophilic) | Variable drug exposure, potential toxicity | [104,107,108] |
| Obesity | Need for adjusted dosing weight | Aminoglycosides, colistin | Risk of nephrotoxicity/ototoxicity if misdosed | [109,110] |
| Hepatic Inflammation | CYP450 dysregulation | CYP450-metabolized agents | Requires hepatic function assessment | [104,108] |
| Critical Illness | PK variability | Multiple antibiotic classes | Benefit from therapeutic drug monitoring (TDM) | [111] |
| Clinical Domain | Personalized Strategy in MetS Context | Rationale and Therapeutic Target | References |
|---|---|---|---|
| Antimicrobial Dosing | Adjust dosing based on BMI and hepatic function | Accounts for altered volume of distribution and metabolism in obesity/MASLD | [106,107,112] |
| Therapeutic Drug Monitoring | Apply TDM for β-lactams, aminoglycosides, and colistin | Addresses PK variability, reduces toxicity, and ensures efficacy | [110] |
| Microbiota Restoration | FMT; targeted probiotics and prebiotics | Corrects dysbiosis, limits resistome spread | [54,92,101] |
| Immunometabolic Modulation | Use polyphenols, SCFAs, and immune checkpoint inhibitors | Mitigates chronic inflammation, enhances host immunity | [93,114] |
| Multidisciplinary Care | Coordinate ID–Internal Medicine–Pharmacology–Nutrition | Facilitates integrated care for complex host–pathogen dynamics | [4,112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acierno, C.; Nevola, R.; Barletta, F.; Rinaldi, L.; Sasso, F.C.; Adinolfi, L.E.; Caturano, A. Multidrug-Resistant Infections and Metabolic Syndrome: An Overlooked Bidirectional Relationship. Biomedicines 2025, 13, 1343. https://doi.org/10.3390/biomedicines13061343
Acierno C, Nevola R, Barletta F, Rinaldi L, Sasso FC, Adinolfi LE, Caturano A. Multidrug-Resistant Infections and Metabolic Syndrome: An Overlooked Bidirectional Relationship. Biomedicines. 2025; 13(6):1343. https://doi.org/10.3390/biomedicines13061343
Chicago/Turabian StyleAcierno, Carlo, Riccardo Nevola, Fannia Barletta, Luca Rinaldi, Ferdinando Carlo Sasso, Luigi Elio Adinolfi, and Alfredo Caturano. 2025. "Multidrug-Resistant Infections and Metabolic Syndrome: An Overlooked Bidirectional Relationship" Biomedicines 13, no. 6: 1343. https://doi.org/10.3390/biomedicines13061343
APA StyleAcierno, C., Nevola, R., Barletta, F., Rinaldi, L., Sasso, F. C., Adinolfi, L. E., & Caturano, A. (2025). Multidrug-Resistant Infections and Metabolic Syndrome: An Overlooked Bidirectional Relationship. Biomedicines, 13(6), 1343. https://doi.org/10.3390/biomedicines13061343







