Abstract
Subjects with inflammatory bowel diseases (IBDs) have a higher opportunity for fractures due to the inflammatory potential of the disorder and because of the glucocorticoid therapy that is often inevitable. The fracture risk can be assessed by dual-energy X-ray absorptiometry and can also be combined with assessing the trabecular bone score (TBS). The evaluation of the TBS offers additional advantages in particular conditions, such as glucocorticoid-induced osteoporosis, and thus optimizes the fracture risk evaluation in the IBD subject group. A limited number of studies involving TBS in other digestive diseases is unlikely to provide sufficient evidence regarding the usefulness of TBS in gastroenterology. Our aim is to review the clinical utility of TBS in digestive diseases.
1. Introduction
Inflammatory bowel diseases (IBDs) are a heterogeneous group of chronic and recurrent intestinal diseases composed of two major forms: Crohn’s disease (CD) and ulcerative colitis (UC) [1], presented with a relapsing and recurrent course [2]. CD features chronic transmural impaction, with interrupted inflamed lesions grasping any part of the digestive tube, characterized by intestinal granulomas, obstruction, strictures and fistulas [3], whereas UC causes continuous mucosal inflammation extending from the rectum toward the colon, but without the above involvement [4]. This debilitating condition can also occur with disorders beyond the digestive tract, known as extra-intestinal manifestations (EIMs), and the main ones are those affecting joints, causing both peripheral and axial arthropathies, skin involvement, causing erythema nodosum, aphthous ulcers, or pyoderma gangrenosum, lesions of the biliary tract, leading to primary sclerosing cholangitis, and eye involvement, causing mainly uveitis, scleritis [5].
Osteoporosis is considered to be a chronic, metabolic skeletal disease, distinguished by reduced bone mass and microarchitectural impairment of bone tissue, with a consequential increase in bone fragility and susceptibility to fracture [6]. Osteoporosis is a highly prevalent disorder considered to affect over 200 million people worldwide, causing a major international social and economic burden [7], and it is followed by a reduction in bone mineral density (BMD) [8]. Recent evidence demonstrated that pro-inflammatory cytokines act on the bone-remodeling flow and represent a pivotal role in the pathogenesis of osteoporosis [9,10]. Also, the participation of both innate and adaptive immunocytes in the development of osteoporosis has been proposed [11,12]; accordingly, osteoporosis can be understood as an inflammatory disorder. It is widely known that any chronic inflammation caused by refractory infections and autoimmune disorders, for example, rheumatoid arthritis, IBD, autoimmune thyroid disease, both type 1 diabetes and type 2 diabetes, and some viral infections (i.e., human immunodeficiency virus), is frequently associated with decreased BMD, and the higher risk of osteoporosis with co-existing progressive inflammation has been firmly established [13,14,15,16,17,18]. Also, elderly individuals with autoimmune rheumatic and gastrointestinal disorders face higher fracture risks when using proton pump inhibitors, especially when there is a simultaneous usage of glucocorticoids [19].
Recent reports have suggested a connection regarding osteoporosis and lifestyle, and dietary and genetic agents unique to particular ethnic groups [20,21,22].
Malnutrition, delayed puberty, reduced physical activity, and 25-hydroxyvitamin D (25-OHD) deficiency have also been proposed in other reports [23,24].
Different gastrointestinal conditions, such as IBD, can negatively influence bone status by deteriorating nutrient and calcium absorption and producing inflammatory factors that interfere with bone resorption and formation, consequently leading to deterioration [25].
The formation of osteoporosis and the incidence of pathological fracture in individuals with IBD has grown over the decade [26]; so, establishing a causal link between IBD and osteoporosis from an epidemiological point of view has been quite challenging.
The etiopathogenesis of skeletal manifestations in IBD is multifactorial and has not been completely explained to date. In addition to systemic inflammation, numerous agents have been determined to influence bone deterioration in individuals with IBD, such as long-term corticosteroid therapy, malabsorption and menopause [27].
Also, it is well known that the levels of many proinflammatory osteoclast activators (such as TNFα and interleukin-1β in the mucosa of the gastrointestinal tract and interleukin-6 in peripheral blood) are increased in individuals with IBD compared to healthy subjects [28].
The literature indicates that osteoporosis is present in 12–42% and osteopenia in up to 77% of individuals with IBD [29,30,31,32]. Numerous reports in adults have clearly indicated a higher risk of vertebral fractures [33,34,35].
The diagnosis of osteoporosis has been based on the BMD assessed by dual-energy X-ray absorptiometry (DXA) since the World Health Organization (WHO) defined osteoporosis diagnosis as a BMD 2.5 standard deviation or more below the average value of healthy young women (T-score) [36]. BMD is influenced by alterations in bone size and can be falsely higher by degenerative changes, showing to be a suboptimal fracture predictor [37].
DXA bone densitometry can be used not only for BMD assessment but also to precisely define bone quality by the means of trabecular bone score (TBS) and hip structural analysis measurement. TBS may elucidate and enrich skeletal information that is not measured by the standard BMD evaluation. TBS is a software-based tool for analysis of DXA images of the lumbar spine by assessing pixel gray-level variations in the image. Increased TBS levels are obtained in more homogeneously textured bone, and decreased TBS levels in less well-textured bone. TBS cut-off points for classifying normal and abnormal TBS values have not been completely proposed yet. In postmenopausal women, the following normal range was suggested: TBS > 1.350 as normal; TBS between 1.200 and 1.350 is shown to be consistent with partially degraded microarchitecture; and TBS ≤ 1.200 defines degraded microarchitecture [38].
Numerous reports have indicated that TBS is significantly linked with direct measurements of bone microarchitecture, and may be a useful adjunct to BMD for the detection and assessment of fragility fractures in primary osteoporosis [38,39,40,41]. Since BMD evaluates bone quantity and TBS evaluates bone quality, these methods have been shown to be complementary in predicting the incidence of fracture and response to treatment [42].
TBS correlates more precisely with trabecular microarchitecture than BMD when used in premenopausal women and in men with idiopathic osteoporosis and low-traumatic fractures [43].
Interestingly, in particular conditions, such as glucocorticoid-induced osteoporosis and in diabetes mellitus, TBS appears to out-perform DXA [44].
TBS has been shown to be an BMD-independent clinical risk tool for developing fragility fracture and could determine individuals who have an enlarged risk of fragility fractures better than BMD [45,46].
Numerous recent studies, as well as meta-analyses, have established the value of TBS in BMD measurements, proposing this new parameter to be included in international guidelines [47,48].
According to the conducted studies, it has been concluded that, in dissimilar individuals with the same BMD, structural bone health is more precisely distinguished by assessing TBS [36,49,50]. The supplemented use of TBS and BMD remarkably enhances the prediction of fractures [51].
Different agents have been proposed for osteoporosis treatment, such as bisphosphonates, denosumab, selective estrogen receptor modulators, and calcitonin [52]. However, the various etiologies of osteoporosis, for example, age-related bone loss, long-term corticosteroid treatment, or chronic inflammatory diseases, challenge the efficacy of the current therapeutic agents. Nowadays, pharmacological modalities for osteoporosis, such as bisphosphonates, antibodies, parathyroid hormone (PTH)-related peptides, and teriparatide, exhibit limitations in their therapeutic efficacy and are linked with consequential side effects. So, there is an urgent need to enable comprehensive therapeutic methods connected with a favorable safety profile. Some reports show that colon-targeted engineered postbiotics, protecting the gut, can decrease chronic inflammation, which rebalances inflammation-disturbed osteoblast–osteoclast levels, resetting bone homeostasis [53].
The goal of our study is to elucidate the use of TBS in clinical practice with precise attention to fracture risk assessment, differential diagnosis and evaluation of treatment outcomes in patients suffering from gastrointestinal diseases.
2. Materials and Methods
A comprehensive literature search was conducted for articles published in MedLine via PubMed using an approach similar to the PRISMA guidelines. Fracture syntax comprised ‘trabecular bone score’ OR ‘TBS’ [search term(Title/Abstract)], AND ‘bone mineral density’ [search term(Title/Abstract)] OR ‘BMD’ [search term (Title/Abstract)] (Figure 1), with no time restrictions. The articles were adequate for review if they met the following general screening criteria: (i) an original, full-text report with TBS as a primary outcome, and (ii) available in the English language. Further eligibility criteria were specific to each of the four topics. A total of 136 papers were reviewed by 2 independent reviewers.
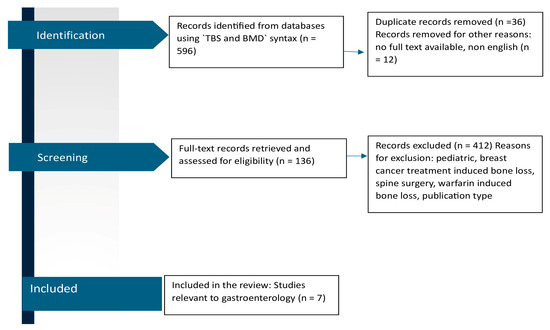
Figure 1.
PRISMA flow diagram of the literature search process for studies investigating TBS and BMD in gastroenterology.
3. Results
A total of 136 studies met the eligibility criteria (prospective study design, conducted in men and/or women aged 18 years or over). Several of these reports were conducted in individuals with rheumatology diseases, and most of them in patients with endocrinology diseases and chronic kidney disease. A small number of studies were conducted in individuals with hematological and neurological disorders, and in those with chronic infectious diseases (AIDS, HCV).
We found only seven studies conducted in individuals with gastrointestinal disorders (Figure 1).
Our choice was to conduct a narrative review of the literature; because this study is not a systematic review and few articles were found, evaluations of methodological quality were not used to exclude papers from this study.
3.1. TBS and IBD
It is well known there is the discordance between low BMD and high frequency of vertebral fractures in a cohort of CD individuals [54], but there are only four studies [55,56,57,58] comparing bone quality measured by TBS in IBD subjects (Table 1).

Table 1.
Study characteristics; studies comparing bone quality measured by TBS in IBD patients.
The study of Soare included both CD and UC individuals. It is a cross-sectional, multicentric study, with 80 IBD individuals, with a positive correlation between BMI and TBS. But there is the lack of a control group and no assessment of disease duration nor of activity [55].
Krajcovicova et al. conducted a study of 50 adults only with CD, and concluded that TBS, but not BMD, was significantly influenced by the severity of CD [56].
In other study, Soare [57] provided an evaluation of disease duration and activity. This study found that decreased BMD status was more predominant in IBD individuals compared with healthy subjects. They found that inflammation activity is negatively associated with TBS in CD individuals. The Harvey–Bradshaw index, a marker of disease activity, is independently correlated with TBS values in CD individuals, while exposure to high-dosage glucocorticoids has an adverse effect on both BMD and TBS values [57]. The limitation of this study is that IBD individuals were not treated with vitamin D supplements, with their mean vitamin D levels being borderline between insufficiency and deficiency, which could have impact on the TBS values. Further prospective studies should be conducted with a prospective follow-up of TBS changes in comparison with BMD changes during different stages of the inflammation and after vitamin D supplementation. Also, they did not find differences in the DXA parameters between CD and UC individuals, and the literature regarding the subject is contradictory. Ezzat [59] and Bjarnason [60] obtained reduced BMD values in CD individuals compared to UC individuals.
In a report of TBS in pediatric subjects with IBD, the TBS values in the pediatric group of patients with CD were reduced compared to healthy children of similar characteristics (age and gender). TBS was significantly decreased in individuals with CD compared to in those with UC. TBS showed a correlation with BMI Z-score, the phosphorus level at diagnosis, and with age at the time of DXA scan [61].
Haschka et al. [58], in their study, evaluated TBS and BMD only in CD individuals, and the subjects were divided into subgroups according to glucocorticoid usage and disease duration. They found decreased TBS in individuals on glucocorticoid treatment.
3.2. TBS and Cirrhosis
Individuals with liver cirrhosis, regardless of its etiology, have an increased prevalence of osteoporosis compared to the general population [62]. There are only two reports on the link between TBS and vertebral fractures in individuals with cirrhosis, which is unusual given the common knowledge that there is a considerable fracture burden in liver transplant recipients [63].
Ogiso et al. [64], in their study, indicated that TBS can help identify individuals with cirrhosis at risk of vertebral fractures, and provided complementary information to BMD in detecting fracture incidence. In fact, their study presents the idea that TBS has a certain advantage over BMD in assessing vertebral fracture risk in cirrhotic individuals, especially when ascites is present.
In their study, Stulic et al. used a comprehensive individualized clinical fracture risk evaluation approach assessing both BMD and TBS and serum bone turnover biomarkers to compare adult male individuals with alcoholic liver cirrhosis, individuals with chronic alcohol abuse, and a healthy age- and sex-matched cohort. In their study, the TBS analysis showed that the vertebral micro-architecture was significantly preserved in the healthy cohort compared to those with alcoholic liver cirrhosis and chronic alcohol abuse [65].
Since there is a lack of studies evaluating the usefulness of TBS in cirrhotic individuals, further prospective studies are required.
An important finding of the study of Yokoyama et al. [66] was that an increased BMI or a large waist circumference was connected to the preservation of BMD in individuals with metabolic-dysfunction-associated steatotic liver disease (MASLD), which is consistent with the phenomenon reported in the literature as the “obesity paradox” [67]. Maybe future trials evaluating TBS instead of only BMD would indicate that there is bone deterioration in these individuals.
3.3. TBS and Chronic Atrophic Gastritis
Chronic atrophic gastritis (CAG) is a long-term inflammatory condition in the gastric mucosa characterized by the deterioration of regular glandular structures and replacement with connective tissue or non-native epithelium [68], and CAG leading to the malabsorption of nutrients, meaning a reduction in vitamin D and calcium absorption [69], consequently causing bone deterioration [70]. Common etiologies of CAG include autoimmunity and Helicobacter pylori infection [71]. H. pylori-related chronic gastritis enhances the risk of osteoporosis, with higher activity of gastritis and more extensive atrophy leading to further enhanced risk [72]. H. pylori infection has been shown to be inversely correlated with vitamin D status, and decreased vitamin D levels lead to a higher risk of fracture [73]. There is only one study exploring using TBS to detect impaired skeletal health in individuals with CAG, and with quite a small number of participants. In spite of those limitations, Aasarød et al. [74] assessed BMD and bone quality in individuals with CAG, and found that both BMD and TBS were decreased in the CAG cohort compared to the healthy subjects.
4. Discussion
Our comparative analysis of the literature about the usage of TBS in gastrointestinal diseases underlined the need for further studies.
BMD is a standard and widely accepted tool for diagnosing osteoporosis and predicting fracture risk. However, research suggests that BMD alone cannot accurately assess bone strength [75], and in order to improve the assessment of bone strength, TBS has been utilized for years [76].
Knowledge regarding the connection between decreased TBS levels in individuals with endocrine and rheumatological diseases is well elucidated in the literature. On the other hand, when it comes to conditions affecting the gastrointestinal tract, the discourse is undoubtedly more complex and requires further studies.
In the gastrointestinal spectrum of diseases, TBS was found to be efficient in the field of IBD individuals. But there is a high heterogeneity between the reports, and differences in study characteristics; so, there is a need for future trials.
Lower BMD is one of the most common extraintestinal symptoms of celiac disease [77]. Data on the incidence of osteopenia and osteoporosis often vary between reports. In other studies, there was not any significant difference between osteopenia and osteoporosis in the femur and spine in patients with celiac disease [78]. Unfortunately, no studies reporting TBS levels in celiac disease were found.
The fracture risk in eosinophilic esophagitis (EoE) patients was not statistically significantly increased compared to that in non-EoE reference subjects according to Garber et al. [79]; however, we cannot exclude the possibility that steroid therapy among individuals with EoE is linked with a moderately increased fracture risk; so, it would be interesting to assess the TBS levels in those individuals.
The International Society for Clinical Densitometry (ISCD) proposes that TBS can be used in assessing together with BMD, though not as a stand-alone tool, to refine fracture risk evaluation [42]. Since BMD evaluates bone quantity and TBS evaluates bone quality, these measurements can be considered complementary in predicting fracture risk and response to therapy in appropriate individuals.
The literature analysis points to the higher use of TBS in clinical practice in the fields of rheumatology, endocrinology and nephrology, but there is a lack of studies using TBS as a predictive tool in gastroenterology.
5. Conclusions
In conclusion, it is valuable to use the DXA along with TBS analysis for improved prediction of fracture risk. TBS has potential as a clinical tool in gastroenterology; however, there is a need for future prospective studies that involve the use of TBS in individuals with gastrointestinal diseases.
Author Contributions
Conceptualization, I.Z. and I.O.; methodology, I.Z. and I.O.; software, P.M.Z.; validation, I.O. and I.T.H.; formal analysis, I.Z.; investigation, I.O.; resources, I.O.; data curation, I.O.; writing—original draft preparation, P.M.Z. and I.Z.; writing—review and editing, I.Z.; visualization, I.T.H.; supervision, P.M.Z. and I.Z.; project administration, I.Z.; funding acquisition, I.T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| IBD | inflammatory bowel disease |
| EIMs | extra-intestinal manifestations |
| BMD | bone mineral density |
| DXA | dual-energy X-ray absorptiometry |
| TBS | trabecular bone score |
| PTH | parathyroid hormone |
| MASLD | metabolic-dysfunction-associated steatotic liver disease |
| CAG | chronic atrophic gastritis |
| UC | ulcerative colitis |
| CD | Crohn’s disease |
| WHO | World Health Organization |
| ISCD | The International Society for Clinical Densitometry |
| EoE | eosinophilic esophagitis |
References
- Larsen, S.; Bendtzen, K.; Nielsen, O.H. Extraintestinal manifestations of inflammatory bowel disease: Epidemiology, diagnosis, and management. Ann. Med. 2010, 42, 97–114. [Google Scholar] [CrossRef]
- Roda, G.; Ng, S.C.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Primers 2020, 6, 22. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.F.; Aurelio, D.M. Extraintestinal Manifestations of Inflammatory Bowel Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Kanis, J.; McCloskey, E.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, X.; Wu, J.; Lin, X.; Zhou, X.; Zhu, Z.; Pan, X.; Xu, J.; Qiao, J.; Zhang, T.; et al. The global burden of osteoporosis, low bone mass, and its related fracture in 204 countries and territories, 1990–2019. Front. Endocrinol. 2022, 13, 882241. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, D.; Zhou, Z.; Zhu, L.; Hu, X.; Lu, J.; Shi, C.; Chen, F.; Chen, A. TNF-α suppresses osteogenic differentiation of MSCs by accelerating P2Y2 receptor in estrogen-deficiency induced osteoporosis. Bone 2018, 117, 161–170. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Gao, Y.; Zhao, L.; Cai, C.; Qi, D.; Zhu, M.; Zhao, L.; Liu, C.; Guo, F.; et al. Hesperetin suppresses RANKL-induced osteoclastogenesis and ameliorates lipopolysaccharide-induced bone loss. J. Cell. Physiol. 2019, 234, 11009–11022. [Google Scholar] [CrossRef]
- Saxena, Y.; Routh, S.; Mukhopadhaya, A. Immunoporosis: Role of innate immune cells in osteoporosis. Front. Immunol. 2021, 12, 687037. [Google Scholar] [CrossRef]
- Wu, D.; Cline-Smith, A.; Shashkova, E.; Perla, A.; Katyal, A.; Aurora, R. T-Cell mediated inflammation in postmenopausal osteoporosis. Front. Immunol. 2021, 12, 687551. [Google Scholar] [CrossRef]
- Kirkham-Wilson, F.; Dennison, E. Osteoporosis and Rheumatoid Arthritis: A Review of Current Understanding and Practice. Br. J. Hosp. Med. 2024, 85, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Penner, J.; Ombajo, L.A.; Nkuranga, J.; Otieno, E.; Nyakoe, D.; Wanjohi, R.; Mbewa, V.; Ndinya, F.; Eshiwani, S.; Wahome, S.; et al. High prevalence of osteoporosis among virally suppressed older people (≥60 years) living with HIV. HIV Med. 2024, 26, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wu, X.; Li, Y.; Zhao, S.; Wang, S.; Yu, D. Association between inflammatory bowel disease and osteoporosis in European and East Asian populations: Exploring causality, mediation by nutritional status, and shared genetic architecture. Front. Immunol. 2024, 15, 1425610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.; Li, J.; Yan, Z.; Yu, X.; Huang, H. Higher prevalence of thyroid-specific autoantibodies (TPOAb and TgAb) is related to a higher prevalence of fractures in females: Results from NHANES 2007–2010. Osteoporos Int. 2024, 35, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Leng, P.; Qiu, Y.; Zhou, M.; Zhu, Y.; Yin, N.; Zhou, M.; Wu, W.; Liu, M. Hypothyroidism correlates with osteoporosis: Potential involvement of lipid mediators. Front. Med. 2024, 11, 1453502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leungsuwan, D.S.; Chandran, M. Bone Fragility in Diabetes and its Management: A Narrative Review. Drugs 2024, 84, 1111–1134. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Lee, Y.H.; Wang, S.I.; Palmowski, A.; Chu, W.M.; Buttgereit, F. Fracture Risk Linked to Proton Pump Inhibitors Versus H2 Receptor Antagonists in Autoimmune Rheumatic and Gastrointestinal Disease Patients. Int. J. Rheum. Dis. 2025, 28, e70055. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.P.; Prasad, P.; Malaty, H.M. Vitamin D deficiency and corticosteroid use are risk factors for low bone mineral density in inflammatory bowel disease patients. Dig. Dis. Sci. 2014, 59, 1878–1884. [Google Scholar] [CrossRef]
- Davis, J.W.; Novotny, R.; Ross, P.D.; Wasnich, R.D. Anthropometric, lifestyle and menstrual factors influencing size-adjusted bone mineral content in a multiethnic population of premenopausal women. J. Nutr. 1996, 126, 2968–2976. [Google Scholar] [CrossRef]
- Patel, D.N.; Pettifor, J.M.; Becker, P.J. The effect of ethnicity on appendicular bone mass in white, coloured and Indian schoolchildren. S. Afr. Med. J. 1993, 83, 847–853. [Google Scholar]
- Dennison, E.; Yoshimura, N.; Hashimoto, T.; Cooper, C. Bone loss in Great Britain and Japan: A comparative longitudinal study. Bone 1998, 23, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Burnham, J.M.; Shults, J.; Semeao, E.; Foster, B.; Zemel, B.S.; Stallings, V.A.; Leonard, M.B. Whole body BMC in pediatric Crohn disease: Independent effects of altered growth, maturation, and body composition. J. Bone Miner. Res. 2004, 19, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Merlotti, D.; Mingiano, C.; Valenti, R.; Cavati, G.; Calabrese, M.; Pirrotta, F.; Bianciardi, S.; Palazzuoli, A.; Gennari, L. Bone Fragility in Gastrointestinal Disorders. Int. J. Mol. Sci. 2022, 23, 2713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adriani, A.; Pantaleoni, S.; Luchino, M.; Ribaldone, D.G.; Reggiani, S.; Sapone, N.; Sguazzini, C.; Isaia, G.; Pellicano, R.; Astegiano, M. Osteopenia and osteoporosis in patients with new diagnosis of inflammatory bowel disease. Panminerva Med. 2014, 56, 145–149. [Google Scholar] [PubMed]
- Targownik, L.E.; Bernstein, C.N.; Leslie, W.D. Risk factors and management of osteoporosis in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2014, 30, 168–174. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Leslie, W.D.; Leboff, M.S. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology 2003, 124, 795–841. [Google Scholar] [CrossRef]
- Schoon, E.; van Nunen, A.; Wouters, R.; Stockbrügger, R.; Russel, M. Osteopenia and osteoporosis in Crohn’s disease: Prevalence in a Dutch population-based cohort. Scand. J. Gastroenterol. Suppl. 2000, 232, 43–47. [Google Scholar]
- Geraci, A.; Tomasello, G.; Damiani, P.; Mazzoccato, G.; Marinato, L.; Gasparo, M. Bone loss in inflammatory Bowel disease: Our multicentric study. Endocrinol. Stud. 2011, 1, e3. [Google Scholar] [CrossRef]
- Boubaker, J.; Feki, M.; Hsairi, M.; Fekih, M.; Kaabachi, N.; Filali, A.; Mebazaa, A. Osteoporosis and inflammatory bowel disease: Prevalence and risk factors in Tunisian patients. Gastroenterol. Clin. Biol. 2003, 27, 901–907. [Google Scholar]
- Miznerova, E.; Hlavaty, T.; Koller, T.; Toth, J.; Holociova, K.; Huorka, M.; Killinger, Z.; Payer, J. The prevalence and risk factors for osteoporosis in patients with inflammatory bowel disease. Bratisl Lek Listy 2012, 114, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Klaus, J.; Armbrecht, G.; Steinkamp, M.; Brückel, J.; Rieber, A.; Adler, G.; Reinshagen, M.; Felsenberg, D.; von Tirpitz, C. High prevalence of osteoporotic vertebral fractures in patients with Crohn’s disease. Gut 2002, 51, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P.; Krogh, K.; Rejnmark, L.; Laurberg, S.; Mosekilde, L. Fracture risk is increased in Crohn’s disease, but not in ulcerative colitis. Gut 2000, 46, 176–181. [Google Scholar] [CrossRef] [PubMed]
- van Staa, T.P.; Cooper, C.; Brusse, L.S.; Leufkens, H.; Javaid, M.K.; Arden, N.K. Inflammatory bowel disease and the risk of fracture. Gastroenterology 2003, 125, 1591–1597. [Google Scholar] [CrossRef]
- Choi, Y.J.; Ock, S.Y.; Chung, Y.S. Trabecular bone score (TBS) and TBS-adjusted fracture risk assessment tool are potential supplementary tools for the discrimination of morphometric vertebral fractures in postmenopausal women with type 2 diabetes. J. Clin. Densitom. 2016, 19, 507–514. [Google Scholar] [CrossRef]
- Jain, R.K.; Vokes, T. Dual-energy X-ray Absorptiometry. J. Clin. Densitom. 2017, 20, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Leslie, W.D.; Resch, H.; Lamy, O.; Lesnyak, O.; Binkley, N.; McCloskey, E.V.; Kanis, J.A.; Bilezikian, J.P. Trabecular bone score: A noninvasive analytical method based upon the DXA image. J. Bone Miner. Res. 2014, 29, 518–530. [Google Scholar] [CrossRef]
- Popp, A.W.; Meer, S.; Krieg, M.A.; Perrelet, R.; Hans, D.; Lippuner, K. Bone mineral density (BMD) and vertebral trabecular bone score (TBS) for the identifcation of elderly women at high risk for fracture: The SEMOF cohort study. Eur. Spine J. 2016, 25, 3432–3438. [Google Scholar] [CrossRef]
- Bonaccorsi, G.; Fila, E.; Messina, C.; Maietti, E.; Ulivieri, F.M.; Caudarella, R.; Greco, P.; Guglielmi, G. Comparison of trabecular bone score and hip structural analysis with FRAX® in postmenopausal women with type 2 diabetes mellitus. Aging Clin. Exp. Res. 2017, 29, 951–957. [Google Scholar] [CrossRef]
- Leslie, W.D.; Johansson, H.; McCloskey, E.V.; Harvey, N.C.; Kanis, J.A.; Hans, D. Comparison of methods for improving fracture risk assessment in diabetes: The Manitoba BMD Registry. J. Bone Miner. Res. 2018, 33, 1923–1930. [Google Scholar] [CrossRef]
- Krohn, K.; Schwartz, E.N.; Chung, Y.S.; Lewiecki, E.M. Dual-energy X-ray absorptiometry monitoring with trabecular bone score: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Muschitz, C.; Kocijan, R.; Haschka, J.; Pahr, D.; Kaider, A.; Pietschmann, P.; Hans, D.; Muschitz, G.K.; Fahrleitner-Pammer, A.; Resch, H. TBS reflects trabecular microarchitecture in premenopausal women and men with idiopathic osteoporosis and low-traumatic fractures. Bone 2015, 79, 259–266. [Google Scholar] [CrossRef]
- Ulivieri, F.M.; Silva, B.C.; Sardanelli, F.; Hans, D.; Bilezikian, J.P.; Caudarella, R. Utility of the trabecular bone score (TBS) in secondary osteoporosis. Endocrine 2014, 47, 435–448. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Odén, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Martineau, P.; Leslie, W. Trabecular bone score (TBS): Method and applications. Bone 2017, 104, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Broy, S.B.; Boutroy, S.; Schousboe, J.T.; Shepherd, J.A.; Leslie, W.D. Fracture risk prediction by non-BMD DXA measures: The 2015 ISCD official positions part 2: Trabecular bone score. J. Clin. Densitom. 2015, 18, 309–330. [Google Scholar] [CrossRef]
- Harvey, N.; Glüer, C.; Binkley, N.; McCloskey, E.; Brandi, M.-L.; Cooper, C.; Kendler, D.; Lamy, O.; Laslop, A.; Camargos, B.; et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015, 78, 216–224. [Google Scholar] [CrossRef]
- Briot, K.; Paternotte, S.; Kolta, S.; Eastell, R.; Reid, D.M.; Felsenberg, D.; Glüer, C.C.; Roux, C. Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: The OPUS study. Bone 2013, 57, 232–236. [Google Scholar] [CrossRef]
- Nassar, K.; Paternotte, S.; Kolta, S.; Fechtenbaum, J.; Roux, C.; Briot, K. Added value of trabecular bone score over bone mineral density for identification of vertebral fractures in patients with areal bone mineral density in the non-osteoporotic range. Osteoporos. Int. 2014, 25, 243–249. [Google Scholar] [CrossRef]
- Chen, W.; Mao, M.; Fang, J.; Xie, Y.; Rui, Y. Fracture risk assessment in diabetes mellitus. Front. Endocrinol. 2022, 13, 961761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Bai, R.; Wang, Z.; Qin, Y.; Wang, J.; Wei, Y.; Zhao, R.; Nie, G.; Han, B. Colon-targeted engineered postbiotics nanoparticles alleviate osteoporosis through the gut-bone axis. Nat. Commun. 2024, 15, 10893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stockbrügger, R.; Schoon, E.; Bollani, S.; Mills, P.; Israeli, E.; Landgraf, L.; Felsenberg, D.; Ljunghall, S.; Nygard, G.; Persson, T.; et al. Discordance between the degree of osteopenia and the prevalence of spontaneous vertebral fractures in Crohn’s disease. Aliment. Pharmacol. Ther. 2002, 16, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Soare, I.; Sirbu, A.; Popa, M.; Martin, S.; Tieranu, C.G.; Mateescu, B.; Diculescu, M.; Barbu, C.; Fica, S. Body Composition as a Modulator of Bone Health Changes in Patients with Inflammatory Bowel Disease. Life 2022, 12, 272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krajcovicova, A.; Kuzma, M.; Hlavaty, T.; Hans, D.; Koller, T.; Jackuliak, P.; Leskova, Z.; Sturdik, I.; Killinger, Z.; Payer, J. Decrease of trabecular bone score reflects severity of Crohn’s disease: Results of a case-control study. Eur. J. Gastroenterol. Hepatol. 2018, 30, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Soare, I.; Sirbu, A.; Martin, S.; Diculescu, M.; Mateescu, B.; Tieranu, C.; Fica, S. Assessment of bone quality with trabecular bone score in patients with inflammatory bowel disease. Sci. Rep. 2021, 11, 20345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haschka, J.; Kraus, D.A.; Behanova, M.; Huber, S.; Bartko, J.; Schanda, J.E.; Meier, P.; Bahrami, A.; Zandieh, S.; Zwerina, J.; et al. Fractal-Based Analysis of Bone Microstructure in Crohn’s Disease: A Pilot Study. J. Clin. Med. 2020, 9, 4116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ezzat, Y.; Hamdy, K. The frequency of low bone mineral density and its associated risk factors in patients with inflammatory bowel diseases. Int. J. Rheum. Dis. 2010, 13, 259–265. [Google Scholar] [CrossRef]
- Bjarnason, I.; Macpherson, A.; Mackintosh, C.; Buxton-Thomas, M.; Forgacs, I.; Moniz, C. Reduced bone density in patients with inflammatory bowel disease. Gut 1997, 40, 228–233. [Google Scholar] [CrossRef]
- Levy-Shraga, Y.; Megnazi, O.; Modan-Moses, D.; Tripto-Shkolnik, L.; Gruber, N.; Haberman, Y.; Shouval, D.S.; Weiss, B. Trabecular Bone Score in Children and Adolescents With Inflammatory Bowel Diseases. J. Clin. Densitom. 2021, 24, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, R.; Di Minno, A.; Spadarella, G.; Ambrosino, P.; Panico, A.; Tarantino, L.; Lupoli, G.; Lupoli, G.; Di Minno, M.N.D. The risk of osteoporosis in patients with liver cirrhosis: A meta-analysis of literature studies. Clin. Endocrinol. 2016, 84, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zavatta, G.; Vitale, G.; Morelli, M.C.; Pianta, P.; Turco, L.; Cappa, F.M.; Ravaioli, M.; Cescon, M.; Piscaglia, F.; Altieri, P.; et al. High bone fracture risk in a large modern cohort of liver transplant recipients. Intern. Emerg. Med. 2024, 20, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, Y.; Hanai, T.; Nishimura, K.; Miwa, T.; Maeda, T.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M. Usefulness of the Trabecular Bone Score in Assessing the Risk of Vertebral Fractures in Patients with Cirrhosis. J. Clin. Med. 2022, 11, 1562. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stulic, M.; Jadzic, J.; Dostanic, N.; Zivkovic, M.; Stojkovic, T.; Aleksic, J.; Stojkovic, S.; Stojkovic Lalosevic, M.; Vojnovic, M.; Vlaisavljevic, Z.; et al. Clinical Indicators of Bone Deterioration in Alcoholic Liver Cirrhosis and Chronic Alcohol Abuse: Looking beyond Bone Fracture Occurrence. Diagnostics 2024, 14, 510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yokoyama, S.; Honda, T.; Ishizu, Y.; Imai, N.; Ito, T.; Yamamoto, K.; Mizuno, K.; Kojima, T.; Kariya, N.; Nakamura, M.; et al. Risk factors for decreased bone mineral density in patients with metabolic dysfunction-associated steatotic liver disease: A cross-sectional study at a health examination center. Clin. Nutr. 2024, 43, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.M.; Joham, A.; Marks, S.; Teede, H. The obesity paradox: An endocrine perspective. Intern. Med. J. 2017, 47, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA clinical practice update on the diagnosis and management of atrophic gastritis: Expert review. Gastroenterology 2021, 161, 1325–1332. [Google Scholar] [CrossRef]
- Massironi, S.; Cavalcoli, F.; Zilli, A.; Del Gobbo, A.; Ciafardini, C.; Bernasconi, S.; Felicetta, I.; Conte, D.; Peracchi, M. Relevance of vitamin D deficiency in patients with chronic autoimmune atrophic gastritis: A prospective study. BMC Gastroenterol. 2018, 18, 172. [Google Scholar] [CrossRef]
- Lee, S.; Yun, J.M.; Park, J.H.; Kwon, H. Association between Chronic Atrophic Gastritis and Bone Mineral Density among Women Older than 40 Years of Age in Korea. Korean J. Fam. Med. 2024, 45, 199–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raza, M.; Bhatt, H. Atrophic Gastritis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Inoue, I.; Yoshimura, N.; Iidaka, T.; Horii, C.; Muraki, S.; Oka, H.; Kawaguchi, H.; Akune, T.; Maekita, T.; Mure, K.; et al. Helicobacter pylori-Related Chronic Gastritis as a Risk Factor for Lower Bone Mineral Density. Calcif. Tissue Int. 2025, 116, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shafrir, A.; Shauly-Aharonov, M.; Katz, L.H.; Paltiel, O.; Pickman, Y.; Ackerman, Z. The association between serum vitamin D levels and Helicobacter pylori presence and eradication. Nutrients 2021, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Aasarød, K.M.; Mosti, M.P.; Stunes, A.K.; Reseland, J.E.; Basso, T.; Syversen, U.; Fossmark, R. Impaired skeletal health in patients with chronic atrophic gastritis. Scand. J. Gastroenterol. 2016, 51, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Slart, R.H.; Ali, D.S.; Bock, O.; Carey, J.J.; Camacho, P.; Engelke, K.; Erba, P.A.; Harvey, N.C.; Lems, W.F.; et al. Osteoporotic Fractures: Diagnosis, Evaluation, and Significance From the International Working Group on DXA Best Practices. Mayo Clin. Proc. 2024, 99, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Kim, K.M. Similarities and differences between bone quality parameters, trabecular bone score and femur geometry. PLoS ONE 2022, 17, e0260924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skoracka, K.; Marciniak, M.D.; Michalak, M.; Zawada, A.; Ratajczak-Pawłowska, A.E.; Dobrowolska, A.; Krela-Kaźmierczak, I. The other side of celiac disease—Assessment of bone mineral density and body composition in patients with celiac disease. Prz Gastroenterol. 2024, 16, 434–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ganji, A.; Esmaeilzadeh, A.; Hatef, M. Prevalence of Osteopenia and Osteoporosis in Patients with Celiac Disease in Northeastern Iran. Govaresh 2012, 16, 223–227. [Google Scholar]
- Garber, J.J.; Roelstraete, B.; Lochhead, P.J.; Uchida, A.M.; Michaëlsson, K.; Olén, O.; Ludvigsson, J.F. Risk of fractures in individuals with eosinophilic esophagitis: Nationwide population-based cohort study. Esophagus. 2022, 19, 542–553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).