Age-Related Decline of Gastric Secretion: Facts and Controversies
Abstract
1. Introduction
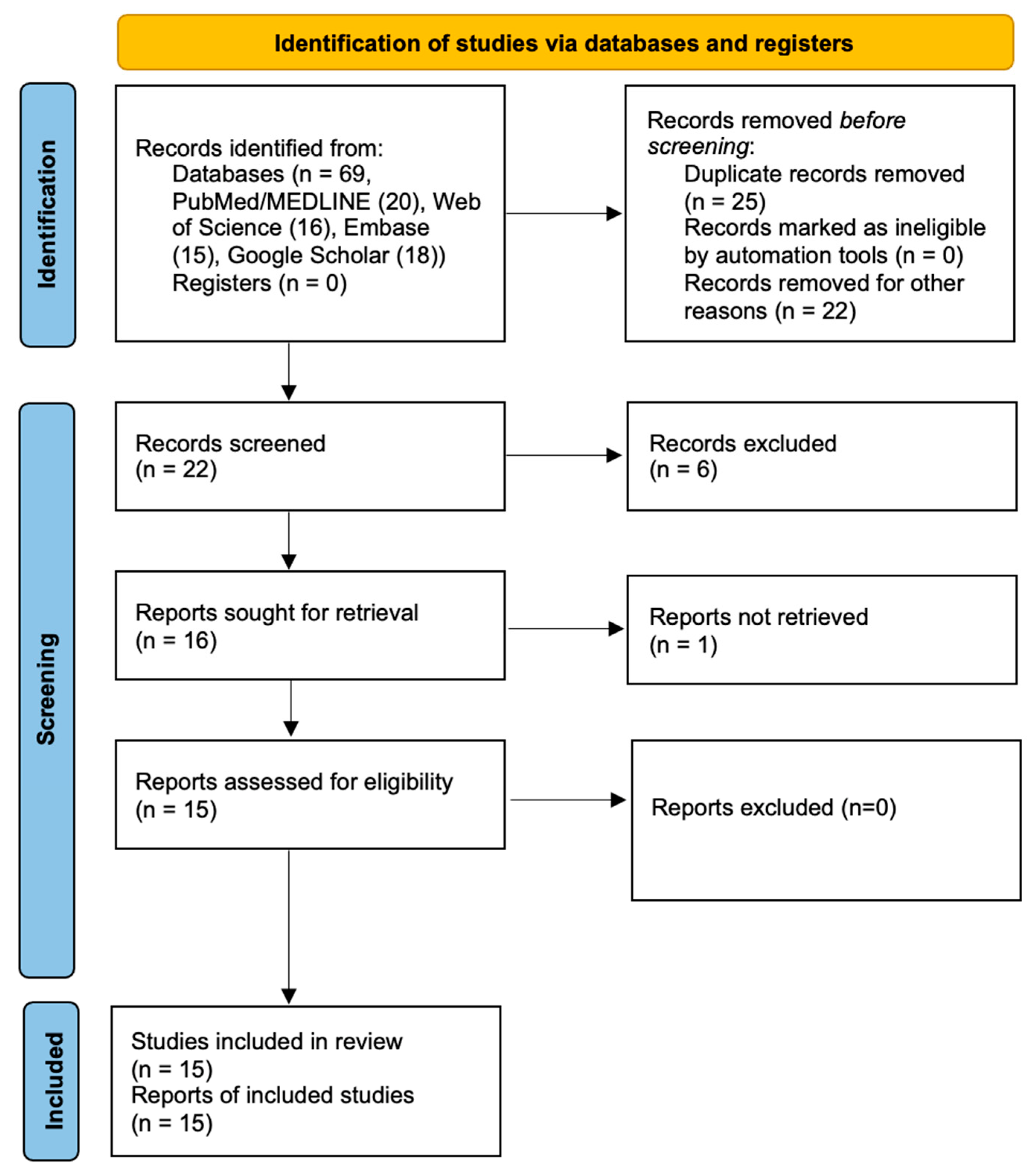
2. Materials and Methods
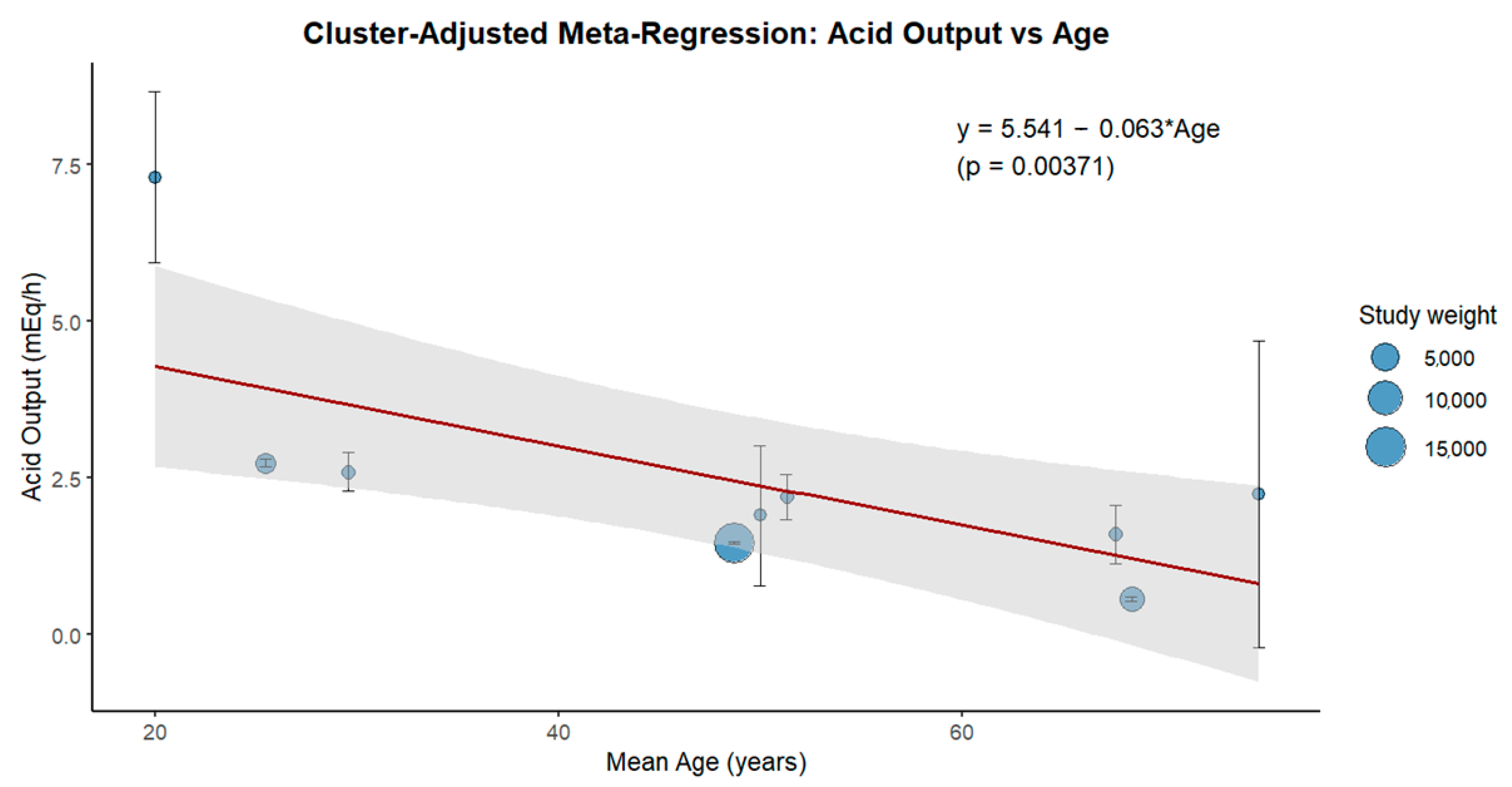
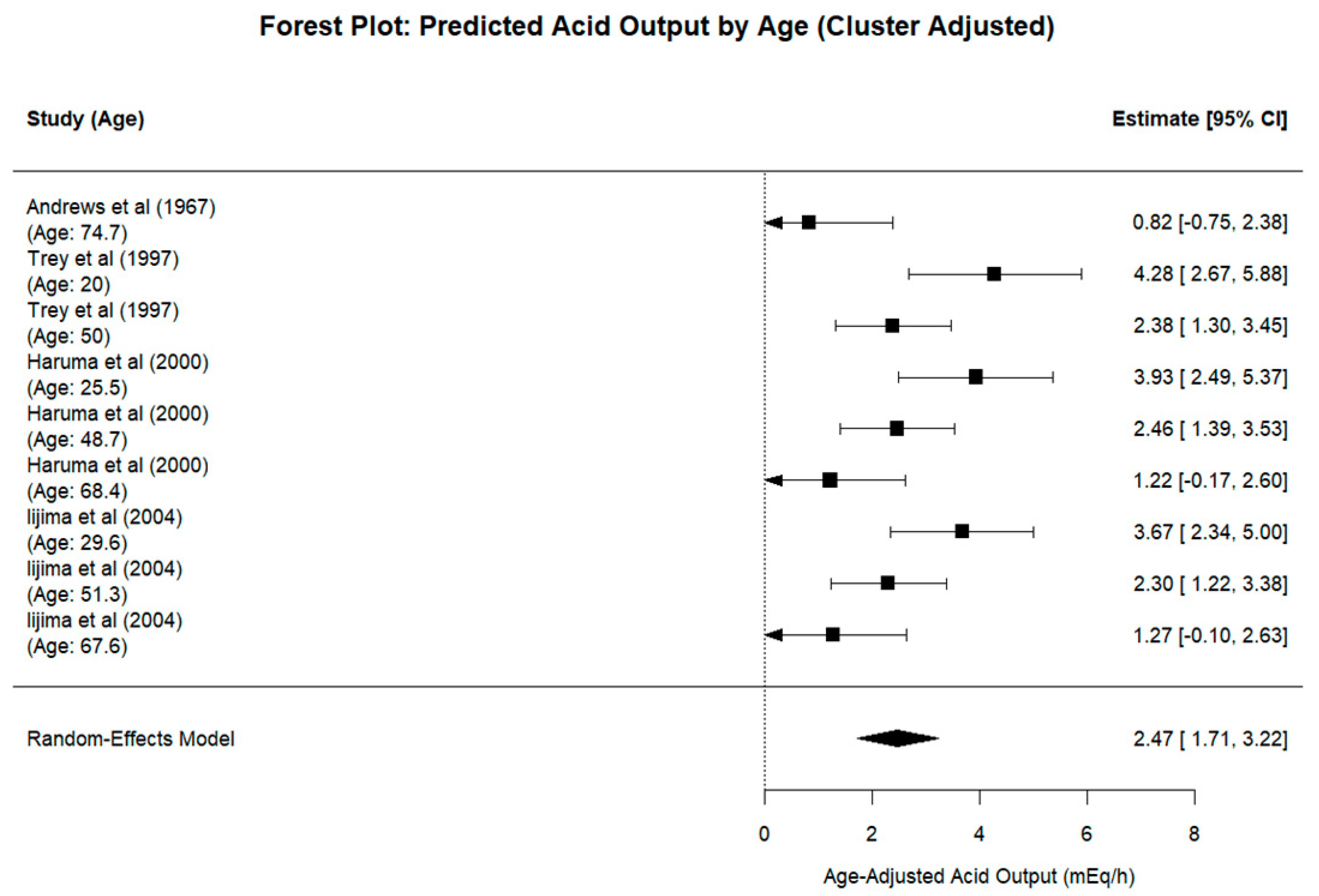
3. Results
4. Discussion
4.1. Impact of Aging on Gastric Secretion
4.1.1. Animal Model Studies
4.1.2. Human Studies
4.2. Impact of Other Factors on Gastric Secretion
4.2.1. Sex, Smoking Factors and Oral Health
4.2.2. Helicobacter Pylori
4.2.3. Chronic Atrophic Gastritis
4.2.4. Antisecretory Therapies
4.3. Clinical Impact of Decline in Gastric Secretion
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAG | Chronic atrophic gastritis |
| H. pylori | Helicobacter pylori |
| PPI | Proton pump inhibitor |
References
- Evert, J.; Lawler, E.; Bogan, H.; Perls, T. Morbidity profiles of centenarians: Survivors, delayers, and escapers. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Vara-Luiz, F.; Mendes, I.; Palma, C.; Mascarenhas, P.; Simas, D.; Gomes, P.; Gonçalves, A.; Simão, I.; Teixeira, M.; Lopes, S.; et al. Upper gastrointestinal bleeding differences between older and younger adults: Should bleeding in non-cirrhotic patients be considered a geriatric syndrome? Therapeutic. Adv. Gastroenterol. 2025, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mathialagan, R.; Illangantilaka, A.; Radhakrishnan, H. Gastroenterology in the elderly. Medicine 2019, 47, 466–469. [Google Scholar] [CrossRef]
- Soenen, S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. The ageing gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Geokas, M.C.; Conteas, C.N.; Majumdar, A.P. The aging gastrointestinal tract, liver, and pancreas. Clin. Geriatr. Med. 1985, 1, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Khalil, T.; Singh, P.; Fujimura, M.; Townsend, C.M., Jr.; Greeley, G.H., Jr.; Thompson, J.C. Effect of aging on gastric acid secretion, serum gastrin, and antral gastrin content in rats. Dig. Dis. Sci. 1988, 33, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Solomons, N.W. Aging, gastric acid secretion, and carotene status. Am. J. Clin. Nutr. 1996, 64, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.R.; Haneman, B.; Arnold, B.J.; Booth, J.C.; Taylor, K. Atrophic gastritis in the aged. Australas. Ann. Med. 1967, 16, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Goldschmiedt, M.; Barnett, C.C.; Schwarz, B.E.; Karnes, W.E.; Redfern, J.S.; Feldman, M. Effect of age on gastric acid secretion and serum gastrin concentrations in healthy men and women. Gastroenterology 1991, 101, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Katelaris, P.H.; Seow, F.; Lin, B.P.; Napoli, J.; Ngu, M.C.; Jones, D.B. Effect of age, Helicobacter pylori infection, and gastritis with atrophy on serum gastrin and gastric acid secretion in healthy men. Gut 1993, 34, 1032–1037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dovjak, P. Polypharmacy in elderly people. Wien. Med. Wochenschr. 2022, 172, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; Rehm, C.D.; Haas, J.S.; Chan, A.T.; Giovannucci, E.L. Trends in Prescription Drug Use Among Adults in the United States From 1999-2012. JAMA 2015, 314, 1818–1831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Maitra, R.S.; Edgerton, E.A.; Majumdar, A.P. Gastric secretion during aging in pyloric-ligated rats and effects of pentagastrin. Exp. Gerontol. 1988, 23, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.H. Studies of basal and peak acid output with an augmented histamine test. Gut 1963, 4, 136–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borgström, S.; Emås, S.; Lilja, B.; Borg, I. Acid response to pentagastrin in relation to age and body stature in male and female ulcer patients. Scand. J. Gastroenterol. 1973, 8, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kekki, M.; Samloff, I.M.; Ihamäki, T.; Varis, K.; Siurala, M. Age- and sex-related behaviour of gastric acid secretion at the population level. Scand. J. Gastroenterol. 1982, 17, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Cryer, B.; McArthur, K.E.; Huet, B.A.; Lee, E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: A prospective study. Gastroenterology 1996, 110, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, A.; Brady, D.A.; Schaal, S.E.; Samloff, I.M.; Dedon, J.; Ruhl, C.E. Gastric acidity in older adults. JAMA 1997, 278, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Trey, G.; Marks, I.N.; Louw, J.A.; Jaskiewicz, K.; Sipponen, P.; Novis, B.H.; Bank, S.; Tigler-Wybrandi, N.A. Changes in acid secretion over the years. A 30-year longitudinal study. J. Clin. Gastroenterol. 1997, 25, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Kawanami, C.; Kishi, K.; Nakata, H.; Seino, Y.; Chiba, T. Helicobacter pylori independent chronological change in gastric acid secretion in the Japanese. Gut 1997, 41, 452–458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haruma, K.; Kamada, T.; Kawaguchi, H.; Okamoto, S.; Yoshihara, M.; Sumii, K.; Inoue, M.; Kishimoto, S.; Kajiyama, G.; Miyoshi, A. Effect of age and Helicobacter pylori infection on gastric acid secretion. J. Gastroenterol. Hepatol. 2000, 15, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Shih, G.L.; Brensinger, C.; Katzka, D.A.; Metz, D.C. Influence of age and gender on gastric acid secretion as estimated by integrated acidity in patients referred for 24-hour ambulatory pH monitoring. Am. J. Gastroenterol. 2003, 98, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Ohara, S.; Koike, T.; Sekine, H.; Shimosegawa, T. Gastric acid secretion of normal Japanese subjects in relation to Helicobacter pylori infection, aging, and gender. Scand. J. Gastroenterol. 2004, 39, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Montgomery, S.C.; Graves, K.A.; Caspary, D.M.; Cox, B.C. The FBN rat model of aging: Investigation of ABR waveforms and ribbon synapse changes. Neurobiol. Aging 2018, 62, 53–63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majumdar, A.P.; Edgerton, E.A.; Dayal, Y.; Murthy, S.N. Gastrin: Levels and trophic action during advancing age. Am. J. Physiol. 1988, 254, G538–G542. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.P.; Johnson, L.R. Gastric mucosal cell proliferation during development in rats and effects of pentagastrin. Am. J. Physiol. 1982, 242, G135–G139. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rae-Venter, B.; Townsend, C.M., Jr.; Khalil, T.; Thompson, J.C. Gastrin receptors in normal and malignant gastrointestinal mucosa: Age-associated changes. Am. J. Physiol. 1985, 249, G761–G769. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.P. Regulation of gastrointestinal mucosal growth during aging. J. Physiol. Pharmacol. 2003, 54 (Suppl. 4), 143–154. [Google Scholar] [PubMed]
- Schuster, M.M. Disorders of the aging GI system. Hosp. Pract. 1976, 11, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Goldschmiedt, M.; Feldman, M. Age-related changes in gastric acid secretion. In Chronic Gastritis and Hypochlorhydria in the Elderly; Holt, P.R., Russell, R.M., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1993; pp. 13–29. [Google Scholar]
- Russell, T.L.; Berardi, R.R.; Barnett, J.L.; Dermentzoglou, L.C.; Jarvenpaa, K.M.; Schmaltz, S.P.; Dressman, J.B. Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharm. Res. 1993, 10, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Collen, M.J.; Abdulian, J.D.; Chen, Y.K. Age does not affect basal gastric acid secretion in normal subjects or in patients with acid-peptic disease. Am. J. Gastroenterol. 1994, 89, 712–716. [Google Scholar] [PubMed]
- He, Y.; Wang, Y.; Luan, F.; Yu, Z.; Feng, H.; Chen, B.; Chen, W. Chinese and global burdens of gastric cancer from 1990 to 2019. Cancer Med. 2021, 10, 3461–3473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, K.; Ogoshi, K.; Makuuchi, H. Influence of aging, gastric mucosal atrophy and dietary habits on gastric secretion. Hepatogastroenterology 2006, 53, 624–628. [Google Scholar] [PubMed]
- Tani, S.; Ohtani, K.; Tanaka, T. Some factors which influence intrinsic factor content and its mRNA level in the rat stomach. Biol. Pharm. Bull. 2000, 23, 390–393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brooks, F.P. Effect of diet on gastric secretion. Am. J. Clin. Nutr. 1985, 42 (Suppl. 5), 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Gondivkar, S.M.; Gadbail, A.R.; Gondivkar, R.S.; Sarode, S.C.; Sarode, G.S.; Patil, S.; Awan, K.H. Nutrition and oral health. Dis. Mon. 2019, 65, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Richardson, C.T.; Walsh, J.H. Sex-related differences in gastrin release and parietal cell sensitivity to gastrin in healthy human beings. J. Clin. Investig. 1983, 71, 715–720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adeniyi, K.O. Gastric acid secretion and parietal cell mass: Effect of sex hormones. Gastroenterology 1991, 101, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Sung, I.K.; Kim, J.H.; Lee, S.Y.; Park, H.S.; Shim, C.S. Risk Factors for the Presence of Symptoms in Peptic Ulcer Disease. Clin. Endosc. 2017, 50, 578–584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cryer, B.; Lee, E.; Feldman, M. Factors influencing gastroduodenal mucosal prostaglandin concentrations: Roles of smoking and aging. Ann. Intern. Med. 1992, 116, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Hogenauer, C.; Hammer, H.F. Gastric secretion. In Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, 10th ed.; Feldman, M., Friedman, L.S., Brandt, L.J., Eds.; Saunders: Philadelphia, PA, USA, 2016; pp. 764–780. [Google Scholar]
- Dibello, V.; Zupo, R.; Sardone, R.; Lozupone, M.; Castellana, F.; Dibello, A.; Daniele, A.; De Pergola, G.; Bortone, I.; Lampignano, L.; et al. Oral frailty and its determinants in older age: A systematic review. Lancet Healthy Longev. 2021, 2, e507–e520. [Google Scholar] [CrossRef] [PubMed]
- Pombo-Lopes, J.; Rodrigues, I.; Costa, J.; Gomes, A.C.; Fonseca, J.; Grillo-Evangelista, J. Health professionals’ perceptions, barriers and knowledge towards oral health care of dependent people in nursing homes: A systematic review. Front. Public Health 2025, 12, 1504542. [Google Scholar] [CrossRef] [PubMed]
- Kivi, M.; Tindberg, Y. Helicobacter pylori occurrence and transmission: A family affair? Scand. J. Infect. Dis. 2006, 38, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.; Coudron, P.E.; McCuen, R.W.; Harrington, L.; Chu, S.; Schubert, M.L.H. pylori acutely inhibits gastric secretion by activating CGRP sensory neurons coupled to stimulation of somatostatin and inhibition of histamine secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G715–G722. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kamiya, S.; Suzuki, T.; Kohda, K.; Muramatsu, S.; Kurumada, T.; Ohta, U.; Miyazawa, M.; Kimura, N.; Mutoh, N.; et al. The effect of Helicobacter pylori on gastric acid secretion by isolated parietal cells from a guinea pig. Association with production of vacuolating toxin by H. pylori. Scand. J. Gastroenterol. 1996, 31, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.S.; King, W.W.; Fox, J.G.; Janik, D.; Cave, D.R. Rabbit and ferret parietal cell inhibition by Helicobacter species. Dig. Dis. Sci. 1995, 40, 147–152. [Google Scholar] [CrossRef] [PubMed]
- El-Omar, E.M. Mechanisms of increased acid secretion after eradication of Helicobacter pylori infection. Gut 2006, 55, 144–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saha, A.; Hammond, C.E.; Gooz, M.; Smolka, A.J. IL-1beta modulation of H,K-ATPase alpha-subunit gene transcription in Helicobacter pylori infection. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1055–G1061. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimatani, T.; Inoue, M.; Iwamoto, K.; Hyogo, H.; Yokozaki, M.; Saeki, T.; Tazuma, S.; Horikawa, Y.; Harada, N. Gastric acidity in patients with follicular gastritis is significantly reduced, but can be normalized after eradication for Helicobacter pylori. Helicobacter 2005, 10, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Chmiela, M.; Gonciarz, W. Molecular mimicry in Helicobacter pylori infections. World J. Gastroenterol. 2017, 23, 3964–3977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moss, S.F.; Legon, S.; Bishop, A.E.; Polak, J.M.; Calam, J. Effect of Helicobacter pylori on gastric somatostatin in duodenal ulcer disease. Lancet 1992, 340, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.S.; Wang, Y.H.; Zeng, Z.R.; Zhang, Z.Y.; Lu, H.; Xu, J.M.; Du, Y.Q.; Li, Y.; Wang, J.B.; Xu, S.P.; et al. Primary antibiotic resistance of Helicobacter pylori in Chinese patients: A multiregion prospective 7-year study. Clin. Microbiol. Infect. 2018, 24, e5–e780. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Pellegrino, S.; Pero, E.; Agnitelli, M.C.; Parlangeli, C.; Landi, F.; Montalto, M. Main Disorders of Gastrointestinal Tract in Older People: An Overview. Gastrointest. Disord. 2024, 6, 313–336. [Google Scholar] [CrossRef]
- Pilotto, A.; Salles, N. Helicobacter pylori infection in geriatrics. Helicobacter 2002, 7 (Suppl. 1), 56–62. [Google Scholar] [CrossRef] [PubMed]
- Karhunen, P.; Tuomisto, S.; Goebeler, S.; Martiskainen, M.; Kok, E. Common occurrence of atrophic gastritis in an ageing non-hospitalised population: An autopsy study. Age Ageing 2025, 54, afaf047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schubert, M.L.; Peura, D.A. Control of gastric acid secretion in health and disease. Gastroenterology 2008, 134, 1842–1860. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Moayyedi, P. Proton Pump Inhibitors in the Elderly, Balancing Risk and Benefit: An Age-Old Problem. Curr. Gastroenterol. Rep. 2019, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Kim, N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J. Neurogastroenterol. Motil. 2013, 19, 25–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013, 310, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; O’Moráin, C. Digestive function of the stomach. Dig. Dis. 2014, 32, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Nandagopalan, P.A.; Magdalene, K.F.; Binu, A. Effect of Aging on the Quantitative Number of Brunner’s Glands. J. Clin. Diagn. Res. 2014, 8, 4–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haboubi, N.Y.; Lee, G.S.; Montgomery, R.D. Duodenal mucosal morphometry of elderly patients with small intestinal bacterial overgrowth: Response to antibiotic treatment. Age Ageing 1991, 20, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Riordan, S.M.; McIver, C.J.; Wakefield, D.; Bolin, T.D.; Duncombe, V.M.; Thomas, M.C. Small intestinal bacterial overgrowth in the symptomatic elderly. Am. J. Gastroenterol. 1997, 92, 47–51. [Google Scholar] [PubMed]
- Sipponen, P.; Maaroos, H.I. Chronic gastritis. Scand. J. Gastroenterol. 2015, 50, 657–667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Correa, P.; Piazuelo, M.B.; Wilson, K.T. Pathology of gastric intestinal metaplasia: Clinical implications. Am. J. Gastroenterol. 2010, 105, 493–498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nilsson-Ehle, H.; Landahl, S.; Lindstedt, G.; Netterblad, L.; Stockbruegger, R.; Westin, J.; Ahren, C. Low serum cobalamin levels in a population study of 70- and 75-year-old subjects. Gastrointestinal causes and hematological effects. Dig. Dis. Sci. 1989, 34, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Genta, R.M. Acid suppression and gastric atrophy: Sifting fact from fiction. Gut 1998, 43 (Suppl. 1), S35–S38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.L.; Fixen, D.R.; Linnebur, S.A. Adverse effects of proton-pump inhibitor use in older adults: A review of the evidence. Ther. Adv. Drug. Saf. 2017, 8, 273–297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, H.J.; Seo, H.J.; Jiang, X.; Jeon, N.; Lee, Y.J.; Ha, I.H. Proton pump inhibitors associated with an increased risk of mortality in elderly: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2024, 80, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Corsonello, A.; Ceda, G.P.; Cattabiani, C.; Lauretani, F.; Buttò, V.; Ferrucci, L.; Bandinelli, S.; Abbatecola, A.M.; Spazzafumo, L.; et al. Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern. Med. 2013, 173, 518–523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freedberg, D.E.; Kim, L.S.; Yang, Y.X. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.A.; Misra, D.N.; Grove, M.; Becich, M.J.; Shao, J.S.; Gordon, M.; Alpers, D.H. Human gastric intrinsic factor expression is not restricted to parietal cells. J. Anat. 1996, 189 Pt 2, 303–313. [Google Scholar] [PubMed] [PubMed Central]
- Carmel, R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch. Intern. Med. 1996, 156, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Ardeman, S.; Chanarin, I. Intrinsic factor secretion in gastric atrophy. Gut 1966, 7, 99–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Schreiber, S.; Bücker, R.; Groll, C.; Azevedo-Vethacke, M.; Scheid, P.; Gatermann, S.; Josenhans, C.; Suerbaum, S. Gastric antibacterial efficiency is different for pepsin A and C. Arch. Microbiol. 2006, 184, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Samloff, I.M. Pepsins, peptic activity, and peptic inhibitors. J. Clin. Gastroenterol. 1981, 3 (Suppl. 2), 91–94. [Google Scholar] [PubMed]
- Montoro-Huguet, M.A.; Belloc, B.; Domínguez-Cajal, M. Small and Large Intestine (I): Malabsorption of Nutrients. Nutrients 2021, 13, 1254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hogenauer, C.; Hammer, H.F. Maldigestion and Malabsorption. In Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, 10th ed.; Feldman, M., Friedman, L.S., Brandt, L.J., Eds.; Saunders: Philadelphia, PA, USA, 2016; pp. 1788–1825. [Google Scholar]
| Authors (Year) | Study Characteristics | Patients | Aging Influence Outcomes | Comments | ||
|---|---|---|---|---|---|---|
| Acid Secretion | Pepsin Secretion | Intrinsic Factor | ||||
| Khalil et al. (1988) [6] | Animal study | 6 young rats (3 months old) 6 old rats (32 months old) | Decrease | Not evaluated | Not evaluated |
|
| Maitra et al. (1988) [14] | Animal study | Young rats (4 months old) Old rats (21 months old) | Decrease | Decrease | Not evaluated |
|
| Baron et al. (1963) [15] | Human study | 40 patients (19–66 years old) | Decrease | Not evaluated | Not evaluated |
|
| Andrews et al. (1967) [8] | Human study | 24 patients (64–87 years old) | Decrease | Not evaluated | Decrease |
|
| Borgström et al. (1973) [16] | Human study | 221 patients < 60 years-old 31 patients > 60 years old | No change | Not evaluated | Not evaluated |
|
| Kekki et al. (1982) [17] | Human study | 437 patients > 14 years old |
| Not evaluated | Not evaluated |
|
| Goldschmiedt et al. (1991) [9] | Human study | 41 patients (23–71 years old) |
| Increase in males (p > 0.05) No difference in females | Not evaluated |
|
| Katelaris et al. (1993) [10] | Human study | 22 patients 18–30 years old 28 patients >65 years-old | No change | Not evaluated | Not evaluated |
|
| Feldman et al. (1996) [18] | Human study | 184 patients 18–64 years old 22 patients ≥ 65 years-old |
| Decrease | Not evaluated |
|
| Hurwitz et al. (1997) [19] | Human study | 248 patients ≥ 65 years old | No change | Not evaluated | Not evaluated |
|
| Trey et al. (1997) [20] | Human study | 11 patients, 48–51 years old | Decrease | Not evaluated | Not evaluated |
|
| Kinoshita et al. (1997) [21] | Human study | 110 patients |
| Not evaluated | Not evaluated |
|
| Haruma et al. (2000) [22] | Human study | 248 patients 16–64 years old 32 patients ≥ 65 years old |
| Not evaluated | Not evaluated |
|
| Shih et al. (2003) [23] | Human study | 645 patients < 65 years old 108 patients ≥ 65 years old | No change | Not evaluated | Not evaluated |
|
| Iijima et al. (2004) [24] | Human study | 156 patients < 60 years old 57 patients > 60 years old |
| Not evaluated | Not evaluated |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vara-Luiz, F.; Mendes, I.; Palma, C.; Mascarenhas, P.; Nunes, G.; Patita, M.; Fonseca, J. Age-Related Decline of Gastric Secretion: Facts and Controversies. Biomedicines 2025, 13, 1546. https://doi.org/10.3390/biomedicines13071546
Vara-Luiz F, Mendes I, Palma C, Mascarenhas P, Nunes G, Patita M, Fonseca J. Age-Related Decline of Gastric Secretion: Facts and Controversies. Biomedicines. 2025; 13(7):1546. https://doi.org/10.3390/biomedicines13071546
Chicago/Turabian StyleVara-Luiz, Francisco, Ivo Mendes, Carolina Palma, Paulo Mascarenhas, Gonçalo Nunes, Marta Patita, and Jorge Fonseca. 2025. "Age-Related Decline of Gastric Secretion: Facts and Controversies" Biomedicines 13, no. 7: 1546. https://doi.org/10.3390/biomedicines13071546
APA StyleVara-Luiz, F., Mendes, I., Palma, C., Mascarenhas, P., Nunes, G., Patita, M., & Fonseca, J. (2025). Age-Related Decline of Gastric Secretion: Facts and Controversies. Biomedicines, 13(7), 1546. https://doi.org/10.3390/biomedicines13071546







