High-Dose Tranexamic Acid Enhances Circulating Neutrophil Extracellular Traps and Thrombus in Thrombosis Mouse Model
Abstract
1. Introduction
2. Materials and Methods
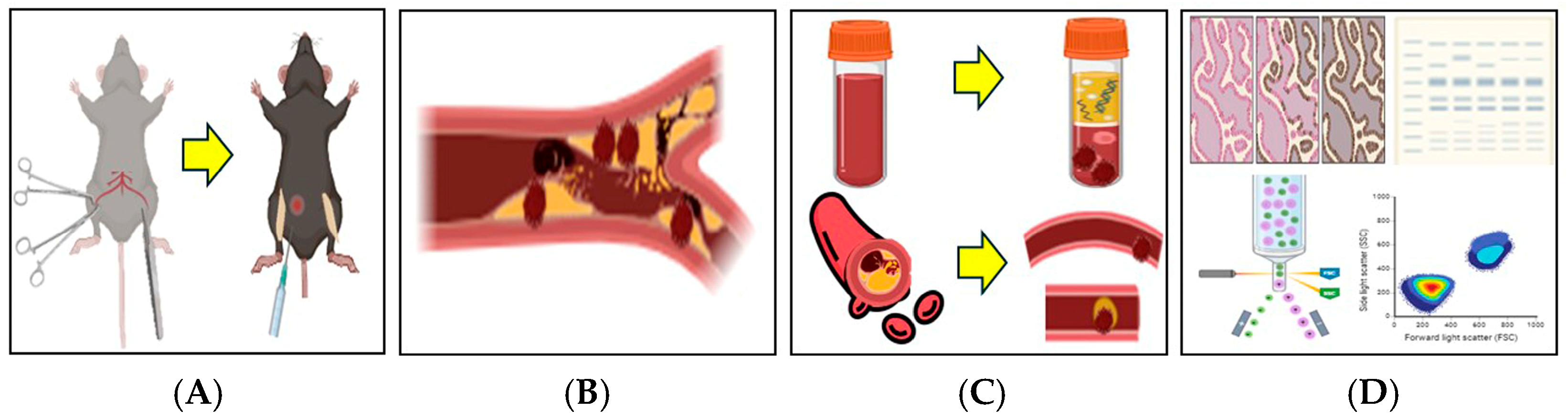
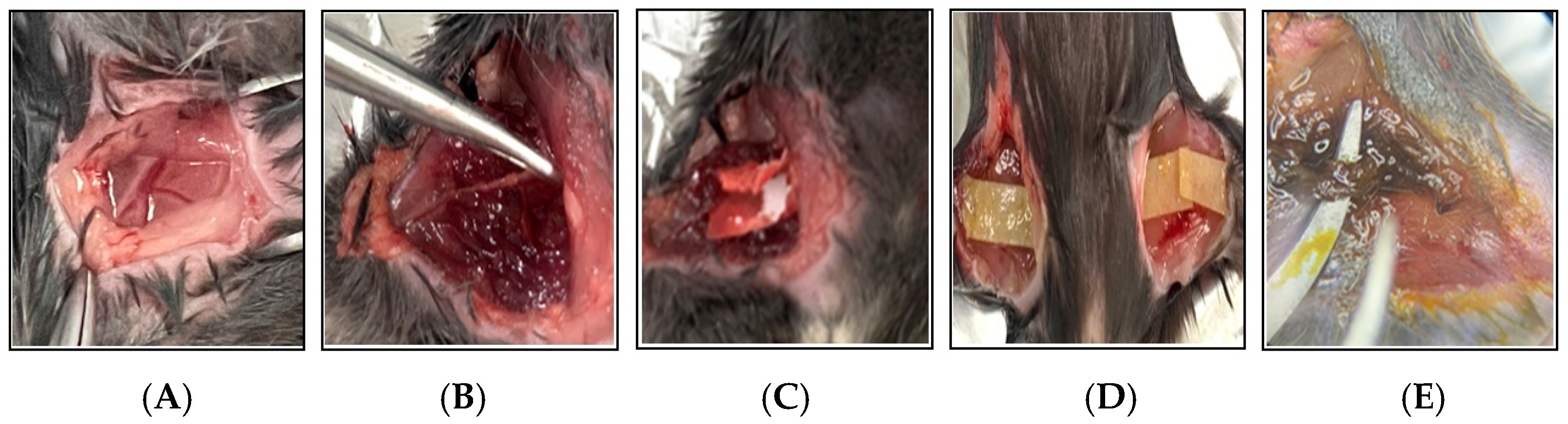
2.1. Preparation of Thrombosis Mouse Model with TXA Administration
2.2. Blood Collection and Peripheral Blood Mononuclear Cell (PBMC) Isolation
2.3. Isolation of Femoral Artery
2.4. Circulating NETs and Neutrophils Assessed by Flow Cytometry
2.5. Localized NETs, Neutrophils, Platelets, and Endothelial Cells in Thrombotic Femoral Artery Assessed by Flow Cytometry
2.6. Localized NETs, Platelets, Thrombus, Neutrophils, and Fibrinogen in Thrombotic Femoral Artery Assessed by Immunohistofluorescence Staining
2.7. Localized Fibrinogen, Neutrophils, and Platelets in Thrombotic Femoral Artery Assessed by Western Blot Analysis
2.8. Statistical Analysis
3. Results
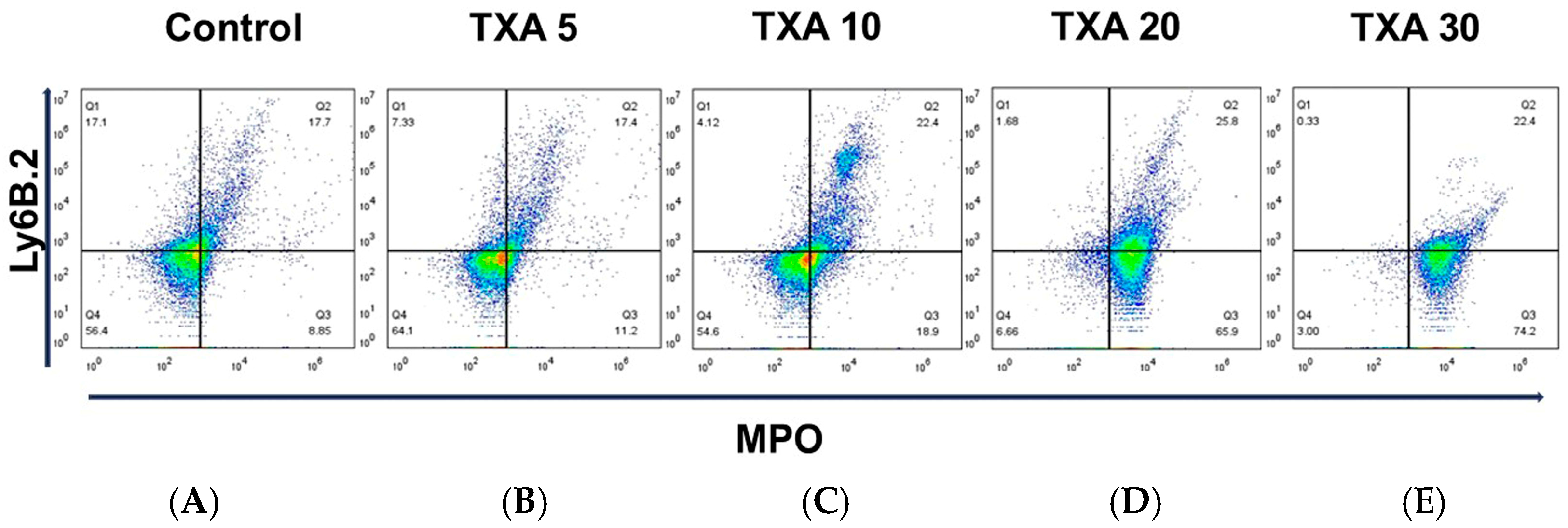
3.1. Circulating NETs and Neutrophils Assessed by Flow Cytometry
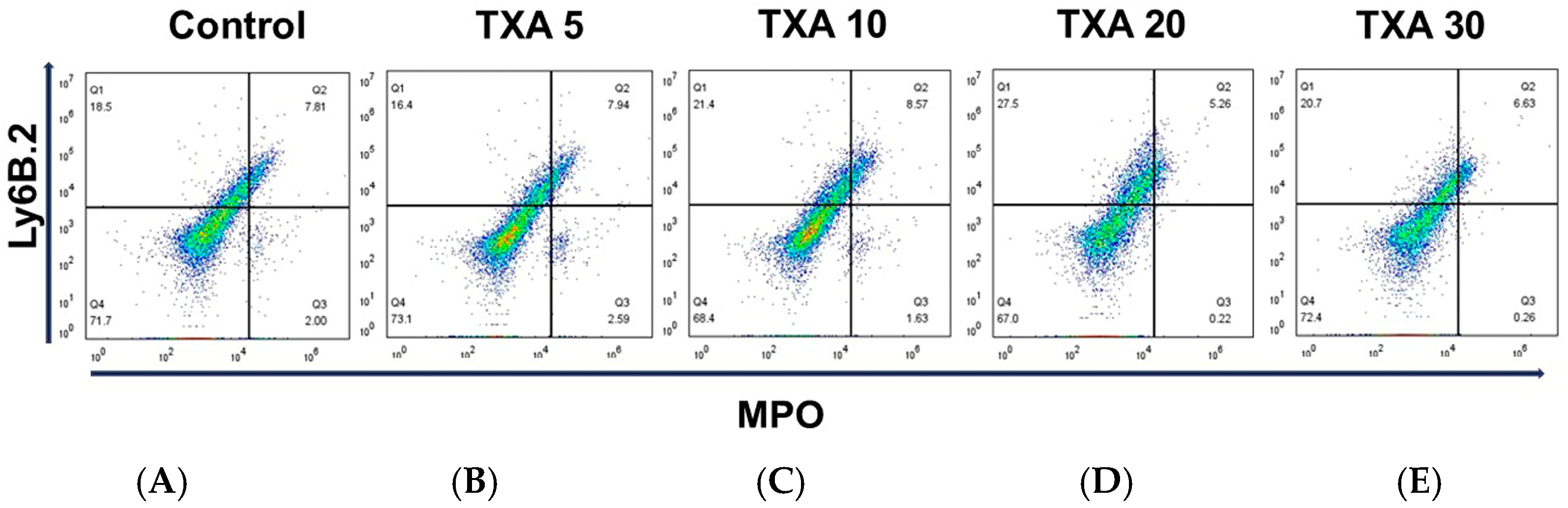
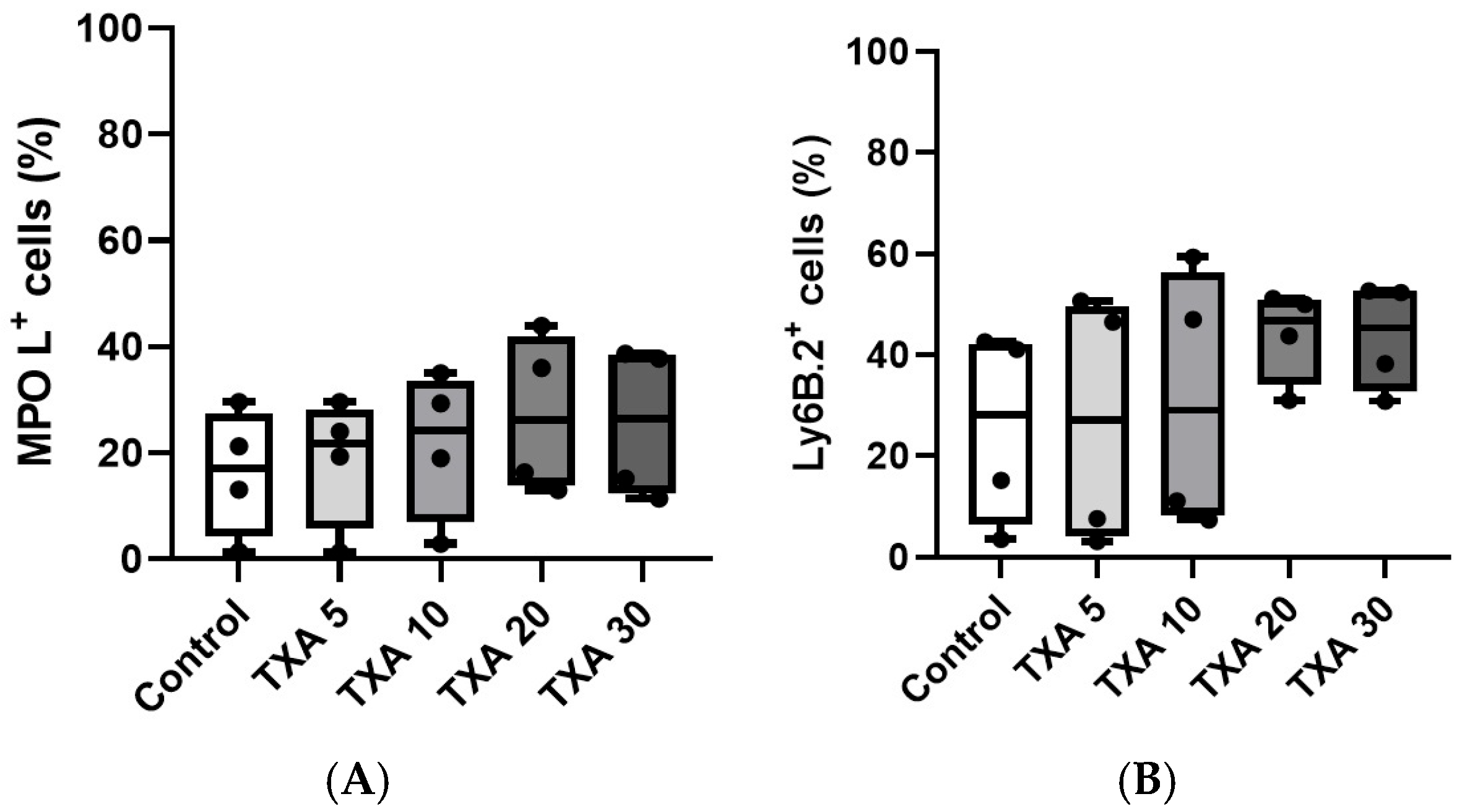
3.2. Localized NETs and Neutrophils in Thrombotic Femoral Artery Assessed by Flow Cytometry
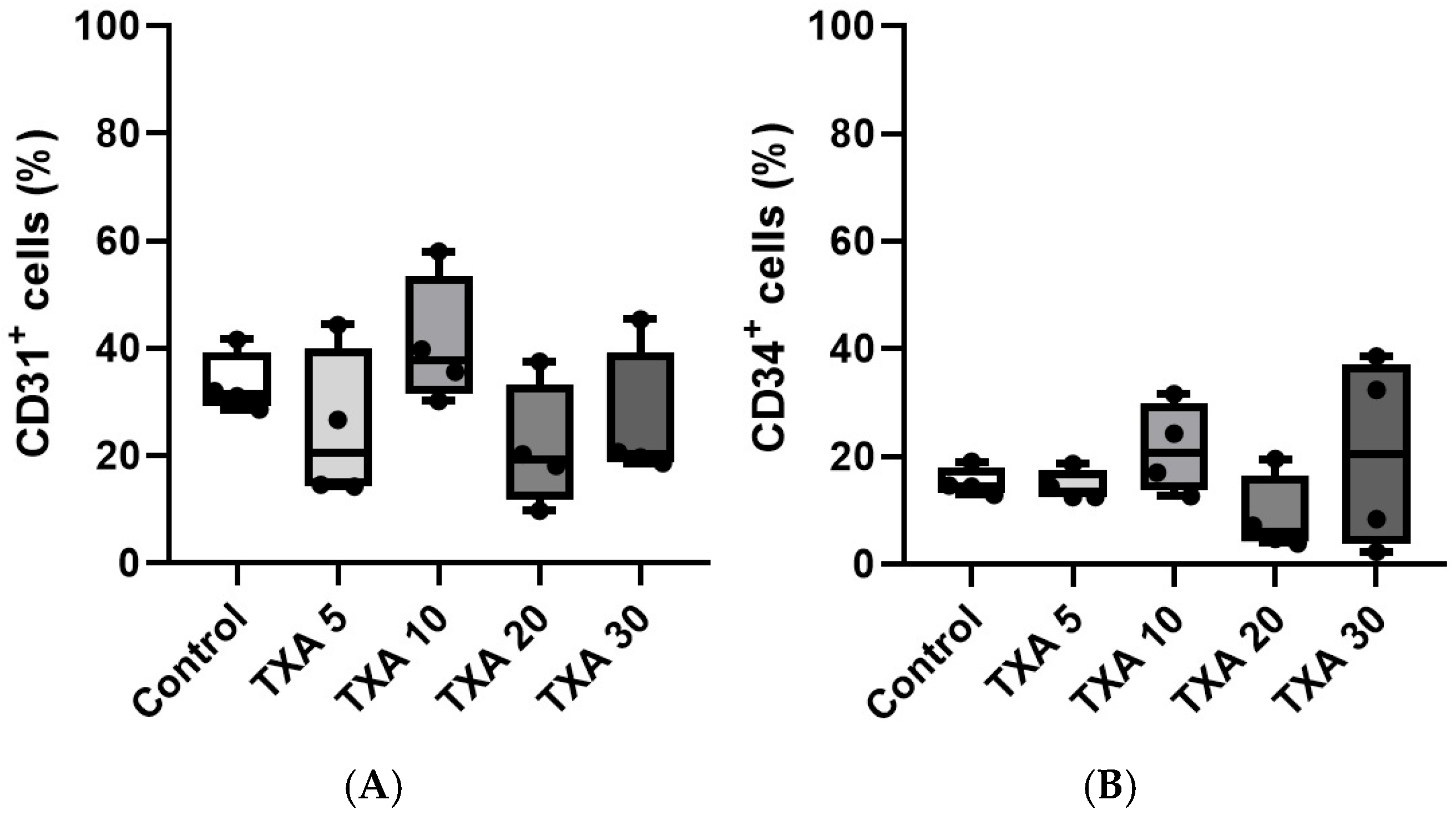
3.3. Localized Platelets and Endothelial Cells in Thrombotic Femoral Artery Assessed by Flow Cytometry
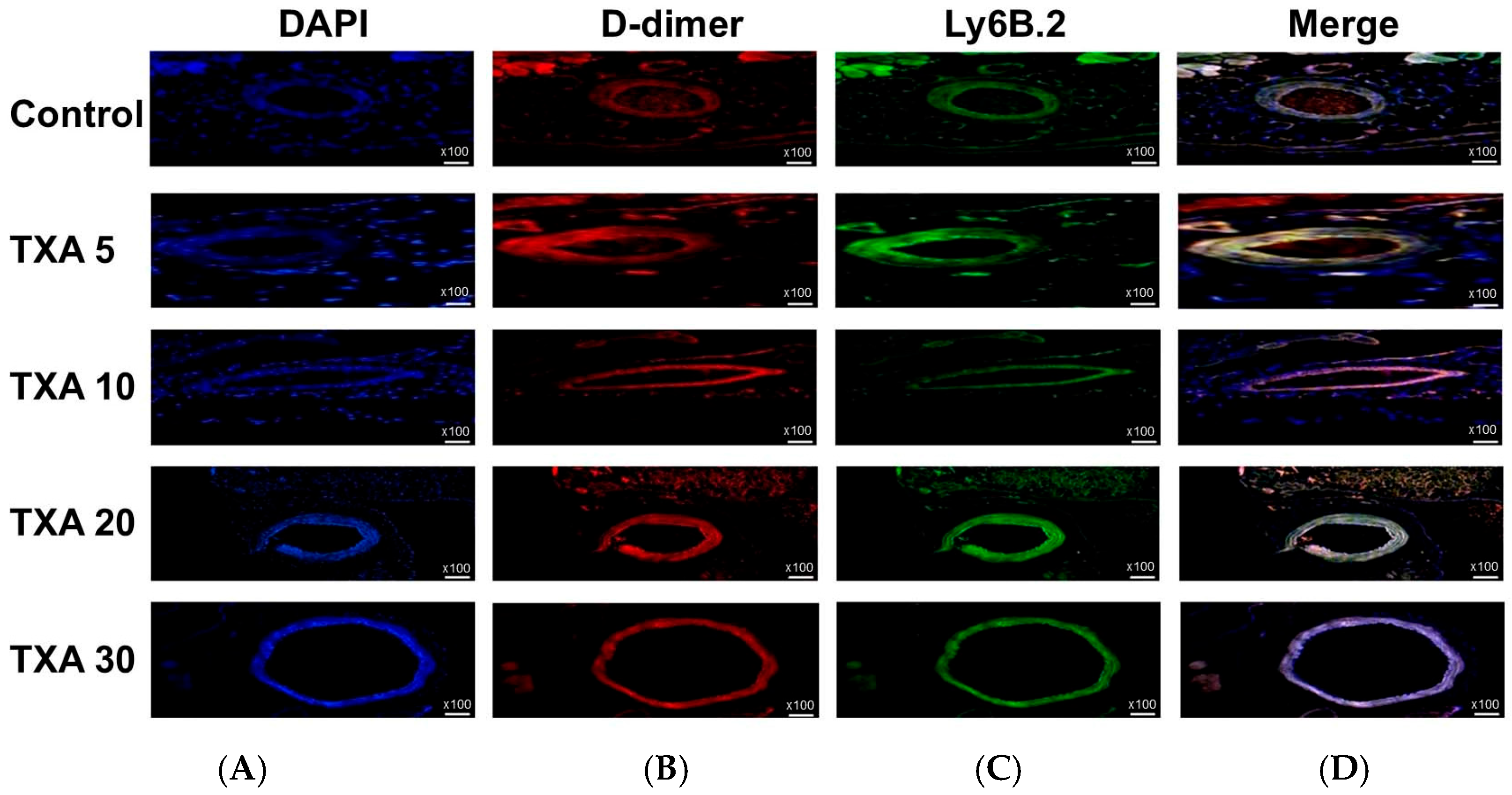
3.4. Localized NETs and Platelets in Thrombotic Femoral Artery Assessed by Immunohistofluorescence Staining
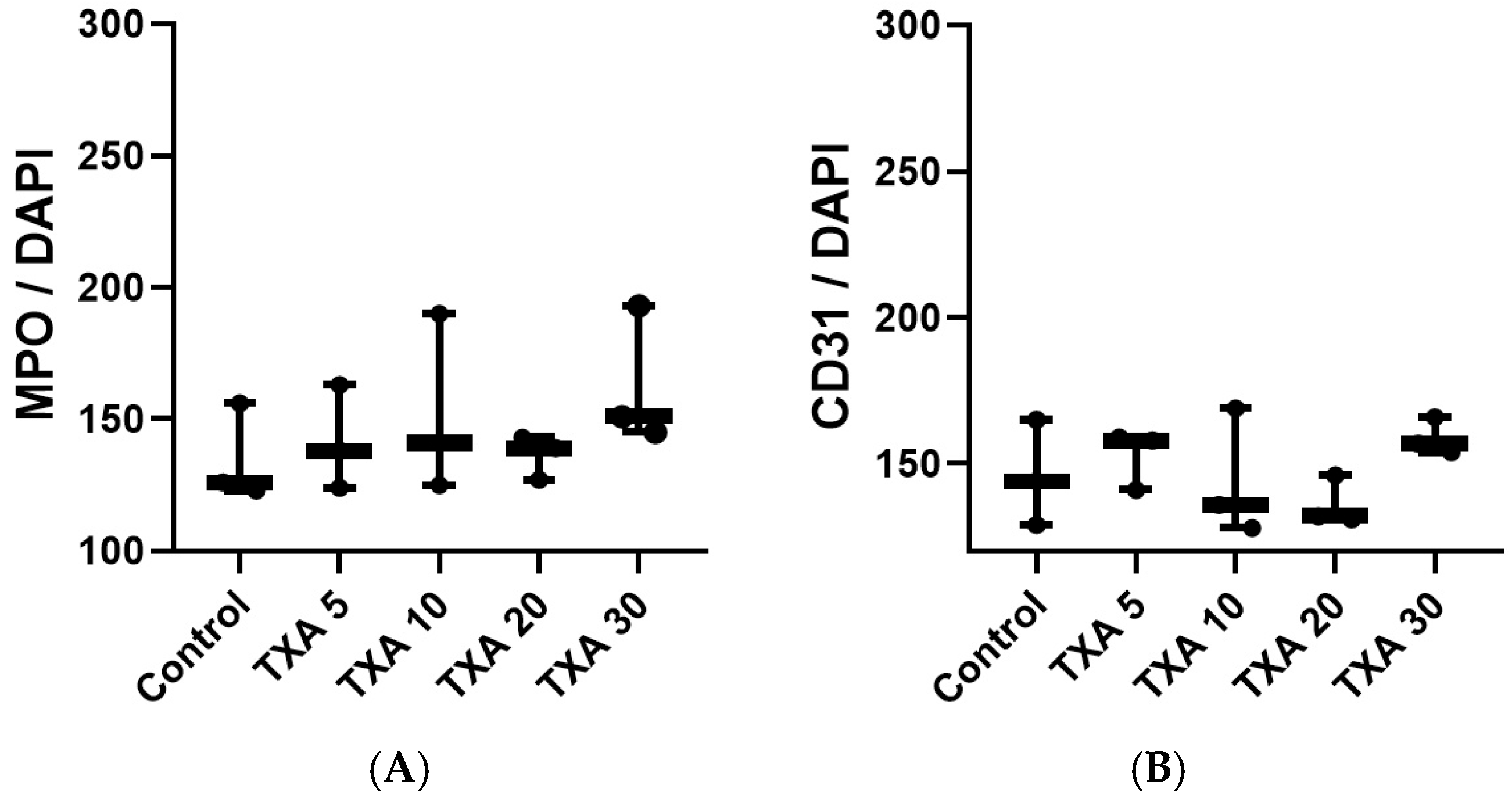
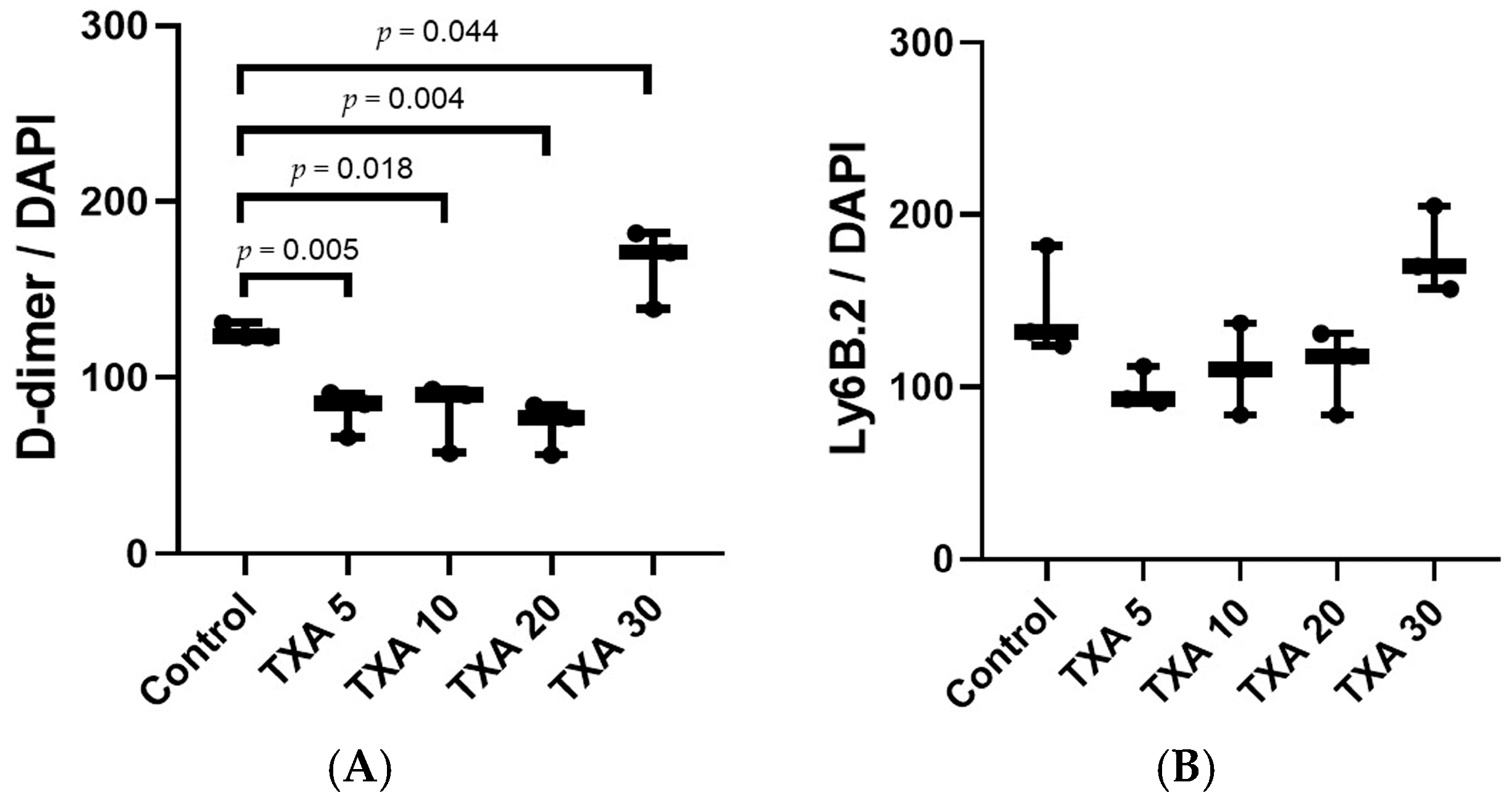
3.5. Localized Thrombus and Neutrophils in Thrombotic Femoral Artery Assessed by Immunohistofluorescence Staining
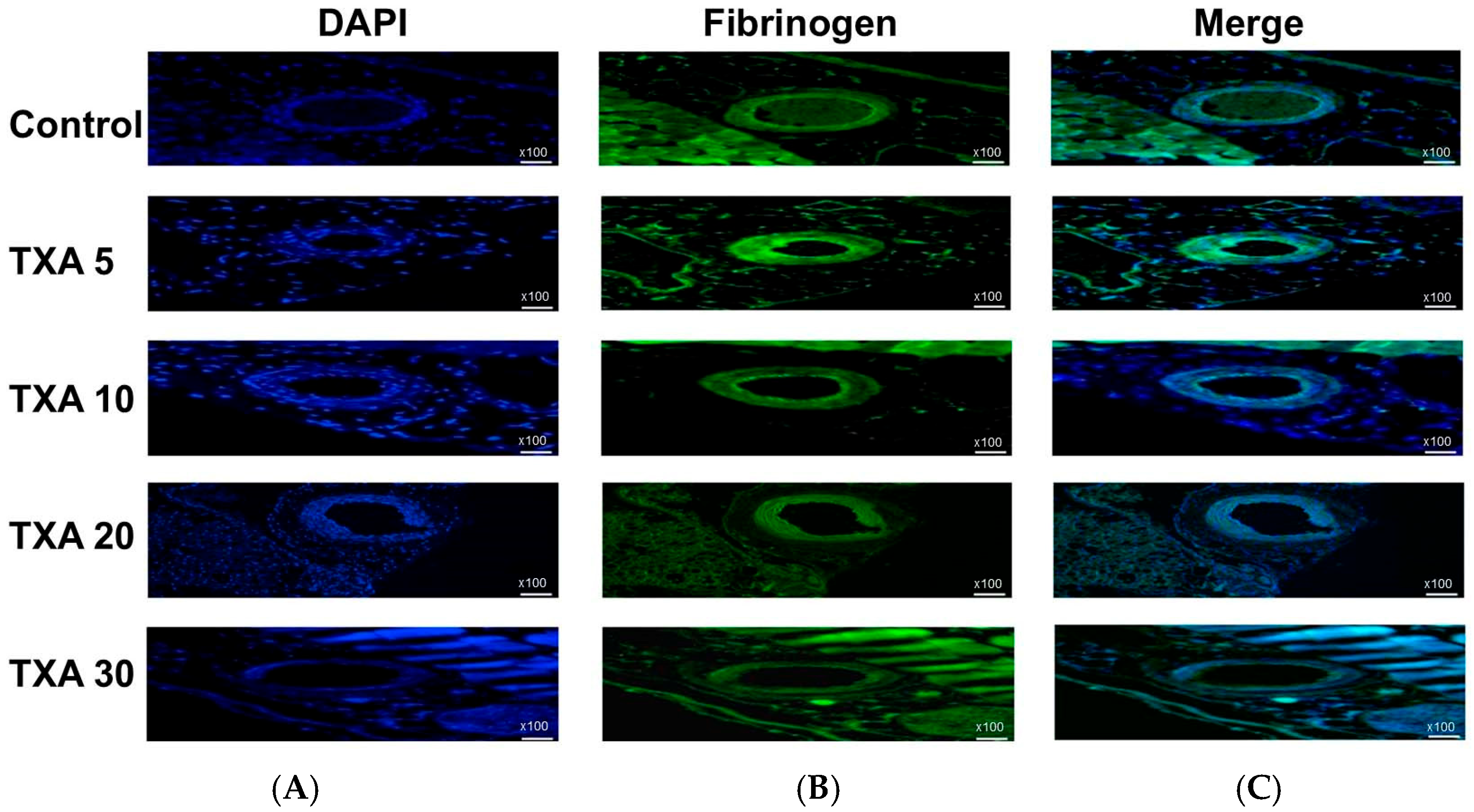
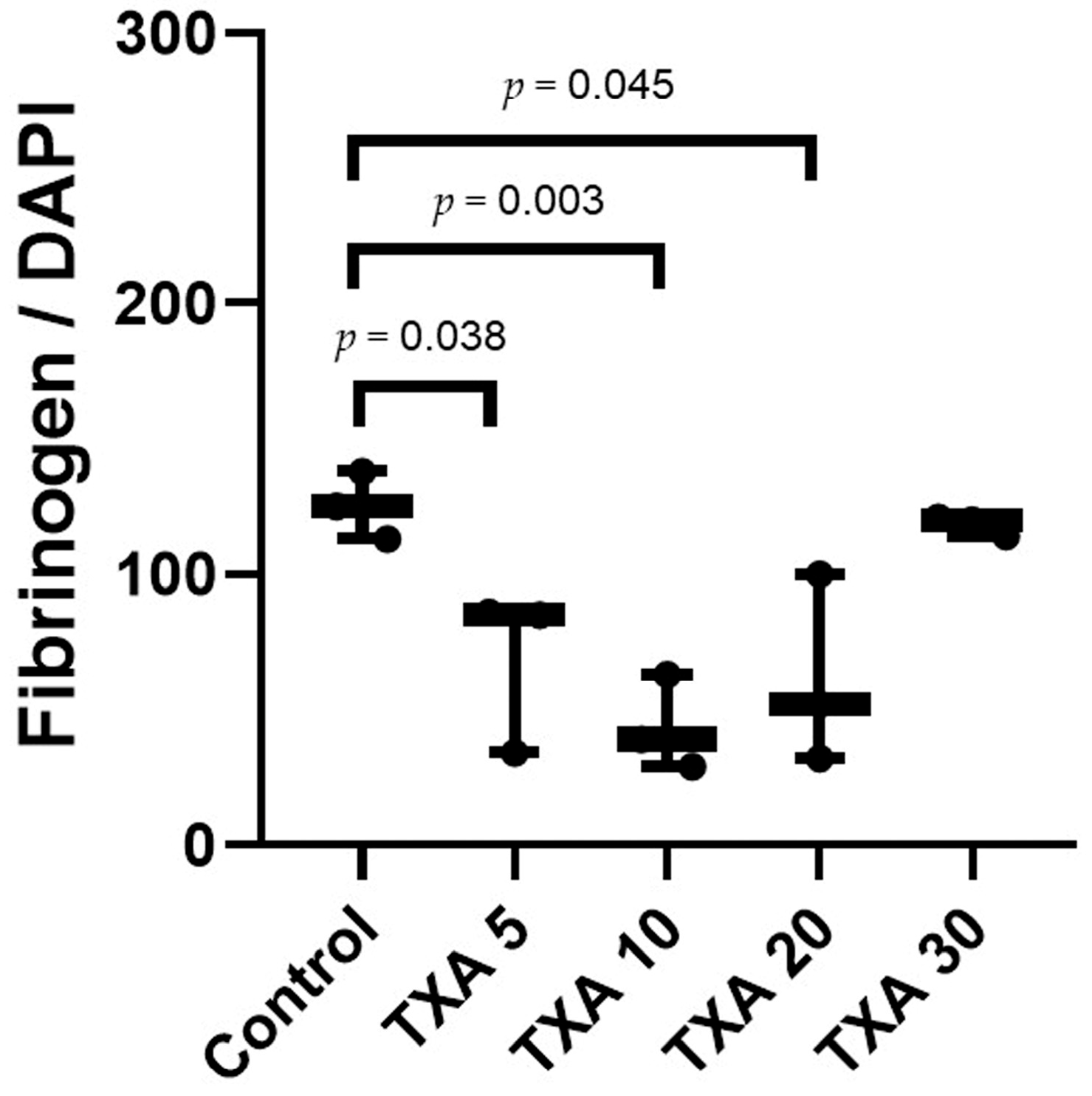
3.6. Localized Fibrinogen in Thrombotic Femoral Artery Assessed by Immunohistofluorescence Staining
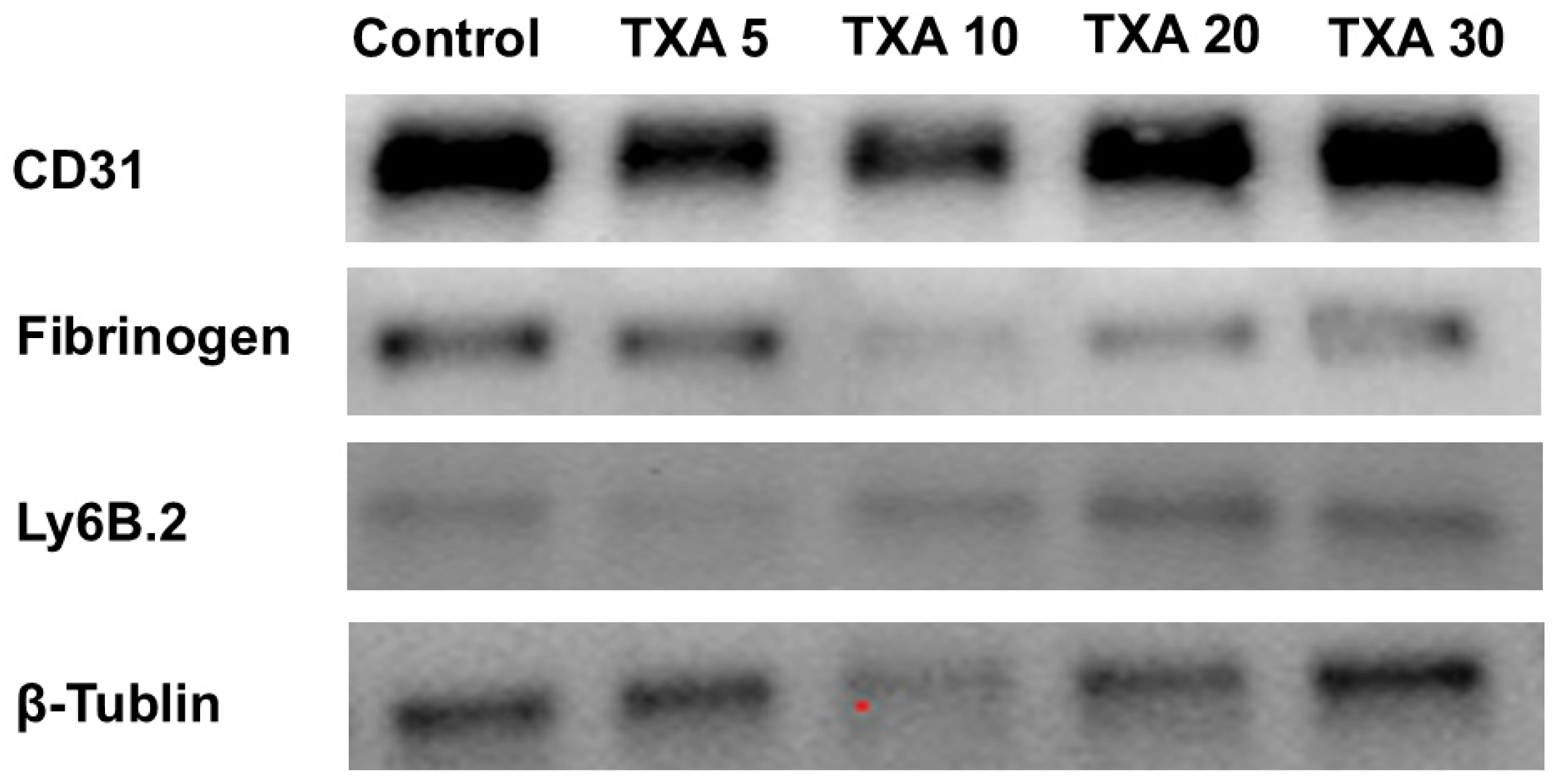
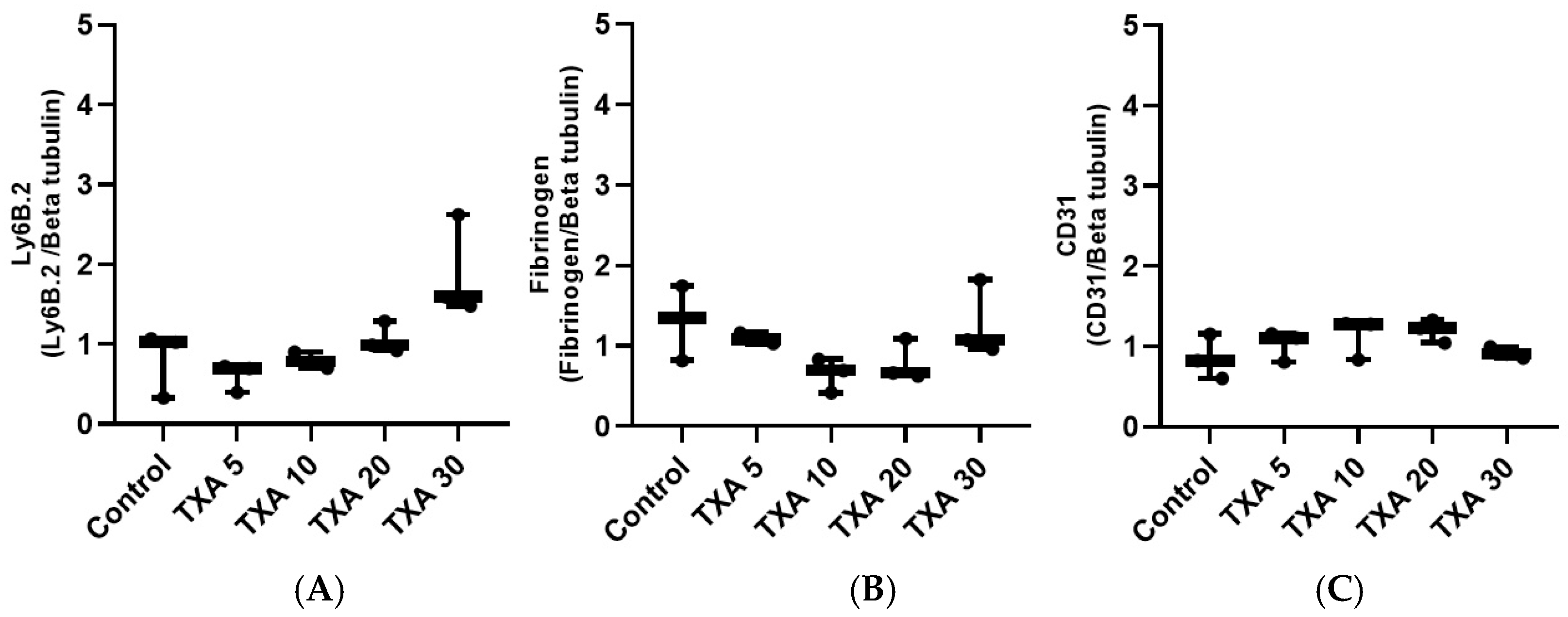
3.7. Localized Neutrophils, Fibrinogen, and Platelets in Thrombotic Femoral Artery Assessed by Western Blot Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TXA | Tranexamic acid |
| NETs | Neutrophil extracellular traps |
| IACUC | Institutional Animal Care and Use Committee |
| FeCl3 | Ferric chloride |
| PBMC | Peripheral blood mononuclear cell |
| DPBS | Dulbecco’s phosphate-buffered saline |
| RBCs | Red blood cells |
| MPO | Myeloperoxidase |
| FBS | Fetal bovine serum |
| CD | Cluster of differentiation |
| PFA | Paraformaldehyde |
| FITC | Fluorescein isothiocyanate |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| PVDF | Polyvinylidene difluoride |
| TBS | Tris-buffered saline |
| BSA | Bovine serum albumin |
| PBS | Phosphate-buffered saline |
| HRP | Horseradish peroxidase |
References
- Rossaint, R.; Afshari, A.; Bouillon, B.; Cerny, V.; Cimpoesu, D.; Curry, N.; Duranteau, J.; Filipescu, D.; Grottke, O.; Grønlykke, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit. Care 2023, 27, 80. [Google Scholar] [CrossRef] [PubMed]
- Ruskin, K.J. Deep vein thrombosis and venous thromboembolism in trauma. Curr. Opin. Anaesthesiol. 2018, 31, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Tilley, R.E.; Key, N.S. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M. Remodeling the blood coagulation cascade. J. Thromb. Thrombolysis 2003, 16, 17–20. [Google Scholar] [CrossRef]
- Kwon, M.A.; Ji, S.M. Revolutionizing trauma care: Advancing coagulation management and damage control anesthesia. Anesth. Pain Med. 2024, 19, 73–84. [Google Scholar] [CrossRef]
- Lier, H.; Maegele, M.; Shander, A. Tranexamic Acid for Acute Hemorrhage: A Narrative Review of Landmark Studies and a Critical Reappraisal of Its Use Over the Last Decade. Anesth. Analg. 2019, 129, 1574–1584. [Google Scholar] [CrossRef]
- Mannucci, P.M. Hemostatic drugs. N. Engl. J. Med. 1998, 339, 245–253. [Google Scholar] [CrossRef]
- Franchini, M.; Focosi, D.; Mannucci, P.M. Tranexamic acid: An evergreen hemostatic agent. Semin. Thromb. Hemost. 2024, 50, 733–738. [Google Scholar] [CrossRef]
- Della Corte, L.; Saccone, G.; Locci, M.; Carbone, L.; Raffone, A.; Giampaolino, P.; Ciardulli, A.; Berghella, V.; Zullo, F. Tranexamic acid for treatment of primary postpartum hemorrhage after vaginal delivery: A systematic review and meta-analysis of randomized controlled trials. J. Matern. Fetal Neonatal Med. 2020, 33, 869–874. [Google Scholar] [CrossRef]
- Meizoso, J.P.; Dudaryk, R.; Mulder, M.B.; Ray, J.J.; Karcutskie, C.A.; Eidelson, S.A.; Namias, N.; Schulman, C.I.; Proctor, K.G. Increased risk of fibrinolysis shutdown among severely injured trauma patients receiving tranexamic acid. J. Trauma. Acute Care Surg. 2018, 84, 426–432. [Google Scholar] [CrossRef]
- Yates, J.; Perelman, I.; Khair, S.; Taylor, J.; Lampron, J.; Tinmouth, A.; Saidenberg, E. Exclusion criteria and adverse events in perioperative trials of tranexamic acid: A systematic review and meta-analysis. Transfusion 2019, 59, 806–824. [Google Scholar] [CrossRef] [PubMed]
- Gall, L.S.; Davenport, R.A. Fibrinolysis and antifibrinolytic treatment in the trauma patient. Curr. Opin. Anaesthesiol. 2018, 31, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Jiao, Y.; An, X.; Tu, Q.; Jiang, Q. Neutrophil extracellular traps and cardiovascular disease: Associations and potential therapeutic approaches. Biomed. Pharmacother. 2024, 180, 117476. [Google Scholar] [CrossRef] [PubMed]
- Swystun, L.L.; Liaw, P.C. The role of leukocytes in thrombosis. Blood J. Am. Soc. Hematol. 2016, 128, 753–762. [Google Scholar] [CrossRef]
- van Montfoort, M.L.; Stephan, F.; Lauw, M.N.; Hutten, B.A.; Van Mierlo, G.J.; Solati, S.; Middeldorp, S.; Meijers, J.C.; Zeerleder, S. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arter. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 147–151. [Google Scholar] [CrossRef]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef]
- Varjú, I.; Longstaff, C.; Szabó, L.; Farkas, Á.Z.; Varga-Szabó, V.J.; Tanka-Salamon, A.; Machovich, R.; Kolev, K. DNA, histones and neutrophil extracellular traps exert anti-fibrinolytic effects in a plasma environment. Thromb. Haemost. 2015, 113, 1289–1298. [Google Scholar] [CrossRef]
- Diaz, J.A.; Fuchs, T.A.; Jackson, T.O.; Kremer Hovinga, J.A.; Lämmle, B.; Henke, P.K.; Myers, D.D., Jr.; Wagner, D.D.; Wakefield, T.W. Plasma DNA is Elevated in Patients with Deep Vein Thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2013, 1, 341–348.e341. [Google Scholar] [CrossRef]
- Lee, I.-S.; Choi, S.-G.; Jeon, W.-K. Optimization of ferric chloride induced carotid artery thrombosis model in a rat: Effect of Ginkgo biloba extracts. Korean J. Clin. Lab. Sci. 2011, 43, 179–187. [Google Scholar]
- Bonnard, T.; Hagemeyer, C.E. Ferric chloride-induced thrombosis mouse model on carotid artery and mesentery vessel. J. Vis. Exp. JoVE 2015, 100, e52838. [Google Scholar] [CrossRef]
- Huttinger, A.L.; Wheeler, D.G.; Gnyawali, S.; Dornbos, D., III; Layzer, J.M.; Venetos, N.; Talentino, S.; Musgrave, N.J.; Jones, C.; Bratton, C. Ferric chloride-induced canine carotid artery thrombosis: A large animal model of vascular injury. J. Vis. Exp. JoVE 2018, 139, 57981. [Google Scholar] [CrossRef]
- Li, W.; Nieman, M.; Sen Gupta, A. Ferric Chloride-induced Murine Thrombosis Models. J. Vis. Exp. JoVE 2016, 115, 54479. [Google Scholar] [CrossRef]
- Lovasova, V.; Bem, R.; Chlupac, J.; Dubsky, M.; Husakova, J.; Nemcova, A.; Fronek, J. Animal experimental models of ischemic limbs—A systematic review. Vasc. Pharmacol. 2023, 153, 107237. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Huynh, H.T.; Shcherbinina, E.; Huang, H.C.; Rezaei, R.; Sarshad, A.A. Biochemical Separation of Cytoplasmic and Nuclear Fraction for Downstream Molecular Analysis. Curr. Protoc. 2024, 4, e1042. [Google Scholar] [CrossRef]
- Kopec, A.M.; Rivera, P.D.; Lacagnina, M.J.; Hanamsagar, R.; Bilbo, S.D. Optimized solubilization of TRIzol-precipitated protein permits Western blotting analysis to maximize data available from brain tissue. J. Neurosci. Methods 2017, 280, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Tsan, S.E.H.; Viknaswaran, N.L.; Cheong, C.C.; Cheah, S.; Ng, K.T.; Mong, S.X.Y.; Wang, C.Y. Prophylactic intravenous tranexamic acid and thromboembolism in non-cardiac surgery: A systematic review, meta-analysis and trial sequential analysis. Anaesthesia 2023, 78, 1153–1161. [Google Scholar] [CrossRef]
- Pacheco, L.D.; Clifton, R.G.; Saade, G.R.; Weiner, S.J.; Parry, S.; Thorp, J.M., Jr.; Longo, M.; Salazar, A.; Dalton, W.; Tita, A.T.N.; et al. Tranexamic Acid to Prevent Obstetrical Hemorrhage after Cesarean Delivery. N. Engl. J. Med. 2023, 388, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Kim, C.-K.; Kim, Y.-C.; Juh, H.-S.; Kim, H.-J.; Kim, H.-S.; Hong, S.J.; Hey, H.W.D. The effectiveness of low-dose and high-dose tranexamic acid in posterior lumbar interbody fusion: A double-blinded, placebo-controlled randomized study. Eur. Spine J. 2017, 26, 2851–2857. [Google Scholar] [CrossRef]
- Shakur, H.; Roberts, I.; Fawole, B.; Chaudhri, R.; El-Sheikh, M.; Akintan, A.; Qureshi, Z.; Kidanto, H.; Vwalika, B.; Abdulkadir, A.; et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 2105–2116. [Google Scholar] [CrossRef]
- Napolitano, L.M. Hemostatic defects in massive transfusion: An update and treatment recommendations. Expert. Rev. Hematol. 2021, 14, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Benipal, S.; Santamarina, J.L.; Vo, L.; Nishijima, D.K. Mortality and Thrombosis in Injured Adults Receiving Tranexamic Acid in the Post-CRASH-2 Era. West. J. Emerg. Med. 2019, 20, 443–453. [Google Scholar] [CrossRef]
- Shakur, H.; Roberts, I.; Bautista, R.; Caballero, J.; Coats, T.; Dewan, Y.; El-Sayed, H.; Gogichaishvili, T.; Gupta, S.; Herrera, J.; et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010, 376, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.; Shakur-Still, H.; Afolabi, A.; Akere, A.; Arribas, M.; Brenner, A.; Chaudhri, R.; Gilmore, I.; Halligan, K.; Hussain, I.; et al. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): An international randomised, double-blind, placebo-controlled trial. Lancet 2020, 395, 1927–1936. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Theusinger, O.M.; Albrecht, R.; Mueller, S.; Spahn, D.R.; Urien, S.; Stein, P. Optimisation of the dosage of tranexamic acid in trauma patients with population pharmacokinetic analysis. Anaesthesia 2018, 73, 719–729. [Google Scholar] [CrossRef]
- Bockenstedt, P. D-dimer in venous thromboembolism. N. Engl. J. Med. 2003, 349, 1203–1204. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.D.; Moore, H.B.; Kong, Y.W.; Chapman, M.P.; Sriram, G.; Lim, D.; Moore, E.E.; Yaffe, M.B. Tranexamic acid mediates proinflammatory and anti-inflammatory signaling via complement C5a regulation in a plasminogen activator-dependent manner. J. Trauma. Acute Care Surg. 2019, 86, 101–107. [Google Scholar] [CrossRef]
- Colling, M.E.; Tourdot, B.E.; Kanthi, Y. Inflammation, Infection and Venous Thromboembolism. Circ. Res. 2021, 128, 2017–2036. [Google Scholar] [CrossRef]
- Li, H.; Shan, W.; Zhao, X.; Sun, W. Neutrophils: Linking Inflammation to Thrombosis and Unlocking New Treatment Horizons. Int. J. Mol. Sci. 2025, 26, 1965. [Google Scholar] [CrossRef]
- Boufenzer, A.; Carrasco, K.; Jolly, L.; Brustolin, B.; Di-Pillo, E.; Derive, M.; Gibot, S. Potentiation of NETs release is novel characteristic of TREM-1 activation and the pharmacological inhibition of TREM-1 could prevent from the deleterious consequences of NETs release in sepsis. Cell Mol. Immunol. 2021, 18, 452–460. [Google Scholar] [CrossRef]
- Zucoloto, A.Z.; Jenne, C.N. Platelet-Neutrophil Interplay: Insights into Neutrophil Extracellular Trap (NET)-Driven Coagulation in Infection. Front. Cardiovasc. Med. 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Itabashi, M.; Tachibana, Y.; Sugihara, T.; Sakashita, Y.; Matsubara, T.; Murayama, S.; Yumura, W.; Shimizu, A.; Takei, T.; et al. Citrullinated histone H3 expression in anti-neutrophil cytoplasmic antibody-associated vasculitis in older Japanese autopsy patients. Geriatr. Gerontol. Int. 2019, 19, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; He, Z.; Rauf, A.; Beikoghli Kalkhoran, S.; Heiestad, C.M.; Stensløkken, K.O.; Parish, C.R.; Soehnlein, O.; Arjun, S.; Davidson, S.M.; et al. Extracellular histones are a target in myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2022, 118, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Brill, A.; Wagner, D.D. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arter. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1777–1783. [Google Scholar] [CrossRef]
- Kumar, R.; Patil, G.; Dayal, S. NLRP3-Induced NETosis: A Potential Therapeutic Target for Ischemic Thrombotic Diseases? Cells 2023, 12, 2709. [Google Scholar] [CrossRef]
- Han, T.; Tang, H.; Lin, C.; Shen, Y.; Yan, D.; Tang, X.; Guo, D. Extracellular traps and the role in thrombosis. Front. Cardiovasc. Med. 2022, 9, 951670. [Google Scholar] [CrossRef]
- Gong, Y.N.; Wang, X.; Wang, J.; Yang, Z.; Li, S.; Yang, J.; Liu, L.; Lei, X.; Shao, F. Chemical probing reveals insights into the signaling mechanism of inflammasome activation. Cell Res. 2010, 20, 1289–1305. [Google Scholar] [CrossRef]
- Varma, M.R.; Varga, A.J.; Knipp, B.S.; Sukheepod, P.; Upchurch, G.R.; Kunkel, S.L.; Wakefield, T.W.; Henke, P.K. Neutropenia impairs venous thrombosis resolution in the rat. J. Vasc. Surg. 2003, 38, 1090–1098. [Google Scholar] [CrossRef]
- Nielsen, J.S.; McNagny, K.M. Novel functions of the CD34 family. J. Cell Sci. 2008, 121, 3683–3692. [Google Scholar] [CrossRef]
- Liu, L.; Shi, G.-P. CD31: Beyond a marker for endothelial cells. Cardiovasc. Res. 2012, 94, 3–5. [Google Scholar] [CrossRef]
- Müller, A.M.; Hermanns, M.I.; Skrzynski, C.; Nesslinger, M.; Müller, K.-M.; Kirkpatrick, C.J. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp. Mol. Pathol. 2002, 72, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.-W.; Seo, E.-H.; Choi, U.Y.; Oh, C.-S.; Kim, A.; Song, K.; Lee, S.-H.; Kim, J.K. High-Dose Tranexamic Acid Enhances Circulating Neutrophil Extracellular Traps and Thrombus in Thrombosis Mouse Model. Biomedicines 2025, 13, 1284. https://doi.org/10.3390/biomedicines13061284
Song J-W, Seo E-H, Choi UY, Oh C-S, Kim A, Song K, Lee S-H, Kim JK. High-Dose Tranexamic Acid Enhances Circulating Neutrophil Extracellular Traps and Thrombus in Thrombosis Mouse Model. Biomedicines. 2025; 13(6):1284. https://doi.org/10.3390/biomedicines13061284
Chicago/Turabian StyleSong, Jung-Wook, Eun-Hye Seo, Un Yung Choi, Chung-Sik Oh, Aram Kim, Keeho Song, Seung-Hyun Lee, and Jin Kook Kim. 2025. "High-Dose Tranexamic Acid Enhances Circulating Neutrophil Extracellular Traps and Thrombus in Thrombosis Mouse Model" Biomedicines 13, no. 6: 1284. https://doi.org/10.3390/biomedicines13061284
APA StyleSong, J.-W., Seo, E.-H., Choi, U. Y., Oh, C.-S., Kim, A., Song, K., Lee, S.-H., & Kim, J. K. (2025). High-Dose Tranexamic Acid Enhances Circulating Neutrophil Extracellular Traps and Thrombus in Thrombosis Mouse Model. Biomedicines, 13(6), 1284. https://doi.org/10.3390/biomedicines13061284




