Abstract
Glucocorticoids (GCs) have revolutionized the treatment of multidisciplinary diseases. Recently, its role in severe infectious diseases has been revisited and discussed since the COVID-19 pandemic. Previous research and discussions have focused more on their anti-inflammatory effects and impact on the immune system, with limited study on other aspects of their action and mechanisms. In recent years, it has been discovered that glucocorticoids can regulate the extracellular matrix by influencing the cellular microenvironment and processes such as fibrosis, thereby exerting regulatory effects on diseases. This article summarizes current research on GC-mediated extracellular matrix (ECM) remodeling. It emphasizes the dual role of the ECM as a therapeutic target and a source of biomarkers, and identifies molecular mechanisms and potential biomarkers for precise glucocorticoid therapy, such as type I collagen (PRO-C1), type III collagen (PRO-C3), fibrillin-C (FBN-C), and type III collagen degradation (C3M). These findings may also contribute to the development of more precise new drugs.
1. Introduction
The therapeutic application of glucocorticoids (GCs) in clinical medicine originated in the late 1950s, representing a revolutionary advancement in treating inflammatory and autoimmune disorders. Subsequent decades have yielded extensive research into their pharmacological effects and molecular mechanisms [1,2,3]. GCs have been widely utilized in COVID-19 treatment due to their anti-inflammatory and immunosuppressive properties, with multiple studies supporting their efficacy in critically ill patients. For instance, a systematic review and network meta-analysis demonstrated that GCs significantly reduce mortality, decrease the need for mechanical ventilation, and improve clinical outcomes in COVID-19 patients [4]. Single-cell RNA sequencing analyses further revealed that GCs suppress type I and II interferon response pathways, while IL-6-associated signatures are additionally downregulated by tocilizumab [5]. A retrospective cohort study also reported that GCs markedly enhance 30-day recovery rates in severe COVID-19 cases [5]. However, their use in non-hypoxemic patients may elevate mortality risks, as evidenced by a meta-analysis showing significantly higher mortality rates in GC-treated individuals compared to controls within this subgroup [6]. While the therapeutic application of GCs in severe and critical COVID-19 has gained broad acceptance [7,8,9,10], their administration during early or mild disease stages remains contentious, necessitating further research to establish optimal treatment protocols and dosing regimens [6,8]. Additionally, individual variability in treatment response and the potential for adverse effects must be carefully considered in clinical decision making [11].
Despite extensive use of GCs and their well-established global anti-inflammatory effects, detailed mechanistic insights—particularly regarding their cell type-specific and context-dependent actions—remain insufficiently explored. In addition to their anti-inflammatory and immunosuppressive properties, GCs critically influence extracellular matrix (ECM) remodeling and cellular microenvironment dynamics.
The ECM is a complex and dynamic component that not only provides structural support to cells but also plays a crucial role in cellular communication, differentiation, and migration [12,13]. In the realm of precision medicine, the ECM is recognized for its critical role in the pathogenesis and progression of various diseases [14]. It serves as a critical determinant in wound healing [15], fibrosis [16], arthritis [17], and cancer metastasis [18]. Understanding the intricacies of ECM dynamics creates opportunities for targeted therapies, enabling more personalized and effective treatment strategies [19].
GCs–ECM interactions constitute a pivotal therapeutic mechanism. GCs modulate ECM remodeling through fibroblast activity, collagen synthesis, and matrix metalloproteinases (MMPs) regulation [20,21]. These bidirectional regulatory effects position the ECM as a potential biomarker for balancing GCs efficacy and adverse effects in precision therapies.
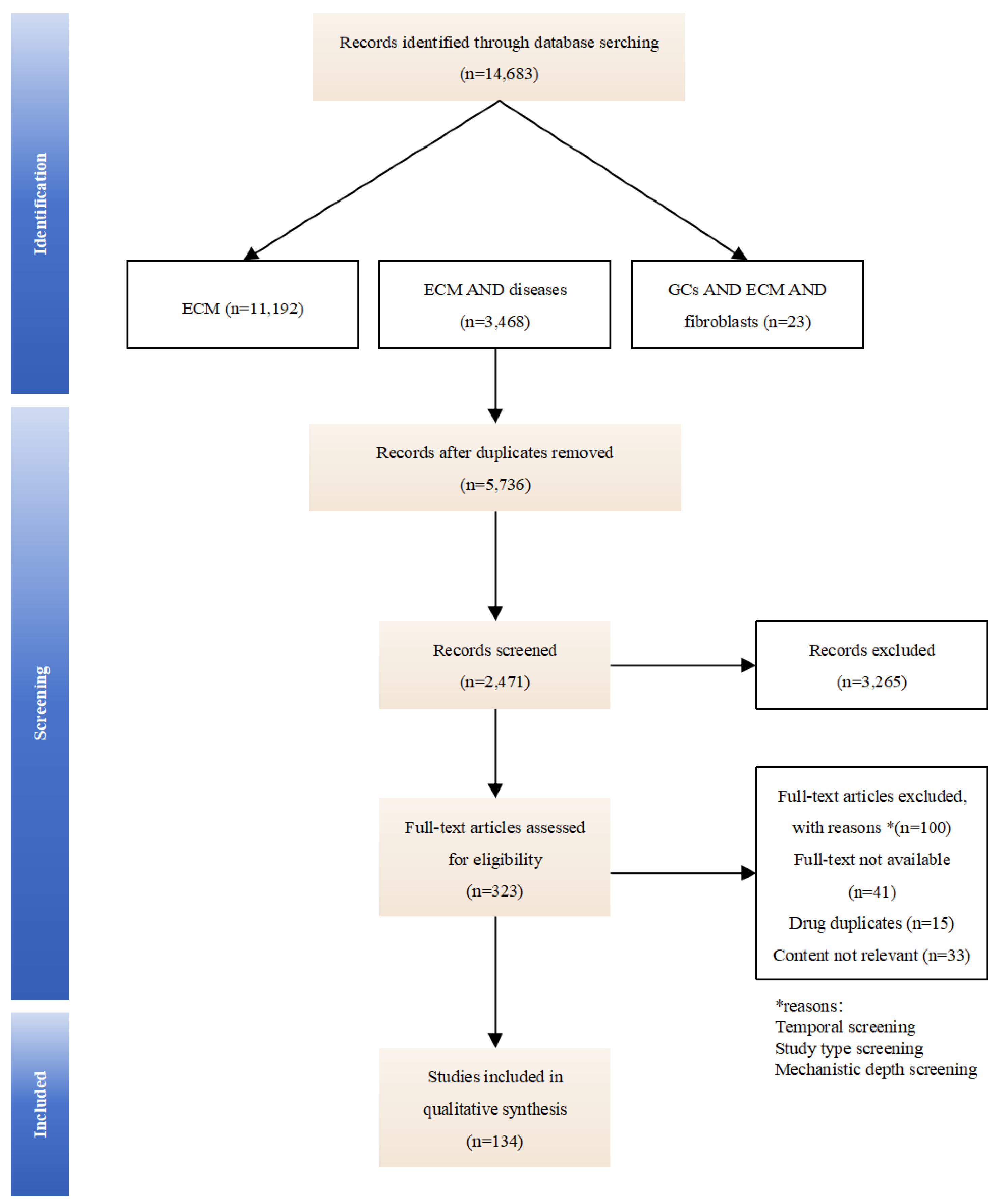
This review synthesizes literature from PubMed, Web of Science, and Google Scholar using the following paired search terms: “extracellular matrix”, “extracellular matrix” with “arthritis, fibrosis, cancer, wound healing”, and “glucocorticoids” with “extracellular matrix, fibroblasts”. We evaluate GCs-induced compositional changes in the ECM and their implications for therapeutic optimization and side-effect mitigation, aiming to clarify how GCs dynamically remodel the ECM and identify ECM-related biomarkers for optimizing therapeutic outcomes. By mapping these mechanisms, we seek to provide insights for precision dosing and targeted drug development (an overview of the studies collection for this study is shown in Figure 1).
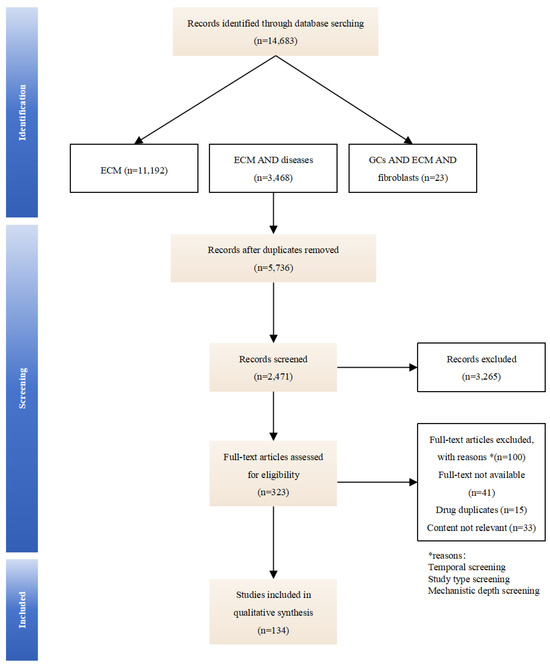
Figure 1.
An overview of the studies collection for this study.
2. What Is the ECM and Its Role in Diseases
The ECM is an intricate network of proteins and polysaccharides that provides structural and biochemical support to the surrounding cells. It is not a static entity; rather, it dynamically interacts with cells, profoundly influencing their behavior and the tissue’s overall function.
2.1. Main Structure of the ECM
The ECM is a complex three-dimensional network structure secreted by cells, primarily composed of two functional components: fibrous proteins and a hydrated ground substance. Fibrous proteins, including collagens, elastin, fibronectin, and laminins, form a cross-linked network that confers tensile strength and elasticity to tissues. The hydrated ground substance, consisting of proteoglycans (such as chondroitin sulfate) and glycosaminoglycans like hyaluronic acid, forms a gel-like structure through highly hydrophilic molecules, mediating compressive resistance and regulating tissue osmotic pressure [22,23]. Beyond providing structural support to cells, the ECM integrates bioactive molecules such as growth factors, thereby synergistically regulating cell migration, proliferation, differentiation, and tissue homeostasis through biochemical and biomechanical signals [24].
2.2. Variability of the ECM Across Tissues
ECM composition exhibits marked tissue specificity, reflecting unique biomechanical demands [25]. The ECM in cartilage predominantly contains collagen II and aggrecan proteoglycans for shock absorption [26,27], whereas the ECM in skin relies on collagen I and elastin networks for dermal flexibility [28]. Pulmonary ECM predominantly contains heparan sulfate, chondroitin sulfate, and hyaluronic acid, which regulate alveolar elasticity and gas exchange efficiency [29]. Renal function critically depends on specialized basement membrane components within the kidney ECM that govern filtration selectivity [30], while mammary gland ECM orchestrates epithelial cell polarization and lactation through dynamic collagen–proteoglycan networks [31]. The liver ECM, primarily composed of collagen, fibronectin, laminin, and proteoglycans, provides structural support, promotes cell adhesion and growth, and regulates growth factor activity, forming a dynamic network essential for liver function and regeneration [32]. These compositional diversities enable the ECM to structurally reinforce tissues while biochemically fine-tuning cellular responses.
Pathological states drive ECM compositional remodeling. In fibrotic diseases and osteoarthritis, dynamic ECM remodeling manifests as excessive deposition or degradation of matrix components, culminating in loss of structural integrity and function [33,34,35,36,37]. Clinically administered GCs (e.g., dexamethasone)—mainstays in connective tissue disease management—induce ECM alterations that exhibit dual therapeutic and adverse effect signatures, necessitating mechanistic dissection.
2.3. Mechanisms of ECM Alteration in Diseases
2.3.1. Arthritis
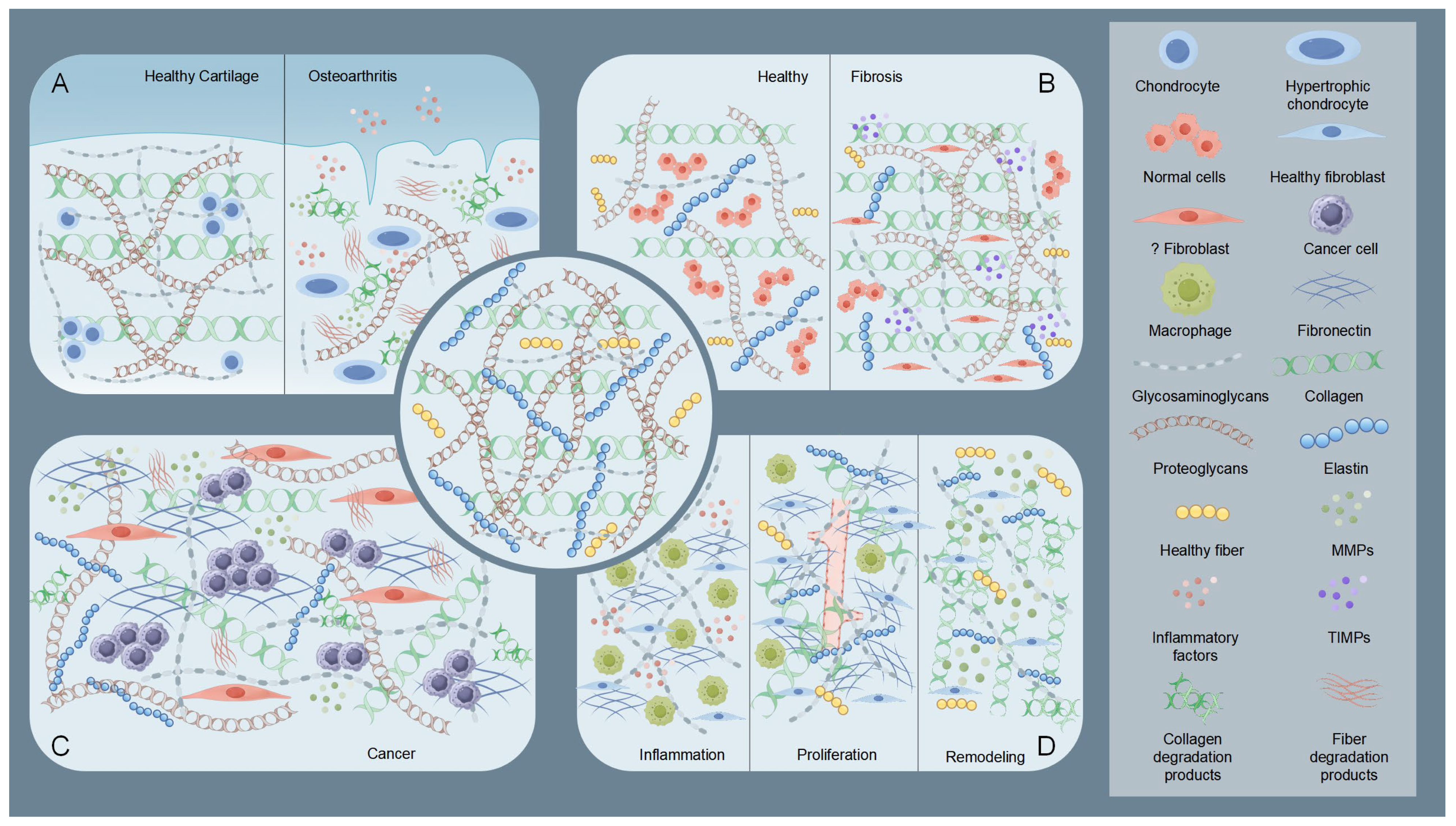
Osteoarthritis (OA) exemplifies the critical role of ECM dysregulation in arthritis pathogenesis. Osteoarthritis involves structural degradation of articular cartilage ECM, characterized by disrupted equilibrium between collagen/proteoglycan synthesis and catabolism [38,39] (Figure 2). Elevated MMPs activity coupled with reduced tissue inhibitor of metalloproteinases (TIMPs) expression drives pathological ECM degradation [20], leading to progressive cartilage erosion, joint dysfunction, and pain [17,40]. Inflammatory cytokines (e.g., IL-1β, TNF-α) stimulate chondrocyte-mediated MMPs production, which targets collagen II and aggrecan [40,41,42]. Insufficient compensatory ECM synthesis by chondrocytes accelerates irreversible cartilage loss [17]. This contrasts with rheumatoid arthritis (RA), where MMP-generated ECM neoepitopes perpetuate autoimmune responses [43].
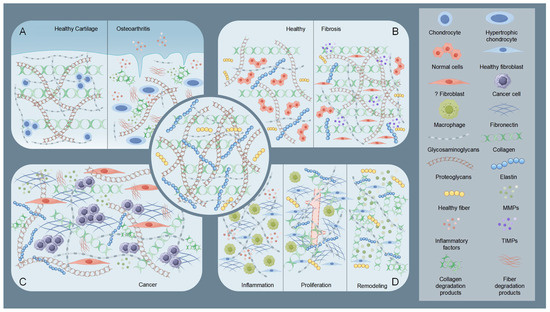
Figure 2.
Schematic illustration of the state of the extracellular matrix (ECM) under pathological conditions. The central part is the normal ECM. (A) Taking osteoarthritis as an example, the diagram shows a comparison of healthy cartilage ECM with osteoarthritic cartilage ECM. In osteoarthritic cartilage ECM, elevated MMPs and inflammatory factors degrade collagen and proteoglycans. Chondrocytes, influenced by these degradation products and inflammatory factors, undergo hypertrophy and lose their fundamental functions. (B) Comparison of ECM in healthy tissue and fibrotic tissue. In fibrotic tissue, abnormal fibroblasts and tissue inhibitor of metalloproteinases (TIMPs) increase, leading to collagen and proteoglycan deposition. (C) Diagram showing tumoral ECM, part of the TME. Here, CAFs trigger a marked rise in matrix components, especially adhesive glycoproteins like fibronectin. This action enhances the ability of tumor cells to adhere, migrate, and invade. (D) During the three stages of wound healing, the ECM undergoes significant changes. In the inflammatory phase, inflammatory factors and macrophages are the main components. During the proliferative phase, collagen and fibronectin levels increase and begin to arrange in a regular pattern, and new blood vessels form. In the remodeling phase, matrix components are altered by MMPs to reduce scar overgrowth. By Figdraw (https://www.figdraw.com).
2.3.2. Fibrosis
Fibrosis features pathological ECM overaccumulation, causing tissue scarring and functional impairment (Figure 2). Chronic injury activates myofibroblasts that overproduce collagen I and other ECM components, disrupting organ architecture [44]. The resultant stiffened ECM establishes a self-sustaining fibrotic cascade via mechanotransduction pathways [45,46]. In hepatic and pulmonary fibrosis, this aberrant remodeling progressively compromises organ function through architectural distortion and altered cellular signaling [45,46].
2.3.3. Cancer
The ECM serves dual roles as a physical constraint and tumor-promoting scaffold in cancer progression [47]. Early-stage tumors are restricted by dense ECM barriers, which are later remodeled via tumor-secreted proteases (e.g., MMPs) and cancer-associated fibroblast (CAF)-derived factors [48,49,50] (Figure 2). Modified collagens (e.g., linearized collagen I) and proteoglycan-rich microenvironments facilitate invasion, metastasis, and immune evasion [49,50,51]. CAF-mediated ECM alterations further promote angiogenesis and activate oncogenic signaling pathways (e.g., PI3K/AKT) [50,51,52].
2.3.4. Wound Healing
During the four phases of wound healing (hemostasis, inflammation, proliferation, and remodeling), the ECM exhibits dynamic regulatory characteristics [53] (Figure 2). The ECM not only serves as a structural scaffold but also coordinates cell behavior through its components (such as fibronectin, hyaluronic acid, and collagen) and signal transmission. During the inflammation phase, the deposition of temporary ECM components (e.g., fibronectin and hyaluronic acid) initiates macrophage polarization and inflammation regulation [54,55,56]. In the proliferation phase, enhanced ECM synthesis (e.g., collagen III and fibronectin fibrillation) supports angiogenesis and granulation tissue formation [56,57,58]. During the remodeling phase, collagen remodeling (replacement of type III with type I) mediated by MMPs and regulation by matrix proteins (e.g., decorin) reduce scarring [59,60]. Studies have shown that ECM dynamics are closely related to wound types (e.g., diabetic wounds, burns). And wound healing often requires intervention to accelerate tissue regeneration and prevent complications [61]. For instance, delayed ECM formation in diabetic wounds requires exogenous supplementation with biomaterials (e.g., OHA-CMC hydrogels) [54].
3. GC-Mediated Fibroblasts Regulation of ECM Dynamics
3.1. Fibroblasts as Central Effectors in ECM Homeostasis
Fibroblasts serve as the principal cellular mediators of ECM synthesis and remodeling during physiological repair and pathological fibrosis [59,62]. Through coordinated biosynthesis of collagens and structural glycoproteins, these cells maintain tissue integrity while dynamically responding to microenvironmental cues [44]. During wound healing, fibroblasts orchestrate granulation tissue formation via ECM deposition and contractile activity, whereas dysregulated activation drives pathological scarring through excessive ECM accumulation.
3.2. Inflammation-Induced Fibroblast Phenotypic Switching
In the context of inflammation and fibrosis, fibroblasts undergo phenotypic changes that alter their function [59]. They can transform into myofibroblasts, characterized by increased contractility and ECM production. This transformation is often driven by cytokines and growth factors released during inflammation [45,63]. The persistence of myofibroblasts and their continued ECM production can contribute to the pathological fibrosis observed in various diseases [45].
3.3. GCs Orchestrate Fibroblast–ECM Dynamics Across Tissue Contexts
GCs are widely used for their anti-inflammatory effects, but their clinical value is often limited by dose-dependent side effects [64]. A key challenge is balancing efficacy with tissue protection, particularly regarding ECM integrity. GCs influence the ECM both indirectly, by modulating immune responses, and directly, by affecting fibroblasts and epithelial cells. During wound healing, GCs suppress inflammatory-phase ECM degradation by downregulating MMP-2 and MMP-9 activity while exhibiting dose-dependent effects on collagen III synthesis—stimulatory at low concentrations but inhibitory at therapeutic doses [63,65,66,67]. The dose-dependent effects of GCs exhibit significant clinical implications across various diseases. According to existing studies, the dosage thresholds of GCs vary considerably among different diseases and research settings. For instance, in RA, studies have shown that low-dose GCs regimens (<5 mg/day prednisone equivalent) do not significantly increase the risk of cardiovascular events (CVE) compared to high-dose regimens (≥10 mg/day prednisone equivalent) [68,69]. However, the administration of high-dose glucocorticoids has been associated with elevated risks of cardiovascular and all-cause mortality [69]. In anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV), low-dose glucocorticoid therapy (<30 mg/day prednisone equivalent) demonstrates comparable efficacy in inducing remission to high-dose regimens (≥30 mg/day prednisone equivalent) while significantly reducing the incidence of infections [70]. These findings collectively indicate that glucocorticoid dosage selection should be individualized based on specific clinical contexts and patient risk profiles.
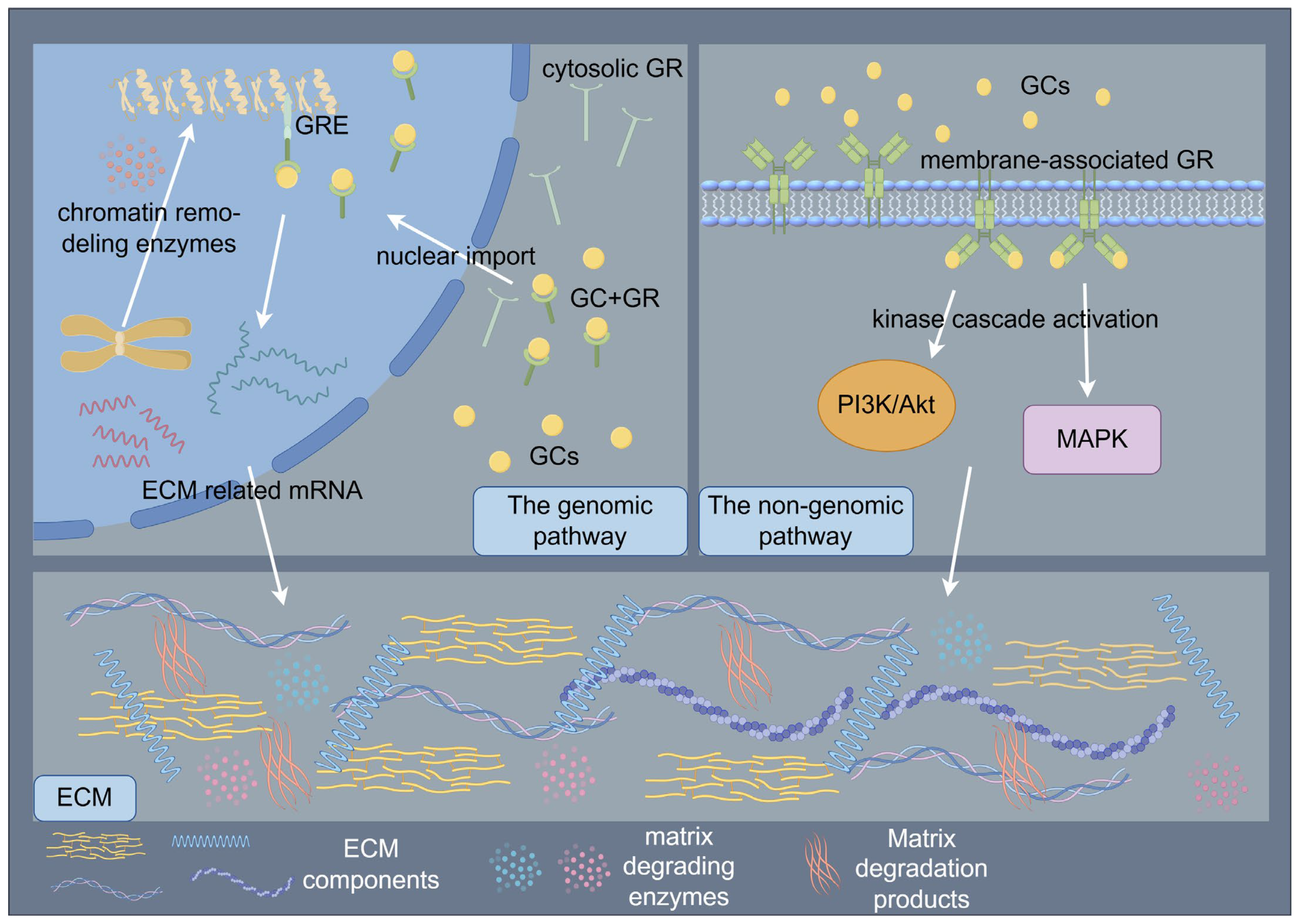
The paradoxical pro- and anti-fibrotic effects of GCs may stem from dose-dependent biphasic regulation of TGF-β signaling and mechanosensitive GC receptor (GR) isoform switching. GC signaling orchestrates ECM regulation through coordinated genomic and non-genomic mechanisms that synergistically modulate cellular behavior and tissue homeostasis [47,71]. The genomic pathway, mediated by cytosolic GR activation, involves ligand-dependent nuclear translocation and subsequent binding to GCs response elements (GREs) in promoter regions, thereby regulating transcriptional programs governing ECM biosynthesis and degradation [71]. Following ligand binding, GRs undergo conformational changes that facilitate their nuclear import, where chromatin remodeling enzymes are recruited to either activate or repress target genes encoding ECM components and modifying enzymes [71]. In contrast, non-genomic mechanisms manifest through rapid membrane-associated GR signaling, which initiates within seconds to minutes via kinase cascade activation (e.g., PI3K/AKT, MAPK pathways), modulating cell migration, proliferation, and ECM remodeling through post-translational regulation of cytoskeletal proteins and matrix metalloproteinases [71]. These temporally distinct yet interconnected pathways converge to dynamically regulate the ECM macromolecular assembly, ultimately determining tissue-specific biomechanical properties and cellular responses to microenvironmental cues [47,71]. (Figure 3) At low doses, GCs activate GR-α to potentiate TGF-β-driven COL1A1 synthesis, whereas higher doses induce miR-29b-mediated TGF-β3 suppression [72,73]. Remodeling-phase ECM maturation is further compromised through GCs-induced MMPs/TIMPs imbalances that impair collagen I crosslinking [20,74]. These multifaceted actions may arise from GR-mediated modulation of AP-1, NF-κB, and TGF-β signaling pathways [75,76,77].
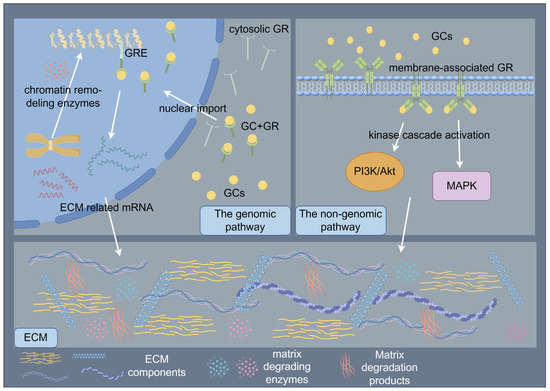
Figure 3.
Schematic illustration of glucocorticoids (GCs) signaling orchestrates the regulation of ECM through coordinated genomic and non-genomic mechanisms. The genomic pathway, mediated by cytosolic GR activation, involves ligand-dependent nuclear translocation and subsequent binding to GCs response elements (GREs) in promoter regions, thereby regulating transcriptional programs governing ECM biosynthesis and degradation. The non-genomic mechanisms manifest through rapid membrane-associated GR signaling, which initiates within seconds to minutes via kinase cascade activation, modulating ECM remodeling. By Figdraw (https://www.figdraw.com).
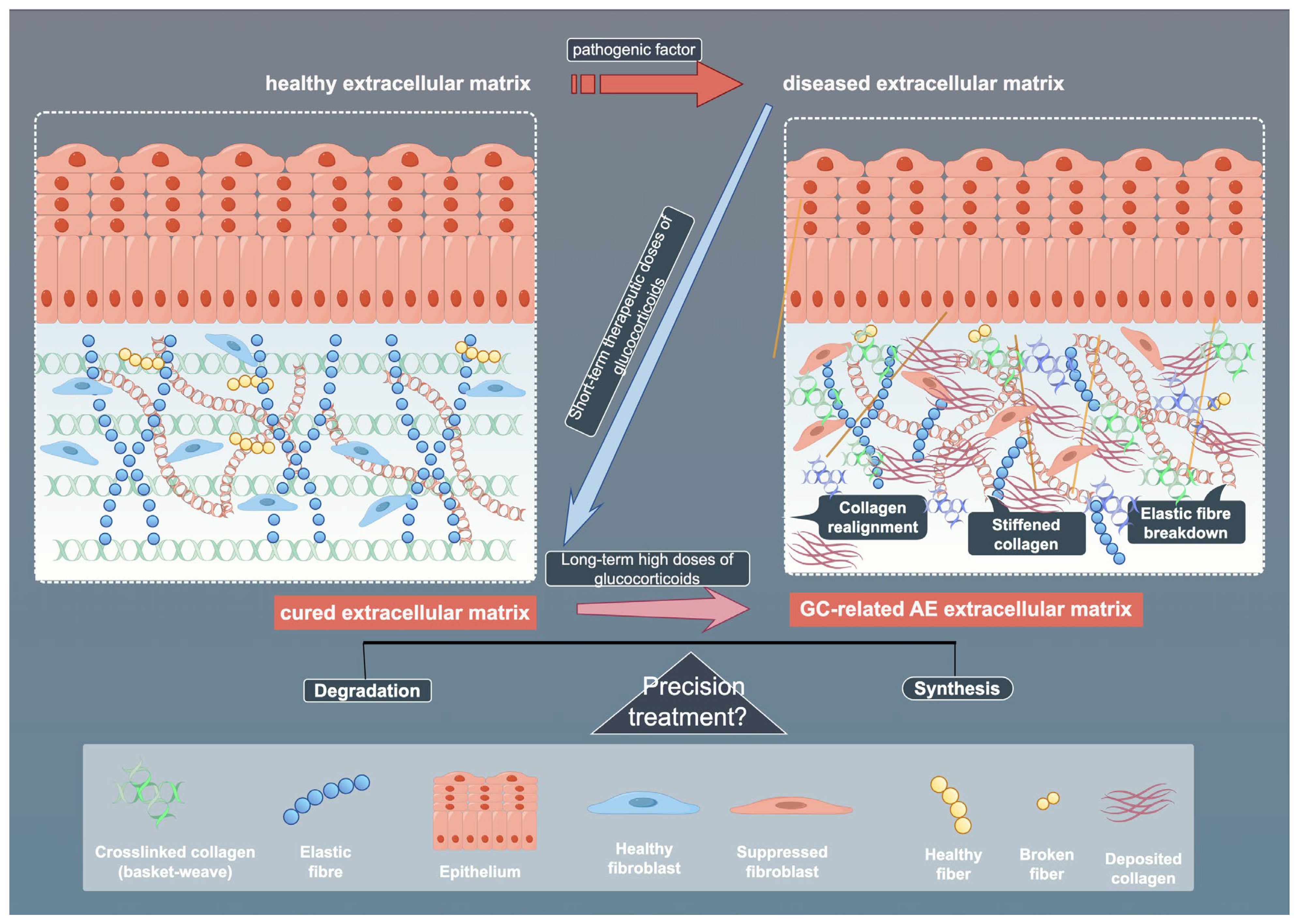
Fibroblast responses to GCs demonstrate pronounced tissue specificity, shaped by receptor interplay, microenvironmental factors, and metabolic regulation. Tissue-specific differences in 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) activity modulate local GCs bioavailability and inflammation. Cross-talk between GR and adrenergic receptor (AR) subtypes further diversifies functional outcomes, with GR activation upregulating specific AR subtypes in human fibroblasts [78,79]. Strategies such as selective GR modulators or targeted delivery systems aim to enhance tissue specificity and reduce ECM-related damage, supporting the development of more precise and personalized GCs therapies [80]. Contaminated vocal fold fibroblasts show aberrant dexamethasone sensitivity, emphasizing the need for microbial screening in experiments [81]. Metabolic reprogramming also plays a role, with adipose fibroblasts regulating leptin and cytokine secretion, processes affected by GCs [82]. While beneficial in acute settings, chronic exposure to GCs may disrupt ECM homeostasis and promote fibrosis [83]. Temporal dynamics further refine these effects: acute GCs exposure rapidly activates MMPs (e.g., MMP-2/9 induction in zebrafish within 72 h) alongside compensatory pathways like SGK signaling [84,85], whereas chronic exposure drives irreversible ECM degradation (e.g., perineuronal net disruption) through oxidative stress-inflammatory feedback loops [86]. Certain agents that adversely affect fibroblasts may also disrupt their DNA molecules. For instance, graphene oxide has been shown to exert negative regulatory effects on the cell cycle of embryonic fibroblasts [87]. However, no relevant studies have been observed regarding such DNA-modifying properties in the application of GCs, suggesting that this mechanism may represent a potential therapeutic target in GC-mediated fibroblast regulation. This spatiotemporal modulation of matrix remodeling program responses highlights GCs’ dual therapeutic potential and the need for precision targeting strategies. (Figure 4)
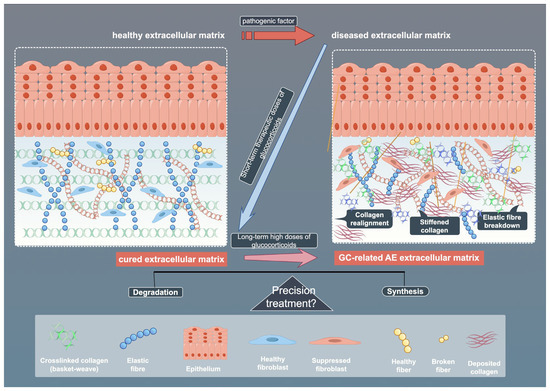
Figure 4.
Schematic illustration of ECM across healthy, diseased, and treated states. The figure shows the effects of pathogenic factors and glucocorticoids (GCs) on ECM components, as well as the potential mechanisms of precision therapy. By Figdraw (https://www.figdraw.com).
3.4. GCs–ECM Interactions in Tumor Microenvironment (TME) Remodeling
GCs critically reshape the TME by modulating ECM dynamics through CAFs activation and immune–ECM cross-talk. GCs enhance CAFs-driven collagen deposition and ECM stiffening via integrin-MMPs signaling, with ovarian cancer models demonstrating DDR2-mediated arginase upregulation that amplifies collagen synthesis [88,89]. This mechanically remodeled ECM promotes tumor invasion while establishing physical barriers to immune infiltration. Thrombospondin-2 (THBS2), a GC-regulated ECM–immune bridge protein, correlates with immunosuppressive TME in gastric/pancreatic cancers through impaired T-cell penetration [90,91].
Clinically, ECM normalization through MMPs inhibition or CAFs depletion enhances chemotherapeutic efficacy in pancreatic cancer, though GCs’ dual roles in immunosuppression and stromal remodeling necessitate tissue-specific strategies [92,93]. Emerging single-cell analyses reveal RUNX2+ myofibroblasts as key GCs–ECM interaction hubs, informing precision approaches to overcome therapy resistance [94,95].
4. Precision Treatment of GCs and the ECM as Clues for Its Biomarkers
Precision medicine is a tailored medical approach that integrates an individual’s genetic makeup, environmental factors, and lifestyle characteristics to deliver personalized medical decisions, treatments, and preventive strategies [96]. While there is evidence-based medical support for glucocorticoid therapy, further research is needed to advance precision medicine approaches based on molecular pharmacological mechanisms [77]. For example, a study shows that three-month tapering and discontinuation of long-term, low-dose glucocorticoids in senior patients with RA is feasible and safe, which is based on a placebo-controlled, double-blind tapering after the GLORIA trial [97]. Another real-world research study revealed that GCs are feasibly discontinued with favorable control of disease activity in real-life settings, mostly without short-term flare. However, the withdrawal time is far from reaching the recommended time frame, indicating the gap between real-world practice and current guidelines [98].
4.1. Classification and Detection of ECM Biomarkers
ECM biomarkers are broadly categorized based on their biological roles: collagen formation markers, collagen degradation markers, elastin breakdown markers, and MMP/TIMP enzyme profiles [99,100]. Collagen formation markers, including PRO-C1 (type I collagen), PRO-C3 (type III collagen), and PRO-C6 (type VI collagen), reflect ECM synthesis activity [99,100]. In contrast, collagen degradation markers such as C1M (type I collagen degradation), C3M (type III collagen degradation), and TUM (type IV collagen degradation) indicate ECM degradation processes [99,100,101]. Elastin breakdown markers like EL-NE reflect ECM degradation mediated by neutrophil elastase [99]. Additionally, MMPs and TIMPs, such as MMP-2, MMP-9, and TIMP-1, reveal the enzymatic mechanisms of ECM remodeling [43,102,103]. These biomarkers capture dynamic ECM turnover processes, with specific clinical relevance in GCs therapy optimization. For instance, elevated BGN (Biglycan) levels may correlate strongly with GCs-induced osteonecrosis risk, providing a quantitative basis for early intervention [104,105]. Collagen degradation markers such as C3M and TUM further serve as sentinels of ECM destabilization in fibrotic diseases, while elastin degradation products like EL-NE reflect neutrophil-driven tissue damage in chronic inflammatory conditions [99,101].
Advanced detection methods like ELISA and mass spectrometry enable precise quantification, while multi-marker panels (e.g., C6M + Pro-C6 + EL-NE) improve diagnostic accuracy in complex diseases such as COPD [99,100,101]. Emerging platforms further expand clinical utility: the BrdU incorporation lymphocyte steroid sensitivity assay (BLISS) achieves 83% sensitivity in identifying GC-resistant patients by measuring lymphocyte proliferation responses ex vivo, offering a non-radioactive alternative to traditional assays [106]. Three-dimensional organoid platforms now faithfully reconstruct tissue-specific ECM architectures, particularly in ovarian cancer models [107,108], where preserved patient-specific ECM components enable systematic screening of GCs sensitivity and chemoresistance prediction [108].
The bidirectional regulation of ECM components by GCs underscores their biomarker potential. Therapeutic GC doses suppress MMP-9 activity in inflamed joints, preserving collagen integrity and reducing cartilage degradation, whereas prolonged high-dose GC exposure disrupts ECM homeostasis, accelerating skin atrophy through collagen I/III imbalance and MMP-mediated elastolysis [109,110]. This duality positions MMP-9/TIMP-1 ratios as dynamic biomarkers to balance GC efficacy and toxicity. Furthermore, proteomic profiling reveals GCs-induced suppression of fibronectin and fibulin-1 in dermal ECM, while paradoxically upregulating collagen IV via YAP pathway activation in endothelial cells [111,112,113,114,115]. It can be a mechanism exploitable for tissue-specific biomarker development.
4.2. ECM-Guided GCs Treatment Strategies
In rheumatic diseases, ECM markers have shown potential for guiding treatment decisions. In RA, transcriptomic profiling of synovial tissue (RNA sequencing and single-cell RNA sequencing), integrated with spatial multi-omics platforms (e.g., 10× Visium), has unveiled fibroblast–T cell interaction networks and dynamic alterations in ECM components, which predict therapeutic responses to GCs [116]. By applying the MITHrIL algorithm to quantify miRNA-regulated immune pathways, combined with gene interaction network analysis (e.g., SOCS2/STAT2 axis), this study identified ECM remodeling-associated biomarkers (e.g., upregulated MMP-3 and downregulated TIMP-1), providing a mechanistic foundation for precision-targeted therapies in RA [117,118]. A study on psoriatic arthritis (PsA) patients found that baseline ECM marker levels can predict the treatment response to guselkumab, an IL-23 inhibitor. Similar strategies may apply to GCs treatment, using ECM markers to identify patient subgroups likely to benefit [43,103,119]. In kidney diseases, human precision-cut kidney slices (PCKSs) models have confirmed that ECM markers like PRO-C1, PRO-C3, and FBN-C reflect fibrosis extent and drug responses [101]. These models can test GCs’ effects on renal ECM remodeling and identify patients likely to benefit from GCs. For pulmonary fibrosis, PRO-C3 and C3M dynamics correlate with disease progression, supporting their use in monitoring GCs effects [100].
Long-term GCs therapy is associated with significant complications requiring vigilant monitoring. Osteonecrosis occurs in 9–40% of adults receiving chronic GCs treatment, independent of osteoporosis development. Adrenal suppression—characterized by hypothalamic–pituitary–adrenal (HPA) axis dysfunction—arises from exogenous GCs exposure, with even short-term high-dose regimens (≥5 days) inducing cortisol insufficiency. Systemic absorption of inhaled/topical GCs and long-acting formulations (e.g., dexamethasone) amplifies this risk, though morning administration may mitigate suppression severity through circadian rhythm alignment [59]. Notably, sex-specific variations in MMPs activity necessitate gender-adjusted biomarker interpretation to optimize GCs dosing [103]. Current research indicates that given the potential adverse effects of GCs, such as skin atrophy and angiogenesis suppression, the exploration of alternative therapeutic strategies has become a critical focus. Selective GR agonists, exemplified by 5α-tetrahydrocorticosterone (5αTHB), demonstrate potential benefits in ECM regulation. Compared to conventional GCs like hydrocortisone, 5αTHB exhibits weaker inhibitory effects on angiogenesis while preserving the expression of genes associated with ECM integrity and inflammatory signaling pathways [120]. However, it should be emphasized that existing studies remain confined to animal models, necessitating further human trials to validate its safety profile and therapeutic efficacy. Concurrently, the development of MMPs inhibitors has advanced, with diverse candidates such as gold nanorods, doxycycline, and natural products under investigation, each operating through distinct mechanisms [121]. Nevertheless, limited understanding of the multifaceted roles of MMPs in disease pathogenesis, coupled with inadequate selectivity of inhibitors, has resulted in the failure of numerous MMP-targeted agents in clinical trials [121,122].
Emerging biomarkers offer solutions for complication management. GCs-induced leucine zipper protein (GILZ) is a GCs-responsive regulator, modulates cellular activation, apoptosis, and inflammation via direct inhibition of NF-κB subunits (p65/p52). It attenuates pro-inflammatory cytokine release and macrophage phagocytosis [123]. This dual functionality positions GILZ as both a predictive biomarker for sepsis outcomes and a therapeutic monitoring target for glucocorticoid regimen optimization.
Understanding how glucocorticoids interact with the ECM at the molecular and cellular levels could lead to more targeted and effective treatments for rheumatic diseases. Healthcare professionals should be aware of these potential biomarkers and management strategies to optimize glucocorticoid treatment plans and minimize adverse patient reactions.
4.3. Challenges and Future Directions
Despite the potential of ECM biomarkers in optimizing GCs therapy, key challenges persist. Longitudinal studies are scarce, with most research relying on cross-sectional designs that fail to capture dynamic ECM changes during long-term GCs treatment [99,100,124]. Standardization issues plague biomarker detection, as inconsistent assay protocols and cutoff values hinder clinical translation [99,101,103]. Mechanistic understanding remains incomplete, particularly regarding how GCs selectively modulate specific ECM components like fibronectin versus collagen [43,100,103]. Additionally, the lack of multi-omics integration (e.g., combining ECM profiles with genomic data) limits predictive model accuracy [124,125].
To address these gaps, researchers should prioritize multi-modal models that integrate ECM biomarkers, clinical parameters, and imaging data to predict GCs responses [125,126]. Patient-derived organoids and 3D culture systems offer physiologically relevant platforms for testing GCs–ECM interactions at the individual level [107,108,127]. Emerging technologies such as nanobiosensors and liquid biopsies could enable real-time ECM monitoring with high sensitivity [128,129]. Clinical trials must validate ECM-guided GCs dosing strategies, particularly for diseases with sex-specific ECM remodeling patterns [100,119,129]. Investigating ECM–immune cell cross-talk may reveal novel therapeutic targets to enhance GCs efficacy [130,131,132].
Artificial intelligence (AI) holds transformative potential, with deep learning algorithms analyzing spatial ECM heterogeneity in tissue samples to improve prognostic accuracy [126,133]. These tools could optimize GCs treatment decisions by quantifying structural ECM changes during therapy [126,133].
5. Conclusions
The field of precision treatment with GCs in rheumatic diseases is rapidly evolving. Current strategies focus on personalized tapering and adverse effect monitoring, while future developments are likely to involve advanced diagnostics and novel therapeutic agents, representing a shift towards more effective, safer, and personalized GCs therapy. The ECM is a complex network of proteins and other molecules that provides structural and biochemical support to surrounding cells. The ECM can play a role in the precision treatment with GCs, particularly in rheumatic diseases. It can emerge as a critical biomarker reservoir for optimizing GCs therapy, bridging molecular mechanisms with clinical outcomes.
In summary, the ECM is an important factor in the pathophysiology of rheumatic diseases and can influence the effectiveness of GCs therapy. Further research into the ECM’s role could enhance the precision of GCs treatment. It can enable more targeted drug delivery and provide new biomarkers for monitoring disease progression and treatment response.
Funding
This research was supported by the Natural Science Foundation of Guangdong Province (2024A1515012856, 2025A1515012408) and the National Natural Science Foundation of China (82474245, 8247140866).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| extracellular matrix | ECM |
| glucocorticoids | GCs |
| matrix metalloproteinases | MMPs |
| tissue inhibitor of metalloproteinases | TIMPs |
| cancer-associated fibroblasts | CAFs |
| α-smooth muscle actin | α-SMA |
| GC receptor | GR |
| rheumatoid arthritis | RA |
| 11β-hydroxysteroid dehydrogenase 1 | 11β-HSD1 |
| adrenergic receptor | AR |
| tumor microenvironment | TME |
| thrombospondin-2 | THBS2 |
| type I collagen | PRO-C1 |
| type III collagen | PRO-C3 |
| type VI collagen | PRO-C6 |
| type I collagen degradation | C1M |
| type III collagen degradation | C3M |
| type IV collagen degradation | TUM |
| psoriatic arthritis | PsA |
| precision-cut kidney slices | PCKS |
| artificial intelligence | AI |
| interleukin-1β | IL-1β |
| interleukin-6 | IL-6 |
| interleukin-23 | IL-23 |
| tumor necrosis factor-α | TNF-α |
| phosphatidylinositol 3-kinase | PI3K |
| protein kinase B | AKT |
| mitogen-activated protein kinase | MAPK |
| transforming growth factor β | TGF-β |
| collagen type II α 1 chain | COL1A1 |
| activator protein 1 | AP-1 |
| nuclear factor κ-B | NF-κB |
| serum and glucocorticoid induced kinase | SGK |
| discoidin domain receptor | DDR2 |
| runt-related transcription factor 2+ | RUNX2+ |
| fibrillin-C | FBN-C |
| elastin degradation by neutrophil elastase | EL-NE |
| chronic obstructive pulmonary disease | COPD |
| biglycan | BGN |
| 5-bromo-2′-deoxyuridine | BrdU |
| BrdU incorporation lymphocyte steroid sensitivity assay | BLISS |
| client-side JavaScript MVC framework | MITHrIL |
| suppressor of cytokine signaling 2 | SOCS2 |
| signal transducer and activator of transcription | STAT2 |
| hypothalamic–pituitary–adrenal | HPA |
| GCs-induced leucine zipper protein | GILZ |
| glucocorticoid response elements | GREs |
| 5α-tetrahydrocorticosterone | 5αTHB |
| cardiovascular events | CVE |
| antibody-associated vasculitis | AAV |
References
- McGonagle, D.; Emery, P. Enthesitis, osteitis, microbes, biomechanics, and immune reactivity in ankylosing spondylitis. J. Rheumatol. 2000, 27, 2302–2304. [Google Scholar] [PubMed]
- Bijlsma, J.W.J. Annals of the Rheumatic Diseases collection on glucocorticoids (2020–2023): Novel insights and advances in therapy. Ann. Rheum. Dis. 2024, 83, 4–8. [Google Scholar] [CrossRef]
- Hardy, R.S.; Raza, K.; Cooper, M.S. Therapeutic glucocorticoids: Mechanisms of actions in rheumatic diseases. Nat. Rev. Rheumatol. 2020, 16, 133–144. [Google Scholar] [CrossRef]
- He, Q.; Wang, C.; Wang, Y.; Chen, G.; Zhou, Y.; Wu, Y.; Zhong, M. Efficacy and safety of glucocorticoids use in patients with COVID-19: A systematic review and network meta-analysis. BMC Infect. Dis. 2023, 23, 896. [Google Scholar] [CrossRef]
- Koh, J.-Y.; Ko, J.-H.; Lim, S.Y.; Bae, S.; Huh, K.; Cho, S.Y.; Kang, C.-I.; Chung, D.R.; Chung, C.R.; Kim, S.-H.; et al. Triple immune modulator therapy for aberrant hyperinflammatory responses in severe COVID-19. Clin. Immunol. 2023, 251, 109628. [Google Scholar] [CrossRef]
- Covello, R.D.; Pasin, L.; Fresilli, S.; Tóth, K.; Damiani, C.; Hajjar, L.A.; Zangrillo, A.; Landoni, G. Meta-Analysis of Glucocorticoids for Covid-19 Patients Not Receiving Oxygen. NEJM Evid. 2023, 2, EVIDoa2200283. [Google Scholar] [CrossRef]
- Alexaki, V.I.; Henneicke, H. The Role of Glucocorticoids in the Management of COVID-19. Horm. Metab. Res. 2021, 53, 9–15. [Google Scholar] [CrossRef]
- Bruscoli, S.; Puzzovio, P.G.; Zaimi, M.; Tiligada, K.; Levi-Schaffer, F.; Riccardi, C. Glucocorticoids and COVID-19. Pharmacol. Res. 2022, 185, 106511. [Google Scholar] [CrossRef]
- Caiazzo, E.; Rezig, A.O.M.; Bruzzese, D.; Ialenti, A.; Cicala, C.; Cleland, J.G.F.; Guzik, T.J.; Maffia, P.; Pellicori, P. Systemic administration of glucocorticoids, cardiovascular complications and mortality in patients hospitalised with COVID-19, SARS, MERS or influenza: A systematic review and meta-analysis of randomised trials. Pharmacol. Res. 2022, 176, 106053. [Google Scholar] [CrossRef]
- Garcia-Cirera, S.; Calvet, J.; Delgado de la Poza, J.F.; Berenguer-Llergo, A.; Orellana, C.; Rusiñol, M.; Llop, M.; Arévalo, M.; Garcia-Pinilla, A.; Costa, E.; et al. Biological and glucocorticoids treatment impair the medium-term immunogenicity to SARS-CoV-2 mRNA vaccines in autoimmune inflammatory rheumatic diseases. Eur. J. Med. Res. 2024, 29, 28. [Google Scholar] [CrossRef]
- Salton, F.; Confalonieri, P.; Meduri, G.U.; Mondini, L.; Trotta, L.; Barbieri, M.; Bozzi, C.; Torregiani, C.; Lerda, S.; Bellan, M.; et al. Theory and Practice of Glucocorticoids in COVID-19: Getting to the Heart of the Matter-A Critical Review and Viewpoints. Pharmaceuticals 2023, 16, 924. [Google Scholar] [CrossRef] [PubMed]
- Arseni, L.; Lombardi, A.; Orioli, D. From Structure to Phenotype: Impact of Collagen Alterations on Human Health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimmetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Herrera, J.; Henke, C.A.; Bitterman, P.B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef]
- Nelson, A.E. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef]
- Yuzhalin, A.E.; Lim, S.Y.; Kutikhin, A.G.; Gordon-Weeks, A.N. Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 207–228. [Google Scholar] [CrossRef]
- Wei, Q.; Zhu, X.; Wang, L.; Zhang, W.; Yang, X.; Wei, W. Extracellular matrix in synovium development, homeostasis and arthritis disease. Int. Immunopharmacol. 2023, 121, 110453. [Google Scholar] [CrossRef]
- Clutterbuck, A.L.; Asplin, K.E.; Harris, P.; Allaway, D.; Mobasheri, A. Targeting matrix metalloproteinases in inflammatory conditions. Curr. Drug Targets 2009, 10, 1245–1254. [Google Scholar] [CrossRef]
- Nakamura, T.; Liu, M.; Mourgeon, E.; Slutsky, A.; Post, M. Mechanical strain and dexamethasone selectively increase surfactant protein C and tropoelastin gene expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L974–L980. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Vallet, S.D. Fragments generated upon extracellular matrix remodeling: Biological regulators and potential drugs. Matrix Biol. 2019, 75–76, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Kollert, M.R.; Krämer, M.; Brisson, N.M.; Schemenz, V.; Tsitsilonis, S.; Qazi, T.H.; Fratzl, P.; Vogel, V.; Reichenbach, J.R.; Duda, G.N. Water and ions binding to extracellular matrix drives stress relaxation, aiding MRI detection of swelling-associated pathology. Nat. Biomed. Eng. 2025, 9, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.E.; Dyer, D.P.; Allen, J.E. The extracellular matrix and the immune system: A mutually dependent relationship. Science 2023, 379, eabp8964. [Google Scholar] [CrossRef]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decorin as a multivalent therapeutic agent against cancer. Adv. Drug Deliv. Rev. 2016, 97, 174–185. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Nyström, A.; Bruckner-Tuderman, L. Matrix molecules and skin biology. Semin. Cell Dev. Biol. 2019, 89, 136–146. [Google Scholar] [CrossRef]
- Hoffman, E.; Song, Y.; Zhang, F.; Asarian, L.; Downs, I.; Young, B.; Han, X.; Ouyang, Y.; Xia, K.; Linhardt, R.J.; et al. Regional and disease-specific glycosaminoglycan composition and function in decellularized human lung extracellular matrix. Acta Biomater. 2023, 168, 388–399. [Google Scholar] [CrossRef]
- Lausecker, F.; Lennon, R.; Randles, M.J. The kidney matrisome in health, aging, and disease. Kidney Int. 2022, 102, 1000–1012. [Google Scholar] [CrossRef]
- Maller, O.; Martinson, H.; Schedin, P. Extracellular matrix composition reveals complex and dynamic stromal-epithelial interactions in the mammary gland. J. Mammary Gland Biol. Neoplasia 2010, 15, 301–318. [Google Scholar] [CrossRef]
- Schuppan, D. Structure of the extracellular matrix in normal and fibrotic liver: Collagens and glycoproteins. Semin. Liver Dis. 1990, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; López de Juan Abad, B.; Cheng, K. Cardiac fibrosis: Myofibroblast-mediated pathological regulation and drug delivery strategies. Adv. Drug Deliv. Rev. 2021, 173, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, R.J.; Puttur, F.; Gaboriau, D.C.A.; Fercoq, F.; Fresquet, M.; Traves, W.J.; Yates, L.L.; Walker, S.A.; Molyneaux, P.L.; Kemp, S.V.; et al. Lung extracellular matrix modulates KRT5(+) basal cell activity in pulmonary fibrosis. Nat. Commun. 2023, 14, 6039. [Google Scholar] [CrossRef]
- Long, Y.; Niu, Y.; Liang, K.; Du, Y. Mechanical communication in fibrosis progression. Trends Cell Biol. 2022, 32, 70–90. [Google Scholar] [CrossRef]
- Cardoneanu, A.; Macovei, L.A.; Burlui, A.M.; Mihai, I.R.; Bratoiu, I.; Rezus, I.I.; Richter, P.; Tamba, B.I.; Rezus, E. Temporomandibular Joint Osteoarthritis: Pathogenic Mechanisms Involving the Cartilage and Subchondral Bone, and Potential Therapeutic Strategies for Joint Regeneration. Int. J. Mol. Sci. 2022, 24, 171. [Google Scholar] [CrossRef]
- Groen, S.S.; Nielsen, S.H.; Bay-Jensen, A.C.; Rasti, M.; Ganatra, D.; Oikonomopoulou, K.; Chandran, V. Investigating protease-mediated peptides of inflammation and tissue remodeling as biomarkers associated with flares in psoriatic arthritis. Arthritis Res. Ther. 2024, 26, 107. [Google Scholar] [CrossRef]
- Otsuki, S.; Brinson, D.C.; Creighton, L.; Kinoshita, M.; Sah, R.L.; D’Lima, D.; Lotz, M. The effect of glycosaminoglycan loss on chondrocyte viability: A study on porcine cartilage explants. Arthritis Rheum. 2008, 58, 1076–1085. [Google Scholar] [CrossRef]
- Arroyo, A.G.; Iruela-Arispe, M.L. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc. Res. 2010, 86, 226–235. [Google Scholar] [CrossRef]
- Molnar, V.; Matisic, V.; Kodvanj, I.; Bjelica, R.; Jelec, Z.; Hudetz, D.; Rod, E.; Cukelj, F.; Vrdoljak, T.; Vidovic, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Dingle, J.T.; Saklatvala, J.; Hembry, R.; Tyler, J.; Fell, H.B.; Jubb, R. A cartilage catabolic factor from synovium. Biochem. J. 1979, 184, 177–180. [Google Scholar] [CrossRef]
- Saklatvala, J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 1986, 322, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Grillet, B.; Pereira, R.V.S.; Damme, J.V.; El-Asrar, A.A.; Proost, P.; Opdenakker, G. Matrix metalloproteinases in arthritis: Towards precision medicine. Nat. Rev. Rheumatol. 2023, 19, 363–377. [Google Scholar] [CrossRef] [PubMed]
- DeLeon-Pennell, K.Y.; Barker, T.H.; Lindsey, M.L. Fibroblasts: The arbiters of extracellular matrix remodeling. Matrix Biol. 2020, 91–92, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B. Extracellular matrix stiffness: Mechanisms in tumor progression and therapeutic potential in cancer. Exp. Hematol. Oncol. 2025, 14, 54. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef]
- Parks, W.C.; Wilson, C.L.; López-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef]
- Kuzet, S.E.; Gaggioli, C. Fibroblast activation in cancer: When seed fertilizes soil. Cell Tissue Res. 2016, 365, 607–619. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 49. [CrossRef]
- Hu, B.; Gao, M.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.; Zhang, X.-Q. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.; Yang, Y.; Zhang, J.; Wang, Y.; Zhang, R.; Yang, L. Versatile Hydrogel Dressings That Dynamically Regulate the Healing of Infected Deep Burn Wounds. Adv. Healthc. Mater. 2023, 12, e2301224. [Google Scholar] [CrossRef]
- Assunção, M.; Yiu, C.H.K.; Wan, H.-Y.; Wang, D.; Ker, D.F.E.; Tuan, R.S.; Blocki, A. Hyaluronic acid drives mesenchymal stromal cell-derived extracellular matrix assembly by promoting fibronectin fibrillogenesis. J. Mater. Chem. B 2021, 9, 7205–7215. [Google Scholar] [CrossRef]
- Seifi, S.; Shamloo, A.; Tavoosi, S.N.; Almasi-Jaf, A.; Shaygani, H.; Sayah, M.R. A novel multifunctional chitosan-gelatin/carboxymethyl cellulose-alginate bilayer hydrogel containing human placenta extract for accelerating full-thickness wound healing. Int. J. Biol. Macromol. 2023, 253, 126929. [Google Scholar] [CrossRef]
- Bhar, B.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Silk-based phyto-hydrogel formulation expedites key events of wound healing in full-thickness skin defect model. Int. J. Biol. Macromol. 2022, 203, 623–637. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef]
- Sylakowski, K.; Hwang, M.P.; Justin, A.; Whaley, D.; Wang, Y.; Wells, A. The matricellular protein decorin delivered intradermally with coacervate improves wound resolution in the CXCR3-deficient mouse model of hypertrophic scarring. Wound Repair Regen. 2022, 30, 436–447. [Google Scholar] [CrossRef]
- Sharifi, E.; Jamaledin, R.; Familsattarian, F.; Nejaddehbashi, F.; Bagheri, M.; Chehelgerdi, M.; Nazarzadeh Zare, E.; Akhavan, O. Bioactive chitosan/poly(ethyleneoxide)/CuFe2O4 nanofibers for potential wound healing. Env. Res. 2023, 239, 117448. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Bignold, R.; Johnson, J.R. Effects of cytokine signaling inhibition on inflammation-driven tissue remodeling. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100023. [Google Scholar] [CrossRef]
- Buttgereit, F.; da Silva, J.A.; Boers, M.; Burmester, G.R.; Cutolo, M.; Jacobs, J.; Kirwan, J.; Köhler, L.; Van Riel, P.; Vischer, T.; et al. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: Current questions and tentative answers in rheumatology. Ann. Rheum. Dis. 2002, 61, 718–722. [Google Scholar] [CrossRef]
- Liu, C.; Tang, J.; Chen, Y.; Zhang, Q.; Lin, J.; Wu, S.; Han, J.; Liu, Z.; Wu, C.; Zhuo, Y.; et al. Intracellular Zn2+ promotes extracellular matrix remodeling in dexamethasone-treated trabecular meshwork. Am. J. Physiol. Physiol. 2024, 326, C1293–C1307. [Google Scholar] [CrossRef]
- Blich, M.; Golan, A.; Arvatz, G.; Sebbag, A.; Shafat, I.; Sabo, E.; Cohen-Kaplan, V.; Petcherski, S.; Avniel-Polak, S.; Eitan, A.; et al. Macrophage activation by heparanase is mediated by TLR-2 and TLR-4 and associates with plaque progression. Arter. Thromb. Vasc. Biol. 2013, 33, e56–e65. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Su, C.; Chen, J.; Zhu, B.; Zhang, H.; Xiao, H.; Zhang, J. Dexamethasone ameliorates H(2)S-induced acute lung injury by alleviating matrix metalloproteinase-2 and -9 expression. PLoS ONE 2014, 9, e94701. [Google Scholar] [CrossRef]
- So, H.; Lam, T.O.; Meng, H.; Lam, S.H.M.; Tam, L.-S. Time and dose-dependent effect of systemic glucocorticoids on major adverse cardiovascular event in patients with rheumatoid arthritis: A population-based study. Ann. Rheum. Dis. 2023, 82, 1387–1393. [Google Scholar] [CrossRef]
- Rincón, I.d.; Battafarano, D.F.; Restrepo, J.F.; Erikson, J.M.; Escalante, A. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 264–272. [Google Scholar] [CrossRef]
- Alchi, M.B.; Lever, R.; Flossmann, O.; Jayne, D. Efficacy and safety of low- versus high-dose glucocorticoid regimens for induction of remission of anti-neutrophil cytoplasm antibody-associated vasculitis: A systematic review and meta-analysis. Scand. J. Rheumatol. 2023, 52, 564–573. [Google Scholar] [CrossRef]
- Giovannelli, P.; Ramaraj, P.; Williams, C. Editorial: Role of Sex Steroids and Their Receptor in Cancers. Front. Endocrinol. 2022, 13, 883229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, S.; Qi, Y.; Gong, Z.; Zhang, H.; Liang, K.; Shen, P.; Huang, Y.-Y.; Zhang, Z.; Ye, W.; et al. Stem cell-homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci. Adv. 2022, 8, eabk0011. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.H.; Shin, J.M.; Yang, H.W.; Lee, H.M.; Park, I.H. TGF-β1-induced HSP47 regulates extracellular matrix accumulation via Smad2/3 signaling pathways in nasal fibroblasts. Sci. Rep. 2019, 9, 15563. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, J.Y.; Montecchi-Palmer, M.; Mao, W.; Clark, A.F. Cross-linked actin networks (CLANs) in glaucoma. Exp. Eye Res. 2017, 159, 16–22. [Google Scholar] [CrossRef]
- Benbow, U.; Brinckerhoff, C.E. The AP-1 site and MMP gene regulation: What is all the fuss about? Matrix Biol. 1997, 15, 519–526. [Google Scholar] [CrossRef]
- Eigentler, A.; Handle, F.; Schanung, S.; Degen, A.; Hackl, H.; Erb, H.H.H.; Fotakis, G.; Hoefer, J.; Ploner, C.; Jöhrer, K.; et al. Glucocorticoid treatment influences prostate cancer cell growth and the tumor microenvironment via altered glucocorticoid receptor signaling in prostate fibroblasts. Oncogene 2024, 43, 235–247. [Google Scholar] [CrossRef]
- Meduri, G.U.; Annane, D.; Confalonieri, M.; Chrousos, G.P.; Rochwerg, B.; Busby, A.; Ruaro, B.; Meibohm, B. Pharmacological principles guiding prolonged glucocorticoid treatment in ARDS. Intensive Care Med. 2020, 46, 2284–2296. [Google Scholar] [CrossRef]
- Hardy, R.; Filer, A.; Cooper, M.S.; Parsonage, G.; Raza, K.; Hardie, D.L.; Rabbitt, E.; Stewart, P.M.; Buckley, C.D.; Hewison, M. Differential expression, function and response to inflammatory stimuli of 11β-hydroxysteroid dehydrogenase type 1 in human fibroblasts: A mechanism for tissue-specific regulation of inflammation. Arthritis Res. Ther. 2018, 8, R108. [Google Scholar] [CrossRef]
- Basarrate, S.; Monzel, A.S.; Smith, J.; Marsland, A.; Trumpff, C.; Picard, M. Glucocorticoid and adrenergic receptor distribution across human organs and tissues: A map for stress transduction. Psychosom. Med. 2022, 86, 89–98. [Google Scholar] [CrossRef]
- Reichardt, S.D.; Amouret, A.; Muzzi, C.; Vettorazzi, S.; Tuckermann, J.P.; Lühder, F.; Reichardt, H.M. The Role of Glucocorticoids in Inflammatory Diseases. Cells 2021, 10, 2921. [Google Scholar] [CrossRef]
- Doyle, C.; Nakamura, R.; Bing, R.; Rousseau, B.; Branski, R. Mycoplasma affects baseline gene expression and the response to glucocorticoids in vocal fold fibroblasts. J. Med. Microbiol. 2021, 70, 001362. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.; Hutson, I.; Tycksen, E.; Pietka, T.A.; Bauerle, K.; Harris, C.A. Role of Mineralocorticoid Receptor in Adipogenesis and Obesity in Male Mice. Endocrinology 2020, 161, bqz010. [Google Scholar] [CrossRef] [PubMed]
- Peinado-Acevedo, J.S.; Rivera-Bustamante, T.; Rivera, J.; Santamaría-Alza, Y. Balancing inflammation and adverse effects of glucocorticoids in clinical practice. Rev. Colomb. Reumatol. 2024, 31, 498–510. [Google Scholar] [CrossRef]
- Hillegass, J.M.; Villano, C.M.; Cooper, K.R.; White, L.A. Glucocorticoids alter craniofacial development and increase expression and activity of matrix metalloproteinases in developing zebrafish (Danio rerio). Toxicol. Sci. 2008, 102, 413–424. [Google Scholar] [CrossRef]
- Do, K. Speaker 1: Nuno Sousa, Portugal. Int. J. Neuropsychopharmacol. 2016, 19, 37. [Google Scholar] [CrossRef]
- Do, K. Speaker 2: Zhen Yan, USA. Int. J. Neuropsychopharmacol. 2016, 19, 37–38. [Google Scholar] [CrossRef][Green Version]
- Hashemi, E.; Akhavan, O.; Shamsara, M.; Ansari Majd, S.; Sanati, M.H.; Daliri Joupari, M.; Farmany, A. Graphene Oxide Negatively Regulates Cell Cycle in Embryonic Fibroblast Cells. Int. J. Nanomed. 2020, 15, 6201–6209. [Google Scholar] [CrossRef]
- Niland, S.; Eble, J. Hold on or Cut? Integrin- and MMP-Mediated Cell–Matrix Interactions in the Tumor Microenvironment. Int. J. Mol. Sci. 2020, 22, 238. [Google Scholar] [CrossRef]
- Akinjiyan, F.A.; Ibitoye, Z.; Zhao, P.; Shriver, L.P.; Patti, G.J.; Longmore, G.; Fuh, K. DDR2-regulated arginase activity in ovarian cancer-associated fibroblasts promotes collagen production and tumor progression. Oncogene 2023, 43, 189–201. [Google Scholar] [CrossRef]
- Liao, X.-x.; Wang, W.; Yu, B.; Tan, S. Thrombospondin-2 acts as a bridge between tumor extracellular matrix and immune infiltration in pancreatic and stomach adenocarcinomas: An integrative pan-cancer analysis. Cancer Cell Int. 2022, 22, 213. [Google Scholar] [CrossRef]
- Siddhartha, R.; Garg, M. Interplay Between Extracellular Matrix Remodeling and Angiogenesis in Tumor Ecosystem. Mol. Cancer Ther. 2023, 22, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, C.; Tong, Y.; Li, Y.; Gao, Y.; Hou, S.; Hao, M.; Han, X.; Wang, B.; Wang, Q.; et al. Novel Nonsecosteroidal Vitamin D Receptor Modulator Combined with Gemcitabine Enhances Pancreatic Cancer Therapy through Remodeling of the Tumor Microenvironment. J. Med. Chem. 2020, 64, 629–643. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kasashima, H.; Fukui, Y.; Tsujio, G.; Yashiro, M.; Maeda, K. The heterogeneity of cancer-associated fibroblast subpopulations: Their origins, biomarkers, and roles in the tumor microenvironment. Cancer Sci. 2022, 114, 16–24. [Google Scholar] [CrossRef]
- Yeo, S.-Y.; Lee, K.-W.; Sohn, I.; Kim, S.-H. Abstract LB282: RUNX2 is a master transcription factor that determines the identity of cancer-associated fibroblast subtypes with abnormal activation in the tumor microenvironment. Cancer Res. 2023, 83, LB282–LB282. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Santiago, T.; Voshaar, M.; de Wit, M.; Carvalho, P.D.; Buttgereit, F.; Cutolo, M.; Paolino, S.; Castelar Pinheiro, G.R.; Boers, M.; Da Silva, J.A.P. Patients’ and rheumatologists’ perspectives on the efficacy and safety of low-dose glucocorticoids in rheumatoid arthritis-an international survey within the GLORIA study. Rheumatol. 2021, 60, 3334–3342. [Google Scholar] [CrossRef]
- Xie, W.; Huang, H.; Li, G.; Hao, Y.; Gui, Y.; Wang, Y.; Deng, X.; Zhao, J.; Geng, Y.; Ji, L.; et al. Dynamical trajectory of glucocorticoids tapering and discontinuation in patients with rheumatoid arthritis commencing glucocorticoids with csDMARDs: A real-world data from 2009 to 2020. Ann. Rheum. Dis. 2021, 80, 997–1003. [Google Scholar] [CrossRef]
- Bihlet, A.R.; Karsdal, M.A.; Sand, J.M.; Leeming, D.J.; Roberts, M.; White, W.; Bowler, R. Biomarkers of extracellular matrix turnover are associated with emphysema and eosinophilic-bronchitis in COPD. Respir. Res. 2017, 18, 22. [Google Scholar] [CrossRef]
- Hesse, C.; Beneke, V.; Konzok, S.; Diefenbach, C.; Bülow Sand, J.M.; Rønnow, S.R.; Karsdal, M.A.; Jonigk, D.; Sewald, K.; Braun, A.; et al. Nintedanib modulates type III collagen turnover in viable precision-cut lung slices from bleomycin-treated rats and patients with pulmonary fibrosis. Respir. Res. 2022, 23, 201. [Google Scholar] [CrossRef]
- Møller, A.L.; Rasmussen, D.; Jensen, M.S.; Kresse, J.C.; Mutsaers, H.; Genovese, F.; Karsdal, M.; Nørregaard, R. MO063: A New Tool for Preclinical Research and Drug Discovery: Extracellular Matrix Remodeling Quantification in Human Precision-Cut Kidney Slices. Nephrol. Dial. Transplant. 2022, 37, gfac063.015. [Google Scholar] [CrossRef]
- Cancemi, P.; Buttacavoli, M.; Roz, E.; Feo, S. Expression of Alpha-Enolase (ENO1), Myc Promoter-Binding Protein-1 (MBP-1) and Matrix Metalloproteinases (MMP-2 and MMP-9) Reflect the Nature and Aggressiveness of Breast Tumors. Int. J. Mol. Sci. 2019, 20, 3952. [Google Scholar] [CrossRef] [PubMed]
- Trentini, A.; Manfrinato, M.C.; Castellazzi, M.; Bellini, T. Sex-Related Differences of Matrix Metalloproteinases (MMPs): New Perspectives for These Biomarkers in Cardiovascular and Neurological Diseases. J. Pers. Med. 2022, 12, 1196. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Tan, Z.; Li, W.; Zhang, H.; Liu, Y.; Yue, C. Infographic: Osteoimmunology mechanism of osteonecrosis of the femoral head. Bone Jt. Res. 2022, 11, 29–31. [Google Scholar] [CrossRef]
- He, Z.; Zhao, S.-B.; Fang, X.; E, J.-F.; Fu, H.-Y.; Song, Y.; Wu, J.-Y.; Pan, P.; Gu, L.; Xia, T.; et al. Prognostic and Predictive Value of BGN in Colon Cancer Outcomes and Response to Immunotherapy. Front. Oncol. 2022, 11, S1046. [Google Scholar] [CrossRef]
- Williams, E.L.; Stimpson, M.L.; Collins, P.L.; Enki, D.G.; Sinha, A.; Lee, R.W.; Dhanda, A.D. Development and validation of a novel bioassay to determine glucocorticoid sensitivity. Biomark. Res. 2016, 4, 26. [Google Scholar] [CrossRef]
- Arjmand, B.; Rabbani, Z.; Soveyzi, F.; Tayanloo-Beik, A.; Rezaei-Tavirani, M.; Biglar, M.; Adibi, H.; Larijani, B. Advancement of Organoid Technology in Regenerative Medicine. Regen. Eng. Transl. Med. 2023, 9, 83–96. [Google Scholar] [CrossRef]
- Spagnol, G.; Sensi, F.; Tommasi, O.D.; Marchetti, M.; Bonaldo, G.; Xhindoli, L.; Noventa, M.; Agostini, M.; Tozzi, R.; Saccardi, C. Patient Derived Organoids (PDOs), Extracellular Matrix (ECM), Tumor Microenvironment (TME) and Drug Screening: State of the Art and Clinical Implications of Ovarian Cancer Organoids in the Era of Precision Medicine. Cancers 2023, 15, 2059. [Google Scholar] [CrossRef]
- Schmidt, J.R.; Vogel, S.; Moeller, S.; Kalkhof, S.; Schubert, K.; von Bergen, M.; Hempel, U. Sulfated hyaluronic acid and dexamethasone possess a synergistic potential in the differentiation of osteoblasts from human bone marrow stromal cells. J. Cell Biochem. 2019, 120, 8706–8722. [Google Scholar] [CrossRef]
- Cheah, J.T.L.; Black, R.J.; Robson, J.C.; Navarro-Millan, I.Y.; Young, S.R.; Richards, P.; Beard, S.; Simon, L.S.; Goodman, S.M.; Mackie, S.L.; et al. Toward a Core Domain Set for Glucocorticoid Impact in Inflammatory Rheumatic Diseases: The OMERACT 2018 Glucocorticoid Impact Working Group. J. Rheumatol. 2019, 46, 1179–1182. [Google Scholar] [CrossRef]
- Gu, Y.C.; Talts, J.F.; Gullberg, D.; Timpl, R.; Ekblom, M. Glucocorticoids down-regulate the extracellular matrix proteins fibronectin, fibulin-1 and fibulin-2 in bone marrow stroma. Eur. J. Haematol. 2001, 67, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Kang, W.; Park, S.; Son, B.; Park, T. Identification of Glucocorticoid Receptor Target Genes That Potentially Inhibit Collagen Synthesis in Human Dermal Fibroblasts. Biomolecules 2023, 13, 978. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; Bravo-Osuna, I.; Subias, M.; Montolío, A.; Cegoñino, J.; Martinez-Rincón, T.; Mendez-Martinez, S.; Aragón-Navas, A.; Garcia-Herranz, D.; Pablo, L.E.; et al. Tunable degrees of neurodegeneration in rats based on microsphere-induced models of chronic glaucoma. Sci. Rep. 2022, 12, 20622. [Google Scholar] [CrossRef]
- Gu, X.; Ge, L.; Ren, B.; Fang, Y.; Li, Y.; Wang, Y.; Xu, H. Glucocorticoids Promote Extracellular Matrix Component Remodeling by Activating YAP in Human Retinal Capillary Endothelial Cells. Front. Cell Dev. Biol. 2021, 9, 738341. [Google Scholar] [CrossRef]
- Sorrentino, G.; Ruggeri, N.; Zannini, A.; Ingallina, E.; Bertolio, R.; Marotta, C.; Neri, C.; Cappuzzello, E.; Forcato, M.; Rosato, A.; et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat. Commun. 2017, 8, 14073. [Google Scholar] [CrossRef]
- Micheroli, R.; Khmelevskaya, A.; Pauli, C.; Vallejo-Yagüe, E.; Elhai, M.; Buerki, K.; Distler, O.; Ciurea, A.; Ospelt, C. POS1011 Different Synovial Macrophage and Fibroblast Subsets, Expression Profiles and Cell-Cell Interactions Characterise Sex Differences in Chronic Inflammatory Joint Diseases. Ann. Rheum. Dis. 2023, 82, 821–822. [Google Scholar] [CrossRef]
- Sciacca, E.; Alaimo, S.; Pulvirenti, A.; Latora, V.; Humby, F.; Ferro, A.; Lewis, M.; Pitzalis, C. P22 Micro-RNA enriched pathway impact analysis applied to synovial RNA-seq in early rheumatoid arthritis identifies response prediction pathways. Rheumatology 2020, 59, keaa111.021. [Google Scholar] [CrossRef]
- Sciacca, E.; Surace, A.E.A.; Alaimo, S.; Pulvirenti, A.; Rivellese, F.; Goldmann, K.; Ferro, A.; Latora, V.; Pitzalis, C.; Lewis, M. Network analysis of synovial RNA sequencing identifies gene-gene interactions predictive of response in rheumatoid arthritis. Arthritis Res. Ther. 2022, 24, 116. [Google Scholar] [CrossRef]
- Nielsen, S.H.; Bay-Jensen, A.; Frederiksen, P.; Karsdal, M.; Shawi, M.; Chakravarty, S.; Kollmeier, A.; Chen, W.; Gao, S. POS0227 Serum Extracellular Matrix Biomarkers Identify Response to Guselkumab in Psoriatic Arthritis: Post-Hoc Analysis from a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study Through 2 Years. Ann. Rheum. Dis. 2023, 82, 342–343. [Google Scholar] [CrossRef]
- Abernethie, A.J.; Gastaldello, A.; Maltese, G.; Morgan, R.A.; McInnes, K.J.; Small, G.R.; Walker, B.R.; Livingstone, D.E.; Hadoke, P.W.; Andrew, R. Comparison of mechanisms of angiostasis caused by the anti-inflammatory steroid 5α-tetrahydrocorticosterone versus conventional glucocorticoids. Eur. J. Pharmacol. 2022, 929, 175111. [Google Scholar] [CrossRef]
- Shi, Y.; Pu, K.; Yao, H.; Chen, Y.; Zheng, X.; Zhao, L.; Ma, X.; Ge, C. Gold Nanorods Inhibit Tumor Metastasis by Regulating MMP-9 Activity: Implications for Radiotherapy. ACS Appl. Mater. Interfaces 2023, 15, 9034–9043. [Google Scholar] [CrossRef]
- Kim, I.-S.; Yang, W.-S.; Kim, C.-H. Physiological Properties, Functions, and Trends in the Matrix Metalloproteinase Inhibitors in Inflammation-Mediated Human Diseases. Curr. Med. Chem. 2023, 30, 2075–2112. [Google Scholar] [CrossRef]
- Mohammadi, S.; Ebadpour, M.R.; Sedighi, S.; Saeedi, M.; Memarian, A. Glucocorticoid-induced leucine zipper expression is associated with response to treatment and immunoregulation in systemic lupus erythematosus. Clin. Rheumatol. 2017, 36, 1765–1772. [Google Scholar] [CrossRef]
- Lamb, C.A.; Saifuddin, A.; Powell, N.; Rieder, F. The Future of Precision Medicine to Predict Outcomes and Control Tissue Remodeling in Inflammatory Bowel Disease. Gastroenterology 2022, 162, 1525–1542. [Google Scholar] [CrossRef]
- Parker, A.L.; Bowman, E.; Zingone, A.; Ryan, B.M.; Cooper, W.A.; Kohonen-Corish, M.; Harris, C.C.; Cox, T.R. Extracellular matrix profiles determine risk and prognosis of the squamous cell carcinoma subtype of non-small cell lung carcinoma. Genome Med. 2022, 14, 126. [Google Scholar] [CrossRef]
- Qiu, L.; Kang, D.; Wang, C.; Guo, W.; Fu, F.; Wu, Q.; Xi, G.; He, J.; Zheng, L.; Zhang, Q.; et al. Intratumor graph neural network recovers hidden prognostic value of multi-biomarker spatial heterogeneity. Nat. Commun. 2022, 13, 4250. [Google Scholar] [CrossRef]
- Li, Z.A.; Shang, J.; Xiang, S.; Li, E.N.; Yagi, H.; Riewruja, K.; Lin, H.; Tuan, R.S. Articular Tissue-Mimicking Organoids Derived from Mesenchymal Stem Cells and Induced Pluripotent Stem Cells. Organoids 2022, 1, 135–148. [Google Scholar] [CrossRef]
- Heimer, B.W.; Tam, B.E.; Minkovsky, A.; Sikes, H.D. Using nanobiotechnology to increase the prevalence of epigenotyping assays in precision medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1407. [Google Scholar] [CrossRef]
- El-Sharkawy, A.; Atef, S.; Abdel-Majeed, A.; Eldaly, U.; Elsherbiny, E.S.; Metwally, F.M.; El-Mezayen, H. Circulating Tumor Cells in Breast Cancer: A Step Toward Precision Medicine for Real-Time Monitoring of Metastasis. Asian Pac. J. Cancer Prev. 2023, 24, 1725–1730. [Google Scholar] [CrossRef]
- Malda, J.; Boere, J.; van de Lest, C.H.; van Weeren, P.; Wauben, M.H. Extracellular vesicles—New tool for joint repair and regeneration. Nat. Rev. Rheumatol. 2016, 12, 243–249. [Google Scholar] [CrossRef]
- Iosef, C.; Martin, C.M.; Slessarev, M.; Gillio-Meina, C.; Cepinskas, G.; Han, V.K.M.; Fraser, D.D. COVID-19 plasma proteome reveals novel temporal and cell-specific signatures for disease severity and high-precision disease management. J. Cell Mol. Med. 2023, 27, 141–157. [Google Scholar] [CrossRef]
- Statzer, C.; Luthria, K.; Sharma, A.; Kann, M.G.; Ewald, C.Y. The Human Extracellular Matrix Diseasome Reveals Genotype-Phenotype Associations with Clinical Implications for Age-Related Diseases. Biomedicines 2023, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jang, J.; Cho, D.W. Recapitulating the Cancer Microenvironment Using Bioprinting Technology for Precision Medicine. Micromachines 2021, 12, 1122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).