Exosomal Protein Biomarkers in Arthritis: Deciphering the Inflammatory Profiles of RA and OA †
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Blood Samples
2.3. Exosome Isolation
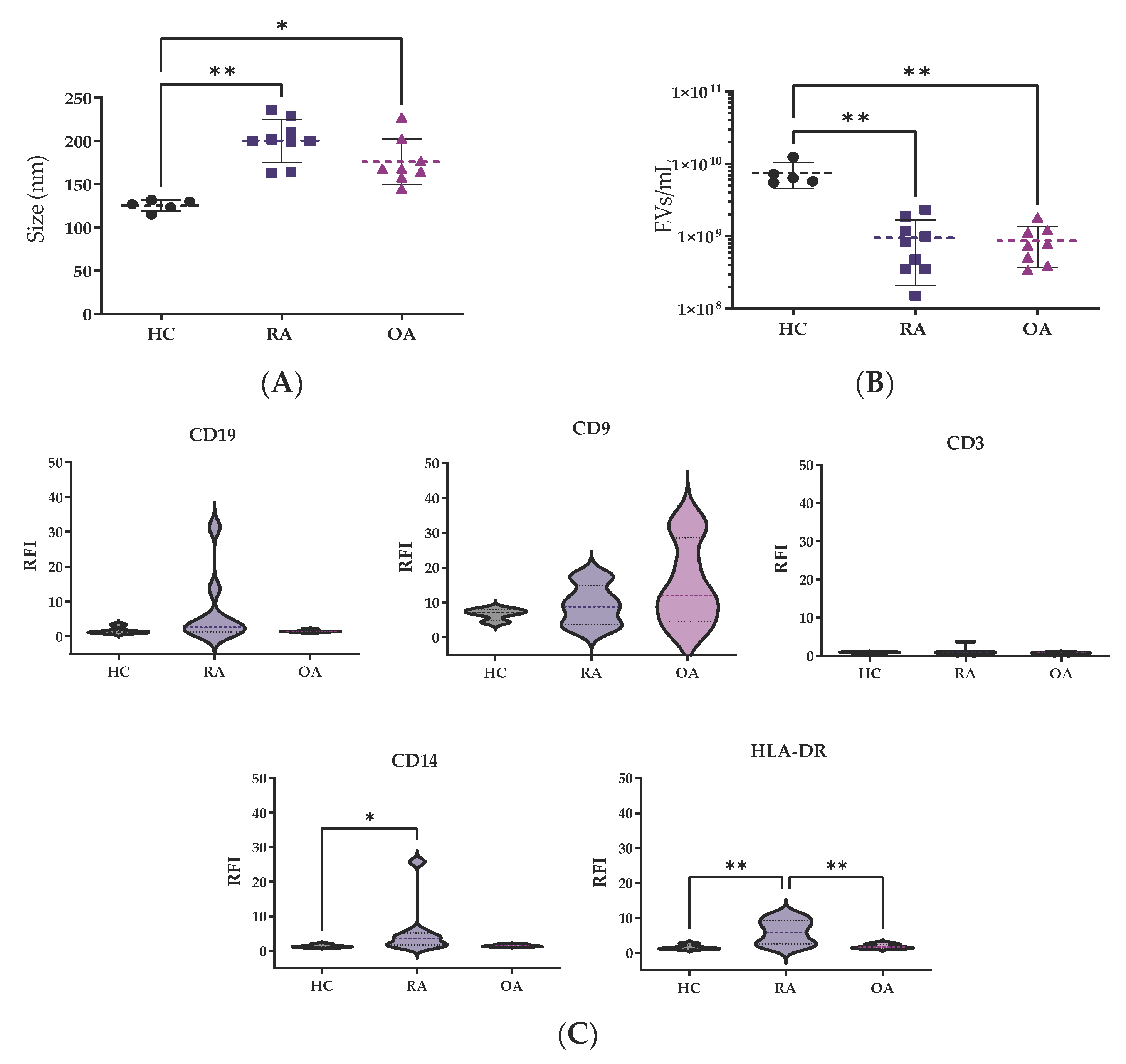
2.4. Nanotracking Analysis
2.5. Flow Cytometry
2.6. Exosome Concentration and Lysis
2.7. Mass Spectrometry
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Matteo, A.D.; Bathon, J.M.; Emery, P. Rheumatoid arthritis. Lancet 2023, 402, 2019–2033. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Peat, G.; Bierma-Zeinstra, M.A.; Arden, N.K.; Bresnihan, B.; Herrero-Beaumont, G.; Kirschner, S.; Leeb, B.F.; Lohmander, L.S.; et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 483–489. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Moseng, T.; Vlieland, T.P.M.V.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update. Ann. Rheum. Dis. 2024, 83, 730–740. [Google Scholar] [CrossRef]
- Mazières, B.; Bannwarth, B.; Dougados, M.; Lequesne, M. EULAR recommendations for the management of knee osteoarthritis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials1. Jt. Bone Spine 2001, 68, 231–240. [Google Scholar] [CrossRef]
- Saraiva, L.; Duarte, C. Barriers to the Diagnosis of Early Inflammatory Arthritis: A Literature Review. Open Access Rheumatol. Res. Rev. 2023, 15, 11–22. [Google Scholar] [CrossRef]
- Burgers, L.E.; Raza, K.; van der Helm-van Mil, A.H. Window of opportunity in rheumatoid arthritis—definitions and supporting evidence: From old to new perspectives. RMD Open 2019, 5, e000870. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Buzás, E.I.; Tóth, E.Á.; Sódar, B.W.; Szabó-Taylor, K.É. Molecular interactions at the surface of extracellular vesicles. Semin. Immunopathol. 2018, 40, 453–464. [Google Scholar] [CrossRef]
- LeClaire, M.; Gimzewski, J.; Sharma, S. A review of the biomechanical properties of single extracellular vesicles. Nano Sel. 2021, 2, 1–15. [Google Scholar] [CrossRef]
- Allenson, K.; Castillo, J.; San Lucas, F.A.; Scelo, G.; Kim, D.U.; Bernard, V.; Davis, G.; Kumar, T.; Katz, M.; Overman, M.J.; et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 2017, 28, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Pang, Y.; Wang, Q.; Qin, W.; Wei, C.; Li, Y.; Li, T.; Li, F.; Wang, Q.; Li, Y.; et al. Proteomic profiling of circulating plasma exosomes reveals novel biomarkers of Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 181. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Navajas, R.; Corrales, F.J.; Paradela, A. Serum Exosome Isolation by Size-Exclusion Chromatography for the Discovery and Validation of Preeclampsia-Associated Biomarkers. Methods Mol. Biol. 2019, 1959, 39–50. [Google Scholar] [CrossRef]
- Hernández-Breijo, B.; Navajas, R.; Brenis, C.M.; Plascencia-Rodríguez, C.; Martínez-Feito, A.; Benavent, D.; Bogas, P.; Tornero, C.; Novella-Navarro, M.; Calvo-Aranda, E.; et al. Protein cargo of serum exosomes reveals the different inflammatory mechanisms between rheumatoid arthritis and osteoarthritis. Reumatol. Clin. 2022, 18, 40–89. Available online: https://www.reumatologiaclinica.org/es-vol-18-num-sc2-sumario-X1699258X22X00C20 (accessed on 16 March 2025).
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Marton, N.; Kovács, O.T.; Baricza, E.; Kittel, Á.; Győri, D.; Mócsai, A.; Meier, F.M.P.; Goodyear, C.S.; McInnes, I.B.; Buzás, E.I.; et al. Extracellular vesicles regulate the human osteoclastogenesis: Divergent roles in discrete inflammatory arthropathies. Cell. Mol. Life Sci. CMLS 2017, 74, 3599–3611. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Green, J.; Bodogai, M.; Mode, N.A.; Bæk, R.; Jørgensen, M.M.; Freeman, D.W.; Witwer, K.W.; Zonderman, A.B.; Biragyn, A.; et al. Age-Related Changes in Plasma Extracellular Vesicle Characteristics and Internalization by Leukocytes. Sci. Rep. 2017, 7, 1342. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Perez-Hernandez, D.; Gutiérrez-Vázquez, C.; Jorge, I.; López-Martín, S.; Ursa, A.; Sánchez-Madrid, F.; Vázquez, J.; Yáñez-Mó, M. The Intracellular Interactome of Tetraspanin-enriched Microdomains Reveals Their Function as Sorting Machineries toward Exosomes. J. Biol. Chem. 2013, 288, 11649–11661. [Google Scholar] [CrossRef]
- Cloutier, N.; Tan, S.; Boudreau, L.H.; Cramb, C.; Subbaiah, R.; Lahey, L.; Albert, A.; Shnayder, R.; Gobezie, R.; Nigrovic, P.A.; et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: The microparticle-associated immune complexes. EMBO Mol. Med. 2013, 5, 235–249. [Google Scholar] [CrossRef]
- Burbano, C.; Rojas, M.; Muñoz-Vahos, C.; Vanegas-García, A.; Correa, L.A.; Vásquez, G.; Castaño, D. Extracellular vesicles are associated with the systemic inflammation of patients with seropositive rheumatoid arthritis. Sci. Rep. 2018, 8, 17917. [Google Scholar] [CrossRef]
- Bresnihan, B.; Gogarty, M.; FitzGerald, O.; Dayer, J.-M.; Burger, D. Apolipoprotein A-I infiltration in rheumatoid arthritis synovial tissue: A control mechanism of cytokine production? Arthritis Res. Ther. 2004, 6, R563. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, M.J.; Choi, J.Y.; Park, J.S.; Park, J.K.; Lee, E.Y.; Lee, E.B.; Pap, T.; Yi, E.C.; Song, Y.W. Apolipoprotein B binds to enolase-1 and aggravates inflammation in rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Soldano, S.; Paolino, S. Potential roles for tenascin in (very) early diagnosis and treatment of rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, e42. [Google Scholar] [CrossRef]
- Page, T.H.; Charles, P.J.; Piccinini, A.M.; Nicolaidou, V.; Taylor, P.C.; Midwood, K.S. Raised circulating tenascin-C in rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R260. [Google Scholar] [CrossRef]
- van Beers, J.J.; Willemze, A.; Stammen-Vogelzangs, J.; Drijfhout, J.W.; Toes, R.E.; Pruijn, M.G.J. Anti-citrullinated fibronectin antibodies in rheumatoid arthritis are associated with human leukocyte antigen-DRB1 shared epitope alleles. Arthritis Res. Ther. 2012, 14, R35. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | Rheumatoid Arthritis (n = 9) | Osteoarthritis (n = 8) | p-Value |
|---|---|---|---|
| Female, n (%) | 8 (89) | 2 (25) | <0.01 |
| Age (yr) | 60 ± 11 | 68 ± 11 | 0.1 |
| BMI (kg/m2) | 30.0 ± 5.3 | 31.0 ± 2.0 | 0.9 |
| Disease duration (yr) | 5 (1–13) | 0 (0–17) | 0.4 |
| CRP (mg/dL) | 5.0 (4.1–92.3) | 6.6 (4.4–43.6) | 0.8 |
| Smokers, n (%) | 4 (44) | 5 (62) | 0.6 |
| Seropositivity, n (%) * | 8 (89) | ||
| RF titer (AU/mL) | 127 (34–465) | ||
| ACPA titer (AU/mL) | 300 (191–748) |
| Increased in OA (Gene Symbol) | Increased in RA (Gene Symbol) | Log2 (RA/OA) | q-Value |
|---|---|---|---|
| ANXA2 | −7.6 | <0.05 | |
| FLG | −6.5 | <0.05 | |
| FABP5 | −6.2 | <0.1 | |
| SPRR1B | −5.0 | <0.1 | |
| ASPRV1 | −4.7 | <0.1 | |
| SFN | −4.7 | <0.05 | |
| JUP | −4.2 | <0.1 | |
| DSG1 | −4.0 | <0.1 | |
| KRT5 | −3.6 | <0.05 | |
| IGHV1-8 | −3.5 | <0.1 | |
| S100A7 | −3.4 | <0.1 | |
| IGKV1D-13 | −3.3 | <0.1 | |
| TUBA1A | −3.1 | <0.1 | |
| DSP | −2.7 | <0.05 | |
| KRT1 | −1.3 | <0.01 | |
| A2M | 0.4 | <0.1 | |
| APOB | 0.4 | <0.01 | |
| FN1 | 0.6 | <0.05 | |
| APOA1 | 0.8 | <0.1 | |
| PROS1 | 1.0 | <0.05 | |
| C4BPA | 1.1 | <0.01 | |
| CLU | 1.2 | <0.1 | |
| F2 | 1.4 | <0.01 | |
| C1S | 1.5 | <0.1 | |
| FCGBP | 1.5 | <0.01 | |
| TNC | 1.6 | <0.1 | |
| SERPINC1 | 1.6 | <0.01 | |
| C1R | 1.7 | <0.01 | |
| VTN | 1.8 | <0.05 | |
| LRP1 | 1.9 | <0.05 | |
| SERPINA1 | 1.9 | <0.1 | |
| HRG | 2.0 | <0.05 | |
| SERPINA3 | 2.4 | <0.1 | |
| MBL2 | 3.0 | <0.1 | |
| PCSK9 | 3.4 | <0.05 | |
| FGB | 3.4 | <0.01 | |
| FGG | 3.6 | <0.05 | |
| SAA2-SAA4 | 3.7 | <0.05 | |
| IGLV3-9 | 3.7 | <0.1 | |
| PZP | 3.8 | <0.05 | |
| IGHV4-34 | 3.9 | <0.1 | |
| SAA2 | 4.6 | <0.1 | |
| SAA1 | 4.7 | <0.01 | |
| IGKV6-21 | 4.7 | <0.1 | |
| LRP10 | 5.4 | <0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brenis Gómez, C.M.; Plasencia-Rodríguez, C.; Novella-Navarro, M.; Martínez-Feito, A.; Balsa, A.; Calvo-Aranda, E.; Hernández-Breijo, B. Exosomal Protein Biomarkers in Arthritis: Deciphering the Inflammatory Profiles of RA and OA. Biomedicines 2025, 13, 1283. https://doi.org/10.3390/biomedicines13061283
Brenis Gómez CM, Plasencia-Rodríguez C, Novella-Navarro M, Martínez-Feito A, Balsa A, Calvo-Aranda E, Hernández-Breijo B. Exosomal Protein Biomarkers in Arthritis: Deciphering the Inflammatory Profiles of RA and OA. Biomedicines. 2025; 13(6):1283. https://doi.org/10.3390/biomedicines13061283
Chicago/Turabian StyleBrenis Gómez, Claudia M., Chamaida Plasencia-Rodríguez, Marta Novella-Navarro, Ana Martínez-Feito, Alejandro Balsa, Enrique Calvo-Aranda, and Borja Hernández-Breijo. 2025. "Exosomal Protein Biomarkers in Arthritis: Deciphering the Inflammatory Profiles of RA and OA" Biomedicines 13, no. 6: 1283. https://doi.org/10.3390/biomedicines13061283
APA StyleBrenis Gómez, C. M., Plasencia-Rodríguez, C., Novella-Navarro, M., Martínez-Feito, A., Balsa, A., Calvo-Aranda, E., & Hernández-Breijo, B. (2025). Exosomal Protein Biomarkers in Arthritis: Deciphering the Inflammatory Profiles of RA and OA. Biomedicines, 13(6), 1283. https://doi.org/10.3390/biomedicines13061283






