Advances in the Treatment of Acute Myeloid Leukemia: Implications for Low- and Middle-Income Countries
Abstract
1. Introduction
2. Diagnostic Work up and Risk Stratification
2.1. Molecular Analysis
2.1.1. Molecular Testing Disparities in AML
2.1.2. Testing Strategies for AML in Low-Resource Environments
| Diagnostic Modality | Common LMIC Limitation | Impact on Care/Recommendations |
|---|---|---|
| Morphology | Variable expertise, inconsistent quality control | Risk of misdiagnosis or delayed diagnosis. Inadequate assessment may hinder appropriate treatment selection. Standardization of morphology training and quality control is essential. |
| Flow Cytometry [30,31] | Limited access to lab centers, high implementation cost, need for trained personnel, difficulty with disease monitoring | Challenges in MRD assessment and risk stratification; limited ability to predict outcomes. If FCM is unavailable, immunophenotyping can be performed using IHC. |
| Cytogenetics | Unavailability, high cost, and limited lab infrastructure and expertise | Inability to perform standard risk stratification; delays when tests must be outsourced. IHC may be used to screen NPM1 and TP53 mutations, pending validation. |
| Molecular Testing [15,16] | Limited access to reagents, equipment, trained personnel, high cost | Inability to identify actionable mutations or perform risk stratification, treatment delays. If NGS is unavailable, use PCR or FISH to detect key mutations or translocations. IHC may be used to screen NPM1 and TP53, pending validation. |
3. Frontline Treatment Strategies
3.1. Standard Induction Chemotherapy
3.2. Alternative Intensive Chemotherapy Regimens
3.2.1. Mutation-Targeted Intensification Strategies
FLT3-Mutated AML
Venetoclax Intensive Combinations
3.3. Are Intensive Chemotherapy Regimens Feasible Across All Resource Settings?
4. Post-Remission Consolidation Therapy
4.1. Allogeneic Stem Cell Transplantation
4.2. Allogeneic Stem Cell Transplant in LMICs
4.2.1. Health System and Logistical Barriers to Access Allo-SCT
4.2.2. Disparities in Donor Availability for Allogeneic Transplantation
4.2.3. Adapting Stem Cell Transplantation to Low-Resource Settings
5. Patients Ineligible for Intensive Treatment
5.1. Targeted Therapies and Mutation-Driven Approaches
5.1.1. Venetoclax-Based Treatments
Venetoclax Dosing
5.1.2. Emerging Venetoclax Combinations Under Investigation
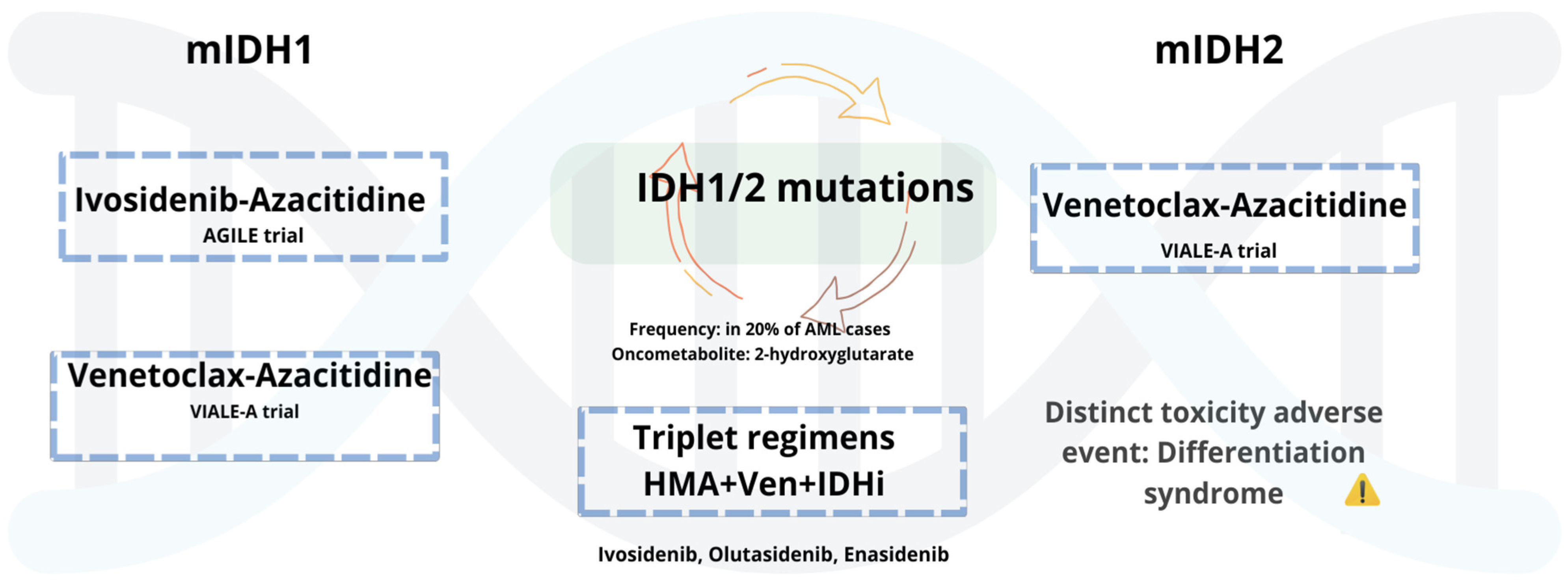
5.1.3. AML with Mutant IDH1/2
5.1.4. Combinations and Emerging Therapeutic Pathways of Mutated IDH-AML
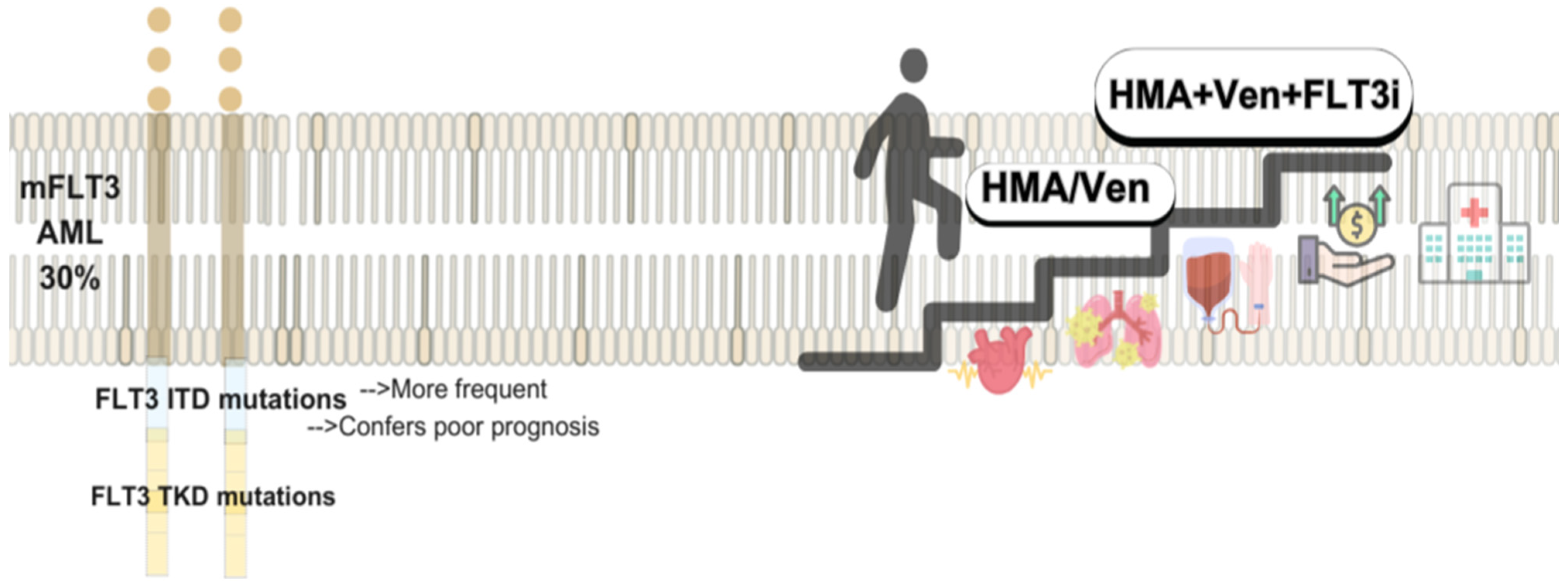
5.2. FLT Mutated AML and FLIT Inhibitors: Low Intensity and Maintenance Therapies
Combinations and Emerging Therapeutic Pathways of mFLT3-AML
6. Biology-Driven Therapeutic Advances: CAR-T Cell Therapy, Menin Inhibitors, and Targeted Strategies for High-Risk Population
7. Alternatives in Constrained Settings for AML Patients with Targetable Mutations
Access to Clinical Trials in LMICs
8. Strategies to Reduce Costs in AML: Our Approach
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, Y.; Huang, G.; Cai, X.; Liu, Y.; Qian, B.; Li, D. Global, regional, and national burden of acute myeloid leukemia, 1990–2021: A systematic analysis for the global burden of disease study 2021. Biomark. Res. 2024, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Nong, T.; Mehra, S.; Taylor, J. Common Driver Mutations in AML: Biological Impact, Clinical Considerations, and Treatment Strategies. Cells 2024, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Jani, C.T.; Ahmed, A.; Singh, H.; Mouchati, C.; Al Omari, O.; Bhatt, P.S.; Sharma, R.; Farooq, M.; Liu, W.; Shalhoub, J.; et al. Burden of AML, 1990-2019: Estimates from the Global Burden of Disease Study. JCO Glob. Oncol. 2023, 9, e2300229. [Google Scholar] [CrossRef] [PubMed]
- Gómez-De León, A.; Demichelis-Gómez, R.; Da Costa-Neto, A.; Gómez-Almaguer, D.; Rego, E.M. Acute myeloid leukemia: Challenges for diagnosis and treatment in Latin America. Hematology 2023, 28, 2158015. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.Z.; Owaidah, T.; Al Sharif, F.; Ahmed, S.Y.; Chaudhri, N.; Aljurf, M. The challenge of risk stratification in acute myeloid leukemia with normal karyotype. Hematol. Oncol. Stem Cell Ther. 2008, 1, 141–158. [Google Scholar] [CrossRef]
- Grimwade, D.; Walker, H.; Oliver, F.; Wheatley, K.; Harrison, C.; Harrison, G.; Rees, J.; Hann, I.; Stevens, R.; Burnett, A. The Importance of Diagnostic Cytogenetics on Outcome in AML: Analysis of 1612 Patients Entered Into the MRC AML 10 Trial. Blood 1998, 92, 2322–2333. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Duncavage, E.J.; Bagg, A.; Hasserjian, R.P.; DiNardo, C.D.; Godley, L.A.; Iacobucci, I.; Jaiswal, S.; Malcovati, L.; Vannucchi, A.M.; Patel, K.P.; et al. Genomic profiling for clinical decision making in myeloid neoplasms and acute leukemia. Blood 2022, 140, 2228–2247. [Google Scholar] [CrossRef]
- Rausch, C.; Rothenberg-Thurley, M.; Dufour, A.; Schneider, S.; Gittinger, H.; Sauerland, C.; Görlich, D.; Krug, U.; Berdel, W.E.; Woermann, B.J.; et al. Validation and refinement of the 2022 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia 2023, 37, 1234–1244. [Google Scholar] [CrossRef]
- Döhner, H.; DiNardo, C.D.; Appelbaum, F.; Craddock, C.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; Larson, R.A.; et al. Genetic risk classification for adults with AML receiving less-intensive therapies: The 2024 ELN recommendations. Blood J. 2024, 144, 2169–2173. [Google Scholar] [CrossRef]
- Nawas, M.T.; Kosuri, S. Utility or futility? A contemporary approach to allogeneic hematopoietic cell transplantation for TP53- mutated MDS/AML. Blood Adv. 2024, 8, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Grob, T.; Al Hinai, A.S.A.; Sanders, M.A.; Kavelaars, F.G.; Rijken, M.; Gradowska, P.L.; Biemond, B.J.; Breems, D.A.; Maertens, J.; Van Marwijk Kooy, M.; et al. Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood 2022, 139, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Mareschal, S.; Palau, A.; Lindberg, J.; Ruminy, P.; Nilsson, C.; Bengtzén, S.; Engvall, M.; Eriksson, A.; Neddermeyer, A.; Marchand, V.; et al. Challenging conventional karyotyping by next-generation karyotyping in 281 intensively treated patients with AML. Blood Adv. 2021, 5, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Duncavage, E.J.; Schroeder, M.C.; O’Laughlin, M.; Wilson, R.; MacMillan, S.; Bohannon, A.; Kruchowski, S.; Garza, J.; Du, F.; Hughes, A.E.O.; et al. Genome Sequencing as an Alternative to Cytogenetic Analysis in Myeloid Cancers. N. Engl. J. Med. 2021, 384, 924–935. [Google Scholar] [CrossRef]
- Bacher, U.; Shumilov, E.; Flach, J.; Porret, N.; Joncourt, R.; Wiedemann, G.; Fiedler, M.; Novak, U.; Amstutz, U.; Pabst, T. Challenges in the introduction of next-generation sequencing (NGS) for diagnostics of myeloid malignancies into clinical routine use. Blood Cancer J. 2018, 8, 113. [Google Scholar] [CrossRef]
- Traina, F.; Figueiredo-Pontes, L.L.; Ayrosa, M.I.; Pagnano, K.B.; Gloria, A.B.F.; Velloso, E.D.R.P.; Nunes, E.C.; Higashi, M.; Duarte, B.K.L.; Fagundes, E.M.; et al. Feasibility and Relevance of Incorporating Genetic Testing by Next-Generation Sequencing for Acute Myeloid Leukemia Patients Treated in Low- and Middle-Income Countries. Blood 2023, 142 (Suppl. 1), 2929. [Google Scholar] [CrossRef]
- Mencia Trinchant, N.; Kohlschmidt, J.; Gomez-Arteaga, A.; Byrd, J.C.; Stock, W.; Mrózek, K.; Carroll, A.J.; Stone, R.M.; Pacheco, E.; Martinez Tovar, A.; et al. Molecular Landscape in Acute Myeloid Leukemia (AML) Patients (pts) from Mexico As an Initial Study to Identify Healthcare Disparities in Hispanic Populations: Alliance for Clinical Trials in Oncology [Alliance]. Blood 2022, 140 (Suppl. 1), 11009–11010. [Google Scholar] [CrossRef]
- Borges Da Silva, F.; De Figueiredo-Pontes, L.L.; Madeira, M.I.A.; Corrêa De Araujo Koury, L.; Gouvêa De Lima, A.S.; Santos Scheucher, P.; Coelho-Silva, J.L.; Pagnano, K.B.B.; Pallota, R.; De Lourdes Chauffaille, M.; et al. The application of an integrated clinical, cytogenetic, and molecular risk stratification for acute myeloid leukemia patients using a central laboratory in a Brazilian multicentric study. Blood Adv. 2017, 1, 86–89. [Google Scholar] [CrossRef]
- Demichelis-Gómez, R.; Zapata-Canto, N.; Leyto-Cruz, F.; Terreros-Muñoz, E.; Carrillo, A.; Montaño-Figueroa, E.; Solís-Poblano, J.C.; Colunga-Pedraza, P.; Díaz-Vargas, G.; Amador-Medina, L.F.; et al. Acute Myeloid Leukemia in Mexico: The Specific Challenges of a Developing Country. Results from a Multicenter National Registry. Clin. Lymphoma Myeloma Leuk. 2020, 20, e295–e303. [Google Scholar] [CrossRef]
- Campos, C.C.D.A.P.; Teixeira, L.; Salvino, M.A. 10-year real-world data on acute myeloid leukemia: The paradigm of a public health center in Brazil. J. Clin. Oncol. 2023, 41 (Suppl. 16), e19022. [Google Scholar] [CrossRef]
- Gálvez-Cárdenas, K.M.; Enciso-Olivera, L.J.; Samanez-Figari, C.A.; Quintana-Truyenque, S.; Castillo-Ríos, B.A.; Quintero-Vega, G.E.; Arrieta-López, E.; Pinto-Gómez, A.J.; Aruachan-Vesga, S.; Durán-Sánchez, M.I.; et al. Treatment patterns and clinical outcomes in acute myeloid leukemia patients who are not eligible for intensive induction chemotherapy: A real-world study from Latin-America. Med. Lab. 2023, 27, 315–332. [Google Scholar] [CrossRef]
- Sossa-Melo, C.; Abello-Polo, V.; Salazar, L.A.; Peña, A.M.; Luna-González, M.; Cuervo-Lozada, D.; Quintero-Vega, G.E.; Daza, J.; Omaña-Orduz, O.P.; Mantilla, W.; et al. Characteristics, outcomes and treatment patterns in acute myeloid leukemia patients 60 years or older in Colombia: A RENEHOC-PETHEMA study. Ann. Hematol. 2025, 104, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, I.; Naser, R.; Abarca, M. Sobrevida en pacientes con leucemia mieloide aguda: Análisis de sobrevida en 120 pacientes con leucemia mieloide aguda con enfoque en pacientes mayores de 60 años del Hospital Sótero del Río. Rev. Médica Chile 2024, 152, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A.; George, T.I.; Abedi, M.; Bejar, R.; Cogle, C.R.; Foucar, K.; Garcia-Manero, G.; Grinblatt, D.L.; Komrokji, R.S.; Maciejewski, J.P.; et al. Diagnostic and molecular testing patterns in patients with newly diagnosed acute myeloid leukemia in the Connect® MDS/AML Disease Registry. eJHaem 2020, 1, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, J.; Capo-Chichi, J.-M.; Tsui, H.; Mahe, E.; Berardi, P.; Minden, M.D.; Brandwein, J.M.; Schuh, A.C. The Clinical Utility of FLT3 Mutation Testing in Acute Leukemia: A Canadian Consensus. Curr. Oncol. 2023, 30, 10410–10436. [Google Scholar] [CrossRef]
- Labrador, J.; Martínez-Cuadrón, D.; Boluda, B.; Serrano, J.; Gil, C.; Pérez-Simón, J.A.; Bernal, T.; Bergua, J.M.; Martínez-López, J.; Rodríguez-Medina, C.; et al. Evolving patterns and clinical outcome of genetic studies performed at diagnosis in acute myeloid leukemia patients: Real life data from the PETHEMA Registry. Cancer 2024, 130, 3436–3451. [Google Scholar] [CrossRef]
- Woolthuis, C.M.; Mulder, A.B.; Verkaik-Schakel, R.N.; Rosati, S.; Diepstra, A.; Van Den Berg, E.; Schuringa, J.J.; Vellenga, E.; Kluin, P.M.; Huls, G. A single center analysis of nucleophosmin in acute myeloid leukemia: Value of combining immunohistochemistry with molecular mutation analysis. Haematologica 2013, 98, 1532–1538. [Google Scholar] [CrossRef]
- Fitzpatrick, M.J.; Boiocchi, L.; Fathi, A.T.; Brunner, A.M.; Hasserjian, R.P.; Nardi, V. Correlation of p53 immunohistochemistry with TP53 mutational status and overall survival in newly diagnosed acute myeloid leukaemia. Histopathology 2022, 81, 496–510. [Google Scholar] [CrossRef]
- Chopra, A.; Soni, S.; Pati, H.; Kumar, D.; Diwedi, R.; Verma, D.; Vishwakama, G.; Bakhshi, S.; Kumar, S.; Gogia, A.; et al. Nucleophosmin mutation analysis in acute myeloid leukaemia: Immunohistochemistry as a surrogate for molecular techniques. Indian J. Med. Res. 2016, 143, 763–768. [Google Scholar] [CrossRef]
- Ross, A.; Rudd, D.; Wight, J. Low flow: Selecting a limited flow cytometry panel where resources are constrained. Blood Rev. 2025, 101284. [Google Scholar] [CrossRef]
- Belkina, A.C.; Roe, C.E.; Tang, V.A.; Back, J.B.; Bispo, C.; Conway, A.; Chakraborty, U.; Daniels, K.T.; De La Cruz, G.; Ferrer-Font, L.; et al. Guidelines for establishing a cytometry laboratory. Cytometry A 2024, 105, 88–111. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.M. The “7+3” regimen in acute myeloid leukemia. Haematologica 2022, 107, 3. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef]
- Teuffel, O.; Leibundgut, K.; Lehrnbecher, T.; Alonzo, T.A.; Beyene, J.; Sung, L. Anthracyclines during induction therapy in acute myeloid leukaemia: A systematic review and meta-analysis. Br. J. Haematol. 2013, 161, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Uy, G.L.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; Bixby, D.L.; et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021, 8, e481–e491. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.D.; Thomas, A.; Thomas, I.; Hills, R.K.; Vyas, P.; Gilkes, A.; Metzner, M.; Jakobsen, N.A.; Kennedy, A.; Moore, R.; et al. Fractionated vs single-dose gemtuzumab ozogamicin with determinants of benefit in older patients with AML: The UK NCRI AML18 trial. Blood 2023, 142, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef]
- Larson, R.A.; Mandrekar, S.J.; Huebner, L.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; et al. Midostaurin reduces relapse in FLT3-mutant acute myeloid leukemia: The Alliance CALGB 10603/RATIFY trial. Leukemia 2021, 35, 2539–2551. [Google Scholar] [CrossRef]
- Erba, H.P.; Montesinos, P.; Kim, H.-J.; Patkowska, E.; Vrhovac, R.; Žák, P.; Wang, P.-N.; Mitov, T.; Hanyok, J.; Kamel, Y.M.; et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1571–1583. [Google Scholar] [CrossRef]
- Wang, H.; Mao, L.; Yang, M.; Qian, P.; Lu, H.; Tong, H.; Xie, W.; Zhou, D.; Huang, X.; Wang, Y.; et al. Venetoclax plus 3 + 7 daunorubicin and cytarabine chemotherapy as first-line treatment for adults with acute myeloid leukaemia: A multicentre, single-arm, phase 2 trial. Lancet Haematol. 2022, 9, e415–e424. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Suciu, S.; Meloni, G.; Labar, B.; Marie, J.-P.; Halkes, C.J.M.; Muus, P.; Mistrik, M.; Amadori, S.; Specchia, G.; et al. High-Dose Cytarabine in Induction Treatment Improves the Outcome of Adult Patients Younger Than Age 46 Years with Acute Myeloid Leukemia: Results of the EORTC-GIMEMA AML-12 Trial. J. Clin. Oncol. 2014, 32, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Hunter, A.E.; Kjeldsen, L.; Yin, J.; Gibson, B.E.S.; Wheatley, K.; Milligan, D. Optimization of Chemotherapy for Younger Patients with Acute Myeloid Leukemia: Results of the Medical Research Council AML15 Trial. J. Clin. Oncol. 2013, 31, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Solh, M.M.; Solomon, S.R.; Morris, L.E.; Zhang, X.; Holland, H.K.; Bashey, A. Improved Post remission survival of non- favorable risk Acute Myelogenous Leukemia (AML) patients following initial remission induction therapy with FLAG+/−Idarubicin versus 3 + 7 (Anthracycline + Cytarabine). Leuk. Res. 2020, 93, 106318. [Google Scholar] [CrossRef]
- Qasrawi, A.; Bahaj, W.; Qasrawi, L.; Abughanimeh, O.; Foxworth, J.; Gaur, R. Cladribine in the remission induction of adult acute myeloid leukemia: Where do we stand? Ann. Hematol. 2019, 98, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Holowiecki, J.; Grosicki, S.; Giebel, T.; Robak, T.; Kyrcz-Krzemien, S.; Kuliczkowski, K.; Skotnicki, A.; Hellmann, A.; Sulek, K.; Dmoszynska, A.; et al. Cladribine, But Not Fludarabine, Added to Daunorubicin and Cytarabine During Induction Prolongs Survival of Patients with Acute Myeloid Leukemia: A Multicenter, Randomized Phase III Study. J. Clin. Oncol. 2012, 30, 2441–2448. [Google Scholar] [CrossRef]
- Boddu, P.; Kantarjian, H.M.; Ravandi, F.; Jabbour, E.J.; Daver, N.; Pemmaraju, N.; DiNardo, C.D.; Verstovsek, S.; Alvarado, Y.; Borthakur, G.; et al. Outcomes by Treatment Setting and Genomic Profile in Patients with AML on Cladribine, Idarubicin, and Cytarabine. Blood 2017, 130 (Suppl. 1), 3898. [Google Scholar] [CrossRef]
- Bataller, A.; Kantarjian, H.M.; Bazinet, A.; Senapati, J.; Borthakur, G.; Short, N.J.; Jabbour, E.; Takahashi, K.; Daver, N.; Issa, G.C.; et al. Phase II Study of Cladribine with Low Dose Cytarabine and Venetoclax Alternating with Azacytidine and Venetoclax for Newly Diagnosed Acute Myeloid Leukemia. Blood 2024, 144 (Suppl. 1), 56. [Google Scholar] [CrossRef]
- Halpern, A.B.; Othus, M.; Huebner, E.M.; Scott, B.L.; Becker, P.S.; Percival, M.-E.M.; Hendrie, P.C.; Gardner, K.M.; Chen, T.L.; Buckley, S.A.; et al. Phase 1/2 trial of GCLAM with dose-escalated mitoxantrone for newly diagnosed AML or other high-grade myeloid neoplasms. Leukemia 2018, 32, 2352–2362. [Google Scholar] [CrossRef]
- Othman, J.; Wilhelm-Benartzi, C.; Dillon, R.; Knapper, S.; Freeman, S.D.; Batten, L.M.; Canham, J.; Hinson, E.L.; Wych, J.; Betteridge, S.; et al. A randomized comparison of CPX-351 and FLAG-Ida in adverse karyotype AML and high-risk MDS: The UK NCRI AML19 trial. Blood Adv. 2023, 7, 4539–4549. [Google Scholar] [CrossRef]
- Yin, P.-Y.; Wang, R.-W.; Jing, R.; Li, X.; Ma, J.-H.; Li, K.-M.; Wang, H. Research progress on molecular biomarkers of acute myeloid leukemia. Front. Oncol. 2023, 13, 1078556. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.E.; Edwards, H.; Meshinchi, S.; Taub, J.W.; Ge, Y. “FLipping” the Story: FLT3-Mutated Acute Myeloid Leukemia and the Evolving Role of FLT3 Inhibitors. Cancers 2022, 14, 3398. [Google Scholar] [CrossRef] [PubMed]
- Röllig, C.; Serve, H.; Hüttmann, A.; Noppeney, R.; Müller-Tidow, C.; Krug, U.; Baldus, C.D.; Brandts, C.H.; Kunzmann, V.; Einsele, H.; et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): A multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015, 16, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Röllig, C.; Serve, H.; Noppeney, R.; Hanoun, M.; Krug, U.; Baldus, C.D.; Brandts, C.H.; Kunzmann, V.; Einsele, H.; Krämer, A.; et al. Sorafenib or placebo in patients with newly diagnosed acute myeloid leukaemia: Long-term follow-up of the randomized controlled SORAML trial. Leukemia 2021, 35, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Pratz, K.W.; Cherry, M.; Altman, J.K.; Cooper, B.W.; Podoltsev, N.A.; Cruz, J.C.; Lin, T.L.; Schiller, G.J.; Jurcic, J.G.; Asch, A.; et al. Gilteritinib in Combination with Induction and Consolidation Chemotherapy and as Maintenance Therapy: A Phase IB Study in Patients with Newly Diagnosed AML. J. Clin. Oncol. 2023, 41, 4236–4246. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3 -Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- Zhao, J.C.; Agarwal, S.; Ahmad, H.; Amin, K.; Bewersdorf, J.P.; Zeidan, A.M. A review of FLT3 inhibitors in acute myeloid leukemia. Blood Rev. 2022, 52, 100905. [Google Scholar] [CrossRef]
- Wang, E.S.; Goldberg, A.D.; Tallman, M.; Walter, R.B.; Karanes, C.; Sandhu, K.; Vigil, C.E.; Collins, R.; Jain, V.; Stone, R.M. Crenolanib and Intensive Chemotherapy in Adults with Newly Diagnosed FLT3-Mutated AML. J. Clin. Oncol. 2024, 42, 1776–1787. [Google Scholar] [CrossRef]
- Jen, W.Y. FLAG-IDA + Venetoclax (Ven) in Newly Diagnosed (ND) or Relapsed/Refractory (RR). In Proceedings of the 2024 ASCO Annual Meeting, Chicago, IL, USA, 31 May–4 June 2024. [Google Scholar]
- Reville, P.K.; Kantarjian, H.; Borthakur, G.; Yilmaz, M.; Daver, N.; Short, N.; DiNardo, C.D.; Kornblau, S.M.; Pemmaraju, N.; Jain, N.; et al. Venetoclax Combined with Cladribine, Idarubicin, Cytarabine (CLIA) As Induction Therapy in Patients with Newly Diagnosed Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome. Blood 2022, 140 (Suppl. 1), 1702–1704. [Google Scholar] [CrossRef]
- Kadia, T.M.; Reville, P.K.; Borthakur, G.; Yilmaz, M.; Kornblau, S.; Alvarado, Y.; Dinardo, C.D.; Daver, N.; Jain, N.; Pemmaraju, N.; et al. Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A cohort from a single-centre, single-arm, phase 2 trial. Lancet Haematol. 2021, 8, e552–e561. [Google Scholar] [CrossRef]
- Pandya, B.J.; Chen, C.-C.; Medeiros, B.C.; McGuiness, C.B.; Wilson, S.D.; Walsh, E.H.; Wade, R.L. Economic and Clinical Burden of Acute Myeloid Leukemia Episodes of Care in the United States: A Retrospective Analysis of a Commercial Payer Database. J. Manag. Care Spec. Pharm. 2020, 26, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Bonifacio, G.; Latremouille-Viau, D.; Guerin, A.; Shi, S.; Gagnon-Sanschagrin, P.; Briggs, O.; Joseph, G.J. Treatment patterns, healthcare resource utilization, and costs in patients with acute myeloid leukemia in commercially insured and Medicare populations. J. Med. Econ. 2018, 21, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Huggar, D.; Knoth, R.L.; Copher, R.; Cao, Z.; Lipkin, C.; McBride, A.; LeBlanc, T.W. Economic burden in US patients with newly diagnosed acute myeloid leukemia receiving intensive induction chemotherapy. Future Oncol. 2022, 18, 3609–3621. [Google Scholar] [CrossRef]
- Giri, S.; Pathak, R.; Aryal, M.R.; Karmacharya, P.; Bhatt, V.R.; Martin, M.G. Impact of hospital volume on outcomes of patients undergoing chemotherapy for acute myeloid leukemia: A matched cohort study. Blood 2015, 125, 3359–3360. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.H.; Halpern, A.B.; Wu, Q.; Voutsinas, J.M.; Walter, R.B.; Yun, S.; Kanaan, M.; Estey, E.H. Impact of region of diagnosis, ethnicity, age, and gender on survival in acute myeloid leukemia (AML). J. Drug Assess. 2018, 7, 51–53. [Google Scholar] [CrossRef]
- Jain, H.; Eipe, T.; Shetty, A.; Nayak, L.; Bagal, B.P.; Sharma, N.; Pawar, A.; Sengar, M. Real-World Analysis Evaluating Treatment Eligibility and Outcomes in Patients with AML Receiving Intensive Chemotherapy: Insights from an Underrepresented Population. JCO Glob. Oncol. 2025, 11, e2400482. [Google Scholar] [CrossRef] [PubMed]
- Dumas, P.-Y.; Bertoli, S.; Bérard, E.; Leguay, T.; Tavitian, S.; Galtier, J.; Alric, C.; Bidet, A.; Delabesse, E.; Rieu, J.B.; et al. Delivering HDAC over 3 or 5 days as consolidation in AML impacts health care resource consumption but not outcome. Blood Adv. 2020, 4, 3840–3849. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, S.; Benner, A.; Krauter, J.; Martin, H.; Kindler, T.; Bentz, M.; Salih, H.; Held, G.; Köhne, C.-H.; Götze, K.; et al. Condensed versus standard schedule of high-dose cytarabine consolidation therapy with pegfilgrastim growth factor support in acute myeloid leukemia. Blood Cancer J. 2017, 7, e564. [Google Scholar] [CrossRef]
- He, P.; Liang, J.; Zhang, W.; Lin, S.; Wu, H.; Li, Q.; Xu, X.; Ji, C. Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia: An Overview of Systematic Reviews. Int. J. Clin. Pract. 2022, 2022, 1828223. [Google Scholar] [CrossRef]
- Dholaria, B.; Savani, B.N.; Hamilton, B.K.; Oran, B.; Liu, H.D.; Tallman, M.S.; Ciurea, S.O.; Holtzman, N.G.; Ii, G.L.P.; Devine, S.M.; et al. Hematopoietic Cell Transplantation in the Treatment of Newly Diagnosed Adult Acute Myeloid Leukemia: An Evidence-Based Review from the American Society of Transplantation and Cellular Therapy. Transplant. Cell. Ther. 2021, 27, 6–20. [Google Scholar] [CrossRef]
- Pei, X.; Huang, X. New approaches in allogenic transplantation in AML. Semin. Hematol. 2019, 56, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chang, Y.-J.; Hong, Y.; Xu, L.-P.; Wang, Y.; Zhang, X.-H.; Wang, M.; Chen, H.; Chen, Y.-H.; Wang, F.-R.; et al. Dynamic immune profiling identifies the stronger graft-versus-leukemia (GVL) effects with haploidentical allografts compared to HLA-matched stem cell transplantation. Cell. Mol. Immunol. 2021, 18, 1172–1185. [Google Scholar] [CrossRef]
- Hourigan, C.S.; Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey, A.; et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia with Genomic Evidence of Residual Disease. J. Clin. Oncol. 2020, 38, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Gilleece, M.H.; Labopin, M.; Yakoub-Agha, I.; Volin, L.; Socié, G.; Ljungman, P.; Huynh, A.; Deconinck, E.; Wu, D.; Bourhis, J.H.; et al. Measurable residual disease, conditioning regimen intensity, and age predict outcome of allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first remission: A registry analysis of 2292 patients by the Acute Leukemia Working Party European Society of Blood and Marrow Transplantation. Am. J. Hematol. 2018, 93, 1142–1152. [Google Scholar] [CrossRef]
- Wong, Z.C.; Dillon, L.W.; Hourigan, C.S. Measurable residual disease in patients undergoing allogeneic transplant for acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2023, 36, 101468. [Google Scholar] [CrossRef] [PubMed]
- Woolfrey, A.; Lee, S.J.; Gooley, T.A.; Malkki, M.; Martin, P.J.; Pagel, J.M.; Hansen, J.A.; Petersdorf, E. HLA-Allele Matched Unrelated Donors Compared to HLA-Matched Sibling Donors: Role of Cell Source and Disease Risk Category. Biol. Blood Marrow Transplant. 2010, 16, 1382–1387. [Google Scholar] [CrossRef]
- Moore, J.; Nivison-Smith, I.; Goh, K.; Ma, D.; Bradstock, K.; Szer, J.; Durrant, S.; Schwarer, A.; Bardy, P.; Herrmann, R.; et al. Equivalent survival for sibling and unrelated donor allogeneic stem cell transplantation for acute myelogenous leukemia. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2007, 13, 601–607. [Google Scholar] [CrossRef]
- Gupta, V.; Tallman, M.S.; He, W.; Logan, B.R.; Copelan, E.; Gale, R.P.; Khoury, H.J.; Klumpp, T.; Koreth, J.; Lazarus, H.M.; et al. Comparable survival after HLA-well-matched unrelated or matched sibling donor transplantation for acute myeloid leukemia in first remission with unfavorable cytogenetics at diagnosis. Blood 2010, 116, 1839–1848. [Google Scholar] [CrossRef]
- Ciurea, S.O.; Zhang, M.-J.; Bacigalupo, A.A.; Bashey, A.; Appelbaum, F.R.; Aljitawi, O.S.; Armand, P.; Antin, J.H.; Chen, J.; Devine, S.M.; et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 2015, 126, 1033–1040. [Google Scholar] [CrossRef]
- Gagelmann, N.; Bacigalupo, A.; Rambaldi, A.; Hoelzer, D.; Halter, J.; Sanz, J.; Bonifazi, F.; Meijer, E.; Itälä-Remes, M.; Marková, M.; et al. Haploidentical Stem Cell Transplantation with Posttransplant Cyclophosphamide Therapy vs Other Donor Transplantations in Adults with Hematologic Cancers: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1739. [Google Scholar] [CrossRef]
- Luznik, L.; O’Donnell, P.V.; Symons, H.J.; Chen, A.R.; Leffell, M.S.; Zahurak, M.; Gooley, T.A.; Piantadosi, S.; Kaup, M.; Ambinder, R.F.; et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biol. Blood Marrow Transplant. 2008, 14, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Eckrich, M.; Pasquini, M. Hematopoietic cell transplantation in Latin America. Hematology 2012, 17, s189–s191. [Google Scholar] [CrossRef] [PubMed]
- Tokaz, M.C.; Baldomero, H.; Cowan, A.J.; Saber, W.; Greinix, H.; Koh, M.B.C.; Kröger, N.; Mohty, M.; Galeano, S.; Okamoto, S.; et al. An Analysis of the Worldwide Utilization of Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia. Transplant. Cell. Ther. 2023, 29, 279.e1–279.e10. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, U.; George, B. Access to hematopoietic stem-cell transplantation in India. J. Postgrad. Med. 2019, 65, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Paulson, K.; Brazauskas, R.; Khera, N.; He, N.; Majhail, N.; Akpek, G.; Aljurf, M.; Buchbinder, D.; Burns, L.; Beattie, S.; et al. Inferior Access to Allogeneic Transplant in Disadvantaged Populations: A Center for International Blood and Marrow Transplant Research Analysis. Biol. Blood Marrow Transplant. 2019, 25, 2086–2090. [Google Scholar] [CrossRef] [PubMed]
- Meillon-Garcia, L.A.; Demichelis-Gómez, R. Access to Therapy for Acute Myeloid Leukemia in the Developing World: Barriers and Solutions. Curr. Oncol. Rep. 2020, 22, 125. [Google Scholar] [CrossRef] [PubMed]
- Jamy, O.; Chen, A.; Battles, K.; Francisco, L.; Salzman, D.; Bal, S.; Di Stasi, A.; Costa, L.; Bhatia, R.; Bhatia, S. Impact of access to care on 1-year mortality following allogeneic blood or marrow transplantation. Bone Marrow Transplant. 2021, 56, 1364–1372. [Google Scholar] [CrossRef]
- Jaimovich, G.; Rolon, J.M.; Baldomero, H.; Rivas, M.; Hanesman, I.; Bouzas, L.; Bonfim, C.; Palma, J.; Kardus-Urueta, A.; Ubidia, D.; et al. Erratum: Latin America: The next region for haematopoietic transplant progress. Bone Marrow Transplant. 2017, 52, 798. [Google Scholar] [CrossRef]
- Jaimovich, G.; Gale, R.P.; Hanesman, I.; Vazquez, A.; Hammerschlak, N.; Simoes, B.P.; Fagundo, J.C.; Jimenez, M.H.; Gomez-Almaguer, D.; Fanilla, E.; et al. The paradox of haematopoietic cell transplant in Latin America. Bone Marrow Transplant. 2021, 56, 2382–2388. [Google Scholar] [CrossRef]
- Noyola-Pérez, A.; Ribas-Muratori, R.; Vargas-Hernández, M.A.; Saavedra-Salazar, L.; Frutos, C.; Bonfim, C.; Barroso-Duarte, F.; Galeano, S.; Jaimovich, G.; Karduss, A.; et al. Training in Transplantation and Cellular Therapy in Latin America: A Cross-Sectional Study of the LABMT. Transplant. Cell. Ther. 2025, 31, 47.e1–47.e9. [Google Scholar] [CrossRef]
- Preussler, J.M.; Mau, L.-W.; Majhail, N.S.; Bevans, M.; Clancy, E.; Messner, C.; Parran, L.; Pederson, K.A.; Stickney Ferguson, S.; Walters, K.; et al. Caregiver availability and patient access to hematopoietic cell transplantation: Social worker perspectives inform practice. Support. Care Cancer 2019, 27, 4253–4264. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthi, T.; Wehrlen, L.; Friedman, E.; Thomas, S.; Bevans, M. Hematopoietic Stem Cell Transplantation Recipient and Caregiver Factors Affecting Length of Stay and Readmission. Oncol. Nurs. Forum 2017, 44, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Frey, P.; Stinson, T.; Siston, A.; Knight, S.; Ferdman, E.; Traynor, A.; O’Gara, K.; Rademaker, A.; Bennett, C.; Winter, J. Lack of caregivers limits use of outpatient hematopoietic stem cell transplant program. Bone Marrow Transplant. 2002, 30, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, N.A.; Aljurf, M.; Almohareb, F.I.; Alzahrani, H.A.; Bashir, Q.; Savani, B.; Gupta, V.; Hashmi, S.K. Establishing an autologous versus allogeneic hematopoietic cell transplant program in nations with emerging economies. Hematol. Oncol. Stem Cell Ther. 2017, 10, 173–177. [Google Scholar] [CrossRef]
- Ganesan, P.; El Cheikh, J.; Isidori, A.; Kuo, S.-H.; Saleh, M.; Nair, R. Editorial: The management of hematologic malignancies in lower-income countries. Front. Oncol. 2023, 13, 1218718. [Google Scholar] [CrossRef]
- Bhatt, V.R.; Shostrom, V.; Giri, S.; Gundabolu, K.; Monirul Islam, K.M.; Appelbaum, F.R.; Maness, L.J. Early mortality and overall survival of acute myeloid leukemia based on facility type. Am. J. Hematol. 2017, 92, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Yerigeri, K.; Glover, J.; Shamshuddin, B.; Kindwall-Keller, T.L. Time to antibiotic administration in patients undergoing HSCT with neutropenic fever. J. Clin. Oncol. 2024, 42 (Suppl. 16), e19501. [Google Scholar] [CrossRef]
- Ji, J.; Klaus, J.; Burnham, J.P.; Michelson, A.; McEvoy, C.A.; Kollef, M.H.; Lyons, P.G. Bloodstream Infections and Delayed Antibiotic Coverage Are Associated with Negative Hospital Outcomes in Hematopoietic Stem Cell Transplant Recipients. Chest 2020, 158, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Almaguer, D.; Gómez-De León, A.; Colunga-Pedraza, P.R.; Cantú-Rodríguez, O.G.; Gutierrez-Aguirre, C.H.; Ruíz-Arguelles, G. Outpatient allogeneic hematopoietic stem-cell transplantation: A review. Ther. Adv. Hematol. 2022, 13, 20406207221080739. [Google Scholar] [CrossRef]
- González, M.J.; Urizar, E.; Urtaran-Laresgoiti, M.; Nuño-Solinís, R.; Lázaro-Pérez, E.; Vázquez, L.; Pascual-Cascón, M.J.; Solano, C.; Kwon, M.; Gallego, C.; et al. Hospital and outpatient models for Hematopoietic Stem Cell Transplantation: A systematic review of comparative studies for health outcomes, experience of care and costs. PLoS ONE 2021, 16, e0254135. [Google Scholar] [CrossRef]
- Guru Murthy, G.S.; Hari, P.N.; Szabo, A.; Pasquini, M.; Narra, R.; Khan, M.; Abedin, S.; Chhabra, S.; Dhakal, B.; D’Souza, A.; et al. Outcomes of Reduced-Intensity Conditioning Allogeneic Hematopoietic Cell Transplantation Performed in the Inpatient versus Outpatient Setting. Biol. Blood Marrow Transplant. 2019, 25, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, J.P.; López-Mora, Y.A.; Salazar-Riojas, R.; Alvarado Navarro, D.M.; Hernández-Navarro, A.K.; Chavez-Estrada, Y.O.; Gómez-De León, A.; Gutierrez-Aguirre, C.H.; Colunga-Pedraza, P.R.; Cantú-Rodríguez, O.G.; et al. Reassessing blood product irradiation in haploidentical transplantation: A single-center perspective. Hematology 2024, 29, 2420144. [Google Scholar] [CrossRef] [PubMed]
- Varela-Constantino, A.L.; Morcos, M.; Noyola-Perez, A.; Méndez-Ramírez, N.; Fuentes-Chavez, E.; Salazar-Riojas, R.; Gutierrez-Aguirre, C.H.; Cantu, O.; Colunga Pedraza, P.R.; Tarin-Arzaga, L.; et al. Optimizing Conditioning Intensity to Disease Burden in Adults with Acute Myeloid Leukemia Undergoing Transplantation: Results of a Prospective Multicenter Study. Blood 2024, 144 (Suppl. 1), 4873. [Google Scholar] [CrossRef]
- Gale, R.P.; Seber, A.; Bonfim, C.; Pasquini, M. Haematopoietic cell transplants in Latin America. Bone Marrow Transplant. 2016, 51, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.; Feinglass, J.; Marfatia, H.; Adekola, K.; Moreira, J. Evaluating Socioeconomic, Racial, and Ethnic Disparities in Survival Among Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplants. J. Racial Ethn. Health Disparities 2024, 11, 1330–1338. [Google Scholar] [CrossRef]
- Gragert, L.; Eapen, M.; Williams, E.; Freeman, J.; Spellman, S.; Baitty, R.; Hartzman, R.; Rizzo, J.D.; Horowitz, M.; Confer, D.; et al. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry. N. Engl. J. Med. 2014, 371, 339–348. [Google Scholar] [CrossRef]
- Pagel, J.M.; Othus, M.; Garcia-Manero, G.; Fang, M.; Radich, J.P.; Rizzieri, D.A.; Marcucci, G.; Strickland, S.A.; Litzow, M.R.; Savoie, M.L.; et al. Rapid Donor Identification Improves Survival in High-Risk First-Remission Patients with Acute Myeloid Leukemia. JCO Oncol. Pract. 2020, 16, e464–e475. [Google Scholar] [CrossRef]
- Solis-Armenta, R.; Hernandez Perez, A.P.; Zapata, N.P.; Delgado, N.; Montano Figueroa, E.H.; Leyto, F.; Solís-Poblano, J.C.; Gomez-De Leon, A.; Amador, L.F.; García-Castillo, C.; et al. Hematopoietic Cell Transplantation in First Remission in AML in Mexico: Very Low Rates Derived from Early Relapses and Lack of Access. Blood 2022, 140 (Suppl. 1), 10830–10832. [Google Scholar] [CrossRef]
- Solís-Armenta, R.; Rodríguez-Rodríguez, S.; Hernández-Pérez, A.P.; Paulina-Zapata, N.; Delgado, N.; Montano-Figueroa, E.H.; Leyto, F.; Solís-Poblano, J.C.; Gómez-De León, A.; Amador, L.F.; et al. Understanding factors limiting hematopoietic cell transplantation for acute myeloid leukemia patients in Mexico: A comprehensive analysis. Ann. Hematol. 2024, 103, 4089–4097. [Google Scholar] [CrossRef]
- Rego, E.M.; Higashi, M.; Romero, M.A.; Enrico, A.I.; Fernandez, I.I.; Jarchum, G.; Aruachan, S.; Quintero, G.; Vicente, A.; Russell-Smith, A.; et al. Real-World Study in Acute Leukemia: Epidemiology, Treatment Patterns and Outcomes for Newly Diagnosed AML in Adult Patients from Latin America—Loyal Study. Blood 2023, 142 (Suppl. 1), 2855. [Google Scholar] [CrossRef]
- Galeano, S.; Bonfim, C.; Karduss, A.; Jaimovich, G.; Gómez-De León, A.; Bettarello, G.; Simione, A.; Correa, C.; Baldomero, H.; Neumann, D.; et al. Results of the Latin American Bone Marrow Transplantation Society (LABMT) activity survey 2019–2022: The impact of the COVID-19 pandemic and the increase in related haploidentical donors. Bone Marrow Transplant. 2025, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Aguirre, C.H.; Gómez-De-León, A.; Alatorre-Ricardo, J.; Cantú-Rodríguez, O.G.; González-Llano, O.; Jaime-Pérez, J.C.; Mancías-Guerra, C.; Flores-Jiménez, J.A.; Gómez-Almaguer, D. Allogeneic peripheral blood stem cell transplantation using reduced-intensity conditioning in an outpatient setting in ABO -incompatible patients: Are survival and graft-versus-host disease different? Transfusion 2014, 54, 1269–1277. [Google Scholar] [CrossRef]
- Gonzalez, A.; Varela Constantino, A.L.; Méndez-Ramírez, N.; Medina-Olivares, F.J.; Fuentes-Chavez, E.; Salazar-Riojas, R.; Gutierrez-Aguirre, C.H.; Cantu, O.; Colunga-Pedraza, P.; Tarin-Arzaga, L.; et al. Lowering Conditioning Intensity in Fit Adolescents and Adults with Lymphoblastic Leukemia without Residual Disease Undergoing Transplantation: A Proof of Concept. Blood 2024, 144 (Suppl. 1), 376. [Google Scholar] [CrossRef]
- Gandhi, A.P.; Lee, C.J. Telemedicine in Hematopoietic Cell Transplantation and Chimeric Antigen Receptor-T Cell Therapy. Cancers 2023, 15, 4108. [Google Scholar] [CrossRef] [PubMed]
- Lübbert, M.; Wijermans, P.W.; Kicinski, M.; Chantepie, S.; Van Der Velden, W.J.F.M.; Noppeney, R.; Griškevičius, L.; Neubauer, A.; Crysandt, M.; Vrhovac, R.; et al. 10-day decitabine versus 3 + 7 chemotherapy followed by allografting in older patients with acute myeloid leukaemia: An open-label, randomised, controlled, phase 3 trial. Lancet Haematol. 2023, 10, e879–e889. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Wei, A.H.; Panayiotidis, P.; Montesinos, P.; Laribi, K.; Ivanov, V.; Kim, I.; Novak, J.; Champion, R.; Fiedler, W.; Pagoni, M.; et al. Long-term follow-up of VIALE-C in patients with untreated AML ineligible for intensive chemotherapy. Blood 2022, 140, 2754–2756. [Google Scholar] [CrossRef] [PubMed]
- Garciaz, S.; Hospital, M.-A.; Collette, Y.; Vey, N. Venetoclax Resistance in Acute Myeloid Leukemia. Cancers 2024, 16, 1091. [Google Scholar] [CrossRef]
- Bataller, A.; Bazinet, A.; DiNardo, C.D.; Maiti, A.; Borthakur, G.; Daver, N.G.; Short, N.J.; Jabbour, E.J.; Issa, G.C.; Pemmaraju, N.; et al. Prognostic risk signature in patients with acute myeloid leukemia treated with hypomethylating agents and venetoclax. Blood Adv. 2024, 8, 927–935. [Google Scholar] [CrossRef]
- Waclawiczek, A.; Leppä, A.-M.; Renders, S.; Stumpf, K.; Reyneri, C.; Betz, B.; Janssen, M.; Shahswar, R.; Donato, E.; Karpova, D.; et al. Combinatorial BCL2 Family Expression in Acute Myeloid Leukemia Stem Cells Predicts Clinical Response to Azacitidine/Venetoclax. Cancer Discov. 2023, 13, 1408–1427. [Google Scholar] [CrossRef] [PubMed]
- Pratz, K.W.; DiNardo, C.D.; Selleslag, D.; Li, J.; Yamamoto, K.; Konopleva, M.; Stevens, D.; Kantarjian, H.; Traina, F.; Venditti, A.; et al. Postremission cytopenia management in patients with acute myeloid leukemia treated with venetoclax and azacitidine in VIALE-A. Am. J. Hematol. 2022, 97, E416–E419. [Google Scholar] [CrossRef] [PubMed]
- Karrar, O.; Abdelmagid, M.; Rana, M.; Iftikhar, M.; McCullough, K.; Al-Kali, A.; Alkhateeb, H.B.; Begna, K.H.; Elliott, M.A.; Mangaonkar, A.; et al. Venetoclax duration (14 vs. 21 vs. 28 days) in combination with hypomethylating agent in newly diagnosed acute myeloid leukemia: Comparative analysis of response, toxicity, and survival. Am. J. Hematol. 2024, 99, E63–E66. [Google Scholar] [CrossRef]
- Cui, J.; Chen, X.; Li, C.; Yan, Q.; Yuan, G. Reduced duration and dosage of venetoclax is efficient in newly diagnosed patients with acute myeloid leukemia. Hematology 2024, 29, 2293512. [Google Scholar] [CrossRef] [PubMed]
- Madarang, E.; Lykon, J.; Zhao, W.; Sekeres, M.A.; Bradley, T.; Chandhok, N.S.; Taylor, J.; Venugopal, S.; Koru-Sengul, T.; Iyer, S.G.; et al. Venetoclax and hypomethylating agents in octogenarians and nonagenarians with acute myeloid leukemia. Blood Neoplasia 2024, 1, 100016. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.-P.; et al. Ivosidenib and Azacitidine in IDH1 -Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- De Botton, S.; Montesinos, P.; Vives Polo, S.; Zarzycka, E.; Wang, J.; Riva, M.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.-P.; et al. Updated efficacy and safety data from the AGILE study in patients with newly diagnosed acute myeloid leukemia treated with ivosidenib + azacitidine compared to placebo + azacitidine. J. Clin. Oncol. 2023, 41 (Suppl. 16), 7012. [Google Scholar] [CrossRef]
- Cortes, J.E.; Jonas, B.A.; Watts, J.M.; Chao, M.M.; De Botton, S. Olutasidenib for mutated IDH1 acute myeloid leukemia: Final five-year results from the phase 2 pivotal cohort. J. Clin. Oncol. 2024, 42, 6528. [Google Scholar] [CrossRef]
- De Botton, S.; Montesinos, P.; Schuh, A.C.; Papayannidis, C.; Vyas, P.; Wei, A.H.; Ommen, H.; Semochkin, S.; Kim, H.-J.; Larson, R.A.; et al. Enasidenib vs conventional care in older patients with late-stage mutant-IDH2 relapsed/refractory AML: A randomized phase 3 trial. Blood 2023, 141, 156–167. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Schuh, A.C.; Stein, E.M.; Montesinos, P.; Wei, A.H.; De Botton, S.; Zeidan, A.M.; Fathi, A.T.; Kantarjian, H.M.; Bennett, J.M.; et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): A single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021, 22, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Loghavi, S.; Zeng, Z.; Tanaka, T.; Kim, Y.J.; Uryu, H.; Turkalj, S.; Jakobsen, N.A.; Luskin, M.R.; Duose, D.Y.; et al. A Phase Ib/II Study of Ivosidenib with Venetoclax ± Azacitidine in IDH1 -Mutated Myeloid Malignancies. Blood Cancer Discov. 2023, 4, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; DiNardo, C.D.; Fathi, A.T.; Mims, A.S.; Pratz, K.W.; Savona, M.R.; Stein, A.S.; Stone, R.M.; Winer, E.S.; Seet, C.S.; et al. Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: A phase 1 study. Blood 2021, 137, 1792–1803. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Montesinos, P.; Minden, M.D.; Lee, J.-H.; Heuser, M.; Naoe, T.; Chou, W.-C.; Laribi, K.; Esteve, J.; Altman, J.K.; et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3 mut+ AML ineligible for intensive chemotherapy. Blood 2022, 140, 1845–1857. [Google Scholar] [CrossRef]
- Brinton, L.T.; Zhang, P.; Williams, K.; Canfield, D.; Orwick, S.; Sher, S.; Wasmuth, R.; Beaver, L.; Cempre, C.; Skinner, J.; et al. Synergistic effect of BCL2 and FLT3 co-inhibition in acute myeloid leukemia. J. Hematol. Oncol. 2020, 13, 139. [Google Scholar] [CrossRef]
- Daver, N.; Perl, A.E.; Maly, J.; Levis, M.; Ritchie, E.; Litzow, M.; McCloskey, J.; Smith, C.C.; Schiller, G.; Bradley, T.; et al. Venetoclax Plus Gilteritinib for FLT3 -Mutated Relapsed/Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2022, 40, 4048–4059. [Google Scholar] [CrossRef]
- Dennis, M.; Thomas, I.F.; Ariti, C.; Upton, L.; Burnett, A.K.; Gilkes, A.; Radia, R.; Hemmaway, C.; Mehta, P.; Knapper, S.; et al. Randomized evaluation of quizartinib and low-dose ara-C vs low-dose ara-C in older acute myeloid leukemia patients. Blood Adv. 2021, 5, 5621–5625. [Google Scholar] [CrossRef] [PubMed]
- Levis, M.J.; Hamadani, M.; Logan, B.; Jones, R.J.; Singh, A.K.; Litzow, M.; Wingard, J.R.; Papadopoulos, E.B.; Perl, A.E.; Soiffer, R.J.; et al. Gilteritinib as Post-Transplant Maintenance for AML with Internal Tandem Duplication Mutation of FLT3. J. Clin. Oncol. 2024, 42, 1766–1775. [Google Scholar] [CrossRef]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Röllig, C.; Wollmer, E.; Wäsch, R.; Bornhäuser, M.; Berg, T.; et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia with FLT3 –Internal Tandem Duplication Mutation (SORMAIN). J. Clin. Oncol. 2020, 38, 2993–3002. [Google Scholar] [CrossRef]
- Yilmaz, M.; Muftuoglu, M.; Short, N.J.; Loghavi, S.; Kadia, T.M.; DiNardo, C.D.; Borthakur, G.; Pemmaraju, N.; Alvarado Valero, Y.; Maiti, A.; et al. Phase I/II Study of Quizartinib, Venetoclax, and Decitabine Triple Combination in FLT3-ITD Mutated AML. Blood 2024, 144 (Suppl. 1), 4263. [Google Scholar] [CrossRef]
- Brown, M.R.; Soto-Feliciano, Y.M. Menin: From molecular insights to clinical impact. Epigenomics 2025, 17, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Revumenib: First Approval. Drugs 2025, 85, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Issa, G.C.; Aldoss, I.; Thirman, M.J.; DiPersio, J.; Arellano, M.; Blachly, J.S.; Mannis, G.N.; Perl, A.; Dickens, D.S.; McMahon, C.M.; et al. Menin Inhibition with Revumenib for KMT2A -Rearranged Relapsed or Refractory Acute Leukemia (AUGMENT-101). J. Clin. Oncol. 2025, 43, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Issa, G.C.; Erba, H.P.; Altman, J.K.; Montesinos, P.; DeBotton, S.; Walter, R.B.; Pettit, K.; Savona, M.R.; Shah, M.V.; et al. Ziftomenib in relapsed or refractory acute myeloid leukaemia (KOMET-001): A multicentre, open-label, multi-cohort, phase 1 trial. Lancet Oncol. 2024, 25, 1310–1324. [Google Scholar] [CrossRef]
- Khalifeh, M.; Hopewell, E.; Salman, H. CAR T cell therapy for treatment of Acute Myeloid Leukemia, Advances and Outcomes. Mol. Ther. 2025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, H.-H. Breakthroughs of CAR T-cell therapy in acute myeloid leukemia: Updates from ASH 2024. Exp. Hematol. Oncol. 2025, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, R.C.; Gibson, C.J.; Murdock, H.M.; Stone, R.M.; Cortes, J.E.; Uy, G.L.; Lin, T.L.; Ritchie, E.K.; Prebet, T.; Ryan, R.J.; et al. Genetic Characteristics and Outcomes By Mutation Status in a Phase 3 Study of CPX-351 Versus 7+3 in Older Adults with Newly Diagnosed, High-Risk/Secondary Acute Myeloid Leukemia (AML). Blood 2019, 134 (Suppl. 1), 15. [Google Scholar] [CrossRef]
- Daver, N.; Vyas, P.; Huls, G.; Dohner, H.; Maury, S.; Novak, J.; Papayannidis, C.; Martinez-Chamorro, C.; Montesinos, P.; Niroula, R.; et al. Magrolimab vs Placebo in Combination with Venetoclax and Azacitidine in Previously Untreated Patients with Acute Myeloid Leukemia Who Are Ineligible for Intensive Chemotherapy: The Enhance-3 Study; EHA Library: Madrid, Spain, 2024. [Google Scholar]
- Heuser, M.; Smith, B.D.; Fiedler, W.; Sekeres, M.A.; Montesinos, P.; Leber, B.; Merchant, A.; Papayannidis, C.; Pérez-Simón, J.A.; Hoang, C.J.; et al. Clinical benefit of glasdegib plus low-dose cytarabine in patients with de novo and secondary acute myeloid leukemia: Long-term analysis of a phase II randomized trial. Ann. Hematol. 2021, 100, 1181–1194. [Google Scholar] [CrossRef]
- Pratz, K.W.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Döhner, H.; Récher, C.; Fiedler, W.; Yamamoto, K.; Wang, J.; et al. Long-term follow-up of VIALE-A: Venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia. Am. J. Hematol. 2024, 99, 615–624. [Google Scholar] [CrossRef]
- Alhajahjeh, A.; Rotter, L.K.; Stempel, J.M.; Grimshaw, A.A.; Bewersdorf, J.P.; Blaha, O.; Kewan, T.; Podoltsev, N.A.; Shallis, R.M.; Mendez, L.; et al. Global Disparities in the Characteristics and Outcomes of Leukemia Clinical Trials: A Cross-Sectional Study of the ClinicalTrials.gov Database. JCO Glob. Oncol. 2024, 10, e2400316. [Google Scholar] [CrossRef]
- INEGI. Población con Afiliación a Servicios de Salud por Entidad Federativa Según Institución. 2020. Available online: https://www.inegi.org.mx/app/tabulados/interactivos/?pxq=Derechohabiencia_Derechohabiencia_02_822ebcc5-ef41-40c1-9901-22e397025c64 (accessed on 11 May 2025).
- Agarwal, S.K.; DiNardo, C.D.; Potluri, J.; Dunbar, M.; Kantarjian, H.M.; Humerickhouse, R.A.; Wong, S.L.; Menon, R.M.; Konopleva, M.Y.; Salem, A.H. Management of Venetoclax-Posaconazole Interaction in Acute Myeloid Leukemia Patients: Evaluation of Dose Adjustments. Clin. Ther. 2017, 39, 359–367. [Google Scholar] [CrossRef] [PubMed]
- De La Garza-Salazar, F.; Colunga-Pedraza, P.R.; Gómez-Almaguer, D. Cytochrome P450 inhibition to decrease dosage and costs of venetoclax and ibrutinib: A proof-of-concept case study. Br. J. Clin. Pharmacol. 2023, 89, 898–902. [Google Scholar] [CrossRef] [PubMed]
- De La Garza-Salazar, F.; Colunga-Pedraza, P.R.; Gómez-Almaguer, D.; García-Zárate, V.A.; Gómez-De León, A. Low dose venetoclax plus itraconazole outpatient induction in newly diagnosed acute myeloid leukemia: A phase 2 study. Leuk. Res. 2023, 133, 107373. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 2020, 135 (Suppl. 1), 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, Y.K.; De La Garza, F.; De La Rosa-Flores, G.A.; Coronado Alejandro, E.U.; Vega-Mateos, A.; Tejada Vasquez, A.C.; Garcia-Salas, G.; García-Zárate, V.; Colunga-Pedraza, P.R.R.; Gomez-Almaguer, D.; et al. Outpatient Low-Dose Venetoclax Plus Azacitidine Vs. Intensive Chemotherapy for Newly Diagnosed Fit Patients with Acute Myeloid Leukemia in a Limited Resource Setting. Blood 2023, 142 (Suppl. 1), 1529. [Google Scholar] [CrossRef]
- Gómez-De León, A.; Demichelis-Gómez, R.; Pinedo-Rodríguez, A.; Enriquez-Vera, D.; Flores-Jiménez, J.A.; Ceballos-López, A.A.; Rodríguez-Mejorada, M.; Herrera Riojas, M.A.; Ovilla-Martínez, R.; Báez-Islas, P.; et al. Venetoclax-based combinations for acute myeloid leukemia: Optimizing their use in Latin-America. Hematology 2022, 27, 249–257. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morcos-Sandino, M.; Quezada-Ramírez, S.I.; Gómez-De León, A. Advances in the Treatment of Acute Myeloid Leukemia: Implications for Low- and Middle-Income Countries. Biomedicines 2025, 13, 1221. https://doi.org/10.3390/biomedicines13051221
Morcos-Sandino M, Quezada-Ramírez SI, Gómez-De León A. Advances in the Treatment of Acute Myeloid Leukemia: Implications for Low- and Middle-Income Countries. Biomedicines. 2025; 13(5):1221. https://doi.org/10.3390/biomedicines13051221
Chicago/Turabian StyleMorcos-Sandino, Michelle, Sofia Isabel Quezada-Ramírez, and Andrés Gómez-De León. 2025. "Advances in the Treatment of Acute Myeloid Leukemia: Implications for Low- and Middle-Income Countries" Biomedicines 13, no. 5: 1221. https://doi.org/10.3390/biomedicines13051221
APA StyleMorcos-Sandino, M., Quezada-Ramírez, S. I., & Gómez-De León, A. (2025). Advances in the Treatment of Acute Myeloid Leukemia: Implications for Low- and Middle-Income Countries. Biomedicines, 13(5), 1221. https://doi.org/10.3390/biomedicines13051221






