Human Placenta Hydrolysate Protects Against Acetaminophen-Induced Liver Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Human Placenta Hydrolysate
2.2. In Vitro Cell Viability Assay
2.3. Animals and Experimental Design
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
2.6. Statistical Analysis
3. Results
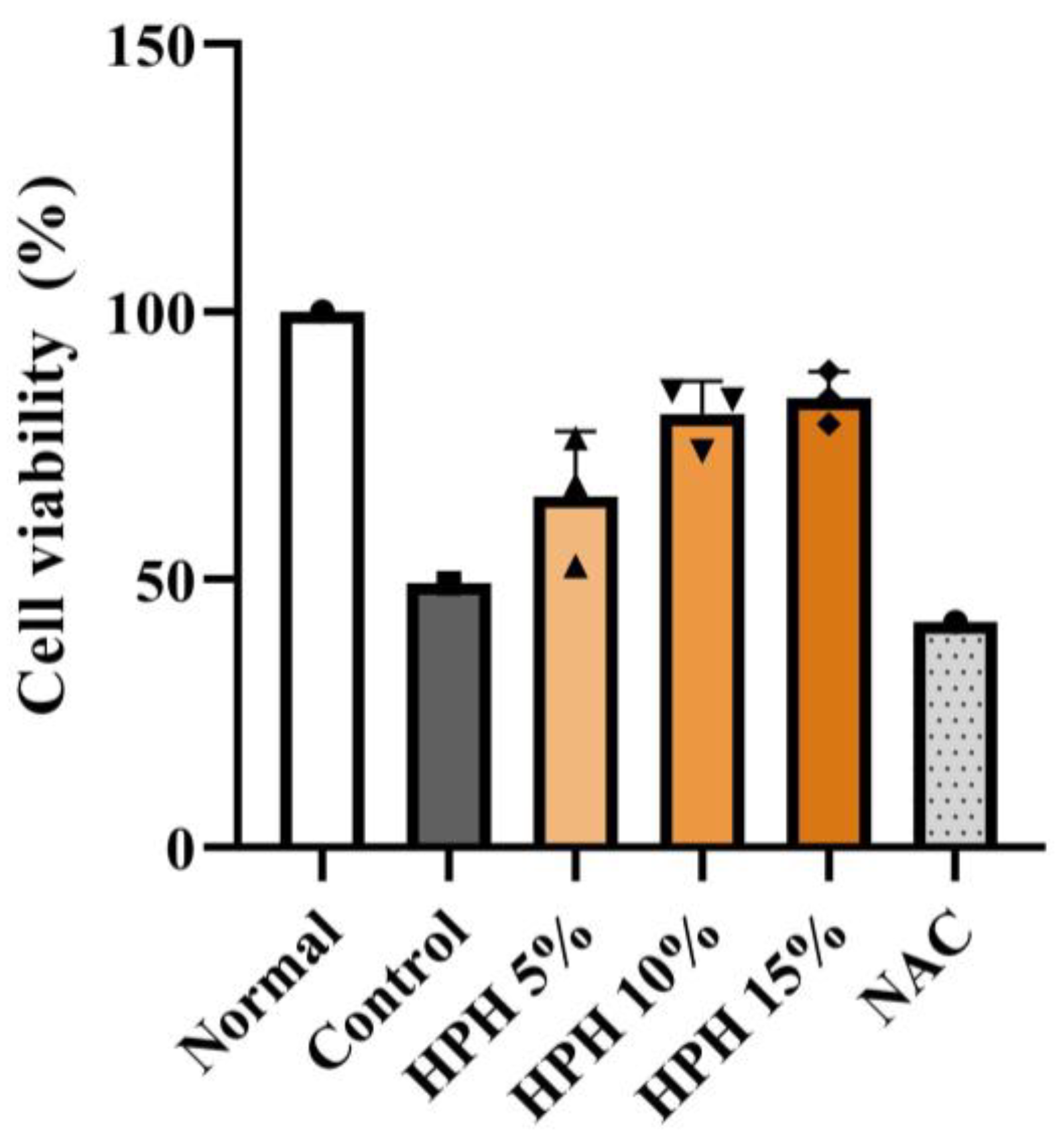
3.1. Effect of HPH on In Vitro Hepatocyte Viability
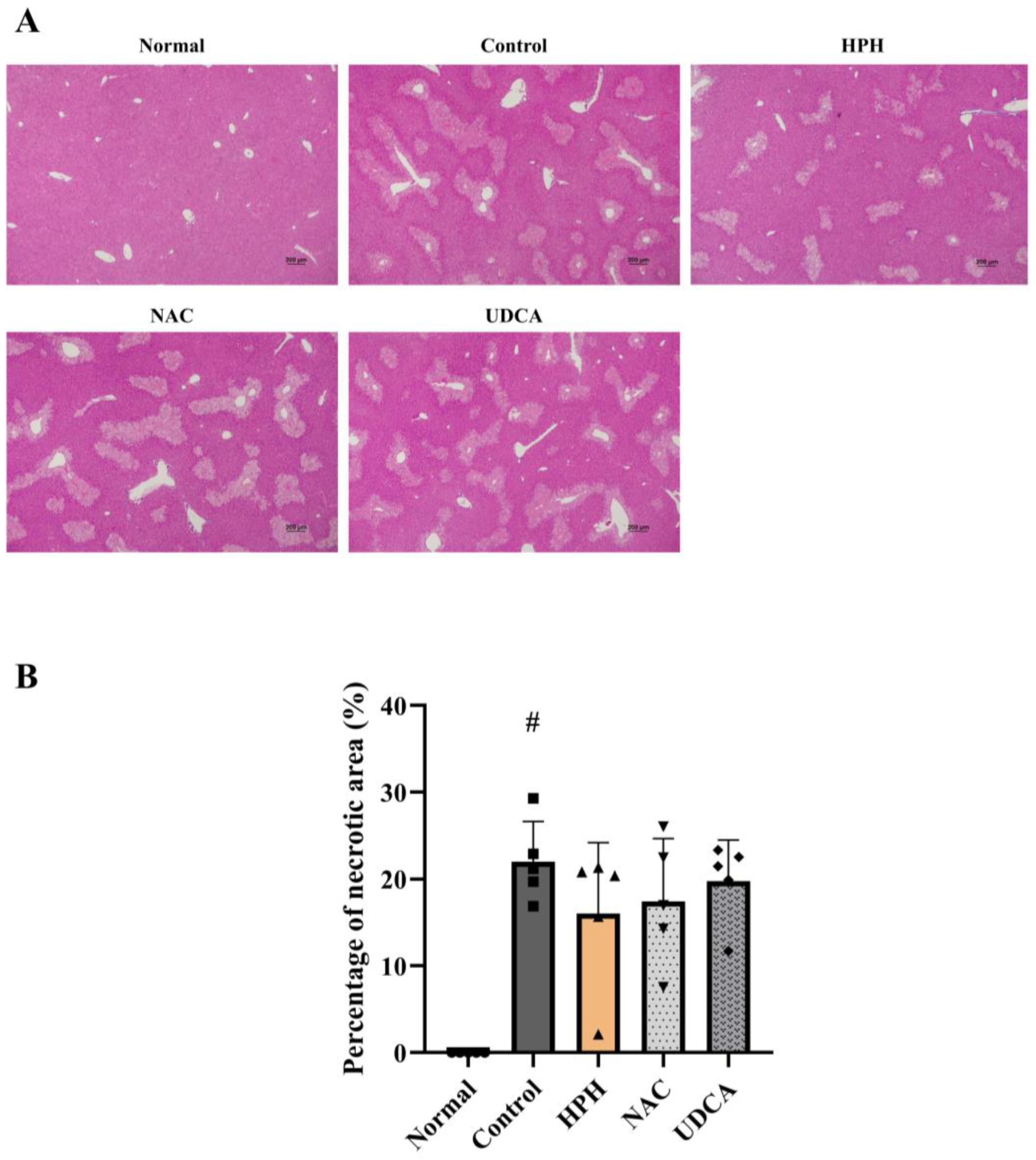
3.2. Effect of HPH on APAP-Induced Acute Liver Injury
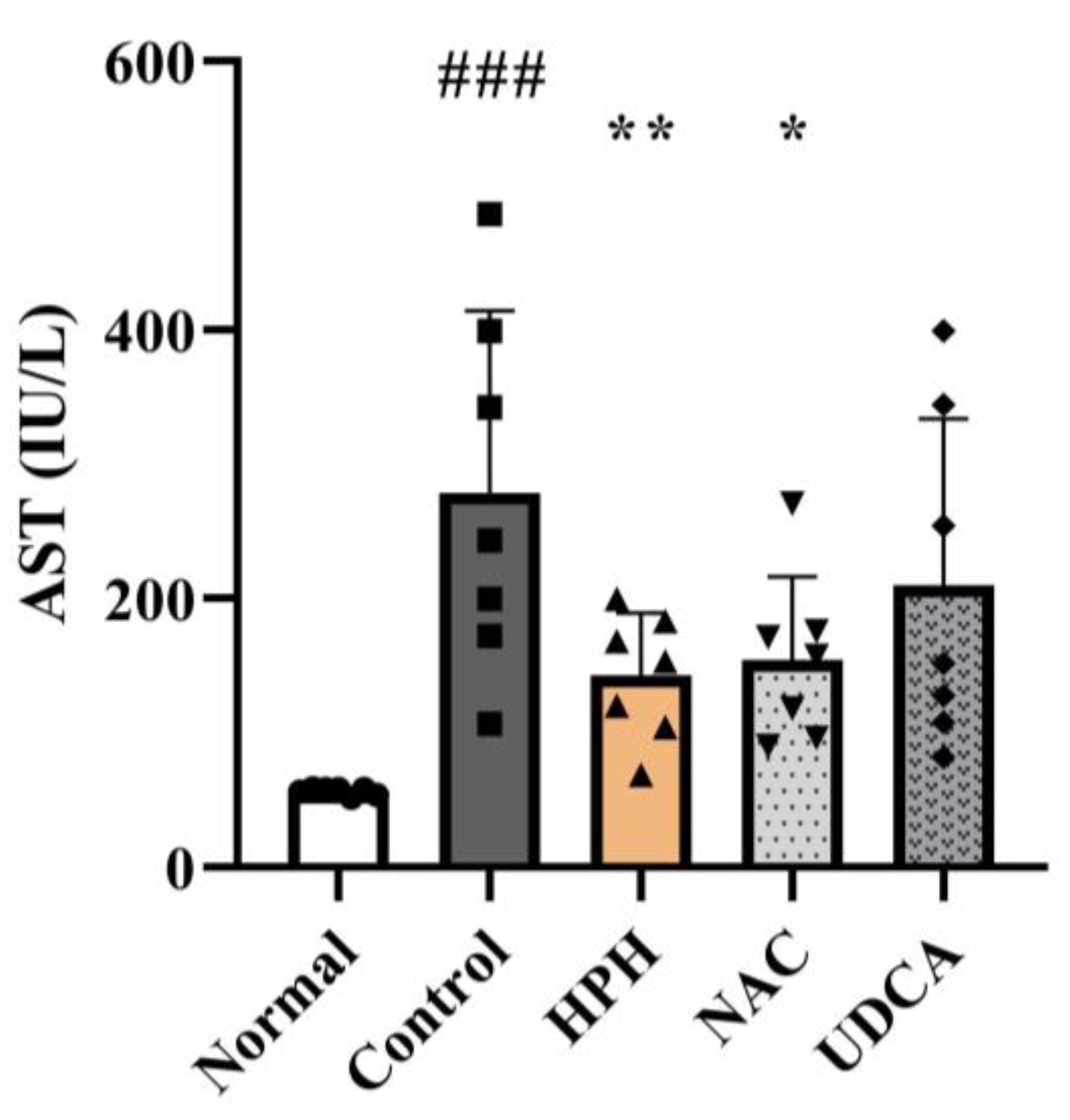
3.3. Effect of HPH on Liver Enzymes
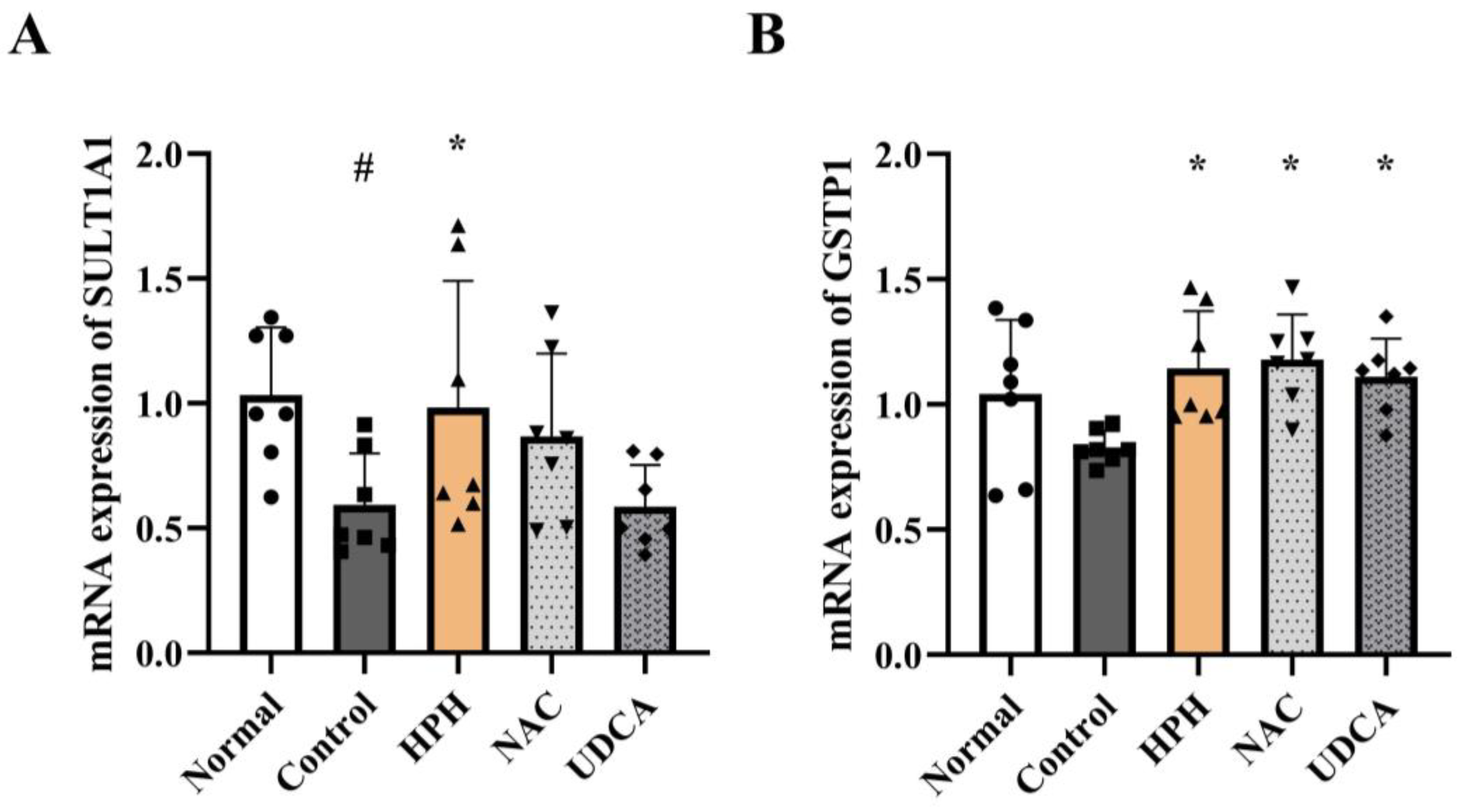
3.4. Effect of HPH on Modulation of Phase Ⅰ/Ⅱ Enzyme Expression
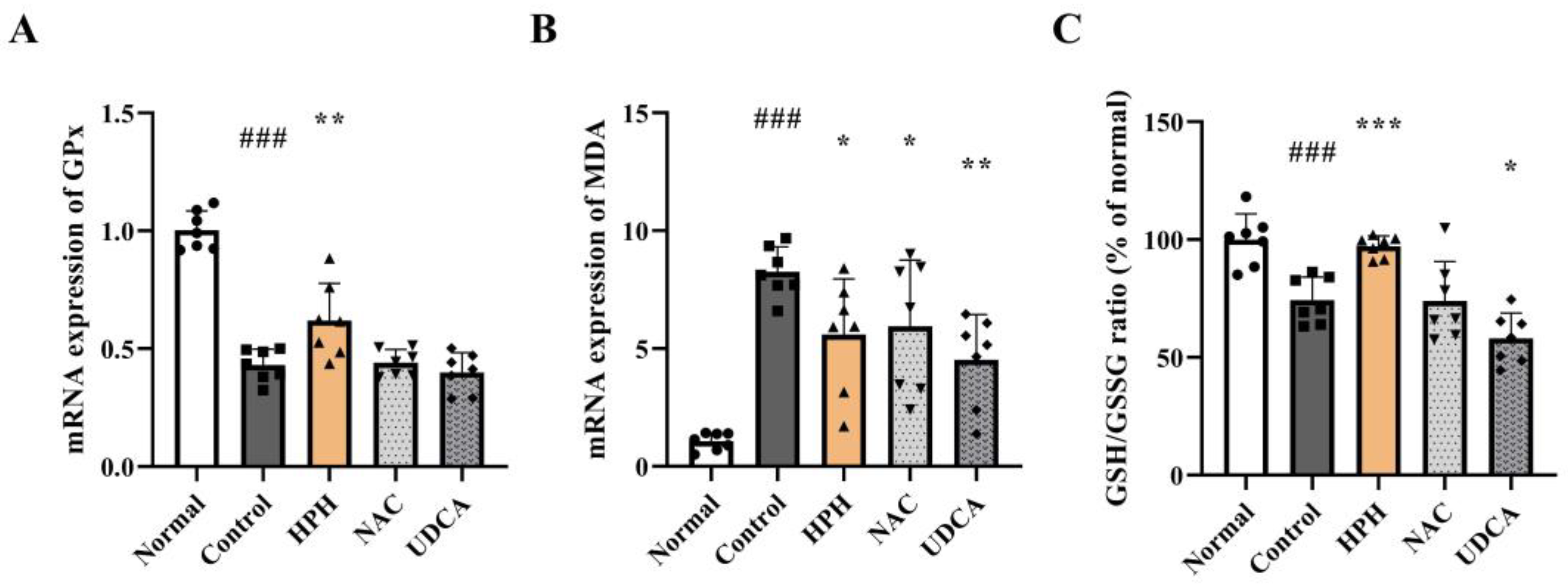
3.5. Effect of HPH on Antioxidant Activity
3.6. Effect of HPH on Inflammatory Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, W.M. Drug-induced acute liver failure. Clin. Liver Dis. 2013, 17, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Huo, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018, 17, 274–283. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Jaeschke, H. Mechanisms of Acetaminophen-Induced Liver Injury. In Cellular Injury in Liver Diseases; Ding, W.-X., Yin, X.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 55–76. [Google Scholar]
- Yoon, E.; Babar, A.; Choudhary, M.; Kutner, M.; Pyrsopoulos, N. Acetaminophen-Induced Hepatotoxicity: A Comprehensive Update. J. Clin. Transl. Hepatol. 2016, 4, 131–142. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen-related hepatotoxicity. Clin. Liver Dis. 2013, 17, 587–607. [Google Scholar] [CrossRef] [PubMed]
- Akakpo, J.Y.; Ramachandran, A.; Jaeschke, H. Novel strategies for the treatment of acetaminophen hepatotoxicity. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1039–1050. [Google Scholar] [CrossRef]
- Corcoran, G.B.; Wong, B.K. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: Studies with N-acetyl-D-cysteine in mice. J. Pharmacol. Exp. Ther. 1986, 238, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, G.B.; Todd, E.L.; Racz, W.J.; Hughes, H.; Smith, C.V.; Mitchell, J.R. Effects of N-acetylcysteine on the disposition and metabolism of acetaminophen in mice. J. Pharmacol. Exp. Ther. 1985, 232, 857–863. [Google Scholar] [CrossRef]
- Smilkstein, M.J.; Knapp, G.L.; Kulig, K.W.; Rumack, B.H. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N. Engl. J. Med. 1988, 319, 1557–1562. [Google Scholar] [CrossRef]
- Whyte, A.J.; Kehrl, T.; Brooks, D.E.; Katz, K.D.; Sokolowski, D. Safety and effectiveness of acetadote for acetaminophen toxicity. J. Emerg. Med. 2010, 39, 607–611. [Google Scholar] [CrossRef]
- Jung, J.; Lee, H.J.; Lee, J.M.; Na, K.H.; Hwang, S.G.; Kim, G.J. Placenta extract promote liver regeneration in CCl4-injured liver rat model. Int. Immunopharmacol. 2011, 11, 976–984. [Google Scholar] [CrossRef]
- Jung, J.; Moon, J.W.; Choi, J.H.; Lee, Y.W.; Park, S.H.; Kim, G.J. Epigenetic Alterations of IL-6/STAT3 Signaling by Placental Stem Cells Promote Hepatic Regeneration in a Rat Model with CCl4-induced Liver Injury. Int. J. Stem Cells 2015, 8, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Jang, Y.; Park, A.Y.; Lee, J.M.; Jeong, K.; Jeon, S.H.; Jin, H.; Im, M.; Kim, J.W.; Kim, B.J. Human Placenta Extract (HPH) Suppresses Inflammatory Responses in TNF-α/IFN-γ-Stimulated HaCaT Cells and a DNCB Atopic Dermatitis (AD)-Like Mouse Model. J. Microbiol. Biotechnol. 2024, 34, 1969–1980. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Park, D.S.; Jang, J.Y.; Lee, I.; Kim, J.M.; Choi, G.S.; Oh, C.T.; Kim, J.Y.; Han, H.J.; Han, B.S.; et al. Human Placenta Hydrolysate Promotes Liver Regeneration Activation of the Cytokine/Growth Factor-Mediated Pathway and Anti-oxidative Effect. Biol. Pharm. Bull. 2019, 42, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Bak, D.H.; Na, J.; Choi, M.J.; Lee, B.C.; Oh, C.T.; Kim, J.Y.; Han, H.J.; Kim, M.J.; Kim, T.H.; Kim, B.J. Anti-apoptotic effects of human placental hydrolysate against hepatocyte toxicity and. Int. J. Mol. Med. 2018, 42, 2569–2583. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010, 196, 369–405. [Google Scholar] [CrossRef]
- Muhammad-Azam, F.; Nur-Fazila, S.H.; Ain-Fatin, R.; Mustapha Noordin, M.; Yimer, N. Histopathological changes of acetaminophen-induced liver injury and subsequent liver regeneration in BALB/C and ICR mice. Vet. World 2019, 12, 1682–1688. [Google Scholar] [CrossRef]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016, 10, 148–156. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Heard, K.J. Acetylcysteine for acetaminophen poisoning. N. Engl. J. Med. 2008, 359, 285–292. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, H.; Wang, X.; Li, J.; Jiang, L. The Dual Role of Innate Immune Response in Acetaminophen-Induced Liver Injury. Biology 2022, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chen, S.; Xie, M.; Zhou, C.; Zheng, M. The complex roles of neutrophils in APAP-induced liver injury. Cell Prolif. 2021, 54, e13040. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Yang, T.; Wang, C.Y.; Liu, Q.; Yao, J.H.; Sun, H.J.; Kaku, T.; Liu, K.X. Laennec Protects Murine from Concanavalin A-Induced Liver Injury through Inhibition of Inflammatory Reactions and Hepatocyte Apoptosis. Biol. Pharm. Bull. 2008, 31, 2040–2044. [Google Scholar] [CrossRef]
- Tonello, G.; Daglio, M.; Zaccarelli, N.; Sottofattori, E.; Mazzei, M.; Balbi, A. Characterization and quantitation of the active polynucleotide fraction (PDRN) from human placenta, a tissue repair stimulating agent. J. Pharm. Biomed. Anal. 1996, 14, 1555–1560. [Google Scholar] [CrossRef]
- Maezono, K.; Mawatari, K.; Kajiwara, K.; Shinkai, A.; Maki, T. Effect of alanine on D-galactosamine-induced acute liver failure in rats. Hepatology 1996, 24, 1211–1216. [Google Scholar] [CrossRef]
- Maezono, K.; Kajiwara, K.; Mawatari, K.; Shinkai, A.; Torii, K.; Maki, T. Alanine protects liver from injury caused by F-galactosamine and CCl4. Hepatology 1996, 24, 185–191. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, E.H. Therapeutic Effects of Amino Acids in Liver Diseases: Current Studies and Future Perspectives. J. Cancer Prev. 2019, 24, 72–78. [Google Scholar] [CrossRef]
- Neo, S.; Makiishi, E.; Fujimoto, A.; Hisasue, M. Human placental hydrolysate promotes the long-term culture of hepatocyte-like cells derived from canine bone marrow. J. Vet. Med. Sci. 2021, 82, 1821–1827. [Google Scholar] [CrossRef]
- Pogozhykh, O.; Prokopyuk, V.; Figueiredo, C.; Pogozhykh, D. Placenta and Placental Derivatives in Regenerative Therapies: Experimental Studies, History, and Prospects. Stem Cells Int. 2018, 2018, 4837930. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.P.; Zhang, B.; Niu, Y.; Jiang, M.; Gao, J.; Zhai, Y.; Hoon Lee, J.; Uppal, H.; Tian, H.; Tortorici, M.A.; et al. Activation of liver X receptor increases acetaminophen clearance and prevents its toxicity in mice. Hepatology 2011, 54, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.H.; Fan, L.; Zhang, Y.; Zhu, Y.K.; Zong, X.L.; Peng, G.N.; Cao, S.Z. Protective Effect and Mechanism of Placenta Extract on Liver. Nutrients 2022, 14, 5071. [Google Scholar] [CrossRef]
- Krenkel, O.; Mossanen, J.C.; Tacke, F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg. Nutr. 2014, 3, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, Y.; Sugiyama, S.; Horie, T. Th1/Th2 cytokine balance as a determinant of acetaminophen-induced liver injury. Chem. Biol. Interact. 2009, 179, 273–279. [Google Scholar] [CrossRef]
- Yamauchi, A.; Kamiyoshi, A.; Koyama, T.; Iinuma, N.; Yamaguchi, S.; Miyazaki, H.; Hirano, E.; Kaku, T.; Shindo, T. Placental extract ameliorates non-alcoholic steatohepatitis (NASH) by exerting protective effects on endothelial cells. Heliyon 2017, 3, e00416. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, I.; Kang, C.-G.; Lim, S.-J.; Kim, H.-J.; Kang, R.; Jeon, S.-H.; Lee, S.-H.; Kim, J.-W.; Kang, J.-S. Human Placenta Hydrolysate Protects Against Acetaminophen-Induced Liver Injury in Mice. Biomedicines 2025, 13, 1219. https://doi.org/10.3390/biomedicines13051219
Hwang I, Kang C-G, Lim S-J, Kim H-J, Kang R, Jeon S-H, Lee S-H, Kim J-W, Kang J-S. Human Placenta Hydrolysate Protects Against Acetaminophen-Induced Liver Injury in Mice. Biomedicines. 2025; 13(5):1219. https://doi.org/10.3390/biomedicines13051219
Chicago/Turabian StyleHwang, Inyoung, Chi-Gu Kang, So-Jung Lim, Hyun-Jin Kim, Ryun Kang, So-Hyun Jeon, Sang-Hoon Lee, Jae-Won Kim, and Ju-Seop Kang. 2025. "Human Placenta Hydrolysate Protects Against Acetaminophen-Induced Liver Injury in Mice" Biomedicines 13, no. 5: 1219. https://doi.org/10.3390/biomedicines13051219
APA StyleHwang, I., Kang, C.-G., Lim, S.-J., Kim, H.-J., Kang, R., Jeon, S.-H., Lee, S.-H., Kim, J.-W., & Kang, J.-S. (2025). Human Placenta Hydrolysate Protects Against Acetaminophen-Induced Liver Injury in Mice. Biomedicines, 13(5), 1219. https://doi.org/10.3390/biomedicines13051219






