Angiogenic Factors and Inflammatory Bowel Diseases
Abstract
1. Introduction
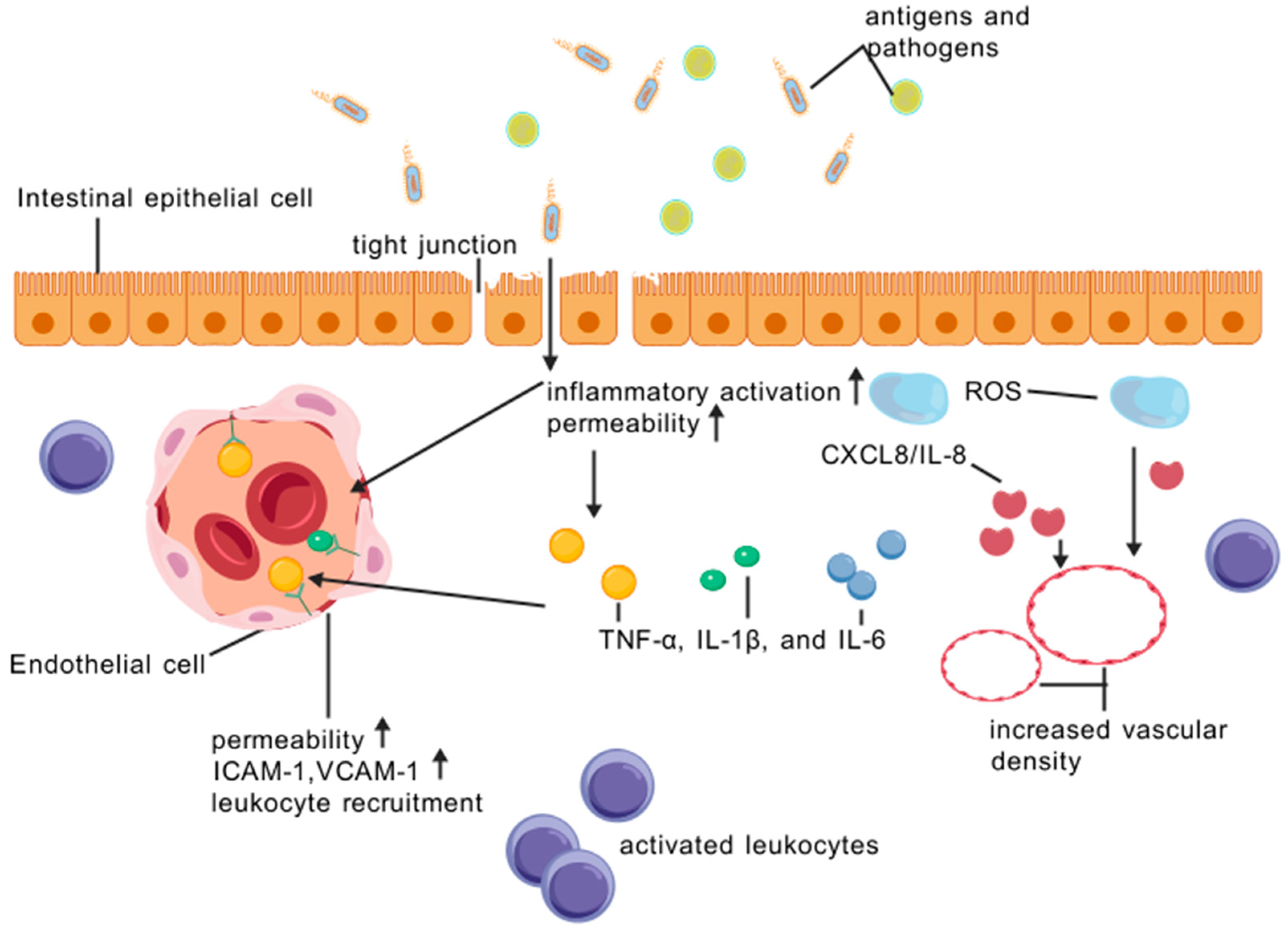
2. The Vascular and Epithelial Barrier
3. Mechanism of Angiogenesis in IBD
3.1. Sprouting Angiogenesis
3.2. Intussusceptive Angiogenesis
4. Inflammation and Angiogenesis in IBD
5. Angiogenic Growth Factors in IBD
5.1. Vascular Endothelial Growth Factor (VEGF)
5.2. Angiopoietins (ANG)
5.3. Platelet-Derived Growth Factor (PDGF)
5.4. Basic Fibroblast Growth Factors (FGFs)
5.5. Inducible Nitric Oxide Synthase (iNOS)
5.6. Other Factors Related to Angiogenesis in IBD
6. Antiangiogenic Therapies in IBD
7. Other Therapies Modulating Angiogenesis in IBD
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ng, S.C.; Tang, W.; Ching, J.Y.; Wong, M.; Chow, C.M.; Lam, C.F.; Leung, W.K. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and Colitis Epidemiology Study. Gastroenterology 2017, 153, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Buie, M.J.; Quan, J.; Windsor, J.W.; Coward, S.; Hansen, T.M.; King, J.A.; Kotze, P.G.; Gearry, R.B.; Ng, S.C.; Mak, J.W.Y.; et al. Global Hospitalization Trends for Crohn’s Disease and Ulcerative Colitis in the 21st Century: A Systematic Review With Temporal Analyses. Clin. Gastroenterol. Hepatol. 2023, 21, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Lin, D.; Jin, Y.; Shao, X.; Xu, Y.; Ma, G.; Jiang, Y.; Hu, D. Global, regional, and national burden of inflammatory bowel disease, 1990–2021: Insights from the global burden of disease 2021. Int. J. Color. Dis. 2024, 39, 139. [Google Scholar] [CrossRef]
- van Linschoten, R.C.A.; van der Woude, C.J.; Visser, E.; van Leeuwen, N.; Bodelier, A.G.L.; Fitzpatrick, C.; de Jonge, V.; Vermeulen, H.; Verweij, K.E.; van der Wiel, S.; et al. Variation Between Hospitals in Outcomes and Costs of IBD Care: Results From the IBD Value Study. Inflamm. Bowel Dis. 2024, 26, izae095. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bernstein, C.N. Environmental risk factors for inflammatory bowel disease. United Eur. Gastroenterol. J. 2022, 10, 1047–1053. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Zielińska, M.; Sokal, A.; Filip, R. Genetic and Epigenetic Etiology of Inflammatory Bowel Disease: An Update. Genes 2022, 13, 2388. [Google Scholar] [CrossRef]
- MaMentella, C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef]
- Kong, L.; Pokatayev, V.; Lefkovith, A.; Carter, G.T.; Creasey, E.A.; Krishna, C.; Subramanian, S.; Kochar, B.; Ashenberg, O.; Lau, H.; et al. The landscape of immune dysregulation in Crohn’s disease revealed through single-cell transcriptomic profiling in the ileum and colon. Immunity 2023, 56, 444–458.e5. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, M.; Peng, Q.; Li, L.; Tian, F.; Guo, Y.; Jing, C. Angiogenesis, a key point in the association of gut microbiota and its metabolites with disease. Eur. J. Med. Res. 2024, 29, 614. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, C.; Hu, S.; Yang, K.; Yu, J.; Sun, X.; Zheng, J. Bioinformatic analysis and in vivo validation of angiogenesis related genes in inflammatory bowel disease. Biocell 2023, 47, 2735–2745. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, Y.; Xu, L.; Liu, H.; Ge, W.; Wu, B.; Duan, H.; Zhang, H.; Fu, Y.; Xu, H. Inhibition of Angiogenesis and Effect on Inflammatory Bowel Disease of Ginsenoside Rg3-Loaded Thermosensitive Hydrogel. Pharmaceutics 2024, 16, 1243. [Google Scholar] [CrossRef] [PubMed]
- Britzen-Laurent, N.; Weidinger, C.; Sturzl, M. Contribution of Blood Vessel Activation, Remodeling and Barrier Function to Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2023, 24, 5517. [Google Scholar] [CrossRef] [PubMed]
- Alkim, C.; Alkim, H.; Koksal, A.R.; Boga, S.; Sen, I. Angiogenesis in Inflammatory Bowel Disease. Int. J. Inflamm. 2015, 2015, 970890. [Google Scholar] [CrossRef]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Nakai, D.; Miyake, M. Intestinal Membrane Function in Inflammatory Bowel Disease. Pharmaceutics 2023, 16, 29. [Google Scholar] [CrossRef]
- Stewart, A.S.; Pratt-Phillips, S.; Gonzalez, L.M. Alterations in Intestinal Permeability: The Role of the “Leaky Gut” in Health and Disease. J. Equine Vet. Sci. 2017, 52, 10–22. [Google Scholar] [CrossRef]
- Hu, J.C.E.; Weiß, F.; Bojarski, C.; Branchi, F.; Schulzke, J.D.; Fromm, M.; Krug, S.M. Expression of tricellular tight junction proteins and the paracellular macromolecule barrier are recovered in remission of ulcerative colitis. BMC Gastroenterol. 2021, 21, 141. [Google Scholar] [CrossRef]
- Hurairah, H.; Ferro, A. The role of the endothelium in the control of vascular function. Int. J. Clin. Pract. 2004, 58, 173–183. [Google Scholar] [CrossRef]
- Duan, Y.; Prasad, R.; Feng, D.; Beli, E.; Li Calzi, S.; Longhini, A.L.F.; Lamendella, R.; Floyd, J.L.; Dupont, M.; Noothi, S.K.; et al. Bone Marrow-Derived Cells Restore Functional Integrity of the Gut Epithelial and Vascular Barriers in a Model of Diabetes and ACE2 Deficiency. Circ. Res. 2019, 125, 969–988. [Google Scholar] [CrossRef]
- Dicks, L.M.T.; Dreyer, L.; Smith, C.; van Staden, A.D. A Review: The Fate of Bacteriocins in the Human Gastro-Intestinal Tract: Do They Cross the Gut-Blood Barrier? Front. Microbiol. 2018, 9, 2297. [Google Scholar]
- Balda, M.S.; Matter, K. Tight junctions at a glance. J. Cell Sci. 2008, 121 Pt 22, 3677–3682. [Google Scholar] [CrossRef] [PubMed]
- Bruewer, M.; Luegering, A.; Kucharzik, T.; Parkos, C.A.; Madara, J.L.; Hopkins, A.M.; Nusrat, A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003, 171, 6164–6172. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Pettersson, S.; Büschenfelde, K.H.M.Z.; Strober, W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-κB abrogates established experimental colitis in mice. Nat. Med. 1996, 2, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.Y.; Osterreicher, C.H.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, J.; Piao, H.; Zhu, Z.; Wei, R.; Liu, K. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar] [CrossRef]
- Haque, M.; Kaminsky, L.; Abdulqadir, R.; Engers, J.; Kovtunov, E.; Rawat, M.; Al-Sadi, R.; Ma, T.Y. Lactobacillus acidophilus inhibits the TNF-α-induced increase in intestinal epithelial tight junction permeability via a TLR-2 and PI3K-dependent inhibition of NF-κB activation. Front. Immunol. 2024, 15, 1348010. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Ayansola, H.; Mayorga, E.J.; Jin, Y. Subepithelial Stromal Cells: Their Roles and Interactions with Intestinal Epithelial Cells during Gut Mucosal Homeostasis and Regeneration. Biomedicines 2024, 12, 668. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef]
- Balda, M.S.; Matter, K. Tight junctions. Curr. Biol. 2023, 33, R1135–R1140. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. The role of the mucosal barrier system in maintaining gut symbiosis to prevent intestinal inflammation. Semin. Immunopathol. 2024, 47, 2. [Google Scholar] [CrossRef]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.P.; Ooi, J.D.; Goldberg, R. The interplay between the microbiota, diet and T regulatory cells in the preservation of the gut barrier in inflammatory bowel disease. Front. Microbiol. 2023, 14, 1291724. [Google Scholar]
- Catalioto, R.-M.; Maggi, C.A.; Giuliani, S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr. Med. Chem. 2011, 18, 398–426. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Panaccione, R.; Ferraz, J.G.; Beck, P. Advances in medical therapy of inflammatory bowel disease. Curr. Opin. Pharmacol. 2005, 5, 566–572. [Google Scholar] [CrossRef]
- Dong, Y.; Fan, H.; Zhang, Z.; Jiang, F.; Li, M.; Zhou, H.; Guo, W.; Zhang, Z.; Kang, Z.; Gui, Y.; et al. Berberine ameliorates DSS-induced intestinal mucosal barrier dysfunction through microbiota-dependence and Wnt/β-catenin pathway. Int. J. Biol. Sci. 2022, 18, 1381–1397. [Google Scholar] [CrossRef]
- Ibrahim, S.; Zhu, X.; Luo, X.; Feng, Y.; Wang, J. PIK3R3 regulates ZO-1 expression through the NF-kB pathway in inflammatorybowel disease. Int. Immunopharmacol. 2020, 85, 106610. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, M.; Yan, Y.; Wang, Y.; Zhao, X.; Yang, J.; Chen, J.; Chen, C.; Tang, L.; Zeng, W.; et al. The miR-7/EGFR axis controls the epithelial cell immunomodulation and regeneration and orchestrates the pathology in inflammatory bowel disease. J. Adv. Res. 2024, 57, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- Pai, Y.-C.; Li, Y.-H.; Turner, J.R.; Yu, L.C.-H. Transepithelial Barrier Dysfunction Drives Microbiota Dysbiosis to Initiate Epithelial Clock-driven Inflammation. J. Crohn’s Colitis 2023, 17, 1471–1488. [Google Scholar] [CrossRef]
- Zhang, J.; Cen, L.; Zhang, X.; Tang, C.; Chen, Y.; Zhang, Y.; Yu, M.; Lu, C.; Li, M.; Li, S.; et al. MPST deficiency promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via AKT. Redox Biol. 2022, 56, 102469. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, M.; Erkert, L.; Becker, C. Cytokine-Mediated Crosstalk between Immune Cells and Epithelial Cells in the Gut. Cells 2021, 10, 111. [Google Scholar] [CrossRef]
- Danese, S.; Sans, M.; de la Motte, C.; Graziani, C.; West, G.; Phillips, M.H.; Pola, R.; Rutella, S.; Willis, J.; Gasbarrini, A.; et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology 2006, 130, 2060–2073. [Google Scholar] [CrossRef]
- Zoroddu, S.; Di Lorenzo, B.; Paliogiannis, P.; Mangoni, A.A.; Carru, C.; Zinellu, A. Vascular endothelial growth factor in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2025, 55, e14361. [Google Scholar] [CrossRef]
- Aksoy, E.K.; Çetinkaya, H.; Savaş, B.; Ensari, A.; Torgutalp, M.; Efe, C. Vascular endothelial growth factor, endostatin levels and clinical features among patients with ulcerative colitis and irritable bowel syndrome and among healthy controls: A cross-sectional analytical study. Sao Paulo Med. J. 2018, 136, 543–550. [Google Scholar] [CrossRef]
- Pousa, I.D.; Maté, J.; Gisbert, J.P. Angiogenesis in inflammatory bowel disease. Eur. J. Clin. Investig. 2008, 38, 73–81. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Neubauer, K.; Matusiewicz, M. Platelet-derived growth factor-BB reflects clinical, inflammatory and angiogenic disease activity and oxidative stress in inflammatory bowel disease. Clin. Biochem. 2009, 42, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Macé, V.; Ahluwalia, A.; Coron, E.; Le Rhun, M.; Boureille, A.; Bossard, C.; Mosnier, J.F.; Matysiak-Budnik, T.; Tarnawski, A.S. Confocal laser endomicroscopy: A new gold standard for the assessment of mucosal healing in ulcerative colitis. J. Gastroenterol. Hepatol. 2015, 30 (Suppl. S1), 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, C.; Colucci, R.; Segnani, C.; Errede, M.; Girolamo, F.; Virgintino, D.; Dolfi, A.; Tirotta, E.; Buccianti, P.; Di Candio, G.; et al. Fibrotic and Vascular Remodelling of Colonic Wall in Patients with Active Ulcerative Colitis. J. Crohn’s Colitis 2016, 10, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Danese, S. Inflammation and the mucosal microcirculation in inflammatory bowel disease: The ebb and flow. Curr. Opin. Gastroenterol. 2007, 23, 384–389. [Google Scholar] [CrossRef]
- Binion, D.G.; Rafiee, P. Is inflammatory bowel disease a vascular disease? Targeting angiogenesis improves chronic inflammation in inflammatory bowel disease. Gastroenterology 2009, 136, 400–403. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Maltezos, E.; Papazoglou, D.; Simopoulos, C.; Gatter, K.C.; Harris, A.L.; Koukourakis, M.I. Hypoxia inducible factor 1α and 2α overexpression in inflammatory bowel disease. J. Clin. Pathol. 2003, 56, 209–213. [Google Scholar] [CrossRef]
- Konerding, M.A.; Turhan, A.; Ravnic, D.J.; Lin, M.; Fuchs, C.; Secomb, T.W.; Tsuda, A.; Mentzer, S.J. Inflammation-induced intussusceptive angiogenesis in murine colitis. Anat. Rec. 2010, 293, 849–857. [Google Scholar] [CrossRef]
- Djonov, V.; Baum, O.; Burri, P.H. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003, 314, 107–117. [Google Scholar] [CrossRef]
- Groppa, E.; Brkic, S.; Uccelli, A.; Wirth, G.; Korpisalo-Pirinen, P.; Filippova, M.; Dasen, B.; Sacchi, V.; Muraro, M.G.; Trani, M.; et al. EphrinB2/EphB4 signaling regulates non-sprouting angiogenesis by VEGF. EMBO Rep. 2018, 19, e45054. [Google Scholar] [CrossRef]
- Ackermann, M.; Tsuda, A.; Secomb, T.W.; Mentzer, S.J.; Konerding, M.A. Intussusceptive remodeling of vascular branch angles in chemically-induced murine colitis. Microvasc. Res. 2013, 87, 75–82. [Google Scholar] [CrossRef][Green Version]
- Esteban, S.; Clemente, C.; Koziol, A.; Gonzalo, P.; Rius, C.; Martínez, F.; Linares, P.M.; Chaparro, M.; Urzainqui, A.; Andrés, V.; et al. Endothelial MT1-MMP targeting limits intussusceptive angiogenesis and colitis via TSP1/nitric oxide axis. EMBO Mol. Med. 2020, 12, e10862. [Google Scholar] [CrossRef] [PubMed]
- Benelli, R.; Lorusso, G.; Albini, A.; Noonan, D.M. Cytokines and chemokines as regulators of angiogenesis in health and disease. Curr. Pharm. Des. 2006, 12, 3101–3115. [Google Scholar] [CrossRef] [PubMed]
- Reenaers, C.; Louis, E. Advances in the management of inflammatory bowel diseases. Rev. Med. Liege 2022, 77, 323–329. [Google Scholar]
- Deban, L.; Correale, C.; Vetrano, S.; Malesci, A.; Danese, S. Multiple pathogenic role of microvasculature in inflammatory bowel disease: A Jack of all trades. Am. J. Pathol. 2008, 172, 1457–1466. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2023, 9, 669–676. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Vetrano, S.; Sans, M.; Arena, V.; Straface, G.; Stigliano, E.; Repici, A.; Sturm, A.; Malesci, A.; Panes, J.; et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology 2009, 136, 585–595.e5. [Google Scholar] [CrossRef]
- Alkim, C.; Sakiz, D.; Alkim, H.; Livaoglu, A.; Kendir, T.; Demirsoy, H.; Erdem, L.; Akbayir, N.; Sokmen, M. Thrombospondin-1 and VEGF in inflammatory bowel disease. Libyan J. Med. 2012, 7, 8942. [Google Scholar] [CrossRef] [PubMed]
- van Beijnum, J.R.; Huijbers, E.J.M.; van Loon, K.; Blanas, A.; Akbari, P.; Roos, A.; Wong, T.J.; Denisov, S.S.; Hackeng, T.M.; Jimenez, C.R.; et al. Extracellular vimentin mimics VEGF and is a target for anti-angiogenic immunotherapy. Nat. Commun. 2022, 13, 2842. [Google Scholar] [CrossRef]
- Tsiolakidou, G.; Koutroubakis, I.E.; Tzardi, M.; Kouroumalis, E.A. Increased expression of VEGF and CD146 in patients with inflammatory bowel disease. Dig. Liver Dis. 2008, 40, 673–679. [Google Scholar] [CrossRef]
- Tolstanova, G.; Deng, X.; Khomenko, T.; Garg, P.; Paunovic, B.; Chen, L.; Sitaraman, S.V.; Shiloach, J.; Szabo, S.; Sandor, Z. Role of anti-angiogenic factor endostatin in the pathogenesis of experimental ulcerative colitis. Life Sci. 2011, 88, 74–81. [Google Scholar] [CrossRef]
- D’Alessio, S.; Correale, C.; Tacconi, C.; Gandelli, A.; Pietrogrande, G.; Vetrano, S.; Genua, M.; Arena, V.; Spinelli, A.; Peyrin-Biroulet, L.; et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J. Clin. Investig. 2014, 124, 3863–3878. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhao, J.; Qin, L.; Qiao, M. Promoting inflammatory lymphangiogenesis by vascular endothelial growth factor-C (VEGF-C) aggravated intestinal inflammation in mice with experimental acute colitis. Braz. J. Med. Biol. Res. 2016, 49, e4738. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Cao, J.; Ye, C. Exosomes from Adipose-Derived Stem Cells Promotes VEGF-C-Dependent Lymphangiogenesis by Regulating miRNA-132/TGF-β Pathway. Cell Physiol. Biochem. 2018, 49, 160–171. [Google Scholar] [CrossRef]
- Harit, A.L.; Ng, S.C. Crohn’s disease. Medicine 2015, 43, 282–290. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 2002, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- De Zoeten, E.F.; Battista, K.D.; Colson, S.B.; Lovell, M.A.; Kessler, B.E.; Isfort, R.W.; Fennimore, B.P.; Onyiah, J.C.; Kao, D.J.; Yeckes, A.; et al. Markers of Hypoxia Correlate with Histologic and Endoscopic Severity of Colitis in Inflammatory Bowel Disease. Hypoxia 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Tolstanova, G.; Deng, X.; Ahluwalia, A.; Paunovic, B.; Prysiazhniuk, A.; Ostapchenko, L.; Tarnawski, A.; Sandor, Z.; Szabo, S. Role of Dopamine and D2 Dopamine Receptor in the Pathogenesis of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 2963–2975. [Google Scholar] [CrossRef]
- Nejabati, H.R.; Latifi, Z.; Ghasemnejad, T.; Fattahi, A.; Nouri, M. Placental growth factor (PlGF) as an angiogenic/inflammatory switcher: Lesson from early pregnancy losses. Gynecol. Endocrinol. 2017, 33, 668–674. [Google Scholar] [CrossRef]
- Zhou, Y.; Tu, C.; Zhao, Y.; Liu, H.; Zhang, S. Placental growth factor enhances angiogenesis in human intestinal microvascular endothelial cells via PI3K/Akt pathway: Potential implications of inflammation bowel disease. Biochem. Biophys. Res. Commun. 2016, 470, 967–974. [Google Scholar] [CrossRef]
- Thapa, K.; Khan, H.; Kaur, G.; Kumar, P.; Singh, T.G. Therapeutic targeting of angiopoietins in tumor angiogenesis and cancer development. Biochem. Biophys. Res. Commun. 2023, 687, 149130. [Google Scholar] [CrossRef]
- Oikonomou, K.A.; Kapsoritakis, A.N.; Kapsoritaki, A.I.; Manolakis, A.C.; Tiaka, E.K.; Tsiopoulos, F.D.; Tsiompanidis, I.A.; Potamianos, S.P. Angiogenin, angiopoietin-1, angiopoietin-2 and endostatin serum levels in inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 7, 963–970. [Google Scholar] [CrossRef]
- Ganta, V.C.; Cromer, W.; Mills, G.L.; Traylor, J.; Jennings, M.; Daley, S.; Clark, B.; Mathis, J.M.; Bernas, M.; Boktor, M.; et al. Angiopoietin-2 in experimental colitis. Inflamm. Bowel Dis. 2010, 16, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, S.; Xue, A.; Shi, J.; Zheng, C.; Huang, Y. Thalidomide Inhibits Angiogenesis via Downregulation of VEGF and Angiopoietin-2 in Crohn’s Disease. Inflammation 2021, 44, 795–807. [Google Scholar] [CrossRef]
- Fang, Z.; Qu, S.; Ji, X.; Zheng, C.; Mo, J.; Xu, J.; Zhang, J.; Shen, H. Correlation between PDGF-BB and M1-type macrophage in inflammatory bowel disease: A case-control study. BMC Gastroenterol. 2024, 24, 417. [Google Scholar] [CrossRef] [PubMed]
- Kataria, J.; Kerr, J.; Lourenssen, S.R.; Blennerhassett, M.G. Nintedanib regulates intestinal smooth muscle hyperplasia and phenotype in vitro and in TNBS colitis in vivo. Sci. Rep. 2022, 12, 10275. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fierro, M.L.; Garza-Veloz, I.; Rocha-Pizaña, M.R.; Cardenas-Vargas, E.; Cid-Baez, M.A.; Trejo-Vazquez, F.; Flores-Morales, V.; Villela-Ramirez, G.A.; Delgado-Enciso, I.; Rodriguez-Sanchez, I.P.; et al. Serum cytokine, chemokine, and growth factor profiles and their modulation in inflammatory bowel disease. Medicine 2019, 98, e17208. [Google Scholar] [CrossRef]
- Rioux, J.D.; Boucher, G.; Forest, A.; Bouchard, B.; Coderre, L.; Daneault, C.; Frayne, I.R.; Legault, J.T.; Consortium, I.; Bitton, A.; et al. A pilot study to identify blood-based markers associated with response to treatment with Vedolizumab in patients with Inflammatory Bowel Disease. medRxiv 2024. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Ciccocioppo, R.; Armellini, E.; Morera, R.; Ricevuti, L.; Cazzola, P.; Fulle, I.; Corazza, G.R. Serum bFGF and VEGF correlate respectively with bowel wall thickness and intramural blood flow in Crohn’s disease. Inflamm. Bowel Dis. 2004, 10, 573–577. [Google Scholar] [CrossRef]
- Bousvaros, A.; Zurakowski, D.; Fishman, S.J.; Keough, K.; Law, T.; Sun, C.; Leichtner, A.M. Serum basic fibroblast growth factor in pediatric Crohn’s disease. Implications for wound healing. Dig. Dis. Sci. 1997, 42, 378–386. [Google Scholar] [CrossRef]
- Sessa, W.C. Molecular control of blood flow and angiogenesis: Role of nitric oxide. J. Thromb. Haemost. 2009, 7 (Suppl. S1), 35–37. [Google Scholar] [CrossRef]
- Aguilar, G.; Koning, T.; Ehrenfeld, P.; Sánchez, F.A. Role of NO and S-nitrosylation in the Expression of Endothelial Adhesion Proteins That Regulate Leukocyte and Tumor Cell Adhesion. Front. Physiol. 2020, 11, 595526. [Google Scholar] [CrossRef] [PubMed]
- Binion, D.G.; Rafiee, P.; Ramanujam, K.S.; Fu, S.; Fisher, P.J.; Rivera, M.T.; Johnson, C.P.; Otterson, M.F.; Telford, G.L.; Wilson, K.T. Deficient iNOS in inflammatory bowel disease intestinal microvascular endothelial cells results in increased leukocyte adhesion. Free Radic. Biol. Med. 2000, 29, 881–888. [Google Scholar]
- Horowitz, S.; Binion, D.G.; Nelson, V.M.; Kanaa, Y.; Javadi, P.; Lazarova, Z.; Andrekopoulos, C.; Kalyanaraman, B.; Otterson, M.F.; Rafiee, P. Increased arginase activity and endothelial dysfunction in human inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1323–G1336. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, O.A.; Binion, D.G.; Otterson, M.F.; Gutterman, D.D. Acquired microvascular dysfunction in inflammatory bowel disease:Loss of nitric oxide-mediated vasodilation. Gastroenterology 2003, 125, 58–69. [Google Scholar] [CrossRef]
- Rana, T. Influence and Implications of the Molecular Paradigm of Nitric Oxide Underlying Inflammatory Reactions of the Gastrointestinal Tract of Dog: A Major Hallmark of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Thibeault, S.; Rautureau, Y.; Oubaha, M.; Faubert, D.; Wilkes, B.C.; Delisle, C.; Gratton, J.P. S-nitrosylation of β-catenin by eNOS-derived NO promotes VEGF-induced endothelial cell permeability. Mol. Cell 2010, 39, 468–476. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Lin, M.I.; Murata, T.; Landskroner-Eiger, S.; Schleicher, M.; Kothiya, M.; Iwakiri, Y.; Yu, J.; Huang, P.L.; Sessa, W.C. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J. Cell Sci. 2013, 126, 5541–5552. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Liu, Z.; Chang, Z. Lipopolysaccharide induces vascular endothelial cell pyroptosis via the SP1/RCN2/ROS signaling pathway. Eur. J. Cell Biol. 2021, 100, 151164. [Google Scholar] [CrossRef]
- Beck, P.L.; Xavier, R.; Wong, J.; Ezedi, I.; Mashimo, H.; Mizoguchi, A.; Mizoguchi, E.; Bhan, A.K.; Podolsky, D.K. Paradoxical roles of different nitric oxide synthase isoforms in colonic injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G137–G147. [Google Scholar] [CrossRef]
- Hokari, R.; Kato, S.; Matsuzaki, K.; Kuroki, M.; Iwai, A.; Kawaguchi, A.; Nagao, S.; Miyahara, T.; Itoh, K.; Sekizuka, E.; et al. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to chronic colitis. Free Radic. Biol. Med. 2001, 31, 153–163. [Google Scholar]
- Sasaki, M.; Bharwani, S.; Jordan, P.; Elrod, J.W.; Grisham, M.B.; Jackson, T.H.; Lefer, D.J.; Alexander, J.S. Increased disease activity in eNOS-deficient mice in experimental colitis. Free Radic. Biol. Med. 2003, 35, 1679–1687. [Google Scholar]
- Vallance, B.A.; Dijkstra, G.; Qiu, B.; van der Waaij, L.A.; van Goor, H.; Jansen, P.L.; Mashimo, H.; Collins, S.M. Relative contributions of NOS isoforms during experimental colitis: Endothelial-derived NOS maintains mucosal integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G865–G874. [Google Scholar] [CrossRef] [PubMed]
- Eder, P.; Korybalska, K.; Linke, K.; Witowski, J. Angiogenesis-related proteins—Their role in the pathogenesis and treatment of inflammatory bowel disease. Curr. Protein Pept. Sci. 2015, 16, 249–258. [Google Scholar] [CrossRef]
- Kumar, M.; Murugesan, S.; Ibrahim, N.; Elawad, M.; Al Khodor, S. Predictive biomarkers for anti-TNF α therapy in IBD patients. J. Transl. Med. 2024, 22, 284. [Google Scholar] [CrossRef]
- Rutella, S.; Vetrano, S.; Correale, C.; Graziani, C.; Sturm, A.; Spinelli, A.; De Cristofaro, R.; Repici, A.; Malesci, A.; Danese, S. Enhanced platelet adhesion induces angiogenesis in intestinal inflammation and inflammatory bowel disease microvasculature. J. Cell Mol. Med. 2011, 15, 625–634. [Google Scholar] [CrossRef]
- Altorjay, I.A.; Veréb, Z.; Serfozo, Z.; Bacskai, I.; Bátori, R.; Erdodi, F.; Udvardy, M.; Sipka, S.; Lányi, Á.; Rajnavölgyi, É.; et al. Anti-TNF-α antibody (infliximab) therapy supports the recovery of eNOS and VEGFR2 protein expression in endothelial cells. Int. J. Immunopathol. Pharmacol. 2011, 24, 323–335. [Google Scholar] [CrossRef]
- Rutella, S.; Fiorino, G.; Vetrano, S.; Correale, C.; Spinelli, A.; Pagano, N.; Arena, V.; Maggiano, N.; Repici, A.; Malesci, A.; et al. Infliximab therapy inhibits inflammation-induced angiogenesis in the mucosa of patients with Crohn’s disease. Am. J. Gastroenterol. 2011, 106, 762–770. [Google Scholar] [CrossRef]
- Jakubowska, K.; Pryczynicz, A.; Iwanowicz, P.; Niewiński, A.; Maciorkowska, E.; Hapanowicz, J.; Jagodzińska, D.; Kemona, A.; Guzińska-Ustymowicz, K. Expressions of Matrix Metalloproteinases (MMP-2, MMP-7, and MMP-9) and Their Inhibitors (TIMP-1, TIMP-2) in Inflammatory Bowel Diseases. Gastroenterol. Res. Pract. 2016, 2016, 2456179. [Google Scholar] [CrossRef]
- Pehrsson, M.; Domislovic, V.; Alexdottir, M.S.; Brinar, M.; Karsdal, M.A.; Barisic, A.; Krznaric, Z.; Mortensen, J.H. Blood-Based Biomarkers Reflecting Protease 3 and MMP-12 Catalyzed Elastin Degradation as Potential Noninvasive Surrogate Markers of Endoscopic and Clinical Disease in Inflammatory Bowel Disease. J. Clin. Med. 2023, 13, 21. [Google Scholar] [CrossRef]
- Herrera-Gómez, R.G.; Grecea, M.; Gallois, C.; Boige, V.; Pautier, P.; Pistilli, B.; Planchard, D.; Malka, D.; Ducreux, M.; Mir, O. Safety and Efficacy of Bevacizumab in Cancer Patients with Inflammatory Bowel Disease. Cancers 2022, 14, 2914. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Scheiner, B.; Peck-Radosavljevic, M. Immunotherapy for advanced hepatocellular carcinoma: A focus on special subgroups. Gut 2021, 70, 204–214. [Google Scholar] [CrossRef]
- Coriat, R.; Mir, O.; Leblanc, S.; Ropert, S.; Brezault, C.; Chaussade, S.; Goldwasser, F. Feasibility of anti-VEGF agent bevacizumab in patients with Crohn’s disease. Inflamm. Bowel Dis. 2011, 17, 1632. [Google Scholar] [CrossRef] [PubMed]
- Pentapati, S.; Caucci, S.; Balmuri, S.; Devarkonda, V. Complete Remission of Advanced Hepatocellular Carcinoma in a Patient With Ulcerative Colitis Treated With Atezolizumab and Bevacizumab: A Case Report. Cureus 2023, 15, e39538. [Google Scholar] [CrossRef]
- Lanzetta, P.; Korobelnik, J.F.; Heier, J.S.; Leal, S.; Holz, F.G.; Clark, W.L.; Eichenbaum, D.; Iida, T.; Xiaodong, S.; Berliner, A.J.; et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-Week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet 2024, 403, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Saoudi Gonzalez, N.; López, D.; Gómez, D.; Ros, J.; Baraibar, I.; Salva, F.; Tabernero, J.; Élez, E. Pharmacokinetics and pharmacodynamics of approved monoclonal antibody therapy for colorectal cancer. Expert Opin. Drug Metab. Toxicol. 2022, 18, 755–767. [Google Scholar] [CrossRef]
- Kim, H.; Kim, C.Y.; Kim, D.; Kim, E.; Ma, L.; Park, K.; Liu, Z.; Huang, K.E.; Wen, W.; Ko, J.; et al. Protective Effects of Imatinib on a DSS-induced Colitis Model Through Regulation of Apoptosis and Inflammation. In Vivo 2024, 38, 2310–2317. [Google Scholar] [CrossRef]
- Teranishi, R.; Takahashi, T.; Nishida, T.; Kurokawa, Y.; Nakajima, K.; Koh, M.; Nishigaki, T.; Saito, T.; Yamamoto, K.; Yamashita, K.; et al. Plasma trough concentration of imatinib and its effect on therapeutic efficacy and adverse events in Japanese patients with GIST. Int. J. Clin. Oncol. 2023, 28, 680–687. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Allegretti, J.R.; Rubin, D.T.; Bressler, B.; Germinaro, M.; Huang, K.G.; Shipitofsky, N.; Zhang, H.; Wilson, R.; Han, C.; et al. Guselkumab in Patients With Moderately to Severely Active Ulcerative Colitis: QUASAR Phase 2b Induction Study. Gastroenterology 2023, 165, 1443–1457. [Google Scholar] [CrossRef]
- de Bruyn, M.; Vandooren, J.; Ugarte-Berzal, E.; Arijs, I.; Vermeire, S.; Opdenakker, G. The molecular biology of matrix metalloproteinases and tissue inhibitors of metalloproteinases in inflammatory bowel diseases. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 295–358. [Google Scholar] [CrossRef]
- Wu, X.X.; Huang, X.L.; Chen, R.R.; Li, T.; Ye, H.J.; Xie, W.; Huang, Z.M.; Cao, G.Z. Paeoniflorin Prevents Intestinal Barrier Disruption and Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in Caco-2 Cell Monolayers. Inflammation 2019, 42, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Wang, X.; Yang, C.; Chen, F.; Shi, L.; Xu, W.; Wang, K.; Liu, B.; Wang, C.; Sun, D.; et al. Neutrophil extracellular traps drive intestinal microvascular endothelialferroptosis by impairing Fundc1-dependent mitophagy. Redox Biol. 2023, 67, 102906. [Google Scholar] [CrossRef] [PubMed]
- Chidlow, J.H., Jr.; Shukla, D.; Grisham, M.B.; Kevil, C.G. Pathogenic angiogenesis in IBD and experimental colitis: New ideas and therapeutic avenues. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G5–G18. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Strategies for targeting cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2024, 24, 559–576. [Google Scholar] [CrossRef]
- Sigall Boneh, R.; Westoby, C.; Oseran, I.; Sarbagili-Shabat, C.; Albenberg, L.G.; Lionetti, P.; Navas-López, V.M.; Martín-de-Carpi, J.; Yanai, H.; Maharshak, N.; et al. The Crohn’s Disease Exclusion Diet: A Comprehensive Review of Evidence, Implementation Strategies, Practical Guidance, and Future Directions. Inflamm. Bowel Dis. 2024, 30, 1888–1902. [Google Scholar] [CrossRef]
- Lasa, J.S.; Olivera, P.A.; Danese, S.; Peyrin-Biroulet, L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: A systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 161–170. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients With Inflammatory Bowel Disease: From Basic Science to Clinical Practice. J. Crohn’s Colitis 2020, 14, 694–709. [Google Scholar] [CrossRef]
| Drug Type | Drug Name | Target | Side Effects |
|---|---|---|---|
| VEGF Inhibitor | Bevacizumab | VEGF-A | Gastrointestinal perforation, bleeding, arterial thromboembolic events, infection, hypertension |
| VEGF Inhibitor | Aflibercept | VEGF-A, VEGF-B, PlGF | Eye stinging, eye inflammation, blurred vision, eye bleeding, intraocular infection, increased eye pressure, retinal detachment |
| VEGF Inhibitor | Ramucirumab | VEGF Receptor-2 | Bleeding, gastrointestinal perforation, Wound healing complications, arterial thromboembolic events, hypertension, infusion-related reactions, proteinuria |
| PDGF Inhibitor | Imatinib | PDGF receptor signaling | Nausea, vomiting, myelosuppression |
| IL-23 Inhibitor | Guselkumab | p19 subunit of IL-23 | Respiratory infection, headache, arthralgia, diarrhea, gastroenteritis, tinea infection, herpes simplex infection. |
| Anti-TNF-α Therapy | Infliximab | TNF-α | Infections, infusion reactions, autoimmune phenomena, headache, nausea, rash |
| Anti-TNF-α Therapy | Adalimumab | TNF-α | Infections, infusion reactions, autoimmune phenomena, headache, musculoskeletal pain |
| Anti-TNF-α Therapy | Golimumab | TNF-α | Infections, infusion reactions, autoimmune phenomena, tumor, cytopenia, allergy, headache, hypertension, rash |
| MMP Inhibitors | (Under study) | Matrix metalloproteinases | In early development (side effects not yet defined) |
| Endothelial Cell-Targeting Therapies | (Under study) | Endothelial cell signaling pathways | In preclinical/clinical validation (side effects not yet defined) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zeng, L.; Huang, W.; Zhang, X.; Zhang, L.; Xie, Q. Angiogenic Factors and Inflammatory Bowel Diseases. Biomedicines 2025, 13, 1154. https://doi.org/10.3390/biomedicines13051154
Li Z, Zeng L, Huang W, Zhang X, Zhang L, Xie Q. Angiogenic Factors and Inflammatory Bowel Diseases. Biomedicines. 2025; 13(5):1154. https://doi.org/10.3390/biomedicines13051154
Chicago/Turabian StyleLi, Zhiru, Li Zeng, Wei Huang, Xinxing Zhang, Li Zhang, and Qin Xie. 2025. "Angiogenic Factors and Inflammatory Bowel Diseases" Biomedicines 13, no. 5: 1154. https://doi.org/10.3390/biomedicines13051154
APA StyleLi, Z., Zeng, L., Huang, W., Zhang, X., Zhang, L., & Xie, Q. (2025). Angiogenic Factors and Inflammatory Bowel Diseases. Biomedicines, 13(5), 1154. https://doi.org/10.3390/biomedicines13051154








