SpO2/FiO2 Correlates with PaO2/FiO2 (P/F) and Radiological Biomarkers of Severity: A Retrospective Study on COVID-19 Pneumonia Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patient Court, and Data Collection
2.2. Variables
2.3. Statistical Analysis
3. Results
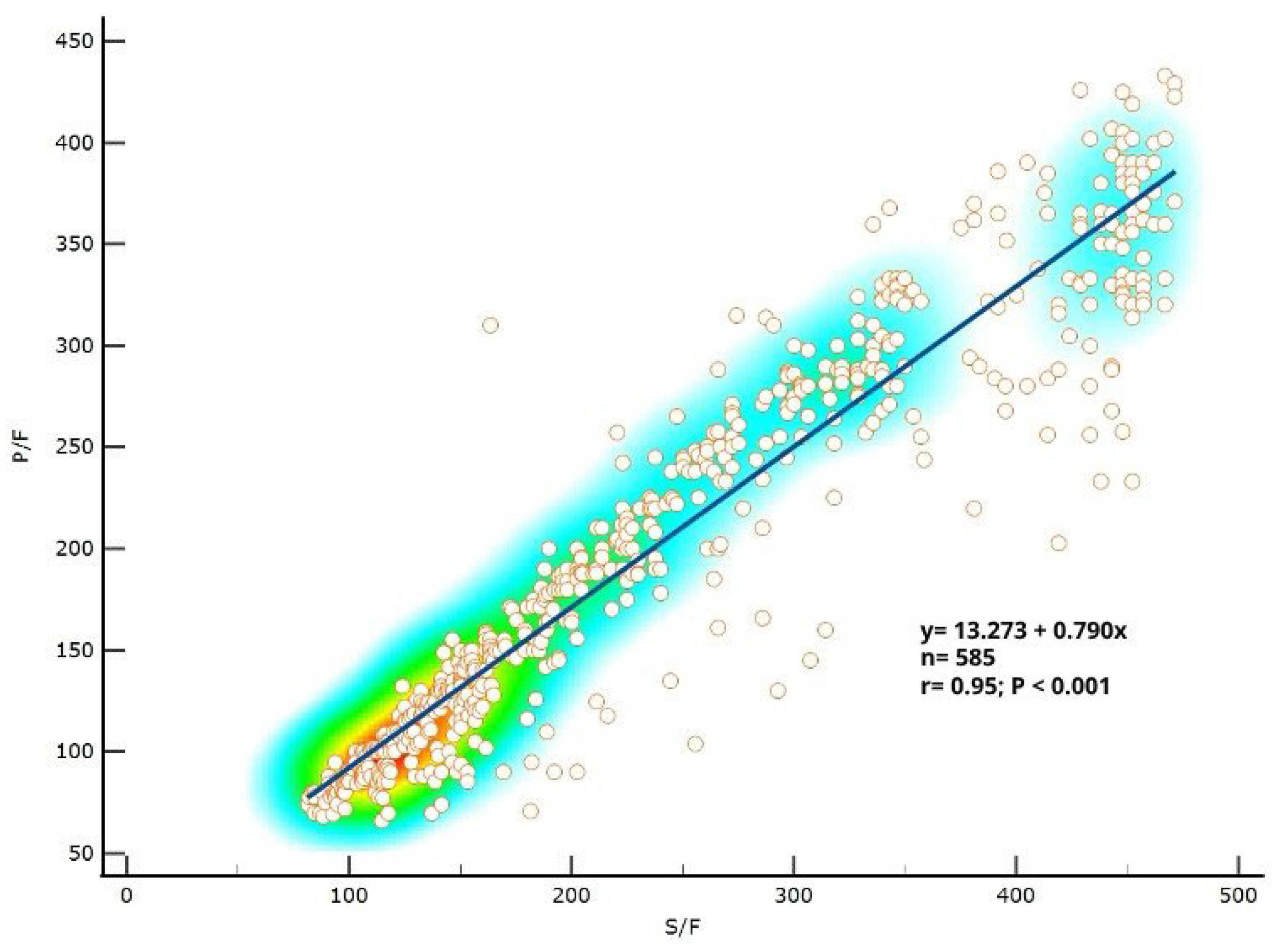
3.1. P/F Ratio Can Be Predicted Well from S/F Ratio in Patients with COVID-19
3.2. S/F Ratio Significantly Correlated with COVID-19 Severity Based on P/F Ratio
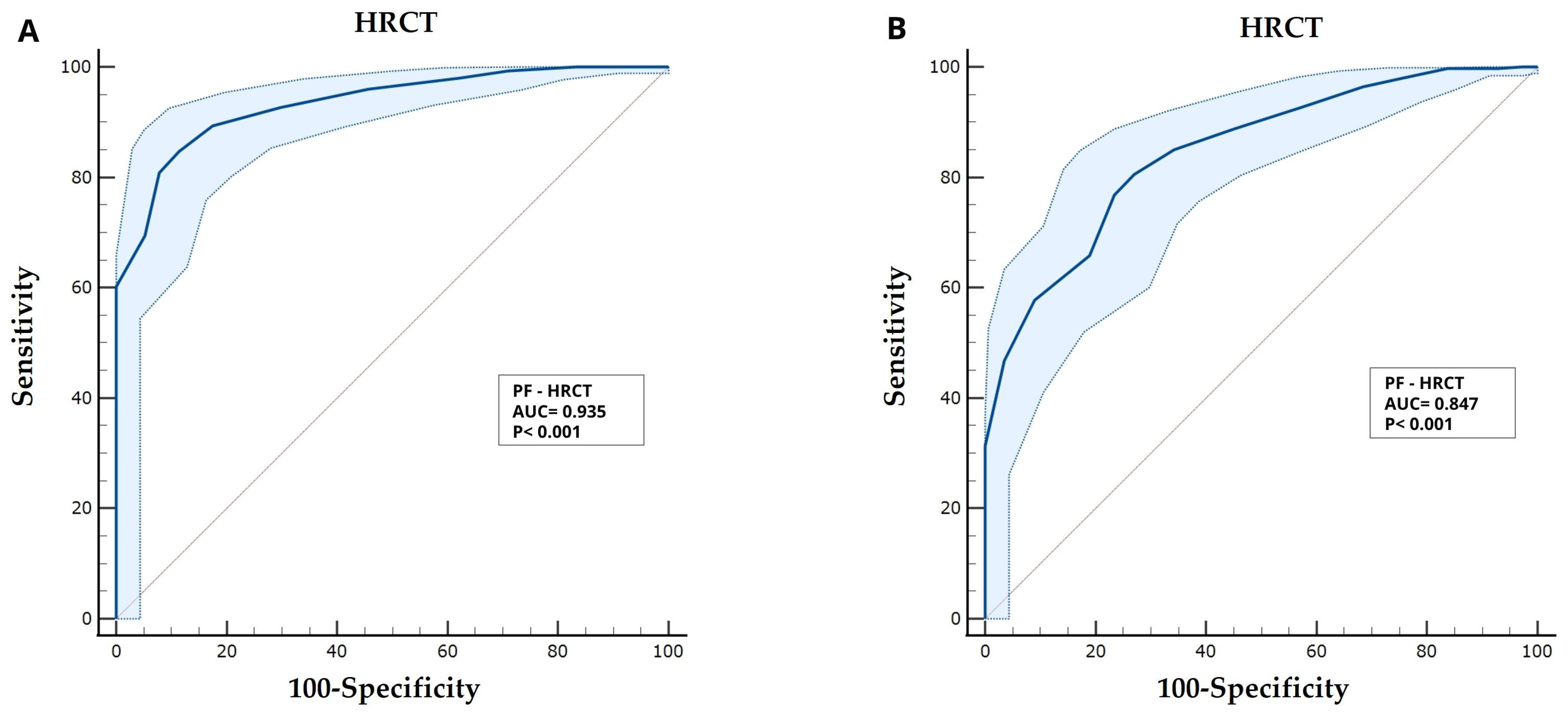
3.3. Severity of Diseases Based on Both P/F and S/F Did Significantly Correlate with HRCT Score
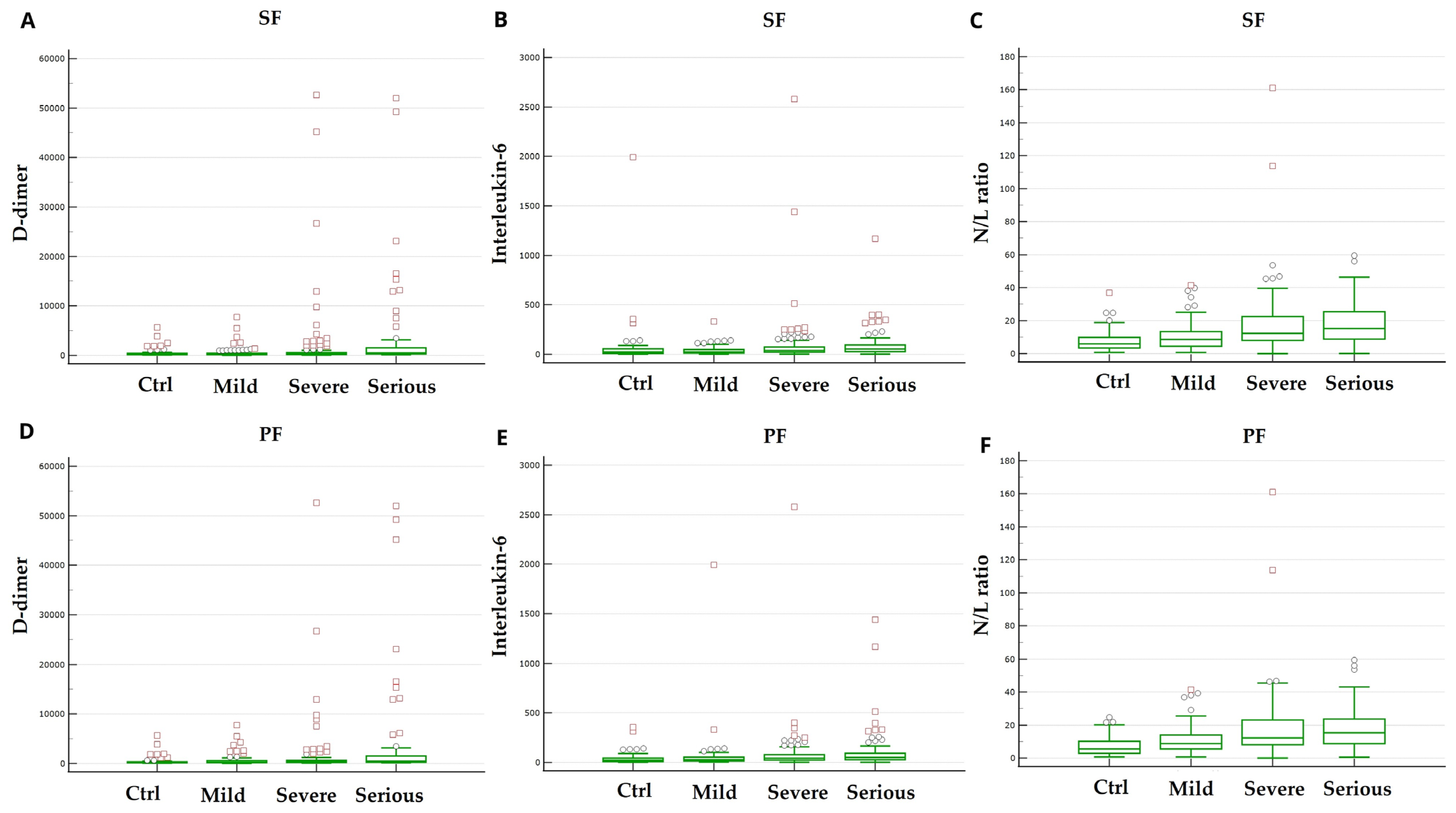
3.4. Laboratory Findings on Admission
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef] [PubMed]
- Boccia, M.; Aronne, L.; Celia, B.; Mazzeo, G.; Ceparano, M.; D’Agnano, V.; Parrella, R.; Valente, T.; Bianco, A.; Perrotta, F. COVID-19 and coagulative axis: Review of emerging aspects in a novel disease. Monaldi Arch. Chest Dis. 2020, 90, 1300. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Hashimoto, S. Peripheral oxygen saturation to inspiratory oxygen fraction ratio-based identification of critically ill coronavirus disease patients for early therapeutic interventions. J. Anesth. 2021, 35, 827–836. [Google Scholar] [CrossRef]
- Alberdi-Iglesias, A.; Martín-Rodríguez, F.; Ortega Rabbione, G.; Rubio-Babiano, A.I.; Núñez-Toste, M.G.; Sanz-García, A.; Del Pozo Vegas, C.; Castro Villamor, M.A.; Martín-Conty, J.L.; Jorge-Soto, C.; et al. Role of SpO2/FiO2 Ratio and ROX Index in Predicting Early Invasive Mechanical Ventilation in COVID-19. A Pragmatic, Retrospective, Multi-Center Study. Biomedicines 2021, 9, 1036. [Google Scholar] [CrossRef]
- Chen, W.; Janz, D.R.; Shaver, C.M.; Bernard, G.R.; Bastarache, J.A.; Ware, L.B. Clinical Characteristics and Outcomes Are Similar in ARDS Diagnosed by Oxygen Saturation/FiO2 Ratio Compared with PaO2/FiO2 Ratio. Chest 2015, 148, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Hayden, D.L.; Schoenfeld, D.A.; Ware, L.B. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007, 132, 410–417. [Google Scholar] [CrossRef]
- Bonaventura, A.; Mumoli, N.; Mazzone, A.; Colombo, A.; Evangelista, I.; Cerutti, S.; Brivio, L.; Vecchié, A.; Bonomi, A.; Para, O.; et al. Correlation of SpO2/FiO2 and PaO2/FiO2 in patients with symptomatic COVID-19: An observational, retrospective study. Intern. Emerg. Med. 2022, 17, 1769–1775. [Google Scholar] [CrossRef]
- Brusselaers, N.; Lagergren, J. The Charlson Comorbidity Index in Registry-based Research. Methods Inf. Med. 2017, 56, 401–406. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A.; et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef]
- Kanne, J.P. Chest CT Findings in 2019 Novel Coronavirus (2019-nCoV) Infections from Wuhan, China: Key Points for the Radiologist. Radiology 2020, 295, 16–17. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19); National Institutes of Health: Bethesda, MD, USA, 2019; pp. 1–243. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_NBK570371.pdf (accessed on 30 March 2025).
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.A.; Onyskiw, J.E.; Prasad, N.G. Meta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adults. Heart Lung 1998, 27, 387–408. [Google Scholar] [CrossRef]
- Pierson, D.J. Pulse oximetry versus arterial blood gas specimens in long-term oxygen therapy. Lung 1990, 168 (Suppl. S1), 782–788. [Google Scholar] [CrossRef] [PubMed]
- Rauniyar, N.; Pujari, S.; Shrestha, P. Study of Oxygen Saturation by Pulse Oximetry and Arterial Blood Gas in ICU Patients: A Descriptive Cross-sectional Study. JNMA J. Nepal Med. Assoc. 2020, 58, 789–793. [Google Scholar] [CrossRef]
- Poorzargar, K.; Pham, C.; Ariaratnam, J.; Lee, K.; Parotto, M.; Englesakis, M.; Chung, F.; Nagappa, M. Accuracy of pulse oximeters in measuring oxygen saturation in patients with poor peripheral perfusion: A systematic review. J. Clin. Monit. Comput. 2022, 36, 961–973. [Google Scholar] [CrossRef]
- Athale, J.; Suffredini, A.F. Pulse Oximetry: A Necessary Imperfect Tool in Critical Care. Crit. Care Med. 2023, 51, 1246–1248. [Google Scholar] [CrossRef]
- De Pauw, C.; Poelaert, J.; Rolly, G.; Colardyn, F. Clinical evaluation of pulse oximetry monitors on critically ill patients. Acta Anaesthesiol. Belg. 1990, 41, 33–39. [Google Scholar]
- Tobin, M.J.; Laghi, F.; Jubran, A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. Am. J. Respir. Crit. Care Med. 2020, 202, 356–360. [Google Scholar] [CrossRef]
- Rinaldi, L.; Milione, S.; Fascione, M.C.; Pafundi, P.C.; Altruda, C.; Di Caterino, M.; Monaco, L.; Reginelli, A.; Perrotta, F.; Porta, G.; et al. Relevance of lung ultrasound in the diagnostic algorithm of respiratory diseases in a real-life setting: A multicentre prospective study. Respirology 2020, 25, 535–542. [Google Scholar] [CrossRef]
- Rubin, G.D.; Ryerson, C.J.; Haramati, L.B.; Sverzellati, N.; Kanne, J.P.; Raoof, S.; Schluger, N.W.; Volpi, A.; Yim, J.-J.; Martin, I.B.K.; et al. The Role of Chest Imaging in Patient Management During the COVID-19 Pandemic: A Multinational Consensus Statement from the Fleischner Society. Chest 2020, 158, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Floridi, C.; Fogante, M.; Agostini, A.; Borgheresi, A.; Cellina, M.; Natella, R.; Bruno, F.; Cozzi, D.; Maggialetti, N.; Palumbo, P.; et al. Radiological diagnosis of Coronavirus Disease 2019 (COVID-19): A Practical Guide. Acta Biomed. 2020, 91, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kwee, T.C.; Kwee, R.M. Chest CT in COVID-19: What the Radiologist Needs to Know. RadioGraphics 2020, 40, 1848–1865. [Google Scholar] [CrossRef]
- Abd El Megid, A.G.I.; El Shabrawy, M.; Abdalla, A.A.E.-H.M. Correlation between chest CT severity scoring system with oxygen saturation and laboratory inflammatory markers in adult patients with COVID-19 infection. Egypt. J. Radiol. Nucl. Med. 2022, 53, 72. [Google Scholar] [CrossRef]
- Qadir, F.I.; Kakamad, F.H.; Abdullah, I.Y.; Abdulla, B.A.; Mohammed, S.H.; Salih, R.Q.; Ali, R.K.; Salh, A.M. The relationship between CT severity infections and oxygen saturation in patients infected with COVID-19, a cohort study. Ann. Med. Surg. 2022, 76, 103439. [Google Scholar] [CrossRef]
- Eskandarian Boroujeni, M.; Sekrecka, A.; Antonczyk, A.; Hassani, S.; Sekrecki, M.; Nowicka, H.; Lopacinska, N.; Olya, A.; Kluzek, K.; Wesoly, J.; et al. Dysregulated Interferon Response and Immune Hyperactivation in Severe COVID-19: Targeting STATs as a Novel Therapeutic Strategy. Front. Immunol. 2022, 13, 888897. [Google Scholar] [CrossRef] [PubMed]
- Lowery, S.A.; Sariol, A.; Perlman, S. Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19. Cell Host Microbe 2021, 29, 1052–1062. [Google Scholar] [CrossRef]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.-G.; Neidleman, J.; Andrew, A.; Young, K.C.; Ma, T.; Hoh, R.; et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 2024, 25, 218–225. [Google Scholar] [CrossRef]
- Siavoshi, F.; Safavi-Naini, S.A.A.; Shirzadeh Barough, S.; Azizmohammad Looha, M.; Hatamabadi, H.; Ommi, D.; Jalili Khoshnoud, R.; Fatemi, A.; Pourhoseingholi, M.A. On-admission and dynamic trend of laboratory profiles as prognostic biomarkers in COVID-19 inpatients. Sci. Rep. 2023, 13, 6993. [Google Scholar] [CrossRef]
- D’Agnano, V.; Scialò, F.; Perna, F.; Atripaldi, L.; Sanduzzi, S.; Allocca, V.; Vitale, M.; Pastore, L.; Bianco, A.; Perrotta, F. Exploring the Role of Krebs von den Lungen-6 in Severe to Critical COVID-19 Patients. Life 2022, 12, 1141. [Google Scholar] [CrossRef]
- Kutlusoy, S.; Koca, E. Routine laboratory parameters in estimating mortality and morbidity in COVID-19 diagnosed cases followed in the intensive care unit. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5885–5892. [Google Scholar] [CrossRef] [PubMed]
- Ghahramani, S.; Tabrizi, R.; Lankarani, K.B.; Kashani, S.M.A.; Rezaei, S.; Zeidi, N.; Akbari, M.; Heydari, S.T.; Akbari, H.; Nowrouzi-Sohrabi, P.; et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: A systematic review and meta-analysis. Eur. J. Med. Res. 2020, 25, 30. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.R.; Basnet, A.; Tamang, B.; Khadka, S.; Maharjan, R.; Maharjan, R.; Chand, A.B.; Thapa, S.; Rai, S.K. Analysis of altered level of blood-based biomarkers in prognosis of COVID-19 patients. PLoS ONE 2023, 18, e0287117. [Google Scholar] [CrossRef] [PubMed]
| Variables | ||
|---|---|---|
| Total patients | n = 585 | |
| Gender (male/female) | 388/197 (66.3%/33.7%) | |
| Age (Years) | 65 [22–95] | |
| Hospitalization (days) | 19 [0–78] | |
| Onset-to-door (days) | 7 [1–37] | |
| Swap Positivity (Days) | 26 [6–80] | |
| Body Temperature (°C) | 36.9 [35.5–39] | |
| Exitus (healed/deceased) | 399/186 (68.2%/31.8%) | |
| PaO2/FiO2 (P/F) | 203 [66–433] | |
| P/F Severity | >300 | 19.5% |
| 200–300 | 25% | |
| 100–200 | 37.1% | |
| <100 | 18.4% | |
| Sat.O2 (%) | 91 [50–100] | |
| Sat.O2/FiO2 (S/F) | 240 [81–471] | |
| HRCT (score) | 13 [2–20] | |
| HRCT Severity | 2–8 | 19.5% |
| 9–14 | 41.9% | |
| 15–20 | 38.6% | |
| Comorbidity (0, 1, 2, 3 or more) | 0 | 24.8% |
| 1 | 41.8% | |
| 2 | 23.3% | |
| ≥3 | 10.1% | |
| Cardiac disease | 59% | |
| Metabolic disease | 22.6% | |
| Pulmonary disease | 10% | |
| Chronic Renal Insufficiency | 9.4% | |
| Cancer | 9.1% | |
| SpO2/FiO2 >350 Class 0 | SpO2/FiO2 236–350 Class 1 | SpO2/FiO2 123–236 Class 2 | SpO2/FiO2 <123 Class 3 | |
|---|---|---|---|---|
| D-dimer (ng/mL) | 240.0 [72–5652] | 232,000 [16.0–7741.0] | 320.0 [63.0–52,673.0] | 514.5 [144.0–52,005.0] |
| Ferritin (ng/mL) | 613.0 [88–3217] | 786.5 [58.0–10,601.0] | 1020.0 145.0–8360.0] | 875.0 [139.0–60,219.0] |
| Haemoglobin (g/L) | 140 [8–175] | 140 [84–187] | 142 [74–180] | 136 [56–184] |
| IL6/Lymph (pg/mL·cells) | 30.1 [0.98–6988] | 31.0 [1.5–906.3] | 58.1 [2.9–2875.3] | 79.6 [1.3–2309.5] |
| IL2R (IU) | 859.0 [127–17,328] | 965,500 [296.0–7050.0] | 1061.0 [100.0–6781.0] | 1296.0 [95.0–7338.0] |
| IL-6 (pg/mL) | 24.2 [2–1995] | 24.7 [2.0–332.0] | 38.3 [2.0–2580.0] | 54.1 [2.0–1167.0] |
| NLR | 5.9 [0.7–36.9] | 8.6 [0.7–41.5] | 12.3 [0–161.1] | 15.1 [0.1–59.4] |
| RCP (mg/dL) | 5.4 [0.4–29] | 5.8 [0.4–213.0] | 8.5 [0.4–124.0] | 9.0 [0.4–26.6] |
| Platelets (×103/uL) | 193.0 [44–634] | 211.0 [13.3–741.0] | 225.0 [51.0–565.0] | 241.0 [5.3–647.0] |
| PCT (ng/mL) | 0.07 [0.02–8.2] | 0.08 [0.006–55.5] | 0.13 [0.006–19.7] | 0.230 [0.03–40.5] |
| Temperature (°C) | 36.5 [35.8–38.5] | 36,800 [35.5–39.5] | 36.8 [36.0–38.5] | 37.6 [0.03–40.5] |
| Comorbidities | ||||
| Cardiovascular | 62 (54%) | 74 (50%) | 130 (59%) | 65 (60%) |
| Diabetes | 24 (21%) | 28 (19%) | 44 (20%) | 31 (28%) |
| Neoplastic | 10 (9%) | 9 (6%) | 21 (10%) | 11 (10%) |
| Respiratory | 12 (10%) | 10 (7%) | 21 (10%) | 13 (12%) |
| Renal | 7 (6%) | 14 (10%) | 15 (7%) | 17 (16%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marra, A.; D’Agnano, V.; Pagliaro, R.; Perrotta, F.; Di Fiore, I.; D’Orologio, A.; Scialò, F.; Schiattarella, A.; Bianco, A.; Parrella, R. SpO2/FiO2 Correlates with PaO2/FiO2 (P/F) and Radiological Biomarkers of Severity: A Retrospective Study on COVID-19 Pneumonia Patients. Biomedicines 2025, 13, 1072. https://doi.org/10.3390/biomedicines13051072
Marra A, D’Agnano V, Pagliaro R, Perrotta F, Di Fiore I, D’Orologio A, Scialò F, Schiattarella A, Bianco A, Parrella R. SpO2/FiO2 Correlates with PaO2/FiO2 (P/F) and Radiological Biomarkers of Severity: A Retrospective Study on COVID-19 Pneumonia Patients. Biomedicines. 2025; 13(5):1072. https://doi.org/10.3390/biomedicines13051072
Chicago/Turabian StyleMarra, Alberto, Vito D’Agnano, Raffaella Pagliaro, Fabio Perrotta, Ilaria Di Fiore, Antonio D’Orologio, Filippo Scialò, Angela Schiattarella, Andrea Bianco, and Roberto Parrella. 2025. "SpO2/FiO2 Correlates with PaO2/FiO2 (P/F) and Radiological Biomarkers of Severity: A Retrospective Study on COVID-19 Pneumonia Patients" Biomedicines 13, no. 5: 1072. https://doi.org/10.3390/biomedicines13051072
APA StyleMarra, A., D’Agnano, V., Pagliaro, R., Perrotta, F., Di Fiore, I., D’Orologio, A., Scialò, F., Schiattarella, A., Bianco, A., & Parrella, R. (2025). SpO2/FiO2 Correlates with PaO2/FiO2 (P/F) and Radiological Biomarkers of Severity: A Retrospective Study on COVID-19 Pneumonia Patients. Biomedicines, 13(5), 1072. https://doi.org/10.3390/biomedicines13051072







