Efficacy of Durvalumab Consolidation Therapy After Sequential Chemoradiotherapy in Patients with Unresectable Stage III Non-Small Cell Lung Cancer—Experience from the Daily Hospital of Clinic for Pulmonology, University Clinical Center of Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orosz, Z.; Kovács, Á. The role of chemoradiotherapy and immunotherapy in stage III NSCLC. Pathol. Oncol. Res. 2024, 30, 1611716. [Google Scholar] [CrossRef] [PubMed]
- Curran, W.J., Jr.; Paulus, R.; Langer, C.J.; Komaki, R.; Lee, J.S.; Hauser, S.; Movsas, B.; Wasserman, T.; Rosenthal, S.A.; Gore, E.; et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J. Natl. Cancer Inst. 2011, 103, 1452–1460, Erratum in J. Natl. Cancer Inst. 2012, 104, 79. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Ahn, Y.C.; Kim, J.H.; Lee, C.G.; Cho, E.K.; Lee, K.C.; Chen, M.; Kim, D.W.; Kim, H.K.; Min, Y.J.; et al. Multinational Randomized Phase III Trial with or without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J. Clin. Oncol. 2015, 33, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Flentje, M.; Huber, R.M.; Engel-Riedel, W.; Andreas, S.; Kollmeier, J.; Staar, S.; Dickgreber, N.; Vaissiere, N.; De Almeida, C.; Edlich, B.; et al. A randomised phase III study of oral vinorelbine and cisplatin with concomitant radiotherapy followed by either consolidation therapy with oral vinorelbine and cisplatin or best supportive care alone in stage III non-small cell lung cancer. Strahlenther. Onkol. 2016, 192, 216–222. [Google Scholar] [CrossRef]
- Hanna, N.; Neubauer, M.; Yiannoutsos, C.; McGarry, R.; Arseneau, J.; Ansari, R.; Reynolds, C.; Govindan, R.; Melnyk, A.; Fisher, W.; et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J. Clin. Oncol. 2008, 26, 5755–5760. [Google Scholar] [CrossRef]
- Gandara, D.R.; Chansky, K.; Albain, K.S.; Leigh, B.R.; Gaspar, L.E.; Lara, P.N., Jr.; Burris, H.; Gumerlock, P.; Kuebler, J.P.; Bearden, J.D. 3rd.; et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: Phase II Southwest Oncology Group Study S9504. J. Clin. Oncol. 2003, 21, 2004–2010. [Google Scholar] [CrossRef]
- Tsujino, K.; Kurata, T.; Yamamoto, S.; Kawaguchi, T.; Kubo, A.; Isa, S.; Hasegawa, Y.; Ou, S.H.; Takada, M.; Ando, M. Is consolidation chemotherapy after concurrent chemo-radiotherapy beneficial for patients with locally advanced non-small-cell lung cancer? A pooled analysis of the literature. J. Thorac. Oncol. 2013, 8, 1181–1189. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Garassino, M.C.; Mazieres, J.; Reck, M.; Chouaid, C.; Bischoff, H.; Reinmuth, N.; Cove-Smith, L.; Mansy, T.; Cortinovis, D.; Migliorino, M.R.; et al. Durvalumab After Sequential Chemoradiotherapy in Stage III, Unresectable NSCLC: The Phase 2 PACIFIC-6 Trial. J. Thorac. Oncol. 2022, 17, 1415–1427. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Spira, A.; Raben, D.; Planchard, D.; Cho, B.C.; Özgüroğlu, M.; Daniel, D.; Villegas, A.; Vicente, D.; Hui, R.; et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann. Oncol. 2020, 31, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J. Thorac. Oncol. 2020, 15, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes from the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Bar, J.; Garrido, P.; Garassino, M.C.; McDonald, F.; Mornex, F.; Filippi, A.R.; Smit, H.J.M.; Peters, S.; Field, J.K.; et al. Treatment Characteristics and Real-World Progression-Free Survival in Patients with Unresectable Stage III NSCLC Who Received Durvalumab After Chemoradiotherapy: Findings from the PACIFIC-R Study. J. Thorac. Oncol. 2023, 18, 181–193. [Google Scholar] [CrossRef]
- Park, C.K.; Oh, H.J.; Kim, Y.C.; Kim, Y.H.; Ahn, S.J.; Jeong, W.G.; Lee, J.Y.; Lee, J.C.; Choi, C.M.; Ji, W.; et al. Korean Real-World Data on Patients with Unresectable Stage III NSCLC Treated with Durvalumab After Chemoradiotherapy: PACIFIC-KR. J. Thorac. Oncol. 2023, 18, 1042–1054. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.J.; Soon, Y.Y.; Wong, A.; Aminkeng, F.; Ang, Y.; Asokumaran, Y.; Low, J.L.; Lee, M.; Choo, J.R.E.; et al. Real-world experience of consolidation durvalumab after concurrent chemoradiotherapy in stage III non-small cell lung cancer. Thorac. Cancer 2022, 13, 3152–3161. [Google Scholar] [CrossRef]
- Chu, C.H.; Chiu, T.H.; Wang, C.C.; Chang, W.C.; Huang, A.C.; Liu, C.Y.; Wang, C.L.; Ko, H.W.; Chung, F.T.; Hsu, P.C.; et al. Consolidation treatment of durvalumab after chemoradiation in real-world patients with stage III unresectable non-small cell lung cancer. Thorac. Cancer 2020, 11, 1541–1549. [Google Scholar] [CrossRef]
- Preti, B.T.B.; Sanatani, M.S.; Breadner, D.; Lakkunarajah, S.; Scott, C.; Esmonde-White, C.; McArthur, E.; Rodrigues, G.; Chaudhary, M.; Mutsaers, A.; et al. Real-World Analysis of Durvalumab after Chemoradiation in Stage III Non-Small-Cell Lung Cancer. Curr. Oncol. 2023, 30, 7713–7721. [Google Scholar] [CrossRef]
- Taugner, J.; Käsmann, L.; Eze, C.; Tufman, A.; Reinmuth, N.; Duell, T.; Belka, C.; Manapov, F. Durvalumab after Chemoradiotherapy for PD-L1 Expressing Inoperable Stage III NSCLC Leads to Significant Improvement of Local-Regional Control and Overall Survival in the Real-World Setting. Cancers 2021, 13, 1613. [Google Scholar] [CrossRef]
- Taugner, J.; Käsmann, L.; Eze, C.; Rühle, A.; Tufman, A.; Reinmuth, N.; Duell, T.; Belka, C.; Manapov, F. Real-world prospective analysis of treatment patterns in durvalumab maintenance after chemoradiotherapy in unresectable, locally advanced NSCLC patients. Invest. New Drugs 2021, 39, 1189–1196. [Google Scholar] [CrossRef]
- Faehling, M.; Schumann, C.; Christopoulos, P.; Hoffknecht, P.; Alt, J.; Horn, M.; Eisenmann, S.; Schlenska-Lange, A.; Schütt, P.; Steger, F.; et al. Durvalumab after definitive chemoradiotherapy in locally advanced unresectable non-small cell lung cancer (NSCLC): Real-world data on survival and safety from the German expanded-access program (EAP). Lung Cancer 2020, 150, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Noh, J.M.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Ahn, M.J.; Pyo, H.; Ahn, Y.C.; Park, K. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer 2020, 146, 23–29. [Google Scholar] [CrossRef] [PubMed]

| Demographic Characteristics | All Patients (N = 24) |
|---|---|
| Age, mean ± SD | 66.3 ± 4.6 |
| Sex | |
| Female sex, n (%) | 9 (37.5) |
| Male sex, n (%) | 15 (62.5) |
| Smoking | |
| Former smokers, n (%) | 13 (54.2) |
| Current smokers, n (%) | 11 (45.8) |
| Tumor histologic type | |
| Adeno, n (%) | 8 (33.3) |
| Squamous, n (%) | 12 (50.0) |
| Large cell, n (%) | 1 (4.2) |
| NOS, n (%) | 3 (12.5) |
| Stage (AJCC 8th edition) | |
| IIIa, n (%) | 11 (45.8) |
| IIIb, n (%) | 11 (45.8) |
| IIIc, n (%) | 2 (8.3) |
| PD-L1 expression | |
| ≥50%, n (%) | 9 (37.5) |
| 1–49%, n (%) | 15 (62.5) |
| Previous chemotherapy | |
| Ethoposide, Platinum, n (%) | 2 (8.3) |
| Vinorelbine, Platinum, n (%) | 1 (4.2) |
| Gemcytabine, Platinum, n (%) | 11 (45.8) |
| Paclitaxel, Platinum, n (%) | 10 (41.7) |
| ECOG PS | |
| ECOG PS 0, n (%) | 2 (8.0) |
| ECOG PS 1, n (%) | 22 (92) |
| PFS | p Value | OS | p Value | |
|---|---|---|---|---|
| Sex | p = 0.617 | p = 0.675 | ||
| Male | 18.5 (13.4–23.6) | 19.0 (14.6–23.4) | ||
| Female | 15.2 (8.7–21.8) | 18.2 (11.6–24.9) | ||
| Smoking status | p = 0.823 | p = 0.607 | ||
| Former smokers | 16.5 (10.7–22.3) | 18.2 (12.8–23.6) | ||
| Current smokers | 18.2 (12.7–23.7) | 19.1 (14.2–24.1) | ||
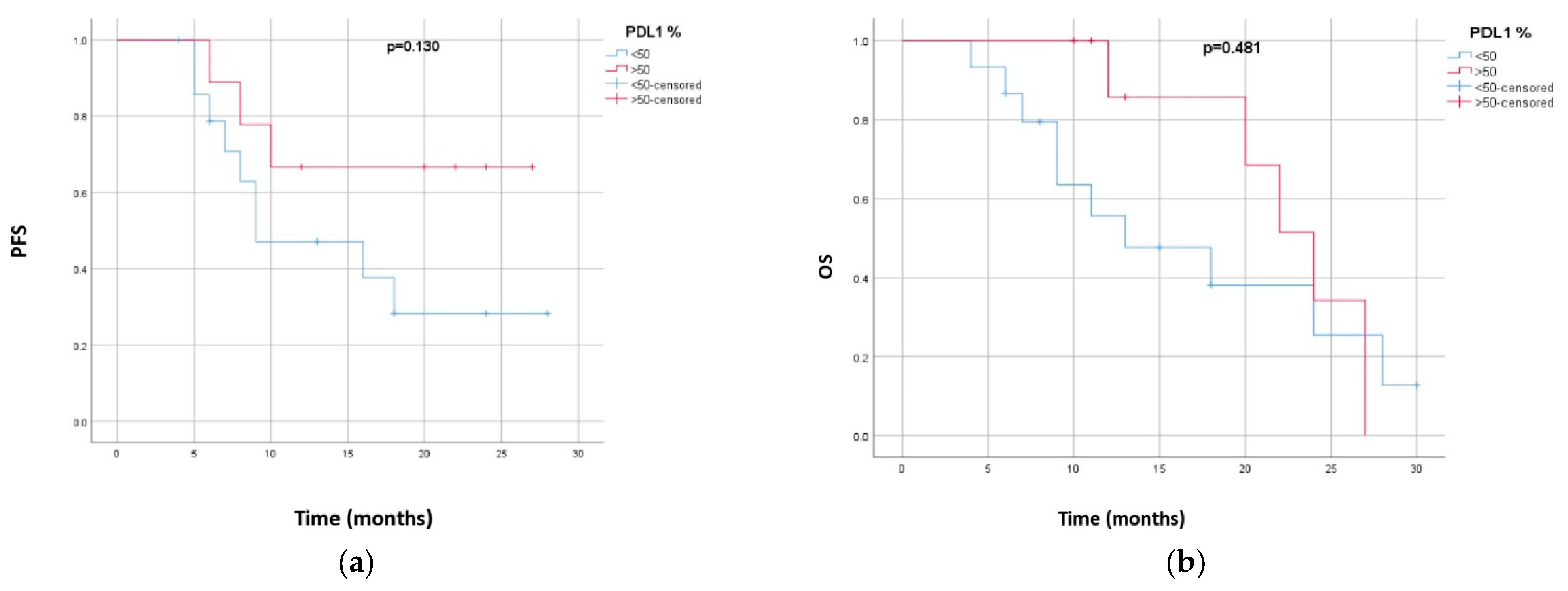
| PD-L1 expression | p = 0.13 | p = 0.481 | ||
| ≥50% | 20.7 (14.8–26.5) | 22.3 (18.2–26.4) | ||
| 1–49% | 14.9 (9.8–19.9) | 16.6 (11.6–21.7) |
| Study | No of Patients | Concurrent/ Sequential RT | PFS (Months) | OS (Months) | 12-Month PFS Rate | 12-Month OS Rate |
|---|---|---|---|---|---|---|
| PACIFIC [8] | 709 | Concurrent | 16.8 | 47.5 | 55.9% | 83.1% |
| PACIFIC-R [14] | 1399 | Both | 21.7 | Not reached | 62.2% | / |
| PACIFIC-KR [15] | 157 | Both | 25.9 | Not reached | 59.4% | 87.8% |
| PACIFIC 6 [9] | 117 | Sequential | 10.9 | / | 49.6% | 84.1% |
| Huang et al. [16] | 39 | Concurrent | 17.5 | Not reached | / | / |
| Chu et al. [17] | 31 | Concurrent | Not reached | / | 56.4% | / |
| Preti et al. [18] | 118 | Concurrent | Not reached >20 months | Not reached >32 months | / | / |
| Taugner et al. [19] | 26 | Both | Not reached | / | 62% | 100% |
| Faehlig et al. [21] | 126 | Concurrent | 20.1 | Not reached | 56% | 78.6% |
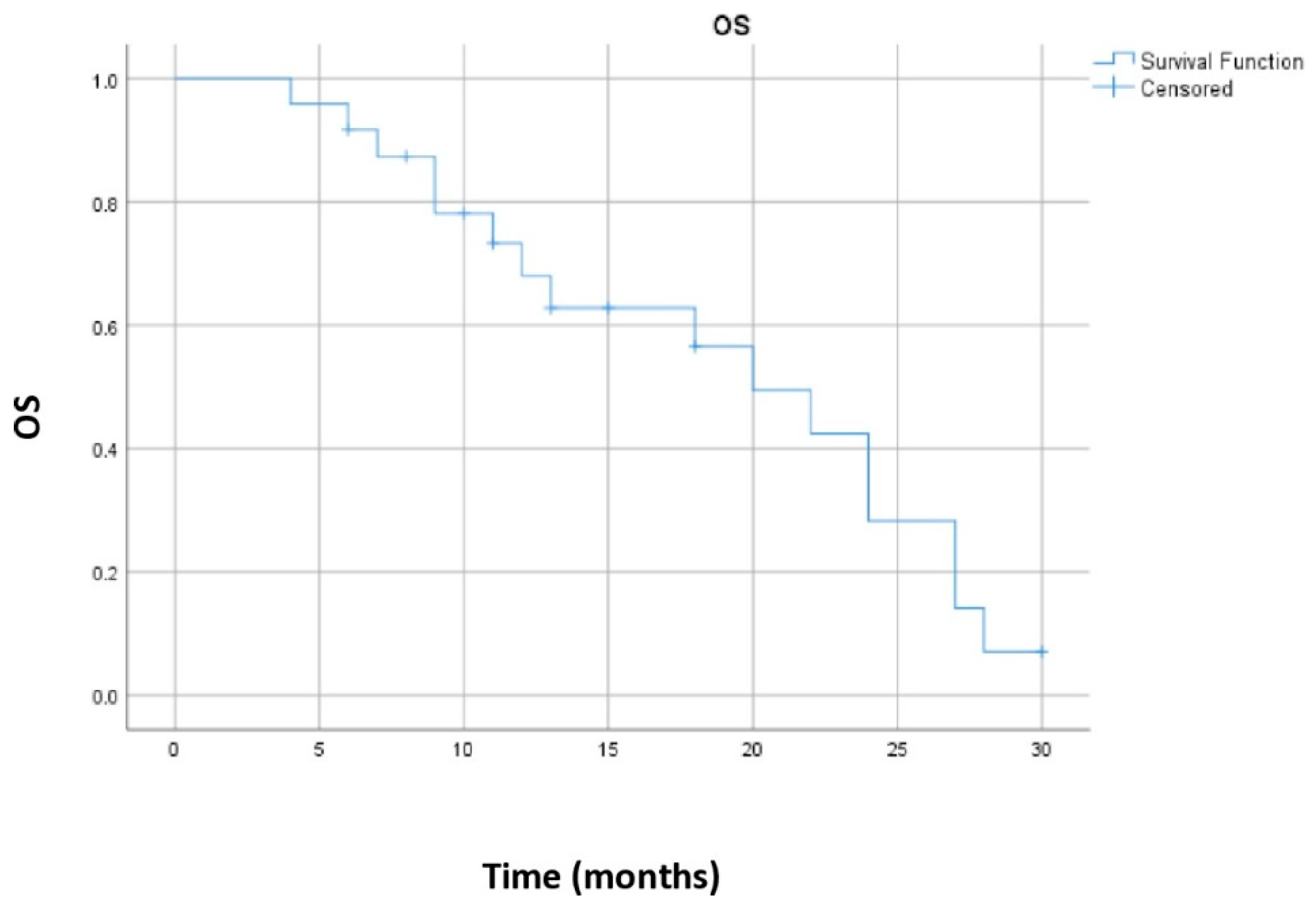
| Ceriman Krstic and Samardzic et al. | 24 | Sequential | 16 | 20 | 55.1% | 68% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćeriman Krstić, V.; Samardžić, N.; Popević, S.; Stević, R.; Ilić, B.; Gajić, M.; Čolić, N.; Lukić, K.; Milošević Maračić, B.; Poparić Banđur, B.; et al. Efficacy of Durvalumab Consolidation Therapy After Sequential Chemoradiotherapy in Patients with Unresectable Stage III Non-Small Cell Lung Cancer—Experience from the Daily Hospital of Clinic for Pulmonology, University Clinical Center of Serbia. Biomedicines 2025, 13, 892. https://doi.org/10.3390/biomedicines13040892
Ćeriman Krstić V, Samardžić N, Popević S, Stević R, Ilić B, Gajić M, Čolić N, Lukić K, Milošević Maračić B, Poparić Banđur B, et al. Efficacy of Durvalumab Consolidation Therapy After Sequential Chemoradiotherapy in Patients with Unresectable Stage III Non-Small Cell Lung Cancer—Experience from the Daily Hospital of Clinic for Pulmonology, University Clinical Center of Serbia. Biomedicines. 2025; 13(4):892. https://doi.org/10.3390/biomedicines13040892
Chicago/Turabian StyleĆeriman Krstić, Vesna, Natalija Samardžić, Spasoje Popević, Ruža Stević, Branislav Ilić, Milija Gajić, Nikola Čolić, Katarina Lukić, Brankica Milošević Maračić, Bojana Poparić Banđur, and et al. 2025. "Efficacy of Durvalumab Consolidation Therapy After Sequential Chemoradiotherapy in Patients with Unresectable Stage III Non-Small Cell Lung Cancer—Experience from the Daily Hospital of Clinic for Pulmonology, University Clinical Center of Serbia" Biomedicines 13, no. 4: 892. https://doi.org/10.3390/biomedicines13040892
APA StyleĆeriman Krstić, V., Samardžić, N., Popević, S., Stević, R., Ilić, B., Gajić, M., Čolić, N., Lukić, K., Milošević Maračić, B., Poparić Banđur, B., Šeha, B., Radončić, D., & Milin Lazović, J. (2025). Efficacy of Durvalumab Consolidation Therapy After Sequential Chemoradiotherapy in Patients with Unresectable Stage III Non-Small Cell Lung Cancer—Experience from the Daily Hospital of Clinic for Pulmonology, University Clinical Center of Serbia. Biomedicines, 13(4), 892. https://doi.org/10.3390/biomedicines13040892






