Pentraxin-3 as a Biomarker in Diabetes Mellitus: Insights into Inflammation, Vascular Complications, and Modulation by Antidiabetic Medications
Abstract
1. Introduction
2. PTX3
3. PTX3 as a Biomarker of Inflammation in DM
3.1. Clinical Studies
3.1.1. Gestational DM
3.1.2. Diabetic Nephropathy (DN)
3.1.3. DR
3.1.4. Foot Ulcer
3.1.5. Cardiovascular (CV) Risk
3.2. Experimental Studies
4. Evidence of the Interplay Between PTX3—DM—Antidiabetic Drugs
4.1. Clinical Studies
- Baseline PTX3 levels (prior to intervention) were higher in the TT/TC group (4570 ± 850 pg/mL) compared to the CC group (3820 ± 560 pg/mL).
- Before intervention, for the overall group, PTX3 was 4170 ± 710 pg/mL, with subgroup values of 4520 ± 840 pg/mL in TT/TC carriers and 3810 ± 560 pg/mL in CC carriers.
- After the dietary intervention, PTX3 levels increased significantly in the TT/TC group (5520 ± 780 pg/mL) compared to 4050 ± 400 pg/mL in the CC group, with the overall PTX3 mean level rising to 4750 ± 630 pg/mL for the entire study population.
- 367 patients remained free of CV events, and the antidiabetic treatment in this subgroup consisted of metformin (n = 239), insulin (n = 108), SFN (n = 110), glitazones (n = 23), DPP-4i (n = 41), and incretin analogs (n = 20).
- 73 patients experienced a CV event, and their antidiabetic therapies were metformin (n = 45), insulin (n = 33), SFN (n = 15), glitazones (n = 2), DPP-4i (n = 3), and incretin analogs (n = 2).
- Among patients with T2DM but without CVD at baseline (n = 496):
- 464 patients remained free of CV events, with treatments including metformin (n = 331), insulin (n = 73), SFN (n = 138), glitazones (n = 33), DPP-4i (n = 52), and incretin analogs (n = 24).
- 32 patients developed a CV event, and their treatment consisted of metformin (n = 26), insulin (n = 8), SFN (n = 7), glitazones (n = 5), DPP-4i (n = 2), and incretin analogs (n = 1).
- Among patients with T2DM and preexisting CVD (n = 327):
- ○
- 276 patients had no CV event, receiving metformin (n = 175), insulin (n = 83), SFN (n = 75), glitazones (n = 13), DPP-4i (n = 37), and incretin analogs (n = 18).
- ○
- 51 patients experienced a CV event, with therapies including metformin (n = 34), insulin (n = 23), SFN (n = 11), DPP-4i (n = 4), and incretin analogs (n = 1). Notably, none of the patients with CV events were receiving glitazones at follow-up.
- Among patients with T2DM but without CVD at baseline (n = 421):
- ○
- 397 patients remained CV event-free, with antidiabetic therapies including metformin (n = 274), insulin (n = 86), SFN (n = 101), glitazones (n = 22), DPP-4i (n = 53), and incretin analogs (n = 23).
- ○
- 24 patients developed a CV event, receiving metformin (n = 20), insulin (n = 8), SFN (n = 7), DPP-4i (n = 4), glitazones (n = 1), and none were treated with incretin analogs.
4.2. Experimental Studies
5. Limitations
6. Future Perspectives and Clinical Implications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AGi | alpha-glucosidase inhibitors |
| AMP-K | activated protein kinase |
| Apo (ApoB, ApoC) | apolipoproteins |
| BGN | biguanides |
| BMI | body mass index |
| C1q | complement component |
| CKD | chronic kidney disease |
| CNS | central nervous system |
| CRP | C-reactive protein |
| CSF | cerebrospinal fluid |
| CV | cardiovascular |
| CVD | cardiovascular diseases |
| DII | dietary insulin index |
| DIL | dietary insulin load |
| DKA | diabetic ketoacidosis |
| DM | diabetes mellitus |
| DN | diabetic nephropathy |
| DPP-4i | dipeptidyl peptidase-4 inhibitors |
| DR | diabetic retinopathy |
| eGFR | estimated glomerular filtration rate |
| ELISA | enzyme-linked immunosorbent assay |
| GLN | meglitinides |
| GLP-1a | glucagon-like peptide-1 agonists |
| GLUT4 | glucose transport proteins |
| GSH | glutathione |
| hsCRP | high-sensitivity CRP |
| IL-1β | interleukin 1β |
| IL-6 | interleukin-6 |
| IQR | interquartile range |
| IT | islet transplantation |
| MDA | malondialdehyde |
| NF-κB | nuclear factor-κB |
| NO | nitric oxide |
| NPDR | non-proliferative DR |
| NPTX1 and NPTX2 | neuronal pentraxins |
| PCT | procalcitonin |
| PDR | proliferative DR |
| PPARγ | peroxisome proliferator-activated receptor γ |
| PTX3 | pentraxin-3 |
| PTX4 | pentraxin-4 |
| PTX3KO | PTX3 knockout |
| ROS | reactive oxygen species |
| SAP/PTX2 | serum amyloid P |
| SFN | sulfonylureas |
| SGLT-2i | sodium–glucose cotransporter-2 inhibitors |
| STZ | streptozotocin |
| SUR-1 | sulfonylurea receptor |
| T1DM | type 1 diabetes mellitus |
| T2DM | type 2 diabetes mellitus |
| TGF-β | transforming growth factor-β |
| TNF-α | tumor necrosis factor-α |
| TsoD | Tsumura Suzuki obese–diabetic |
| TZD | thiazolidinediones |
| UACR | urinary albumin/creatinine ratio |
References
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Nawroth, P.P.; Herzig, S.; Ekim Üstünel, B. Emerging Targets in Type 2 Diabetes and Diabetic Complications. Adv. Sci. 2021, 8, e2100275. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, Y.; Feng, L.; Sun, S.; Sun, G. Role of Glucagon and Its Receptor in the Pathogenesis of Diabetes. Front. Endocrinol. 2022, 13, 928016. [Google Scholar] [CrossRef]
- Kane, J.P.; Pullinger, C.R.; Goldfine, I.D.; Malloy, M.J. Dyslipidemia and diabetes mellitus: Role of lipoprotein species and interrelated pathways of lipid metabolism in diabetes mellitus. Curr. Opin. Pharmacol. 2021, 61, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Boyko, E.J. Chapter 3, Global picture. In IDF Diabetes Atlas, 10th ed.; Magliano, D.J., Boyko, E.J., Eds.; Scientific Committee International Diabetes Federation: Brussels, Belgium, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK581940/ (accessed on 29 January 2025).
- World Health Organization. Diabetes. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 6 February 2025).
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2019; Available online: https://iris.who.int/bitstream/handle/10665/325182/9789241515702-eng.pdf?sequence=1 (accessed on 20 January 2025).
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- van Sloten, T.T.; Sedaghat, S.; Carnethon, M.R.; Launer, L.J.; Stehouwer, C.D.A. Cerebral microvascular complications of type 2 diabetes: Stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020, 8, 325–336. [Google Scholar] [CrossRef]
- Yu, M.G.; Gordin, D.; Fu, J.; Park, K.; Li, Q.; King, G.L. Protective Factors and the Pathogenesis of Complications in Diabetes. Endocr. Rev. 2024, 45, 227–252. [Google Scholar] [CrossRef]
- Koufakis, T.; Dimitriadis, G.; Metallidis, S.; Zebekakis, P.; Kotsa, K. The role of autoimmunity in the pathophysiology of type 2 diabetes: Looking at the other side of the moon. Obes. Rev. 2021, 22, e13231. [Google Scholar] [CrossRef]
- Taylor, S.I.; Yazdi, Z.S.; Beitelshees, A.L. Pharmacological treatment of hyperglycemia in type 2 diabetes. J. Clin. Investig. 2021, 131, e142243. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Januzzi, J.L.; Bruemmer, D.; Butalia, S.; Green, J.B.; Horton, W.B.; Knight, C.; Levi, M.; Rasouli, N.; Richardson, C.R. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care 2022, 45, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Koussih, L.; Atoui, S.; Tliba, O.; Gounni, A.S. New Insights on the Role of pentraxin-3 in Allergic Asthma. Front. Allergy 2021, 2, 678023. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Wang, Z.; Wu, W.; Zhang, N.; Zhang, L.; Hu, J.; Luo, P.; Zhang, J.; Liu, Z.; et al. Molecular insight into pentraxin-3: Update advances in innate immunity, inflammation, tissue remodeling, diseases, and drug role. Biomed. Pharmacother. 2022, 156, 113783. [Google Scholar] [CrossRef]
- Zajkowska, M.; Mroczko, B. The Role of Pentraxin 3 in Gastrointestinal Cancers. Cancers 2023, 15, 5832. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yu, Y.; Lu, L. The Role of Pentraxin 3 in Aspergillosis: Reality and Prospects. Mycobiology 2020, 48, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zou, H.; Dai, Z.; Feng, S.; Zhang, M.; Xiao, G.; Liu, Z.; Cheng, Q. The Basic Characteristics of the Pentraxin Family and Their Functions in Tumor Progression. Front. Immunol. 2020, 11, 1757. [Google Scholar] [CrossRef]
- Qiu, C.; Han, Y.; Zhang, H.; Liu, T.; Hou, H.; Luo, D.; Yu, M.; Bian, K.; Zhao, Y.; Xiao, X. Perspectives on long pentraxin 3 and rheumatoid arthritis: Several potential breakthrough points relying on study foundation of the past. Int. J. Med. Sci. 2021, 18, 1886–1898. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Ardizzone, A.; Salako, A.E.; Pantò, G.; De Luca, F.; Esposito, E.; Capra, A.P. Pentraxin 3: A Main Driver of Inflammation and Immune System Dysfunction in the Tumor Microenvironment of Glioblastoma. Cancers 2024, 16, 1637. [Google Scholar] [CrossRef]
- Capra, A.P.; Ardizzone, A.; Pantò, G.; Paterniti, I.; Campolo, M.; Crupi, L.; Squeri, R.; Esposito, E. The Prognostic Value of Pentraxin-3 in COVID-19 Patients: A Systematic Review and Meta-Analysis of Mortality Incidence. Int. J. Mol. Sci. 2023, 24, 3537. [Google Scholar] [CrossRef]
- Smole, U.; Kratzer, B.; Pickl, W.F. Soluble pattern recognition molecules: Guardians and regulators of homeostasis at airway mucosal surfaces. Eur. J. Immunol. 2020, 50, 624–642. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, T.; Wang, J.; Huang, X.; Deng, X.; Cao, Y.; Zhou, M.; Zhao, C. An update on potential biomarkers for diagnosing diabetic foot ulcer at an early stage. Biomed. Pharmacother. 2021, 133, 110991. [Google Scholar] [CrossRef]
- Stravalaci, M.; Ferrara, M.; Pathak, V.; Davi, F.; Bottazzi, B.; Mantovani, A.; Medina, R.J.; Romano, M.R.; Inforzato, A. The Long Pentraxin PTX3 as a New Biomarker and Pharmacological Target in Age-Related Macular Degeneration and Diabetic Retinopathy. Front. Pharmacol. 2022, 12, 811344. [Google Scholar] [CrossRef]
- Adam, C.A.; Șalaru, D.L.; Prisacariu, C.; Marcu, D.T.M.; Sascău, R.A.; Stătescu, C. Novel Biomarkers of Atherosclerotic Vascular Disease-Latest Insights in the Research Field. Int. J. Mol. Sci. 2022, 23, 4998. [Google Scholar] [CrossRef]
- Popescu, D.M.; Gheorghe, D.N.; Turcu-Stiolica, A.; Soancă, A.; Roman, A.; Ionele, C.M.; Ciucă, E.M.; Boldeanu, V.M.; Boldeanu, L.; Pitru, A.; et al. Evaluation of Pentraxin 3 and Serum Amyloid A in the Gingival Crevicular Fluid of Patients with Periodontal Disease and Obesity. J. Clin. Med. 2023, 12, 3523. [Google Scholar] [CrossRef] [PubMed]
- Rauten, A.M.; Silosi, I.; Stratul, S.I.; Foia, L.; Camen, A.; Toma, V.; Cioloca, D.; Surlin, V.; Surlin, P.; Bogdan, M. Expression of Pentraxin 3 and Thrombospondin 1 in Gingival Crevicular Fluid during Wound Healing after Gingivectomy in Postorthodontic Patients. J. Immunol. Res. 2016, 2016, 4072543. [Google Scholar] [CrossRef] [PubMed]
- Surlin, P.; Rauten, A.M.; Silosi, I.; Foia, L. Pentraxin-3 levels in gingival crevicular fluid during orthodontic tooth movement in young and adult patients. Angle Orthod. 2012, 82, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Zhang, X.; Wang, T.; Zhang, X. Progress in the study of pentraxin-3(PTX-3) as a biomarker for sepsis. Front. Med. 2024, 11, 1398024. [Google Scholar] [CrossRef]
- Badran, H.; Elsabaawy, M.; Magdy, M.; Ghanem, S.; Said, M.; Torky, M.H.; Samir, T. The utility of pentraxin 3 and platelet-derived growth factor receptor beta as non-invasive biomarkers for prediction of cardiovascular risk in MAFLD patients. Egypt. J. Intern. Med. 2024, 36, 86. [Google Scholar] [CrossRef]
- Wu, Q.; Cao, F.; Tao, J.; Li, X.; Zheng, S.G.; Pan, H.F. Pentraxin 3: A promising therapeutic target for autoimmune diseases. Autoimmun. Rev. 2020, 19, 102584. [Google Scholar] [CrossRef]
- Bogdan, M.; Meca, A.D.; Turcu-Stiolica, A.; Oancea, C.N.; Kostici, R.; Surlin, M.V.; Florescu, C. Insights into the Relationship between Pentraxin-3 and Cancer. Int. J. Mol. Sci. 2022, 23, 15302. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Z.; Lei, W.; Shen, M.; Tang, J.; Xu, X.; Yang, Y.; Zhang, H. Pentraxin 3: A promising therapeutic target for cardiovascular diseases. Ageing Res. Rev. 2024, 93, 102163. [Google Scholar] [CrossRef]
- Tamura, Y.; Ono, T.; Kuwana, M.; Inoue, K.; Takei, M.; Yamamoto, T.; Kawakami, T.; Fujita, J.; Kataoka, M.; Kimura, K.; et al. Human pentraxin 3 (PTX3) as a novel biomarker for the diagnosis of pulmonary arterial hypertension. PLoS ONE 2012, 7, e45834. [Google Scholar] [CrossRef] [PubMed]
- Kume, N.; Mitsuoka, H.; Hayashida, K.; Tanaka, M. Pentraxin 3 as a biomarker for acute coronary syndrome: Comparison with biomarkers for cardiac damage. J. Cardiol. 2011, 58, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Scavello, F.; Brunetta, E.; Mapelli, S.N.; Nappi, E.; García Martín, I.D.; Sironi, M.; Leone, R.; Solano, S.; Angelotti, G.; Supino, D.; et al. The long Pentraxin PTX3 serves as an early predictive biomarker of co-infections in COVID-19. EBioMedicine 2024, 105, 105213, Erratum in EBioMedicine 2024, 108, 105372. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, H.; Mostafa, M.G.; Madkour, S. Pentraxin 3: A novel biomarker in pediatric central nervous system infections. BMC Pediatr. 2025, 25, 7. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kang, J.; Han, K.M.; Kim, H. Prognostic Value of Pentraxin3 Protein Expression in Human Malignancies: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 3754. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Jamialahmadi, T.; Sahebkar, A. Anti-inflammatory benefits of semaglutide: State of the art. J. Clin. Transl. Endocrinol. 2024, 36, 100340. [Google Scholar] [CrossRef]
- Doni, A.; Sironi, M.; Del Prete, A.; Pasqualini, F.; Valentino, S.; Cuccovillo, I.; Parente, R.; Calvi, M.; Tosoni, A.; Vago, G.; et al. PTX3 is expressed in terminal lymphatics and shapes their organization and function. Front. Immunol. 2024, 15, 1426869. [Google Scholar] [CrossRef]
- Ma, Y.J.; Parente, R.; Zhong, H.; Sun, Y.; Garlanda, C.; Doni, A. Complement-pentraxins synergy: Navigating the immune battlefield and beyond. Biomed. Pharmacother. 2023, 169, 115878. [Google Scholar] [CrossRef]
- Lin, T.Y.; Guo, H.; Chen, X. Unraveling mechanisms of pentraxin 3 secretion in adipocytes during inflammation. J. Mol. Endocrinol. 2021, 67, 55–69. [Google Scholar] [CrossRef]
- Chen, F.W.; Wu, Y.L.; Cheng, C.C.; Hsiao, Y.W.; Chi, J.Y.; Hung, L.Y.; Chang, C.P.; Lai, M.D.; Wang, J.M. Inactivation of pentraxin 3 suppresses M2-like macrophage activity and immunosuppression in colon cancer. J. Biomed. Sci. 2024, 31, 10. [Google Scholar] [CrossRef]
- Banfi, C.; Brioschi, M.; Vicentini, L.M.; Cattaneo, M.G. The Effects of Silencing PTX3 on the Proteome of Human Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 13487. [Google Scholar] [CrossRef]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in Cardiovascular Disease. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef]
- Doni, A.; Mantovani, A.; Bottazzi, B.; Russo, R.C. PTX3 Regulation of Inflammation, Hemostatic Response, Tissue Repair, and Resolution of Fibrosis Favors a Role in Limiting Idiopathic Pulmonary Fibrosis. Front. Immunol. 2021, 12, 676702. [Google Scholar] [CrossRef]
- Bottazzi, B.; Inforzato, A.; Messa, M.; Barbagallo, M.; Magrini, E.; Garlanda, C.; Mantovani, A. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J. Hepatol. 2016, 64, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Fornai, F.; Carrizzo, A.; Ferrucci, M.; Damato, A.; Biagioni, F.; Gaglione, A.; Puca, A.A.; Vecchione, C. Brain diseases and tumorigenesis: The good and bad cops of pentraxin3. Int. J. Biochem. Cell Biol. 2015, 69, 70–74. [Google Scholar] [CrossRef]
- Garlanda, C.; Bottazzi, B.; Magrini, E.; Inforzato, A.; Mantovani, A. PTX3, a Humoral Pattern Recognition Molecule, in Innate Immunity, Tissue Repair, and Cancer. Physiol. Rev. 2018, 98, 623–639. [Google Scholar] [CrossRef]
- Pan, L.; Hong, S.; Li, Y.; Yuan, L.; Zhao, L.; Wen, J. The causal relationship between 91 inflammatory cytokines and Gestational Diabetes mellitus: A bidirectional two-sample Mendelian randomization study. Diabetes Res. Clin. Pract. 2024, 216, 111838. [Google Scholar] [CrossRef]
- Zhao, B.; Han, X.; Meng, Q.; Luo, Q. Early second trimester maternal serum markers in the prediction of gestational diabetes mellitus. J. Diabetes Investig. 2018, 9, 967–974. [Google Scholar] [CrossRef]
- Qu, X.; Zhuang, J.; Xu, C.; Ai, Z.; Yuan, L.; Tang, Y.; Shu, Q.; Bao, Y.; Han, H.; Ying, H. Maternal serum pentraxin 3 level in early pregnancy for prediction of gestational diabetes mellitus. Ann. Transl. Med. 2019, 7, 722. [Google Scholar] [CrossRef]
- Dawood, A.A.; Kamel, M.A.; Omar, T.A.; Agaba, A.A.M. Study of serum pentraxin 3 level in patients with diabetic nephropathy. Egypt. J. Intern. Med. 2020, 32, 3. [Google Scholar] [CrossRef]
- Negeem, Z.R.; Moneim, A.A.; Mahmoud, B.; Ahmed, A.E.; Hasona, N.A. Association of microRNA-192, pentraxin-3, and transforming growth factor-beta1 with estimated glomerular filtration rate in adults with diabetic nephropathy. Int. J. Diabetes Dev. Ctries. 2024, 44, 812–821. [Google Scholar] [CrossRef]
- Dixit, A.; Unni, S.N.; Prabhu, S.; Krishnan, S.; Ravindran, G.C. Role of serum pentraxin-3 levels in patients with and without diabetic nephropathy. Int. J. Diabetes Dev. Ctries. 2024, 45, 227–233. [Google Scholar] [CrossRef]
- Das, S.; Ramanathan, G. Assessing the Inhibitory Potential of Pregnenolone Sulfate on Pentraxin 3 in Diabetic Kidney Disease: A Molecular Docking and Simulation Study. J. Cell. Biochem. 2025, 126, e30661. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Hu, W. Association of Serum Pentraxin 3 Concentrations with Diabetic Nephropathy. J. Investig. Med. 2016, 64, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, A.; Nouh, M.Z.; Mogahed, M. Evaluation of pentraxin-3 level in patients with diabetic retinopathy. Menoufia Med. J. 2018, 31, 35. [Google Scholar] [CrossRef]
- Mutlu, M.; Yuksel, N.; Takmaz, T.; Dincel, A.S.; Bilgihan, A.; Altınkaynak, H. Aqueous humor pentraxin-3 levels in patients with diabetes mellitus. Eye 2017, 31, 1463–1467. [Google Scholar] [CrossRef][Green Version]
- Zhao, L.; Hu, H.; Zhang, L.; Liu, Z.; Huang, Y.; Liu, Q.; Jin, L.; Zhu, M.; Zhang, L. Inflammation in diabetes complications: Molecular mechanisms and therapeutic interventions. MedComm 2024, 5, e516. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Q.; Liu, K.; Wang, S.; Yan, Y.; Zhang, B.; Zhao, L. The association between interleukin family and diabetes mellitus and its complications: An overview of systematic reviews and meta-analyses. Diabetes Res. Clin. Pract. 2024, 210, 111615. [Google Scholar] [CrossRef]
- Magda, S.M.; Maram, M.M.M.; Hanan Mahmoud, A.; Bedir, F.B.M. Plasma Levels of Pentraxin 3 and Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus. QJM Int. J. Med. 2023, 116, hcad069.400. [Google Scholar] [CrossRef]
- Elbana, K.A.; Salem, H.M.; Abdel Fattah, N.R.; Etman, E. Serum Pentraxin 3 level as a recent biomarker of diabetic retinopathy in Egyptian patients with diabetes. Diabetes Metab. Syndr. 2019, 13, 2361–2364. [Google Scholar] [CrossRef] [PubMed]
- Ardelean, A.; Balta, D.-F.; Neamtu, C.; Neamtu, A.A.; Rosu, M.; Pilat, L.; Moldovan, S.; Tarta, C.; Totolici, B. Pentraxin-3 and Other Inflammatory Markers for an Infected Diabetic Foot Ulcer Diagnosis: A Prospective Study. Diagnostics 2023, 13, 2366. [Google Scholar] [CrossRef]
- Waluś-Miarka, M.; Trojak, A.; Miarka, P.; Kapusta, M.; Kawalec, E.; Idzior-Waluś, B.; Małecki, M.T. Correlates of pentraxin 3 serum concentration in men and women with type 2 diabetes. Innate Immun. 2020, 26, 351–357. [Google Scholar] [CrossRef]
- Ahmed, M.M.M.; Hossam Eldin, A.A.; Ahmed, I.M.E.; Tari, M.A.G.; Mohamed, N.B.A.; Mostafa, A.A.F. Serum Pentraxin 3 Level in Egyptian Patients with Nonalcoholic Fatty Liver Disease and Type 2 Diabetes. QJM Int. J. Med. 2024, 117, hcae175.345. [Google Scholar] [CrossRef]
- Trojak, A.; Waluś-Miarka, M.; Kapusta, M.; Miarka, P.; Kawalec, E.; Idzior-Waluś, B.; Małecki, M.T. Serum pentraxin 3 concentration in patients with type 2 diabetes and nonalcoholic fatty liver disease. Pol. Arch. Intern. Med. 2019, 129, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, V.; Köse Özlece, H.; Eroglu, H.A.; Aktuğ, H.; Erbaş, O.; Taşkıran, D. Accumulation of α-Synuclein in Cerebellar Purkinje Cells of Diabetic Rats and Its Potential Relationship with Inflammation and Oxidative Stress Markers. Neurol. Res. Int. 2017, 2017, 5952149. [Google Scholar] [CrossRef]
- Chen, X.; Luo, J.; Wu, M.; Pan, Z.; Xie, Y.; Wang, H.; Chen, B.; Zhu, H. Study on Association of Pentraxin 3 and Diabetic Nephropathy in a Rat Model. J. Diabetes Res. 2018, 2018, 8968573. [Google Scholar] [CrossRef]
- Pathak, V.; Bertelli, P.M.; Pedrini, E.; Harkin, K.; Peixoto, E.; Allen, L.D.; Mcloughlin, K.; Chavda, N.D.; Hamill, K.J.; Guduric-Fuchs, J.; et al. Modulation of diabetes-related retinal pathophysiology by PTX3. Proc. Natl. Acad. Sci. USA 2024, 121, e2320034121. [Google Scholar] [CrossRef]
- Miyaki, A.; Choi, Y.; Maeda, S. Pentraxin 3 production in the adipose tissue and the skeletal muscle in diabetic-obese mice. Am. J. Med. Sci. 2014, 347, 228–233. [Google Scholar] [CrossRef]
- Uzun, S.; Ozari, M.; Gursu, M.; Karadag, S.; Behlul, A.; Sari, S.; Koldas, M.; Demir, S.; Karaali, Z.; Ozturk, S. Changes in the inflammatory markers with advancing stages of diabetic nephropathy and the role of pentraxin-3. Ren. Fail. 2016, 38, 1193–1198. [Google Scholar] [CrossRef]
- Tsurutani, Y.; Omura, M.; Matsuzawa, Y.; Saito, J.; Higa, M.; Taniyama, M.; Nishikawa, T. SINGLE-Y investigation group. Efficacy and Safety of the Dipeptidyl Peptidase-4 Inhibitor Sitagliptin on Atherosclerosis, β-Cell Function, and Glycemic Control in Japanese Patients with Type 2 Diabetes Mellitus Who are Treatment Naïve or Poorly Responsive to Antidiabetes Agents: A Multicenter, Prospective Observational, Uncontrolled Study. Curr. Ther. Res. Clin. Exp. 2017, 84, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Keramat, L.; Sadrzadeh-Yeganeh, H.; Sotoudeh, G.; Zamani, E.; Eshraghian, M.; Mansoori, A.; Koohdani, F. Apolipoprotein A2 -265 T>C polymorphism interacts with dietary fatty acids intake to modulate inflammation in type 2 diabetes mellitus patients. Nutrition 2017, 37, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Mahmoudi, M.; Saedisomeolia, A.; Mansournia, M.A.; Zahirihashemi, R.; Koohdani, F. Study of the relationship between APOA-II -265T>C polymorphism and HDL function in response to weight loss in overweight and obese type 2 diabetic patients. Clin. Nutr. 2018, 37, 965–969. [Google Scholar] [CrossRef]
- Shore, A.C.; Colhoun, H.M.; Natali, A.; Palombo, C.; Khan, F.; Östling, G.; Aizawa, K.; Kennbäck, C.; Casanova, F.; Persson, M.; et al. Use of Vascular Assessments and Novel Biomarkers to Predict Cardiovascular Events in Type 2 Diabetes: The SUMMIT VIP Study. Diabetes Care 2018, 41, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Takashi, Y.; Koga, M.; Matsuzawa, Y.; Saito, J.; Omura, M.; Nishikawa, T. Circulating pentraxin 3 is positively associated with chronic hyperglycemia but negatively associated with plasma aldosterone concentration. PLoS ONE 2018, 13, e0196526. [Google Scholar] [CrossRef]
- Bala, C.; Rusu, A.; Ciobanu, D.M.; Craciun, A.E.; Roman, G. The association study of high-sensitivity C-reactive protein, pentraxin 3, nitrotyrosine, and insulin dose in patients with insulin-treated type 2 diabetes mellitus. Ther. Clin. Risk Manag. 2018, 14, 955–963. [Google Scholar] [CrossRef]
- Yin, C.; Lu, S.; Wei, D.; Xiong, J.; Zhu, L.; Yan, S.; Meng, R. Effects of nutritional support combined with insulin therapy on serum proteins, inflammatory factors, pentraxin-3, and serum amylase levels in patients with diabetic ketoacidosis complicated with acute pancreatitis. Medicine 2021, 100, e27920. [Google Scholar] [CrossRef]
- Abaj, F.; Rafiee, M.; Koohdani, F. A personalised diet approach study: Interaction between PPAR-γ Pro12Ala and dietary insulin indices on metabolic markers in diabetic patients. J. Hum. Nutr. Diet. 2022, 35, 663–674. [Google Scholar] [CrossRef]
- Demirkılıç, O.; Eski, İ.; Çiftçi Öztürk, E.; Yasun, Ö.; Aydın, B.; Birkan, C.; Özsoy, A.; Şen, S. Association Between Dipeptidyl Peptidase-4 Inhibitor Use and Cognitive Functions, Brain-Derived Neurotrophic Factor, and Pentraxin-3 Levels in Patients With Type 2 Diabetes. Cureus 2024, 16, e54440. [Google Scholar] [CrossRef]
- Hachuła, M.; Kosowski, M.; Basiak, M.; Okopień, B. Influence of Dulaglutide on Serum Biomarkers of Atherosclerotic Plaque Instability: An Interventional Analysis of Cytokine Profiles in Diabetic Subjects-A Pilot Study. Medicina 2024, 60, 908. [Google Scholar] [CrossRef] [PubMed]
- Artunc-Ulkumen, B.; Pala, H.G.; Pala, E.E.; Yavasoglu, A.; Yigitturk, G.; Erbas, O. Exenatide improves ovarian and endometrial injury and preserves ovarian reserve in streptozocin induced diabetic rats. Gynecol. Endocrinol. 2015, 31, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Elkazzaz, S.K.; Khodeer, D.M.; El Fayoumi, H.M.; Moustafa, Y.M. Role of sodium glucose cotransporter type 2 inhibitors dapagliflozin on diabetic nephropathy in rats; Inflammation, angiogenesis and apoptosis. Life Sci. 2021, 280, 119018. [Google Scholar] [CrossRef] [PubMed]
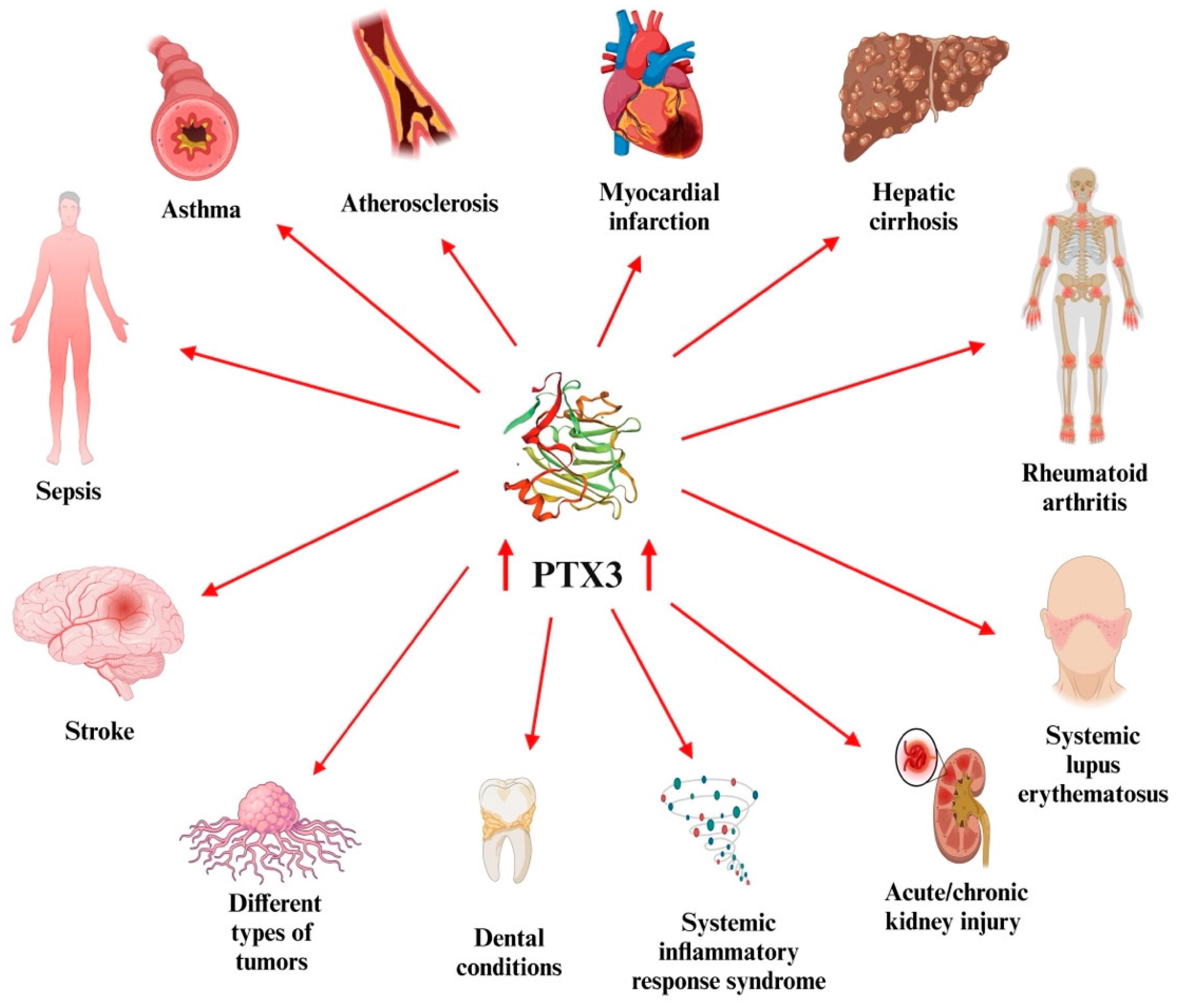
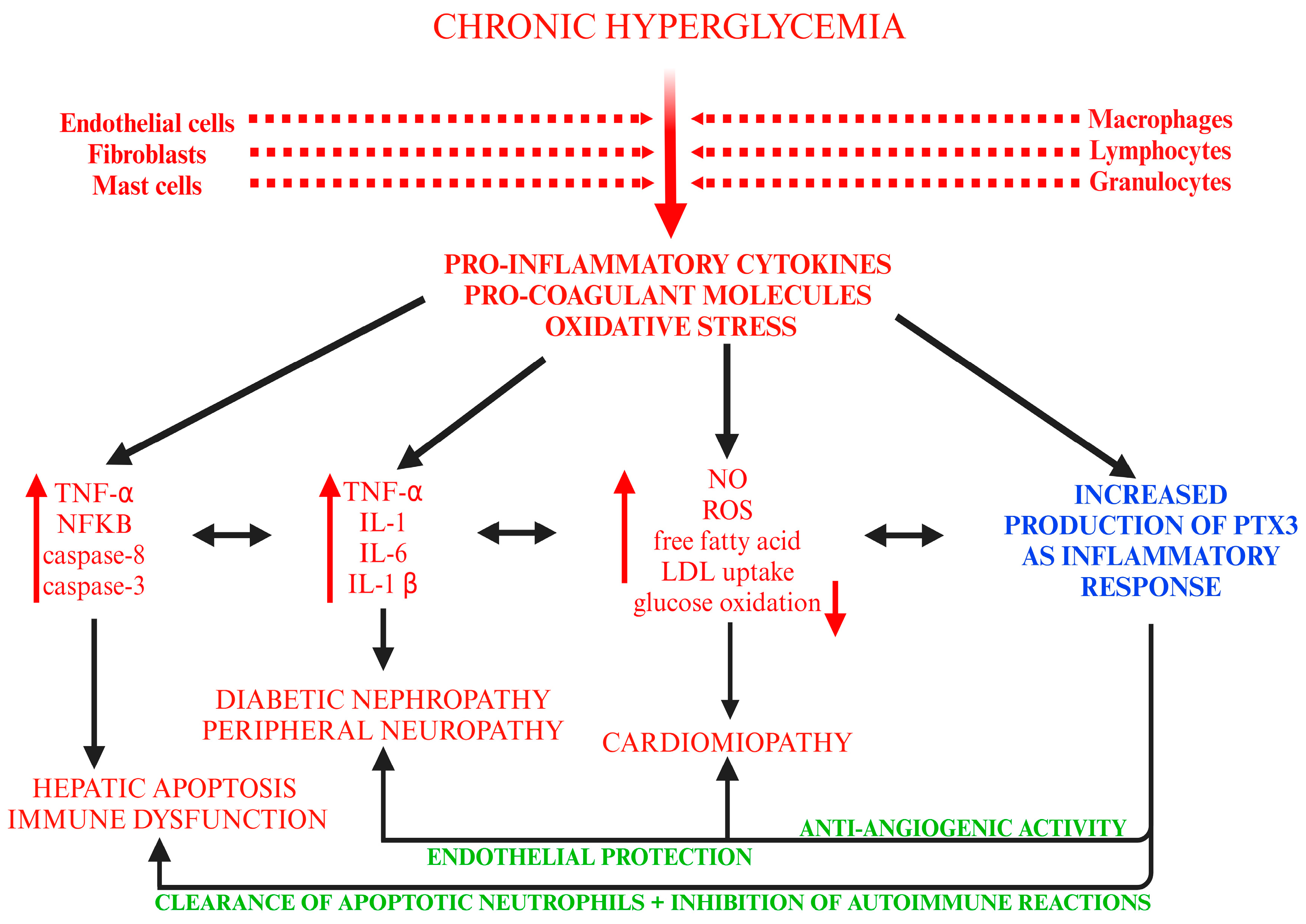
| Author, Year, Country | Number of Patients, Group | Gender/Age Years (Mean ± SD) | Drug (Dose)/ Number of Patients | Period of Administration | Serum PTX-3 Levels (pg/mL) | ||
|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | ||||||
| Uzun, 2016 Turkey [75] | N = 106 group 1: eGFR > 60 mL/min and albuminuria between 30 and 300 mg/day on two of the three measurements within the last 3 months group 2: eGFR > 60 mL/min and albuminuria >300 mg/day on two of the three measurements within the last 3 months. group 3: eGFR < 60 mL/min and albuminuria >300 mg/day on two of the three measurements within the last 3 months. | 61 female 45 male 19 female 18 male 22 female 12 male 20 female 15 male | 55.88 ± 8.7 54.5 ± 10.5 54.4 ± 8.2 58.8 ± 7.3 | 13 SFN (-) 3 insulin secretagogues (-) 1 glitazones (-) 18 insulin (-) 31 metformin (-) 11 acarbose (-) 3 SFN (-) 2 insulin secretagogues (-) 2 glitazones (-) 20 insulin (-) 20 metformin (-) 7 acarbose (-) 3 SFN (-) 1 insulin secretagogues (-) 2 glitazones (-) 29 insulin (-) 7 metformin (-) 3 acarbose (-) | ------------------- | ------------------ | N = 37 810 ± 250 N = 34 940 ± 260 N = 35 1350 ± 1550 |
| Tsurutani, 2016 Japan [76] | N = 270 at the start of the study N = 257 at 3 months N = 211 at 12 months | 105 female 165 male | 64.3 ± 12.4 | - oral administration of sitagliptin (50 mg/day13) for 3 months. - after 3 months, adjustments were allowed (sitagliptin: 25–100 mg/day). - SFN doses were adjusted according to the Japan Diabetes Society recommendations: -glimepiride: ≤ 2 mg/day -glibenclamid: ≤ 1.25 mg/day -gliclazide: ≤ 40 mg/day | 12 months, PTX3 level is presented only at 3 months | N = 34 baseline: 1880 ± 780 | N = 34 3 months: 1630 ± 630 |
| Keramat, 2017 Iran [77] | N = 180 TT/TC N = 120 (obese + non-obese) CC N = 60 (obese + non-obese) | 78 female 42 male 38 female 22 male | 52.98 ± 6.80 56.00 ± 5.89 | 8 without medications 42 metformin (-) 6 glibenclamid (-) 64 metformin + glibenclamid (-) 5 without medications 21 metformin (-) 3 glibenclamid (-) 31 metformin + glibenclamid (-) | --------------- | TT/TC N = 120 obese 2590 ± 380 non-obese 2760 ± 520 CC N = 60 obese 2440 ± 510 non-obese 2590 ± 370 | ------------ |
| Moradi, 2017 Iran [78] | N = 44 22 people in TT/TC group metformin (n = 7) glybenclamid (n = 1) metformin+ glybenclamid (n = 12) other drugs (n = 2) 22 people in CC group metformin (n = 9) glybenclamide (n = 1) metformine+ glybenclamide (n = 12) other drugs (n = 0) | 15 female/group 7 male/group | 56.68 ± 5.9 56.27± 6.51 57.09 ± 5.45 | metformin (-) glybenclamid (-) metformin + glybenclamid (-) other drugs (-) +dietary program | 6 weeks | baseline: N = 22 TT/TC 4570 ± 850 N = 22 CC 3820 ± 560 before intervention: N = 44 4170 ± 710 TT/TC N = 22 4520 ± 840 CC N = 22 3810 ± 560 | N = 44 4750 ± 630 TT/TC N = 22 5520 ± 780 CC N = 22 4050 ± 400 |
| Shore, 2018 U.K., Italy, Sweden, Germany [79] | N = 936 at baseline: - 440 with T2DM and: - CVD (myocardial infarction, stroke, or lower extremity arterial disease): -No CV event (n = 367): glitazones (n = 23) metformin (n = 239) insulin (n = 108) SFN (n = 110) DPP-4i (n = 41) incretin analogs: (n = 20). -CV event (n = 73) glitazones (n = 2) metformin (n = 45) insulin (n = 33) SFN (n = 15) DPP-4i (n = 3) incretin analogs: (n = 2). -496 with T2DM but without clinically manifest CVD: -No CVD event (n = 464): glitazones (n = 33) metformin (n = 331) insulin (n = 73) SFN (n = 138) DPP-4i (n = 52) incretin analogs: (n = 24). -CV event (n = 32): glitazones (n = 5) metformin (n = 26) insulin (n = 8) SFN (n = 7) DPP-4i (n = 2) incretin analogs: (n = 1). N = 648 at 3-year follow-up: 327 with T2DM and: -CVD (myocardial infarction, stroke, or lower extremity arterial disease): -No CV event (n = 276): glitazones (n = 13) metformin (n = 175) insulin (n = 83) SFN (n = 75) DPP-4i (n = 37) incretin analogs: (n = 18). -CV event (n = 51) glitazones (n = 0) metformin (n = 34) insulin (n = 23) SFN (n = 11) DPP-4i (n = 4) incretin analogs: (n = 1). -421 with T2DM but without clinically manifest CVD: -No CVD event (n = 397): glitazones (n = 22) metformin (n = 274) insulin (n = 86) SFN (n = 101) DPP-4i (n = 53) incretin analogs: (n = 23). -CV event (n = 24): glitazones (n = 1) metformin (n = 20) insulin (n = 8) SFN (n = 7) DPP-4i (n = 4) incretin analogs: (n = 0). | baseline: - 440 with T2DM and: - CVD (myocardial infarction, stroke, or lower extremity arterial disease): -No CV event (n = 367): 98 female 269 male -CV event (n = 73): 25 female 48 male 496 with T2DM but without clinically manifest CVD: -No CVD event (n = 464): 164 female 290 male -CV event (n = 32) 12 female 20 male | CVD at baseline (n = 440) No CV event (n = 367) 69.4 ± 8.5 CV event (n = 73) 69.3 ± 8.7 No CVD at baseline (n = 496) No CV event (n = 464) 66.5 ± 8.7 CV event (n = 32) 68.2 ± 6.1 CVD at baseline (n = 234) No CV event (n = 200) 69.1 ± 8.4 CV event (n = 34) 71.1 ± 7.1 No CVD at baseline (n = 253) No CV event (n = 238) 64.6 ± 10.6 CV event (n = 15) 70.0 ± 7.2 | glitazones (-) insulin (-) metformin (-) SFN (-) DPP-4i (-) incretin analogs (-) | November 2010– June 2013 | CVD at baseline (n = 440) No CV event (n = 367) 2100 (1700–2600) CV event (n = 73) 2300 (2000–2700) No CVD at baseline (n = 496) No CV event (n = 464) 2100 (1700–2600) CV event (n = 32) 2100 (1800–2600) CVD at baseline (n = 234) No CV event (n = 200) 2000 (1700–2400) CV event (n = 34) 1900 (1700–2700) No CVD at baseline (n = 253) No CV event (n = 238) 2000 (1700–2400) CV event (n = 15) 1900 (1600–2300) | -------------------- |
| Takashi, 2018 Japan [80] | N = 75 28 non-diabetes 47 diabetes (43 T2DM and 4 T1DM) insulin (n = 9) DPP-4i (n = 11) metformin (n = 21) thiazolidines (n = 4) sulfonylureas (n = 19) glinides (n = 4) α-GIs (n = 13) | 30 female 45 male | total 55.1 ± 13.4 non-diabetes 52.4 ± 12.3 diabetes 56.8 ± 13.9 | insulin (-) DPP4i (-) metformin (-) thiazolidines (-) SFN (-) glinides (-) α-GIs (-) | ---------------- | 2810 ± 1017 2150 ± 1040 3200 ± 1910 | - --------------------- |
| Bala, 2018 Romania [81] | N = 80 1st tertile of insulin dose/kg of body weight (0.18–0.57 IU/kg of body weight) N = 26 2nd tertile of insulin dose/kg of body weight (0.58–0.89 IU/kg of body weight) N = 27 3rd tertile of insulin dose/kg of body weight (0.90–2.35 IU/kg of body weight) N = 27 | 47 female 33 male 14 female 12 male 12 female 15 male 21 female 6 male | 63.8 ± 9.0 65.0 ± 8.1 61.6 ± 9.9 64.8 ± 8.9 | 53 metformin (-) 4 SFN (-) 7 incretins (-) 70 insulin (0.7 insulin dose/kg of body weight, IU/kg/day) 16 metformin (-) 1 SFN (-) 2 incretins (-) 31 insulin (0.4 insulin dose/kg of body weight, IU/kg/day) 20 metformin (-) 2 SFN (-) 3 incretins (-) 70 insulin (0.7 insulin dose/kg of body weight, IU/kg/day) 17 metformin (-) 1 SFN (-) 2 incretins (-) 93 insulin (1.1. insulin dose/kg of body weight, IU/kg/day) | >6 months | N = 80 1000 ± 200 N = 26 1000 ± 200 N = 27 1000 ± 300 N = 27 900 ± 200 | ------------ |
| Walus-Miarka, 2020 Poland [68] | N = 116 28 female + 36 male insulin 36 female + 46 male metformin | 49 female 67 male | 59.1 ± 11.07 62 ± 11.68 57 ± 10.17 | 115 insulin (-) 113 metformin (-) | 2014–2017 | --------------------- | N = 116 4110 ± 245 N = 49 F: 4530 ± 331 N = 67 M: 4020 ± 199 |
| Yin, 2021 China [82] | N = 64 32 nutritional support combined with insulin therapy 32 insulin | 14 female 18 male 15 female 17 male | 51.03 ± 5.21 50.98 ± 5.19 | nutritional support + insulin (0.1 U/(kg h) once every 4 to 6 h). insulin (0.1 U / (kg h) once every 4 to 6 h). | 7 days | N = 32 nutritional support + insulin 9570 ± 410 N = 32 insulin 9540 ± 510 | 230 ± 80 440 ± 60 |
| Abaj, 2022 Iran [83] | N = 393 251 CC 142 CG + GG | CC 52.97 ± 6.37 CG + GG 52.81 ± 7.45 | metformin + glybenclamid (-) other medications (-) tertile of DIL: metformin + glybenclamid (-) N = 131 T1 92 T2 102 T3 118 other medications (-) T1 18 T2 19 T3 25 tertile of DII metformin + glybenclamid (-) N = 131 T1 84 T2 112 T3 116 other medications (-) T1 16 T2 15 T3 31 | -------------------- | 2680 ± 440 2650 ± 480 tertile of DIL: N = 131 T1 2660 ± 430 T2 2710 ± 390 T3 2680 ± 520 tertile of DII N = 131 T1 2690 ± 410 T2 2690 ± 510 T3 2650 ± 410 | ||
| Demirkilic, 2024 Turkey [84] | N = 50 25 metformin ± DPP-4i: vildagliptin (n = 7) sitagliptin (n = 9) linagliptin (n = 9) 25 only metformin | 31 female 19 male 17 female 8 male 14 female 11 male | 51.38 ± 8.42 51.56 ± 9.26 51.20 ± 7.68 | metformin (-) vildagliptin (-) sitagliptin (-) linagliptin (-) | >3 months | ---------------------- | N = 25 metformin ± DPP-4i 5470 ± 3440 N = 25 only metformin 3790 ± 2530 |
| Hachuła, 2024 Poland [85] | N = 34 34 dulaglutide | 19 female 15 male | 61 ± 10.4 | dulaglutide (1.5–3 mg) -treatment was initiated with a dose of 1.5 mg; patients who did not achieve significant glycemic control after 4 weeks had their dose increased to 3 mg. 36 metformin (-) 16 SFN (-) 7 SGLT2i (-) 1 DPP-4i (-) 9 insulin (-) | 6 months | N = 34 dulaglutide 1288 Q1 1174.5 Q3 1381.5 Q1—first quartile; Q3—third quartile | N = 34 1023.5 Q1 1008 Q3 1173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobriceanu, R.-C.; Meca, A.D.; Boboc, I.K.S.; Mititelu-Tartau, L.; Naidin, M.S.; Turcu-Stiolica, A.; Bogdan, M. Pentraxin-3 as a Biomarker in Diabetes Mellitus: Insights into Inflammation, Vascular Complications, and Modulation by Antidiabetic Medications. Biomedicines 2025, 13, 891. https://doi.org/10.3390/biomedicines13040891
Dobriceanu R-C, Meca AD, Boboc IKS, Mititelu-Tartau L, Naidin MS, Turcu-Stiolica A, Bogdan M. Pentraxin-3 as a Biomarker in Diabetes Mellitus: Insights into Inflammation, Vascular Complications, and Modulation by Antidiabetic Medications. Biomedicines. 2025; 13(4):891. https://doi.org/10.3390/biomedicines13040891
Chicago/Turabian StyleDobriceanu, Roxana-Cristina, Andreea Daniela Meca, Ianis Kevyn Stefan Boboc, Liliana Mititelu-Tartau, Mihaela Simona Naidin, Adina Turcu-Stiolica, and Maria Bogdan. 2025. "Pentraxin-3 as a Biomarker in Diabetes Mellitus: Insights into Inflammation, Vascular Complications, and Modulation by Antidiabetic Medications" Biomedicines 13, no. 4: 891. https://doi.org/10.3390/biomedicines13040891
APA StyleDobriceanu, R.-C., Meca, A. D., Boboc, I. K. S., Mititelu-Tartau, L., Naidin, M. S., Turcu-Stiolica, A., & Bogdan, M. (2025). Pentraxin-3 as a Biomarker in Diabetes Mellitus: Insights into Inflammation, Vascular Complications, and Modulation by Antidiabetic Medications. Biomedicines, 13(4), 891. https://doi.org/10.3390/biomedicines13040891










