Traumatic Brain Injury and Chronic Traumatic Encephalopathy: Not Only Trigger for Neurodegeneration but Also for Cerebral Amyloid Angiopathy?
Abstract
1. Introduction
2. Definitions: Traumatic Brain Injury and Traumatic Brain Injury-Induced Encephalopathy
3. Traumatic Brain Injury and Dementia
4. Traumatic Brain Injury and Amyloid-Beta Pathology
4.1. Tau Pathology in TBI
- -
- Whether a certain injury velocity or location is required to initiate chronic tau aggregation.
- -
- The number of impacts necessary to trigger tau pathology.
- -
- Practical issues such as skin deflection, tissue necrosis, and repeat anesthesia, which complicate model development.
4.2. Polypathology in TBI
- -
- Age at the time of injury: Younger individuals may have greater resilience, while older individuals may be more vulnerable to long-term neurodegeneration.
- -
- Duration of survival post-injury: Longer survival times allow for the development and progression of neuropathology.
- -
- Repeated head trauma or increased injury severity: More frequent or severe injuries may increase the likelihood of developing neurodegenerative pathology.
- -
- APOE4 genotype: The presence of the APOE4 allele is a known risk factor for Alzheimer’s disease and has been linked to worse outcomes following TBI.
5. Traumatic Brain Injury and Cerebral Amyloid Angiopathy
6. Pathophysiological Issues
6.1. Diffuse Axonal Injury
6.2. Synapse Loss
6.3. Oxidative Stress
6.4. Apoptosis
6.5. Neuroinflammation
6.6. Loss of Pyramidal Cells
7. Structural Neuroimaging Markers
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allsop, D.; Haga, S.; Bruton, C.; Ishii, T.; Roberts, G.W. Neurofibrillary tangles in some cases of dementia pugilistica share antigens with amyloid beta-protein of Alzheimer’s disease. Am. J. Pathol. 1990, 136, 255–260. [Google Scholar] [PubMed]
- Chen, X.H.; Johnson, V.E.; Uryu, K.; Trojanowski, J.Q.; Smith, D.H. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009, 19, 214–223. [Google Scholar] [CrossRef]
- Chen, X.H.; Siman, R.; Iwata, A.; Meaney, D.F.; Trojanowski, J.Q.; Smith, D.H. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am. J. Pathol. 2004, 165, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.C.; Burke, J.F.; Nettiksimmons, J.; Kaup, A.; Barnes, D.E.; Yaffe, K. Dementia risk after traumatic brain injury vs nonbrain trauma: The role of age and severity. JAMA Neurol. 2014, 71, 1490–1497. [Google Scholar] [CrossRef]
- Fleminger, S.; Oliver, D.L.; Lovestone, S.; Rabe-Hesketh, S.; Giora, A. Head injury as a risk factor for Alzheimer’s disease: The evidence 10 years on; a partial replication. J. Neurol. Neurosurg. Psychiatry 2003, 74, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Raymont, V.; Greathouse, A.; Reding, K.; Lipsky, R.; Salazar, A.; Grafman, J. Demographic, structural and genetic predictors of late cognitive decline after penetrating head injury. Brain 2008, 131, 543–558. [Google Scholar] [CrossRef]
- Rosso, S.M.; Landweer, E.J.; Houterman, M.; Donker Kaat, L.; van Duijn, C.M.; van Swieten, J.C. Medical and environmental risk factors for sporadic frontotemporal dementia: A retrospective case-control study. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1574–1576. [Google Scholar] [CrossRef]
- Kahriman, A.; Bouley, J.; Smith, T.W.; Bosco, D.A.; Woerman, A.L.; Henninger, N. Mouse closed head traumatic brain injury replicates the histological tau pathology pattern of human disease: Characterization of a novel model and systematic review of the literature. Acta Neuropathol. Commun. 2021, 9, 118. [Google Scholar] [CrossRef]
- Washington, P.M.; Villapol, S.; Burns, M.P. Polypathology and dementia after brain trauma: Does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp. Neurol. 2016, 275 Pt 3, 381–388. [Google Scholar] [CrossRef]
- VanItallie, T.B. Traumatic brain injury (TBI) in collision sports: Possible mechanisms of transformation into chronic traumatic encephalopathy (CTE). Metabolism 2019, 100, 153943. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Frosch, M.P.; Baron, J.C.; Pasi, M.; Albucher, J.F.; Banerjee, G.; Barbato, C.; Bonneville, F.; Brandner, S.; et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022, 21, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Hamaguchi, T.; Sakai, K. Acquired cerebral amyloid angiopathy: An emerging concept. Prog. Mol. Biol. Transl. Sci. 2019, 168, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Yamada, M. Transmission of Cerebral β-Amyloidosis Among Individuals. Neurochem. Res. 2022, 47, 2469–2477. [Google Scholar] [CrossRef]
- Adams, J.W.; Alvarez, V.E.; Mez, J.; Huber, B.R.; Tripodis, Y.; Xia, W.; Meng, G.; Kubilus, C.A.; Cormier, K.; Kiernan, P.T.; et al. Lewy Body Pathology and Chronic Traumatic Encephalopathy Associated with Contact Sports. J. Neuropathol. Exp. Neurol. 2018, 77, 757–768. [Google Scholar] [CrossRef]
- McKee, A.C.; Stern, R.A.; Nowinski, C.J.; Stein, T.D.; Alvarez, V.E.; Daneshvar, D.H.; Lee, H.S.; Wojtowicz, S.M.; Hall, G.; Baugh, C.M.; et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013, 136, 43–64. [Google Scholar] [CrossRef]
- Montenigro, P.H.; Alosco, M.L.; Martin, B.M.; Daneshvar, D.H.; Mez, J.; Chaisson, C.E.; Nowinski, C.J.; Au, R.; McKee, A.C.; Cantu, R.C.; et al. Cumulative Head Impact Exposure Predicts Later-Life Depression, Apathy, Executive Dysfunction, and Cognitive Impairment in Former High School and College Football Players. J. Neurotrauma 2017, 34, 328–340. [Google Scholar] [CrossRef]
- Montenigro, P.H.; Corp, D.T.; Stein, T.D.; Cantu, R.C.; Stern, R.A. Chronic traumatic encephalopathy: Historical origins and current perspective. Annu. Rev. Clin. Psychol. 2015, 11, 309–330. [Google Scholar] [CrossRef]
- Stein, T.D.; Montenigro, P.H.; Alvarez, V.E.; Xia, W.; Crary, J.F.; Tripodis, Y.; Daneshvar, D.H.; Mez, J.; Solomon, T.; Meng, G.; et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015, 130, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Walt, G.S.; Burris, H.M.; Brady, C.B.; Spencer, K.R.; Alvarez, V.E.; Huber, B.R.; Guilderson, L.; Abdul Rauf, N.; Collins, D.; Singh, T.; et al. Chronic Traumatic Encephalopathy Within an Amyotrophic Lateral Sclerosis Brain Bank Cohort. J. Neuropathol. Exp. Neurol. 2018, 77, 1091–1100. [Google Scholar] [CrossRef]
- Martland, H.S. Punch Drunk. J. Am. Med. Assoc. 1928, 91, 1103–1107. [Google Scholar]
- Osnato, M.; Giliberti, V. Postconcussion Neurosis—Traumatic Encephalitis: A conception of postconcussion phenomena. Arch. Neurol. Psychiatry 1927, 18, 181–214. [Google Scholar]
- Millspaugh, H.S. Dementia pugilistica. United States Med. Bulliten 1937, 35, 297–303. [Google Scholar]
- McCown, I.A. Boxing injuries. Am. J. Surg. 1959, 98, 509–516. [Google Scholar]
- Smith, D.H.; Johnson, V.E.; Stewart, W. Chronic neuropathologies of single and repetitive TBI: Substrates of dementia? Nat. Rev. Neurol. 2013, 9, 211–221. [Google Scholar]
- Castellani, R.J.; Perry, G. Dementia pugilisitica revisited. J. Alzheimers Dis. 2017, 60, 1209–1221. [Google Scholar] [PubMed]
- McKee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 227, 45–66. [Google Scholar]
- McKee, A.C.; Cairns, N.J.; Dickson, D.W.; Folkerth, R.D.; Keene, C.D.; Litvan, I.; Perl, D.P.; Stein, T.D.; Vonsattel, J.-P.; Stewart, W.; et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016, 131, 75–86. [Google Scholar] [PubMed]
- Perl, D.P. Neuropathology of Alzheimer’s disease. Mt. Sinai J. Med. 2010, 77, 32–42. [Google Scholar]
- Josephs, K.A. Current understanding of neurodegenerative diseases associated with the protein tau. Mayo Clin. Proc. 2017, 92, 1291–1303. [Google Scholar]
- Veech, R.L.; Valeri, C.R.; VanItallie, T.B. The mitochondrial permeability transition pore provides a key to the diagnosis and treatment of traumatic brain injury. IUBMB Life 2012, 64, 203–207. [Google Scholar]
- Glenn, T.C.; Kelly, D.F.; Boscardin, W.J.; McArthur, D.L.; Vespa, P.; Oertel, M.; Hovda, D.A.; Bergsneider, M.; Hillered, L.; Martin, N.A. Energy dysfunction as a predictor of outcome after moderate or severe head injury: Indices of oxygen, glucose, and lactate metabolism. J. Cereb. Blood Flow Metab. 2003, 23, 1239–1259. [Google Scholar] [CrossRef] [PubMed]
- Asken, B.M.; Rabinovici, G.D. Identifying degenerative effects of repetitive head trauma with neuroimaging: A clinically-oriented review. Acta Neuropathol. Commun. 2021, 9, 96. [Google Scholar] [CrossRef]
- Shalat, S.L.; Seltzer, B.; Pidcock, C.; Baker, E.L., Jr. Risk factors for Alzheimer’s disease: A case-control study. Neurology 1987, 37, 1630–1633. [Google Scholar] [CrossRef]
- Katzman, R.; Aronson, M.; Fuld, P.; Kawas, C.; Brown, T.; Morgenstern, H.; Frishman, W.; Gidez, L.; Eder, H.; Ooi, W.L. Development of dementing illnesses in an 80-year-old volunteer cohort. Ann. Neurol. 1989, 25, 317–324. [Google Scholar] [CrossRef]
- Fratiglioni, L.; Ahlbom, A.; Viitanen, M.; Winblad, B. Risk factors for late-onset Alzheimer’s disease: A population-based, case-control study. Ann. Neurol. 1993, 33, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Broe, G.A.; Henderson, A.S.; Creasey, H.; McCusker, E.; Korten, A.E.; Jorm, A.F.; Longley, W.; Anthony, J.C. A case-control study of Alzheimer’s disease in Australia. Neurology 1990, 40, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, M.; Fountoulakis, K.; Chantzi, E.; Kazis, A. Risk factors for clinically diagnosed Alzheimer’s disease: A case-control study of a Greek population. Int. Psychogeriatr. 1997, 9, 327–341. [Google Scholar] [CrossRef]
- Mortimer, J.A.; van Duijn, C.M.; Chandra, V.; Fratiglioni, L.; Graves, A.B.; Heyman, A.; Jorm, A.F.; Kokmen, E.; Kondo, K.; Rocca, W.A.; et al. Head trauma as a risk factor for Alzheimer’s disease: A collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int. J. Epidemiol. 1991, 20 (Suppl. 2), S28–S35. [Google Scholar] [CrossRef]
- Bachman, D.L.; Wolf, P.A.; Linn, R.; Knoefel, J.E.; Cobb, J.; Belanger, A.; D’Agostino, R.B.; White, L.R. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology 1992, 42, 115–119. [Google Scholar] [CrossRef]
- Plassman, B.L.; Havlik, R.J.; Steffens, D.C.; Helms, M.J.; Newman, T.N.; Drosdick, D.; Phillips, C.; Gau, B.A.; Welsh-Bohmer, K.A.; Burke, J.R.; et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 2000, 55, 1158–1166. [Google Scholar] [CrossRef]
- Kalkonde, Y.V.; Jawaid, A.; Qureshi, S.U.; Shirani, P.; Wheaton, M.; Pinto-Patarroyo, G.P.; Schulz, P.E. Medical and environmental risk factors associated with frontotemporal dementia: A case-control study in a veteran population. Alzheimers Dement. 2012, 8, 204–210. [Google Scholar]
- Wang, H.K.; Lee, Y.C.; Huang, C.Y.; Liliang, P.C.; Lu, K.; Chen, H.J.; Li, Y.C.; Tsai, K.J. Traumatic brain injury causes frontotemporal dementia and TDP-43 proteolysis. Neuroscience 2015, 300, 94–103. [Google Scholar]
- Graves, A.B.; White, E.; Koepsell, T.D.; Reifler, B.V.; van Belle, G.; Larson, E.B.; Raskind, M. The association between head trauma and Alzheimer’s disease. Am. J. Epidemiol. 1990, 131, 491–501. [Google Scholar] [PubMed]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R.; Ottman, R.; Maestre, G.; Ngai, C.; Tang, M.X.; Ginsberg, H.; Chun, M.; Tycko, B.; Shelanski, M. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology 1995, 45, 555–557. [Google Scholar] [PubMed]
- O’Meara, E.S.; Kukull, W.A.; Sheppard, L.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Pfanschmidt, M.; Thompson, J.D.; Schellenberg, G.D.; Larson, E.B. Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am. J. Epidemiol. 1997, 146, 373–384. [Google Scholar]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef]
- Uryu, K.; Chen, X.H.; Martinez, D.; Browne, K.D.; Johnson, V.E.; Graham, D.I.; Lee, V.M.; Trojanowski, J.Q.; Smith, D.H. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp. Neurol. 2007, 208, 185–192. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012, 22, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.W.; Gentleman, S.M.; Lynch, A.; Graham, D.I. beta A4 amyloid protein deposition in brain after head trauma. Lancet 1991, 338, 1422–1423. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol. Aging 1997, 18, S85–S88. [Google Scholar] [CrossRef]
- Roberts, G.W.; Gentleman, S.M.; Lynch, A.; Murray, L.; Landon, M.; Graham, D.I. Beta amyloid protein deposition in the brain after severe head injury: Implications for the pathogenesis of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Ikonomovic, M.D.; Uryu, K.; Abrahamson, E.E.; Ciallella, J.R.; Trojanowski, J.Q.; Lee, V.M.; Clark, R.S.; Marion, D.W.; Wisniewski, S.R.; Dekosky, S.T. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp. Neurol. 2004, 190, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, W. Traumatic brain injury: Age at injury influences dementia risk after TBI. Nat. Rev. Neurol. 2015, 11, 128–130. [Google Scholar] [CrossRef]
- Hong, Y.T.; Veenith, T.; Dewar, D.; Outtrim, J.G.; Mani, V.; Williams, C.; Pimlott, S.; Hutchinson, P.J.; Tavares, A.; Canales, R.; et al. Amyloid imaging with carbon 11-labeled Pittsburgh compound B for traumatic brain injury. JAMA Neurol. 2014, 71, 23–31. [Google Scholar] [CrossRef]
- Smith, D.H.; Chen, X.H.; Iwata, A.; Graham, D.I. Amyloid beta accumulation in axons after traumatic brain injury in humans. J. Neurosurg. 2003, 98, 1072–1077. [Google Scholar] [CrossRef]
- Gatson, J.W.; Warren, V.; Abdelfattah, K.; Wolf, S.; Hynan, L.S.; Moore, C.; Diaz-Arrastia, R.; Minei, J.P.; Madden, C.; Wigginton, J.G. Detection of beta-amyloid oligomers as a predictor of neurological outcome after brain injury. J. Neurosurg. 2013, 118, 1336–1342. [Google Scholar] [CrossRef]
- Laskowitz, D.T.; Song, P.; Wang, H.; Mace, B.; Sullivan, P.M.; Vitek, M.P.; Dawson, H.N. Traumatic brain injury exacerbates neurodegenerative pathology: Improvement with an apolipoprotein E-based therapeutic. J. Neurotrauma 2010, 27, 1983–1995. [Google Scholar] [CrossRef]
- Loane, D.J.; Pocivavsek, A.; Moussa, C.E.; Thompson, R.; Matsuoka, Y.; Faden, A.I.; Rebeck, G.W.; Burns, M.P. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat. Med. 2009, 15, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Washington, P.M.; Morffy, N.; Parsadanian, M.; Zapple, D.; Burns, M.P. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer’s disease mouse model. J. Neurotrauma 2013, 31, 125–134. [Google Scholar] [PubMed]
- Bogoslovsky, T.; Wilson, D.; Chen, Y.; Hanlon, D.; Gill, J.; Jeromin, A.; Song, L.; Moore, C.; Gong, Y.; Kenney, K.; et al. Increases of Plasma Levels of Glial Fibrillary Acidic Protein, Tau, and Amyloid β up to 90 Days after Traumatic Brain Injury. J. Neurotrauma 2017, 34, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; LaFerla, F.M.; Holtzman, D.M.; Brody, D.L. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 9513–9525. [Google Scholar]
- Winston, C.N.; Chellappa, D.; Wilkins, T.; Barton, D.J.; Washington, P.M.; Loane, D.J.; Zapple, D.N.; Burns, M.P. Controlled cortical impact results in an extensive loss of dendritic spines that is not mediated by injury-induced amyloid-beta accumulation. J. Neurotrauma 2013, 30, 1966–1972. [Google Scholar]
- Johnson, V.E.; Stewart, W.; Graham, D.I.; Stewart, J.E.; Praestgaard, A.H.; Smith, D.H. A neprilysin polymorphism and amyloid-beta plaques after traumatic brain injury. J. Neurotrauma 2009, 26, 1197–1202. [Google Scholar] [CrossRef]
- Dardiotis, E.; Fountas, K.N.; Dardioti, M.; Xiromerisiou, G.; Kapsalaki, E.; Tasiou, A.; Hadjigeorgiou, G.M. Genetic association studies in patients with traumatic brain injury. Neurosurg. Focus 2010, 28, E9. [Google Scholar] [CrossRef]
- Gentleman, S.M.; Greenberg, B.D.; Savage, M.J.; Noori, M.; Newman, S.J.; Roberts, G.W.; Griffin, W.S.; Graham, D.I. A beta 42 is the predominant form of amyloid beta-protein in the brains of short-term survivors of head injury. Neuroreport 1997, 8, 1519–1522. [Google Scholar]
- Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118, Erratum in: Nat. Rev. Neurol. 2013, 10, e0130507. https://doi.org/10.1038/nrneurol.2013.32. [Google Scholar] [CrossRef]
- Crane, P.K.; Gibbons, L.E.; Dams-O’Connor, K.; Trittschuh, E.; Leverenz, J.B.; Keene, C.D.; Sonnen, J.; Montine, T.J.; Bennett, D.A.; Leurgans, S.; et al. Association of Traumatic Brain Injury with Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol. 2016, 73, 1062–1069. [Google Scholar] [CrossRef]
- Mondragón-Rodríguez, S.; Perry, G.; Luna-Muñoz, J.; Acevedo-Aquino, M.C.; Williams, S. Phosphorylation of tau protein at sites Ser(396-404) is one of the earliest events in Alzheimer’s disease and Down syndrome. Neuropathol. Appl. Neurobiol. 2014, 40, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, J.G.; Burford, C.; Nagappan, P.; Adegboyega, G.; Rajkumar, S.; Kolias, A.; Helmy, A.; Hutchinson, P.J. Is dementia more likely following traumatic brain injury? A systematic review. J. Neurol. 2023, 270, 3022–3051. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, A.; Takeuchi, H.; Tanaka, F. Tau Pathology in Chronic Traumatic Encephalopathy and Alzheimer’s Disease: Similarities and Differences. Front. Neurol. 2019, 10, 980. [Google Scholar] [CrossRef]
- Kulbe, J.R.; Hall, E.D. Chronic traumatic encephalopathy-integration of canonical traumatic brain injury secondary injury mechanisms with tau pathology. Prog. Neurobiol. 2017, 158, 15–44. [Google Scholar] [CrossRef]
- Maroon, J.C.; Winkelman, R.; Bost, J.; Amos, A.; Mathyssek, C.; Miele, V. Chronic traumatic encephalopathy in contact sports: A systematic review of all reported pathological cases. PLoS ONE 2015, 10, e0117338, Erratum in: PLoS ONE 2015, 10, e0130507. https://doi.org/10.1371/journal.pone.0130507. [Google Scholar] [CrossRef]
- Hof, P.R.; Bouras, C.; Buee, L.; Delacourte, A.; Perl, D.P.; Morrison, J.H. Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer’s disease cases. Acta Neuropathol. 1992, 85, 23–30. [Google Scholar]
- Schmidt, M.L.; Zhukareva, V.; Newell, K.L.; Lee, V.M.; Trojanowski, J.Q. Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer’s disease. Acta Neuropathol. 2001, 101, 518–524. [Google Scholar]
- Li, L.; Liang, J.; Fu, H. An update on the association between traumatic brain injury and Alzheimer’s disease: Focus on Tau pathology and synaptic dysfunction. Neurosci. Biobehav. Rev. 2021, 120, 372–386. [Google Scholar] [CrossRef]
- Ojo, J.O.; Mouzon, B.; Greenberg, M.B.; Bachmeier, C.; Mullan, M.; Crawford, F. Repetitive mild traumatic brain injury augments tau pathology and glial activation in aged hTau mice. J. Neuropathol. Exp. Neurol. 2013, 72, 137–151. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Uryu, K.; Higuchi, M.; Longhi, L.; Hoover, R.; Fujimoto, S.; McIntosh, T.; Lee, V.M.; Trojanowski, J.Q. Enhanced neurofibrillary tangle formation, cerebral atrophy, and cognitive deficits induced by repetitive mild brain injury in a transgenic tauopathy mouse model. J. Neurotrauma 2005, 22, 1134–1141. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Gurol, M.E.; Ayata, C.; Bacskai, B.J.; Frosch, M.P.; Viswanathan, A.; Greenberg, S.M. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain 2017, 140, 1829–1850. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Gang, Q.; Werring, D.J. Sporadic cerebral amyloid angiopathy revisited: Recent insights into pathophysiology and clinical spectrum. J. Neurol. Neurosurg. Psychiatry 2012, 83, 124–137. [Google Scholar] [CrossRef]
- Attems, J.; Lauda, F.; Jellinger, K.A. Unexpectedly low prevalence of intracerebral hemorrhages in sporadic cerebral amyloid angiopathy: An autopsy study. J. Neurol. 2008, 255, 70–76. [Google Scholar] [CrossRef]
- O’Donnell, H.C.; Rosand, J.; Knudsen, K.A.; Furie, K.L.; Segal, A.Z.; Chiu, R.I.; Ikeda, D.; Greenberg, S.M. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N. Engl. J. Med. 2000, 342, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Rannikmae, K.; Kalaria, R.N.; Greenberg, S.M.; Chui, H.C.; Schmitt, F.A.; Samarasekera, N.; Al-Shahi Salman, R.; Sudlow, C.L. APOE associations with severe CAA-associated vasculopathic changes: Collaborative meta-analysis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 300–305. [Google Scholar] [CrossRef]
- Pfeifer, L.A.; White, L.R.; Ross, G.W.; Petrovitch, H.; Launer, L.J. Cerebral amyloid angiopathy and cognitive function: The HAAS autopsy study. Neurology 2002, 58, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Vinters, H.V. Cerebral amyloid angiopathy. A critical review. Stroke 1987, 18, 311–324. [Google Scholar] [CrossRef]
- Vinters, H.V.; Gilbert, J.J. Cerebral amyloid angiopathy: Incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke 1983, 14, 924–928. [Google Scholar] [CrossRef]
- Keage, H.A.; Carare, R.O.; Friedland, R.P.; Ince, P.G.; Love, S.; Nicoll, J.A.; Wharton, S.B.; Weller, R.O.; Brayne, C. Population studies of sporadic cerebral amyloid angiopathy and dementia: A systematic review. BMC Neurol. 2009, 9, 3. [Google Scholar] [CrossRef]
- Nordstrom, A.; Nordstrom, P. Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLoS Med. 2018, 15, e1002496. [Google Scholar] [CrossRef]
- Tagge, C.A.; Fisher, A.M.; Minaeva, O.V.; Gaudreau-Balderrama, A.; Moncaster, J.A.; Zhang, X.L.; Wojnarowicz, M.W.; Casey, N.; Lu, H.; Kokiko-Cochran, O.N.; et al. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 2018, 141, 422–458. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Mez, J.; Tripodis, Y.; Kiernan, P.T.; Abdolmohammadi, B.; Murphy, L.; Kowall, N.W.; Stein, T.D.; Huber, B.R.; Goldstein, L.E.; et al. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann. Neurol. 2018, 83, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Mez, J.; Crary, J.F.; Tripodis, Y.; Alvarez, V.E.; Mahar, I.; Huber, B.R.; Alosco, M.L.; Nicks, R.; Abdolmohammadi, B.; et al. Variation in TMEM106B in chronic traumatic encephalopathy. Acta Neuropathol. Commun. 2018, 6, 115. [Google Scholar] [CrossRef]
- Broglio, S.P.; Sosnoff, J.J.; Shin, S.; He, X.; Alcaraz, C.; Zimmerman, J. Head impacts during high school football: A biomechanical assessment. J. Athl. Train 2009, 44, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Crisco, J.J.; Fiore, R.; Beckwith, J.G.; Chu, J.J.; Brolinson, P.G.; Duma, S.; McAllister, T.W.; Duhaime, A.C.; Greenwald, R.M. Frequency and location of head impact exposures in individual collegiate football players. J. Athl. Train 2010, 45, 549–559. [Google Scholar] [CrossRef]
- Ghajari, M.; Hellyer, P.J.; Sharp, D.J. Computational modelling of traumatic brain injury predicts the location of chronic traumatic encephalopathy pathology. Brain 2017, 140, 333–343. [Google Scholar] [CrossRef]
- Leclercq, P.D.; Murray, L.S.; Smith, C.; Graham, D.I.; Nicoll, J.A.; Gentleman, S.M. Cerebral amyloid angiopathy in traumatic brain injury: Association with apolipoprotein E genotype. J. Neurol. Neurosurg. Psychiatry 2005, 76, 229–233. [Google Scholar] [CrossRef]
- Weissberg, I.; Veksler, R.; Kamintsky, L.; Saar-Ashkenazy, R.; Milikovsky, D.Z.; Shelef, I.; Friedman, A. Imaging blood-brain barrier dysfunction in football players. JAMA Neurol. 2014, 71, 1453–1455. [Google Scholar] [CrossRef]
- Standring, O.J.; Friedberg, J.; Tripodis, Y.; Chua, A.S.; Cherry, J.D.; Alvarez, V.E.; Huber, B.R.; Xia, W.; Mez, J.; Alosco, M.L.; et al. Contact sport participation and chronic traumatic encephalopathy are associated with altered severity and distribution of cerebral amyloid angiopathy. Acta Neuropathol. 2019, 138, 401–413. [Google Scholar] [CrossRef]
- Mez, J.; Solomon, T.M.; Daneshvar, D.H.; Murphy, L.; Kiernan, P.T.; Montenigro, P.H.; Kriegel, J.; Abdolmohammadi, B.; Fry, B.; Babcock, K.J.; et al. Assessing clinicopathological correlation in chronic traumatic encephalopathy: Rationale and methods for the UNITE study. Alzheimers Res. Ther. 2015, 7, 62. [Google Scholar] [CrossRef]
- Vonsattel, J.P.; Myers, R.H.; Hedley-Whyte, E.T.; Ropper, A.H.; Bird, E.D.; Richardson, E.P., Jr. Cerebral amyloid angiopathy without and with cerebral hemorrhages: A comparative histological study. Ann. Neurol. 1991, 30, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef]
- Hay, J.R.; Johnson, V.E.; Young, A.M.; Smith, D.H.; Stewart, W. Blood-Brain Barrier Disruption Is an Early Event That May Persist for Many Years After Traumatic Brain Injury in Humans. J. Neuropathol. Exp. Neurol. 2015, 74, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.; Aherne, S.; O’Riordan, S.; O’Keeffe, E.; Greene, C.; Campbell, M. Blood-brain barrier dysfunction in a boxer with chronic traumatic encephalopathy and schizophrenia. Clin. Neuropathol. 2019, 38, 51–58. [Google Scholar] [CrossRef]
- Scott, G.; Ramlackhansingh, A.F.; Edison, P.; Hellyer, P.; Cole, J.; Veronese, M.; Leech, R.; Greenwood, R.J.; Turkheimer, F.E.; Gentleman, S.M.; et al. Amyloid pathology and axonal injury after brain trauma. Neurology 2016, 86, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef]
- Mamourian, A.C.; Hoopes, P.J.; Lewis, L.D. Visualization of intravenously administered contrast material in the CSF on fluid-attenuated inversion-recovery MR images: An in vitro and animalmodel investigation. AJNR Am. J. Neuroradiol. 2000, 21, 105–111. [Google Scholar]
- Russo, M.V.; Latour, L.L.; McGavern, D.B. Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat. Immunol. 2018, 19, 442–452. [Google Scholar] [CrossRef]
- Goldstein, L.E.; Fisher, A.M.; Tagge, C.A.; Zhang, X.L.; Velisek, L.; Sullivan, J.A.; Upreti, C.; Kracht, J.M.; Ericsson, M.; Wojnarowicz, M.W.; et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012, 4, 134ra60. [Google Scholar] [CrossRef]
- Tian, J.; Shi, J.; Bailey, K.; Mann, D.M.A. Negative association between amyloid plaques and cerebral amyloid angiopathy in Alzheimer’s disease. Neurosci. Lett. 2003, 352, 137–140. [Google Scholar] [CrossRef]
- Tian, J.; Shi, J.; Bailey, K.; Mann, D.M.A. Relationships between arteriosclerosis, cerebral amyloid angiopathy and myelin loss from cerebral cortical white matter in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2004, 30, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Hecht, M.; Kramer, L.M.; von Arnim, C.A.F.; Otto, M.; Thal, D.R. Capillary cerebral amyloid angiopathy in Alzheimer’s disease: Association with allocortical/hippocampal microinfarcts and cognitive decline. Acta Neuropathol. 2018, 135, 681–694. [Google Scholar] [CrossRef]
- Thal, D.R.; Papassotiropoulos, A.; Saido, T.C.; Griffin, W.S.; Mrak, R.E.; Kolsch, H.; Del Tredici, K.; Attems, J.; Ghebremedhin, E. Capillary cerebral amyloid angiopathy identifies a distinct APOE epsilon4-associated subtype of sporadic Alzheimer’s disease. Acta Neuropathol. 2010, 120, 169–183. [Google Scholar] [CrossRef]
- Hawkes, C.A.; Jayakody, N.; Johnston, D.A.; Bechmann, I.; Carare, R.O. Failure of perivascular drainage of beta-amyloid in cerebral amyloid angiopathy. Brain Pathol. 2014, 24, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Merlini, M.; Wanner, D.; Nitsch, R.M. Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer’s disease-related microvascular cerebral amyloid angiopathy. Acta Neuropathol. 2016, 131, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Buki, A.; Povlishock, J.T. All roads lead to disconnection?—Traumatic axonal injury revisited. Acta Neurochir. 2006, 148, 181–193. [Google Scholar] [CrossRef]
- Stone, J.R.; Okonkwo, D.O.; Singleton, R.H.; Mutlu, L.K.; Helm, G.A.; Povlishock, J.T. Caspase-3-mediated cleavage of amyloid precursor protein and formation of amyloid Beta peptide in traumatic axonal injury. J. Neurotrauma 2002, 19, 601–614. [Google Scholar] [CrossRef]
- Faul, M.; Coronado, V. Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 3–13. [Google Scholar]
- Ryu, J.; Horkayne-Szakaly, I.; Xu, L.; Pletnikova, O.; Leri, F.; Eberhart, C.; Troncoso, J.C.; Koliatsos, V.E. The problem of axonal injury in the brains of veterans with histories of blast exposure. Acta Neuropathol. Commun. 2014, 2, 153. [Google Scholar] [CrossRef]
- Presson, N.; Beers, S.R.; Morrow, L.; Wagener, L.M.; Bird, W.A.; Van Eman, G.; Krishnaswamy, D.; Penderville, J.; Borrasso, A.J.; Benso, S.; et al. An exploratory analysis linking neuropsychological testing to quantification of tractography using High Definition Fiber Tracking (HDFT) in military TBI. Brain Imaging Behav. 2015, 9, 484–499. [Google Scholar] [CrossRef]
- Eisenmenger, L.B.; Huo, E.J.; Hoffman, J.M.; Minoshima, S.; Matesan, M.C.; Lewis, D.H.; Lopresti, B.J.; Mathis, C.A.; Okonkwo, D.O.; Mountz, J.M. Advances in PET imaging of degenerative, cerebrovascular, and traumatic causes of dementia. Semin. Nucl. Med. 2016, 46, 57–87. [Google Scholar] [PubMed]
- Kawai, N.; Kawanishi, M.; Kudomi, N.; Maeda, Y.; Yamamoto, Y.; Nishiyama, Y.; Tamiya, T. Detection of brain amyloid beta deposition in patients with neuropsychological impairment after traumatic brain injury: PET evaluation using Pittsburgh compound-B. Brain Inj. 2013, 27, 1026–1031. [Google Scholar]
- Mielke, M.M.; Savica, R.; Wiste, H.J.; Weigand, S.D.; Vemuri, P.; Knopman, D.S.; Lowe, V.J.; Roberts, R.O.; Machulda, M.M.; Geda, Y.E.; et al. Head trauma and in vivo measures of amyloid and neurodegeneration in a population-based study. Neurology 2014, 82, 70–76. [Google Scholar]
- Povlishock, J.T.; Katz, D.I. Update of neuropathology and neurological recovery after traumatic brain injury. J. Head Trauma Rehabil. 2005, 20, 76–94. [Google Scholar] [PubMed]
- Povlishock, J.T.; Erb, D.E.; Astruc, J. Axonal response to traumatic brain injury: Reactive axonal change, deafferentation, and neuroplasticity. J. Neurotrauma 1992, 9 (Suppl. 1), S189–S200. [Google Scholar]
- Cotman, C.W.; Nieto-Sampedro, M.; Harris, E.W. Synapse replacement in the nervous system of adult vertebrates. Physiol. Rev. 1981, 61, 684–784. [Google Scholar]
- Hoff, S.F.; Scheff, S.W.; Benardo, L.S.; Cotman, C.W. Lesion-induced synaptogenesis in the dentate gyrus of aged rats: I. Loss and reacquisition of normal synaptic density. J. Comp. Neurol. 1982, 205, 246–252. [Google Scholar] [PubMed]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Roberts, K.N.; Ikonomovic, M.D.; Mufson, E.J. Synapse stability in the precuneus early in the progression of Alzheimer’s disease. J. Alzheimers Dis. 2013, 35, 599–609. [Google Scholar]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Rowan, M.J.; Selkoe, D.J. Amyloid-beta oligomers: Their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 2002, 30, 552–557. [Google Scholar]
- Indo, H.P.; Yen, H.C.; Nakanishi, I.; Matsumoto, K.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T.; et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Uryu, K.; Laurer, H.; McIntosh, T.; Pratico, D.; Martinez, D.; Leight, S.; Lee, V.M.; Trojanowski, J.Q. Repetitive mild brain trauma accelerates abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 2002, 22, 446–454. [Google Scholar] [PubMed]
- Zhao, M.; Su, J.; Head, E.; Cotman, C.W. Accumulation of caspase cleaved amyloid precursor protein represents an early neurodegenerative event in aging and in Alzheimer’s disease. Neurobiol. Dis. 2003, 14, 391–403. [Google Scholar] [PubMed]
- Scheff, S.W.; Ansari, M.A.; Mufson, E.J. Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer’s disease pathology. Neurobiol. Aging 2016, 42, 1–12. [Google Scholar] [CrossRef]
- Ansari, M.A.; Rao, M.S.; Al-Jarallah, A. Insights into early pathogenesis of sporadic Alzheimer’s disease: Role of oxidative stress and loss of synaptic proteins. Front. Neurosci. 2024, 17, 1273626. [Google Scholar] [CrossRef]
- Abrahamson, E.E.; Ikonomovic, M.D.; Ciallella, J.R.; Hope, C.E.; Paljug, W.R.; Isanski, B.A.; Flood, D.G.; Clark, R.S.B.; DeKosky, S.T. Caspase inhibition therapy abolishes brain trauma-induced increases in Abeta peptide: Implications for clinical outcome. Exp. Neurol. 2006, 197, 437–450. [Google Scholar]
- Ansari, M.A.; Rao, M.S.; Al-Jarallah, A.; Babiker, F.M. Early time course of oxidative stress in hippocampal synaptosomes and cognitive loss following impaired insulin signaling in rats: Development of sporadic Alzheimer’s disease. Brain Res. 2023, 1798, 148134. [Google Scholar] [CrossRef]
- Akhtar, A.; Dhaliwal, J.; Sah, S.P. 7,8-Dihydroxyflavone improves cognitive functions in ICV-STZ rat model of sporadic Alzheimer’s disease by reversing oxidative stress, mitochondrial dysfunction, and insulin resistance. Psychopharmacology 2021, 238, 1991–2009. [Google Scholar] [CrossRef]
- Ishrat, T.; Parveen, K.; Khan, M.M.; Khuwaja, G.; Khan, M.B.; Yousuf, S.; Ahmad, A.; Shrivastav, P.; Islam, F. Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2009, 1281, 117–127. [Google Scholar] [CrossRef]
- Loane, D.J.; Kumar, A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp. Neurol. 2016, 275, 316–327. [Google Scholar]
- Norden, D.M.; Muccigrosso, M.M.; Godbout, J.P. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 2015, 96, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Floden, A.M.; Combs, C.K. Microglia demonstrate age-dependent interaction with amyloid-beta fibrils. J. Alzheimers Dis. 2011, 25, 279–293. [Google Scholar] [CrossRef]
- Morganti, J.M.; Riparip, L.K.; Chou, A.; Liu, S.; Gupta, N.; Rosi, S. Age exacerbates the CCR2/5-mediated neuroinflammatory response to traumatic brain injury. J. Neuroinflamm. 2016, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.S.; Sharp, D.J. Understanding neurodegeneration after traumatic brain injury: From mechanisms to clinical trials in dementia. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1221–1233. [Google Scholar] [CrossRef]
- Maxwell, W.L.; Dhillon, K.; Harper, L.; Espin, J.; MacIntosh, T.K.; Smith, D.H.; Graham, D.I. There is differential loss of pyramidal cells from the human hippocampus with survival after blunt head injury. J. Neuropathol. Exp. Neurol. 2003, 62, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Lesman-Segev, O.H.; La Joie, R.; Stephens, M.L.; Sonni, I.; Tsai, R.; Bourakova, V.; Visani, A.V.; Edwards, L.; O’Neil, J.P.; Baker, S.L.; et al. Tau PET and multimodal brain imaging in patients at risk for chronic traumatic encephalopathy. Neuroimage Clin. 2019, 24, 102025. [Google Scholar] [CrossRef]
- Goswami, R.; Dufort, P.; Tartaglia, M.C.; Green, R.E.; Crawley, A.; Tator, C.H.; Wennberg, R.; Mikulis, D.J.; Keightley, M.; Davis, K.D. Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Struct Funct. 2016, 221, 1911–1925. [Google Scholar] [CrossRef]
- Adler, C.M.; DelBello, M.P.; Weber, W.; Williams, M.; Duran, L.R.P.; Fleck, D.; Boespflug, E.; Eliassen, J.; Strakowski, S.M.; Divine, J. MRI Evidence of Neuropathic Changes in Former College Football Players. Clin. J. Sport Med. 2018, 28, 100–105. [Google Scholar] [CrossRef]
- Lepage, C.; Muehlmann, M.; Tripodis, Y.; Hufschmidt, J.; Stamm, J.; Green, K.; Wrobel, P.; Schultz, V.; Weir, I.; Alosco, M.L.; et al. Limbic system structure volumes and associated neurocognitive functioning in former NFL players. Brain Imaging Behav. 2019, 13, 725–734. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Wang, Y.; Munro, C.A.; Ma, S.; Yue, C.; Chen, S.; Airan, R.; Kim, P.K.; Adams, A.V.; Garcia, C.; et al. Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiol. Dis. 2015, 74, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Strain, J.F.; Womack, K.B.; Didehbani, N.; Spence, J.S.; Conover, H.; Hart, J.; Kraut, M.A.; Cullum, C.M. Imaging Correlates of Memory and Concussion History in Retired National Football League Athletes. JAMA Neurol. 2015, 72, 773–780. [Google Scholar] [CrossRef]
- Misquitta, K.; Dadar, M.; Tarazi, A.; Hussain, M.W.; Alatwi, M.K.; Ebraheem, A.; Multani, N.; Khodadadi, M.; Goswami, R.; Wennberg, R.; et al. The relationship between brain atrophy and cognitive-behavioural symptoms in retired Canadian football players with multiple concussions. Neuroimage Clin. 2018, 19, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Wu, J.; Bullen, J.; Banks, S.; Bernick, C.; Modic, M.T.; Ruggieri, P.; Bennett, L.; Jones, S.E. Association of Cavum Septum Pellucidum and Cavum Vergae with Cognition, Mood, and Brain Volumes in Professional Fighters. JAMA Neurol. 2020, 77, 35–42. [Google Scholar] [CrossRef]
- Bernick, C.; Shan, G.; Zetterberg, H.; Banks, S.; Mishra, V.R.; Bekris, L.; Leverenz, J.B.; Blennow, K. Longitudinal change in regional brain volumes with exposure to repetitive head impacts. Neurology 2020, 94, e232–e240. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Wang, Y.; Minn, I.; Bienko, N.; Ambinder, E.B.; Xu, X.; Peters, M.E.; Dougherty, J.W.; Vranesic, M.; Koo, S.M.; et al. Imaging of Glial Cell Activation and White Matter Integrity in Brains of Active and Recently Retired National Football League Players. JAMA Neurol. 2017, 74, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zivadinov, R.; Polak, P.; Schweser, F.; Bergsland, N.; Hagemeier, J.; Dwyer, M.G.; Ramasamy, D.P.; Baker, J.G.; Leddy, J.J.; Willer, B.S. Multimodal Imaging of Retired Professional Contact Sport Athletes Does Not Provide Evidence of Structural and Functional Brain Damage. J. Head Trauma Rehabil. 2018, 33, E24–E32. [Google Scholar] [CrossRef]
- Casson, I.R.; Viano, D.C.; Haacke, E.M.; Kou, Z.; LeStrange, D.G. Is There Chronic Brain Damage in Retired NFL Players? Neuroradiology, Neuropsychology, and Neurology Examinations of 45 Retired Players. Sports Health 2014, 6, 384–395. [Google Scholar] [CrossRef]
- Bang, S.A.; Song, Y.S.; Moon, B.S.; Lee, B.C.; Lee, H.Y.; Kim, J.M.; Kim, S.E. Neuropsychological, Metabolic, and GABAA Receptor Studies in Subjects with Repetitive Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1005–1014. [Google Scholar] [CrossRef]
- Terry, D.P.; Miller, L.S. Repeated mild traumatic brain injuries is not associated with volumetric differences in former high school football players. Brain Imaging Behav. 2018, 12, 631–639. [Google Scholar] [PubMed]
- Tremblay, S.; De Beaumont, L.; Henry, L.C.; Boulanger, Y.; Evans, A.C.; Bourgouin, P.; Poirier, J.; Théoret, H.; Lassonde, M. Sports concussions and aging: A neuroimaging investigation. Cereb. Cortex 2013, 23, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Henry, L.C.; Bedetti, C.; Larson-Dupuis, C.; Gagnon, J.F.; Evans, A.C.; Théoret, H.; Lassonde, M.; De Beaumont, L. Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain 2014, 137, 2997–3011. [Google Scholar] [CrossRef]
- Hart, J., Jr.; Kraut, M.A.; Womack, K.B.; Strain, J.; Didehbani, N.; Bartz, E.; Conover, H.; Mansinghani, S.; Lu, H.; Cullum, C.M. Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: A cross-sectional study. JAMA Neurol. 2013, 70, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Born, C.M.; Meisenzahl, E.M.; Frodl, T.; Pfluger, T.; Reiser, M.; Möller, H.J.; Leinsinger, G.L. The septum pellucidum and its variants. An MRI study. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 295–302. [Google Scholar] [CrossRef]
- Mez, J.; Daneshvar, D.H.; Kiernan, P.T.; Abdolmohammadi, B.; Alvarez, V.E.; Huber, B.R.; Alosco, M.L.; Solomon, T.M.; Nowinski, C.J.; McHale, L.; et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA 2017, 318, 360–370. [Google Scholar] [CrossRef]
- Koerte, I.K.; Hufschmidt, J.; Muehlmann, M.; Tripodis, Y.; Stamm, J.M.; Pasternak, O.; Giwerc, M.Y.; Coleman, M.J.; Baugh, C.M.; Fritts, N.G.; et al. Cavum Septi Pellucidi in Symptomatic Former Professional Football Players. J. Neurotrauma 2016, 33, 346–353. [Google Scholar] [CrossRef]
- Gardner, R.C.; Hess, C.P.; Brus-Ramer, M.; Possin, K.L.; Cohn-Sheehy, B.I.; Kramer, J.H.; Berger, M.S.; Yaffe, K.; Miller, B.; Rabinovici, G.D. Cavum Septum Pellucidum in Retired American Pro-Football Players. J. Neurotrauma 2016, 33, 157–161. [Google Scholar] [CrossRef]
- Arfanakis, K.; Evia, A.M.; Leurgans, S.E.; Cardoso, L.F.; Kulkarni, A.; Alqam, N.; Lopes, L.F.; Vieira, D.; Bennett, D.A.; Schneider, J.A. Neuropathologic Correlates of White Matter Hyperintensities in a Community-Based Cohort of Older Adults. J. Alzheimers Dis. 2020, 73, 333–345. [Google Scholar] [CrossRef]
- De Havenon, A.; Majersik, J.J.; Tirschwell, D.L.; McNally, J.S.; Stoddard, G.; Rost, N.S. Blood pressure, glycemic control, and white matter hyperintensity progression in type 2 diabetics. Neurology 2019, 92, e1168–e1175. [Google Scholar] [CrossRef]
- Shim, Y.S.; Yang, D.-W.; Roe, C.M.; Coats, M.A.; Benzinger, T.L.; Xiong, C.; Galvin, J.E.; Cairns, N.J.; Morris, J.C. Pathological correlates of white matter hyperintensities on magnetic resonance imaging. Dement. Geriatr. Cogn. Disord. 2015, 39, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Lehman, E.J.; Hein, M.J.; Baron, S.L.; Gersic, C.M. Neurodegenerative causes of death among retired National Football League players. Neurology 2012, 79, 1970–1974. [Google Scholar] [PubMed]
- Pokharel, Y.; Basra, S.; Lincoln, A.E.; Tucker, A.M.; Nambi, V.; Nasir, K.; Vogel, R.A.; Wong, N.D.; Boone, J.L.; Roberts, A.J.; et al. Association of body mass index and waist circumference with subclinical atherosclerosis in retired NFL players. South Med. J. 2014, 107, 633–639. [Google Scholar] [CrossRef]
- Tucker, A.M.; Vogel, R.A.; Lincoln, A.E.; Dunn, R.E.; Ahrensfield, D.C.; Allen, T.W.; Castle, L.W.; Heyer, R.A.; Pellman, E.J.; Strollo, P.J., Jr.; et al. Prevalence of cardiovascular disease risk factors among National Football League players. JAMA 2009, 301, 2111–2119. [Google Scholar] [CrossRef]
- Willeumier, K.; Taylor, D.V.; Amen, D.G. Elevated body mass in National Football League players linked to cognitive impairment and decreased prefrontal cortex and temporal pole activity. Transl. Psychiatry 2012, 2, e68. [Google Scholar]
- Grashow, R.; Weisskopf, M.G.; Baggish, A.; Speizer, F.E.; Whittington, A.J.; Nadler, L.; Connor, A.; Keske, R.; Taylor, H.; Zafonte, R.; et al. Premortem Chronic Traumatic Encephalopathy Diagnoses in Professional Football. Ann. Neurol. 2020, 88, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.L.; Zafonte, R.D.; Speizer, F.E.; Baggish, A.; Taylor, H.A.; Nadler, L.; Weisskopf, M.G. Modifiable Risk Factors for Poor Cognitive Function in Former American-Style Football Players: Findings from the Harvard Football Players Health Study. J. Neurotrauma 2021, 38, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Tripodis, Y.; Alvarez, V.E.; Huber, B.; Kiernan, P.T.; Daneshvar, D.H.; Mez, J.; Montenigro, P.H.; Solomon, T.M.; Alosco, M.L.; et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol. Commun. 2016, 4, 112. [Google Scholar] [CrossRef]
- Hasiloglu, Z.; Albayram, S.; Selcuk, H.; Ceyhan, E.; Delil, S.; Arkan, B.; Baskoy, L. Cerebral microhemorrhages detected by susceptibility-weighted imaging in amateur boxers. AJNR Am. J. Neuroradiol. 2011, 32, 99–102. [Google Scholar] [CrossRef]
- Knudsen, K.A.; Rosand, J.; Karluk, D.; Greenberg, S.M. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston criteria. Neurology 2001, 56, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.D.; Turtzo, L.C.; Parikh, G.Y.; Tolpygo, A.; Lodato, Z.; Moses, A.D.; Nair, G.; Perl, D.P.; A Edwards, N.; Dardzinski, B.J.; et al. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain 2019, 142, 3550–3564. [Google Scholar] [CrossRef]
- Shah, B.; Holcomb, J.; Davenport, E.; Lack, C.; McDaniel, J.; Imphean, D.; Xi, Y.; Rosenbaum, D.; Urban, J.; Wagner, B.; et al. Prevalence and Incidence of Microhemorrhages in Adolescent Football Players. AJNR Am. J. Neuroradiol. 2020, 41, 1263–1268. [Google Scholar] [CrossRef]
- Gardner, R.C.; Possin, K.L.; Hess, C.P.; Huang, E.J.; Grinberg, L.T.; Nolan, A.L.; Cohn-Sheehy, B.I.; Ghosh, P.M.; Lanata, S.; Merrilees, J.; et al. Evaluating and treating neurobehavioral symptoms in professional American football players: Lessons from a case series. Neurol. Clin. Pract. 2015, 5, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Multani, N.; Goswami, R.; Khodadadi, M.; Ebraheem, A.; Davis, K.D.; Tator, C.H.; Wennberg, R.; Mikulis, D.J.; Ezerins, L.; Tartaglia, M.C. The association between white-matter tract abnormalities, and neuropsychiatric and cognitive symptoms in retired professional football players with multiple concussions. J. Neurol. 2016, 263, 1332–1341. [Google Scholar] [CrossRef]
- Hampshire, A.; MacDonald, A.; Owen, A.M. Hypoconnectivity and hyperfrontality in retired American football players. Sci. Rep. 2013, 3, 2972. [Google Scholar] [CrossRef] [PubMed]
- Terry, D.P.; Adams, T.E.; Ferrara, M.S.; Miller, L.S. FMRI hypoactivation during verbal learning and memory in former high school football players with multiple concussions. Arch Clin. Neuropsychol. 2015, 30, 341–355. [Google Scholar] [CrossRef]
- Ford, J.H.; Giovanello, K.S.; Guskiewicz, K.M. Episodic memory in former professional football players with a history of concussion: An event-related functional neuroimaging study. J. Neurotrauma 2013, 30, 1683–1701. [Google Scholar] [CrossRef]
- Amen, D.G.; Willeumier, K.; Omalu, B.; Newberg, A.; Raghavendra, C.; Raji, C.A. Perfusion Neuroimaging Abnormalities Alone Distinguish National Football League Players from a Healthy Population. J. Alzheimers Dis. 2016, 53, 237–241. [Google Scholar] [CrossRef]
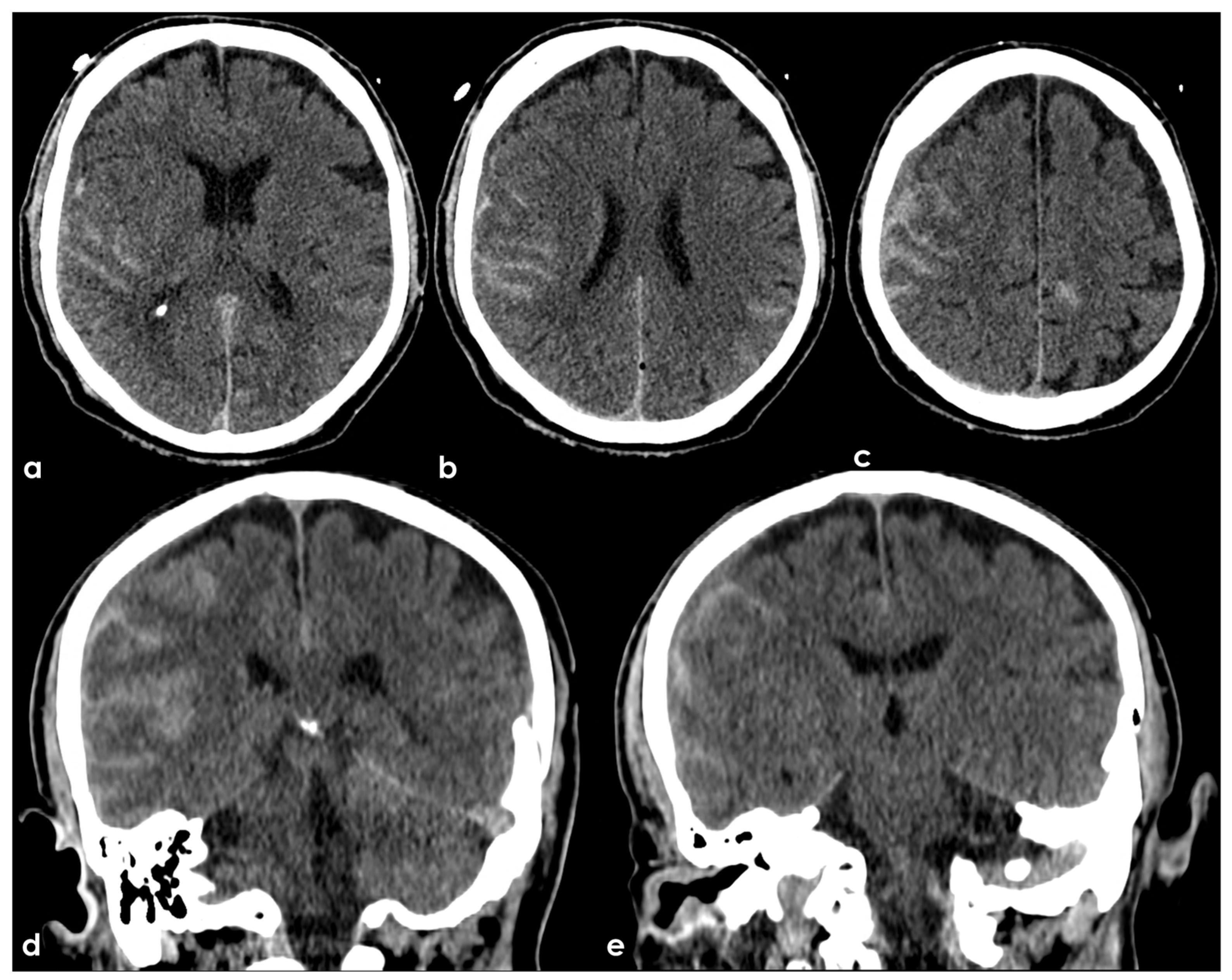
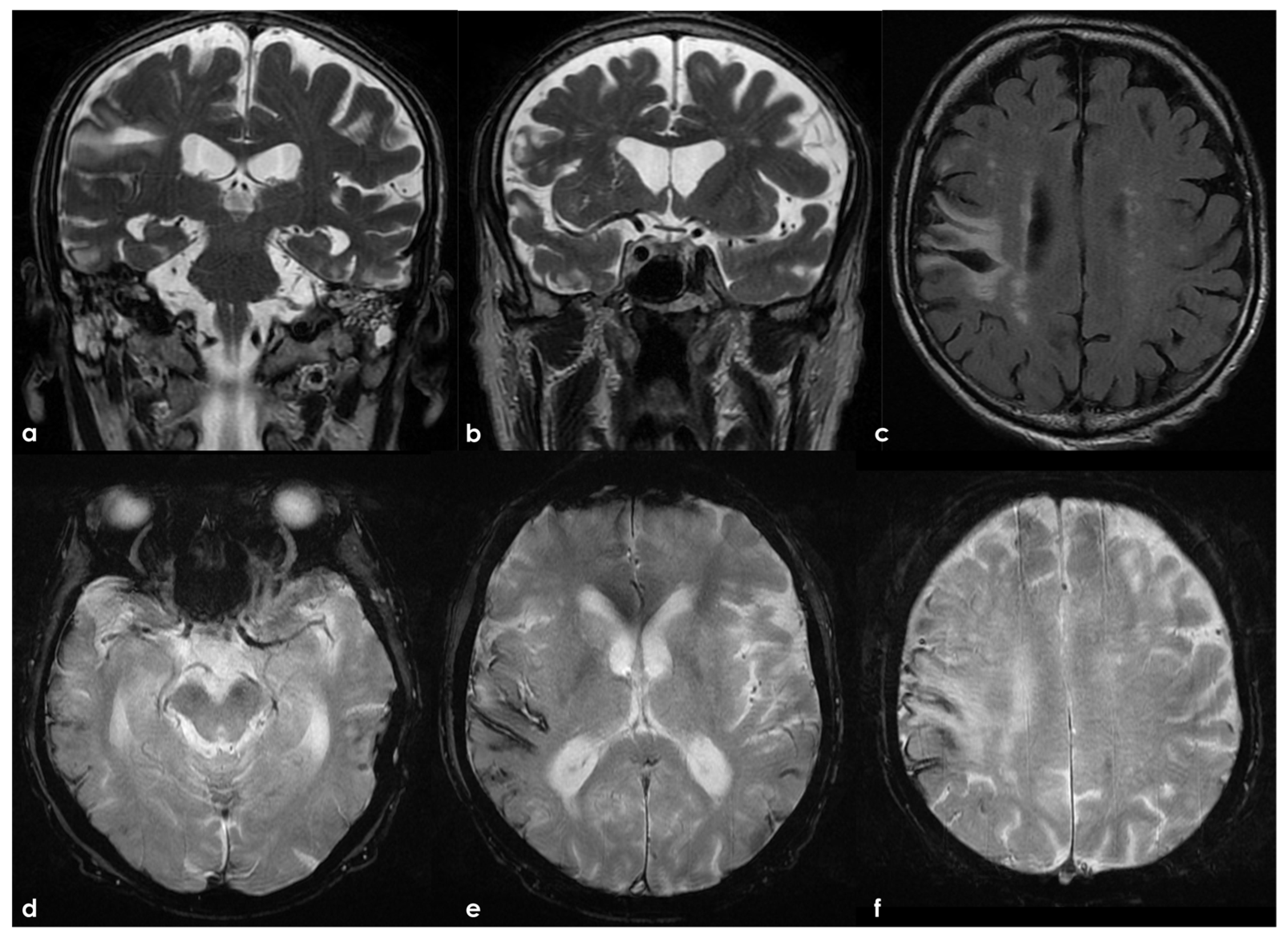
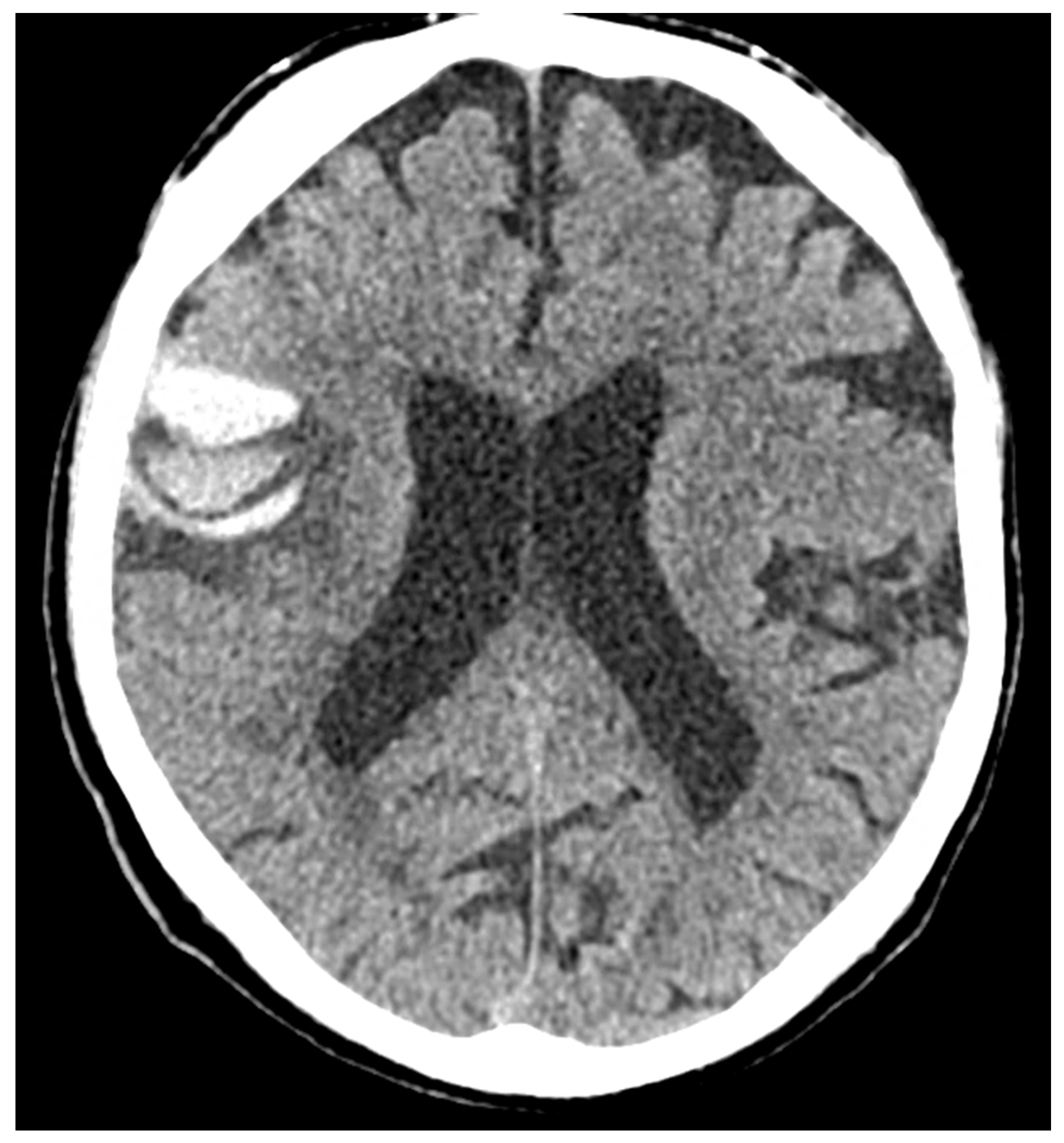

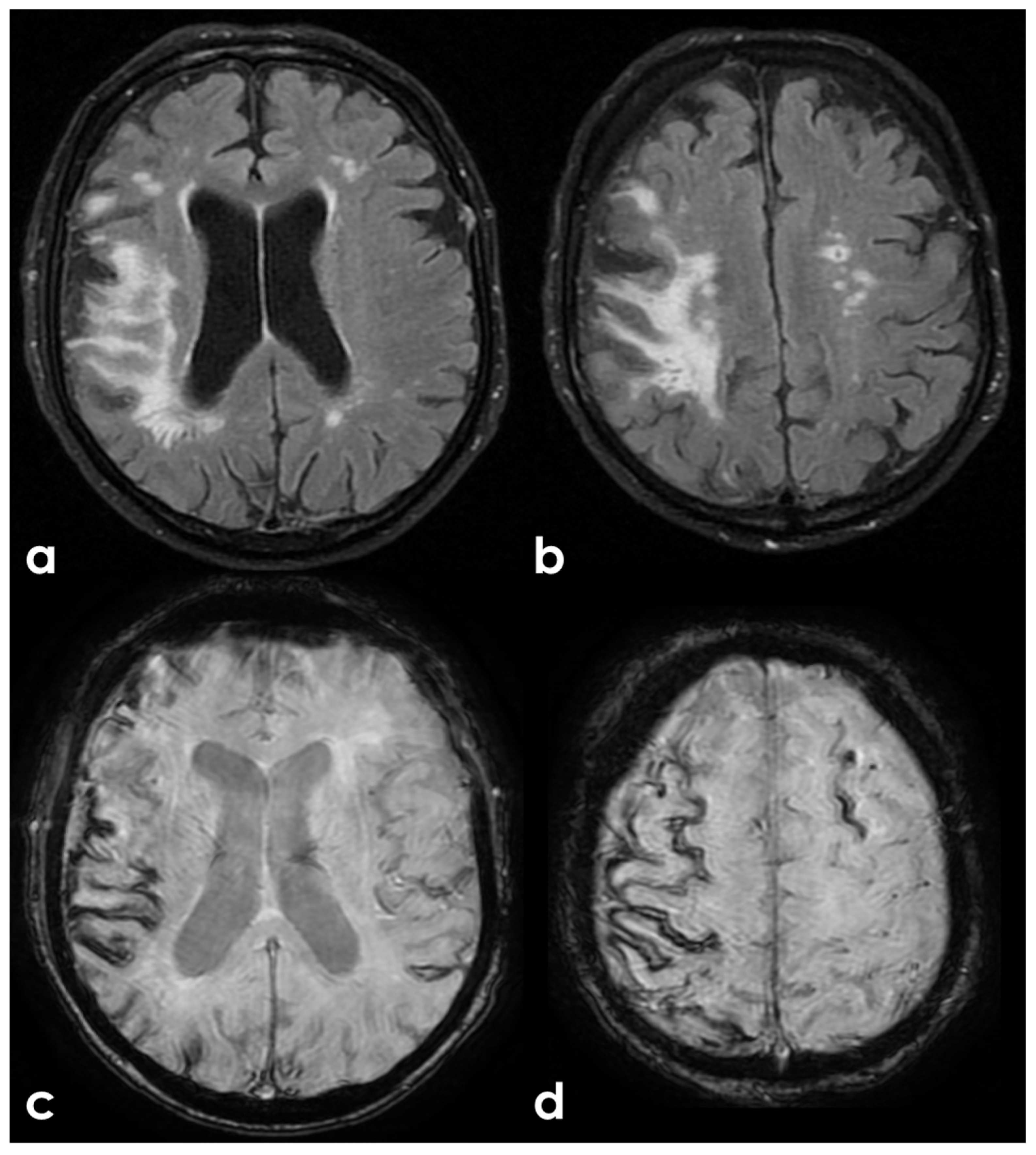
| Definitions | Features |
|---|---|
| Traumatic brain injury (TBI) | Both loss of consciousness (LOC) and post-traumatic amnesia (PTA) should be present in symptomatic cases. The duration of LOC or PTA, and the Glasgow Coma Scale score are used to grade the severity (“mild”, “moderate”, or “severe”, depending on. This framework is more frequently applied in studies involving civilian populations in emergency department settings or among military personnel and veterans, rather than in cases of sports-related head trauma. |
| Concussion | It is considered synonymous with mild TBI. However, in sports contexts, concussion diagnoses are frequently established on the presence of head trauma and headaches, dizziness, impaired balance, nausea, or abnormalities in eye movement, but not LOC or PTA. |
| Subconcussive trauma | It is a common occurrence in collision sports (e.g., American football) as asymptomatic head impacts Similarly, in military environments, service members might face subconcussive exposure due to repeated blast incidents or training exercises (such as breacher or combat training). |
| Traumatic encephalopathy syndrome (TES) | Research criteria have been proposed for classifying cognitive and neuro-behavioral symptoms thought to be linked to repetitive head trauma, with symptoms often emerging years after the last exposure to head trauma. They have high sensitivity but low specificity regarding the underlying neuropathology of chronic traumatic encephalopathy (CTE). |
| Chronic traumatic encephalopathy (CTE) | The consensus diagnostic criteria about neuropathological findings include phosphorylated tau protein aggregates located in neurons around blood vessels at the depths of cortical sulci. The diagnosis of CTE is established independently of the patient’s symptoms during their lifetime. |
| Age and survival time | Two key factors affecting Aβ deposition post-TBI are the age at the time of injury and the length of survival. The incidence of Aβ plaques is higher in older patients after acute severe TBI [52,54], and longer survival appears to correlate with more extensive and mature Aβ pathology [4]. This suggests that, given sufficient time, Aβ deposition may progress from diffuse accumulations to more structured plaques. |
| Genetic variations in Aβ clearance | Neprilysin:
|
Apolipoprotein E (APOE):
|
| Plaque morphology | Aβ deposits in TBI brains are more diffuse than the dense-core neuritic plaques typically seen in advanced AD [52,54,55]. This suggests that post-TBI plaques form rapidly and recently, rather than undergoing the prolonged maturation process seen in AD. |
| Neuritic plaques | While neuritic plaques have been identified in some TBI brains, they are primarily found in older individuals [52,54]. Thioflavin-S staining—used to detect β-sheet structures in mature amyloid plaques—was positive in only 1 of 18 acute TBI cases [55]. In long-term TBI survivors, a higher prevalence of fibrillar Thioflavin-S-positive plaques has been reported [4], suggesting that plaque maturation may require extended post-injury survival. |
| TBI Stages | Features |
|---|---|
| Acute tau pathology after single TBI | Within 24 h of injury, phosphorylation of tau at the Ser396/Ser404 epitope is observed in axons and white matter of excised TBI brain tissue [71]. However, somatodendritic tau accumulation is rare, suggesting that while hyperphosphorylation occurs acutely, neurofibrillary tangle formation does not. Only 11% of acute postmortem TBI brains show p-tau immunoreactivity, and tau-positive glial cells are present in up to 20% of severe TBI cases. |
| Chronic tau pathology in long-term TBI survivors | A study of 39 severe TBI cases with survival times ranging from 1 to 47 years compared to 47 control brains found tau pathology in 34% of TBI brains aged under 60, compared to only 9% of controls [72]. Additionally, the distribution of tau pathology in TBI brains differed from controls, with abnormal tau staining appearing in sulcal depths and superficial cortical layers, rather than being restricted to the entorhinal cortex and hippocampus, as seen in control brains. Widespread tau pathology was detected in the cingulate gyrus, superior frontal gyrus, and insular cortex. |
| Repetitive mTBI and CTE | Most reports of tau pathology post-TBI come from cases of repetitive mTBI associated with CTE [73]. Initially, CTE was primarily documented in boxers. However, in recent years, multiple cases have been identified in athletes from various contact sports, as well as military personnel with a history of blast and military-related concussions [74,75]. |
| Issues | Features |
|---|---|
| Morphology | Tau-positive somatodendritic inclusions in CTE resemble those in AD, but CTE features a significantly greater degree of astrocytic tau deposition. |
| Neocortical distribution | In long-term single-TBI survivors and CTE cases, tau preferentially accumulates in layers II and III of the cortex [4], whereas in AD, tau deposition is typically concentrated in layers V and VI [77]. |
| Biochemical characterization | Limited studies have examined the biochemical profiles of tau post-TBI. The inclusion type (paired helical filaments, straight, or ribbon filaments), primary phosphorylation sites, and roles of different tau isoforms remain unclear. Only two studies have assessed the ratio of 4-repeat (4R) to 3-repeat (3R) tau in CTE. One study found that both isoforms were hyperphosphorylated in brain extracts from two boxers [78]. Another reported both 4R and 3R tau immunostaining in a human CTE case, with 4R tau predominantly found in astrocytic tau inclusions [15]. However, no biochemical analyses have been conducted on the chronic tau pathology observed in long-term survivors of a single TBI. |
| Issues | Features |
|---|---|
| Acute tau phosphorylation | In 3xTg-AD mice (harboring a human P301L tau mutation), cortical impact injury led to punctate, primarily axonal, p-tau accumulation across multiple brain regions, as well as increased somatodendritic p-tau in contralateral CA1 neurons. Endogenous mouse tau phosphorylation at multiple sites has been observed following blast injury [78,79] and closed-head injury. |
| Chronic tau accumulation | Inducing sustained tau pathology in animal models has been difficult, even with tau transgenic mice. In one study using T44 mice (overexpressing the shortest tau isoform), animals received four mTBIs per day, once a week for four weeks (16 impacts total), followed by a 9-month recovery period. Only one mouse exhibited accelerated tau deposition [80]. Another study using aged (18-month-old) human tau (hTau) mice subjected to five mTBIs over nine days found increased tau pathology three weeks post-injury compared to sham or single-mTBI mice [81]. |
| Issues | Features |
|---|---|
| Incidence and overlooked pathologies | Current studies that focus on individual pathological markers report that amyloid-β (Aβ) and tau pathology are present in approximately 30% of TBI cases. However, the true incidence of neurodegenerative pathology post-TBI could be considerably higher if multiple disease-related proteins—such as TDP-43, α-synuclein, and phosphorylated tau—were examined systematically within the same cohorts [9,70]. A broader investigation would provide a more accurate understanding of the pathological burden following TBI. |
| Animal models and the importance of multi-pathology analysis | Experimental models of TBI could also benefit from simultaneous investigation of multiple neurodegenerative markers within individual animals. This would help determine whether different pathological processes follow distinct temporal progressions after injury and whether certain pathologies are more strongly associated with specific types of injury. Studies by Tran et al. examined both Aβ and tau pathology following cortical impact injury in 3xTg-AD mice, which carry mutations linked to AD [82]. Future studies should incorporate additional factors that influence neurodegenerative pathology phenotypes, including the following ones. |
| Individual issues | Injury severity; Age at the time of injury; Duration of survival post-injury. |
| Genetic background | APOE genotype |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedde, M.; Piazza, F.; Pascarella, R. Traumatic Brain Injury and Chronic Traumatic Encephalopathy: Not Only Trigger for Neurodegeneration but Also for Cerebral Amyloid Angiopathy? Biomedicines 2025, 13, 881. https://doi.org/10.3390/biomedicines13040881
Zedde M, Piazza F, Pascarella R. Traumatic Brain Injury and Chronic Traumatic Encephalopathy: Not Only Trigger for Neurodegeneration but Also for Cerebral Amyloid Angiopathy? Biomedicines. 2025; 13(4):881. https://doi.org/10.3390/biomedicines13040881
Chicago/Turabian StyleZedde, Marialuisa, Fabrizio Piazza, and Rosario Pascarella. 2025. "Traumatic Brain Injury and Chronic Traumatic Encephalopathy: Not Only Trigger for Neurodegeneration but Also for Cerebral Amyloid Angiopathy?" Biomedicines 13, no. 4: 881. https://doi.org/10.3390/biomedicines13040881
APA StyleZedde, M., Piazza, F., & Pascarella, R. (2025). Traumatic Brain Injury and Chronic Traumatic Encephalopathy: Not Only Trigger for Neurodegeneration but Also for Cerebral Amyloid Angiopathy? Biomedicines, 13(4), 881. https://doi.org/10.3390/biomedicines13040881






