Transcriptomic Changes in the Frontal Cortex of Juvenile Pigs with Diet-Induced Metabolic Dysfunction-Associated Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Pen Activity and Novel Object Recognition Test
2.3. Immunofluorescence Analysis
2.4. Transcriptomics Analysis
2.5. Statistical Methods
3. Results
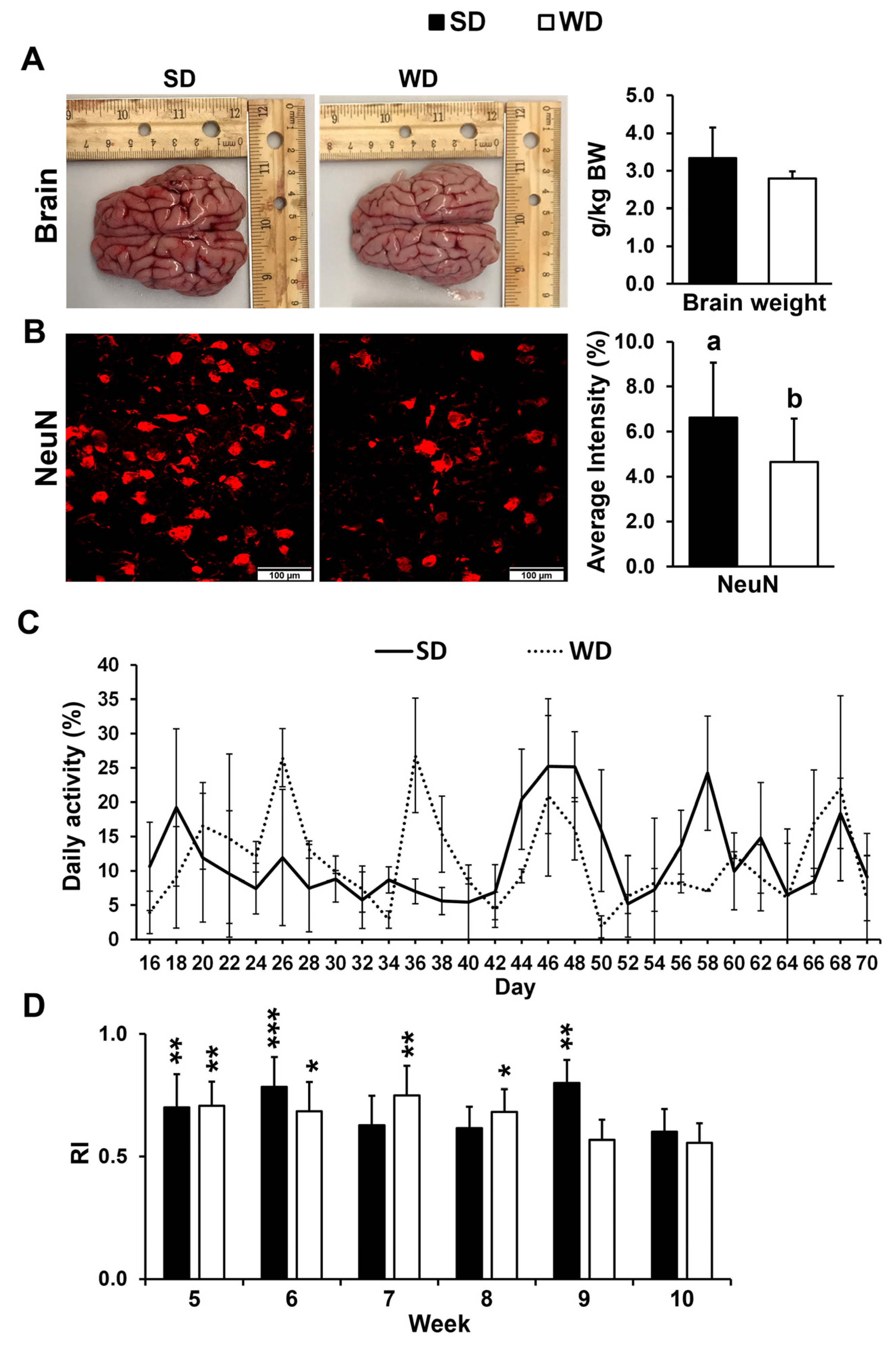
3.1. Western Diet-Fed Pigs Had Increased Frontal Cortex Neuronal Loss Without Changes in Brain Weight, Physical Activity, or Cognitive Function
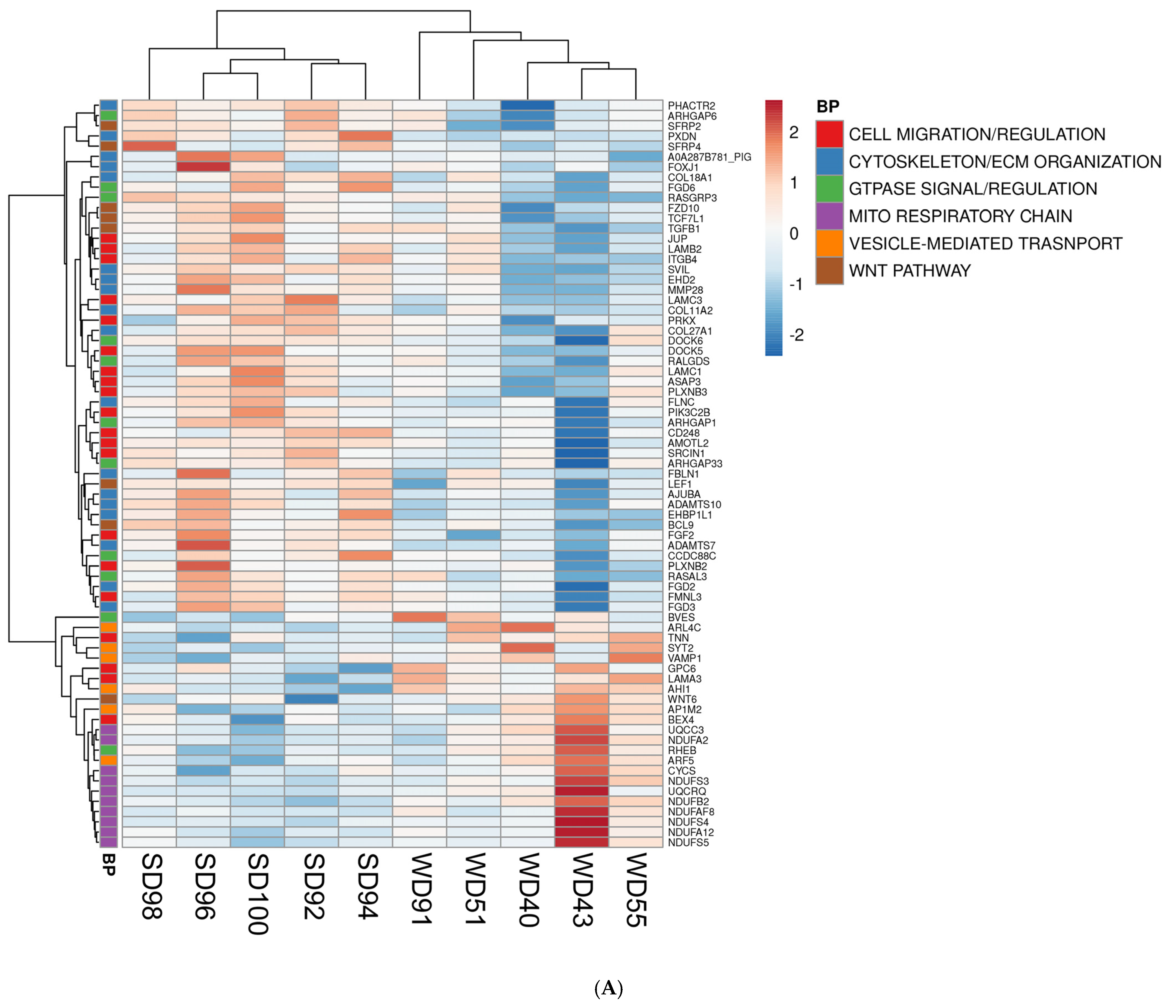
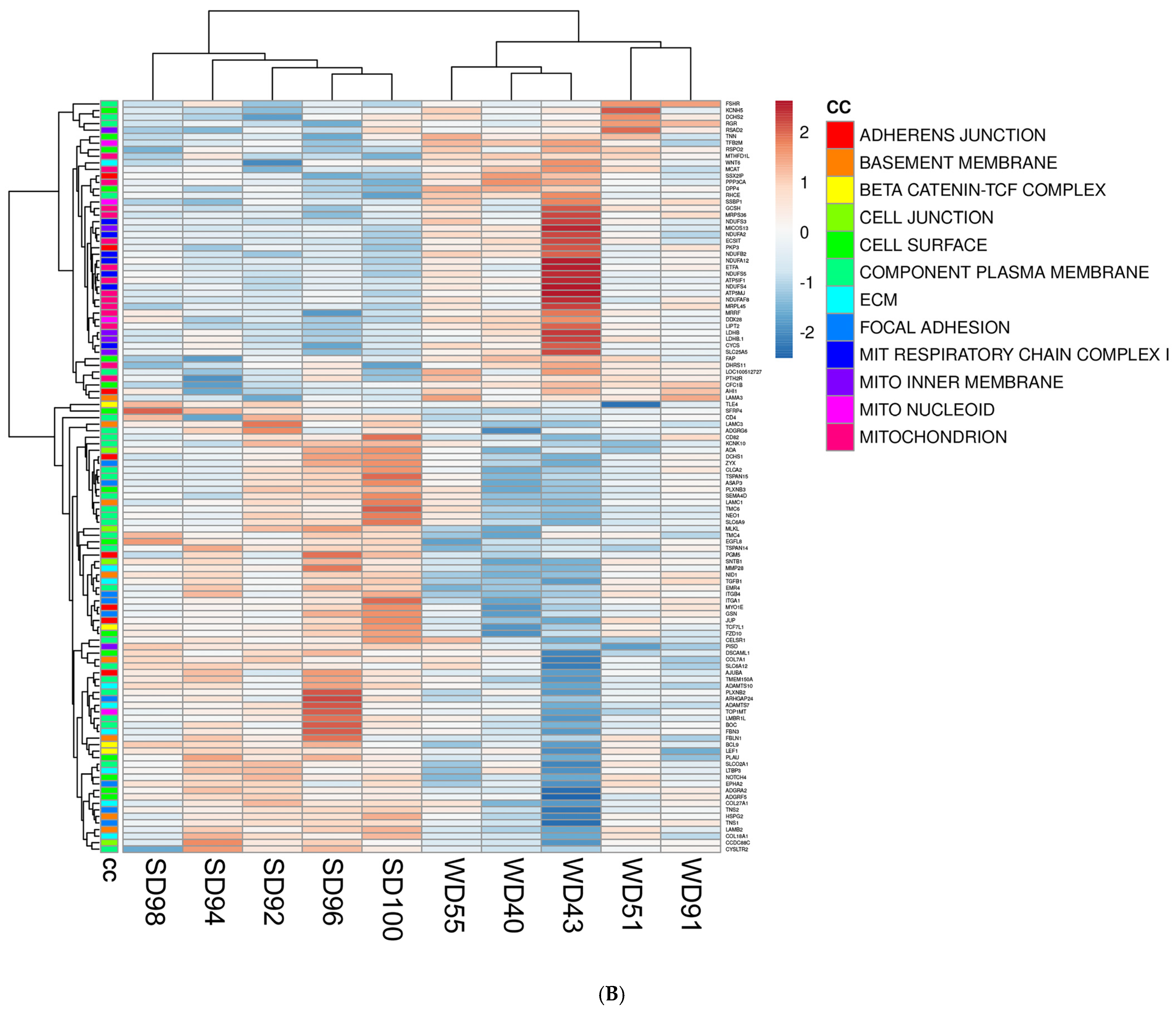
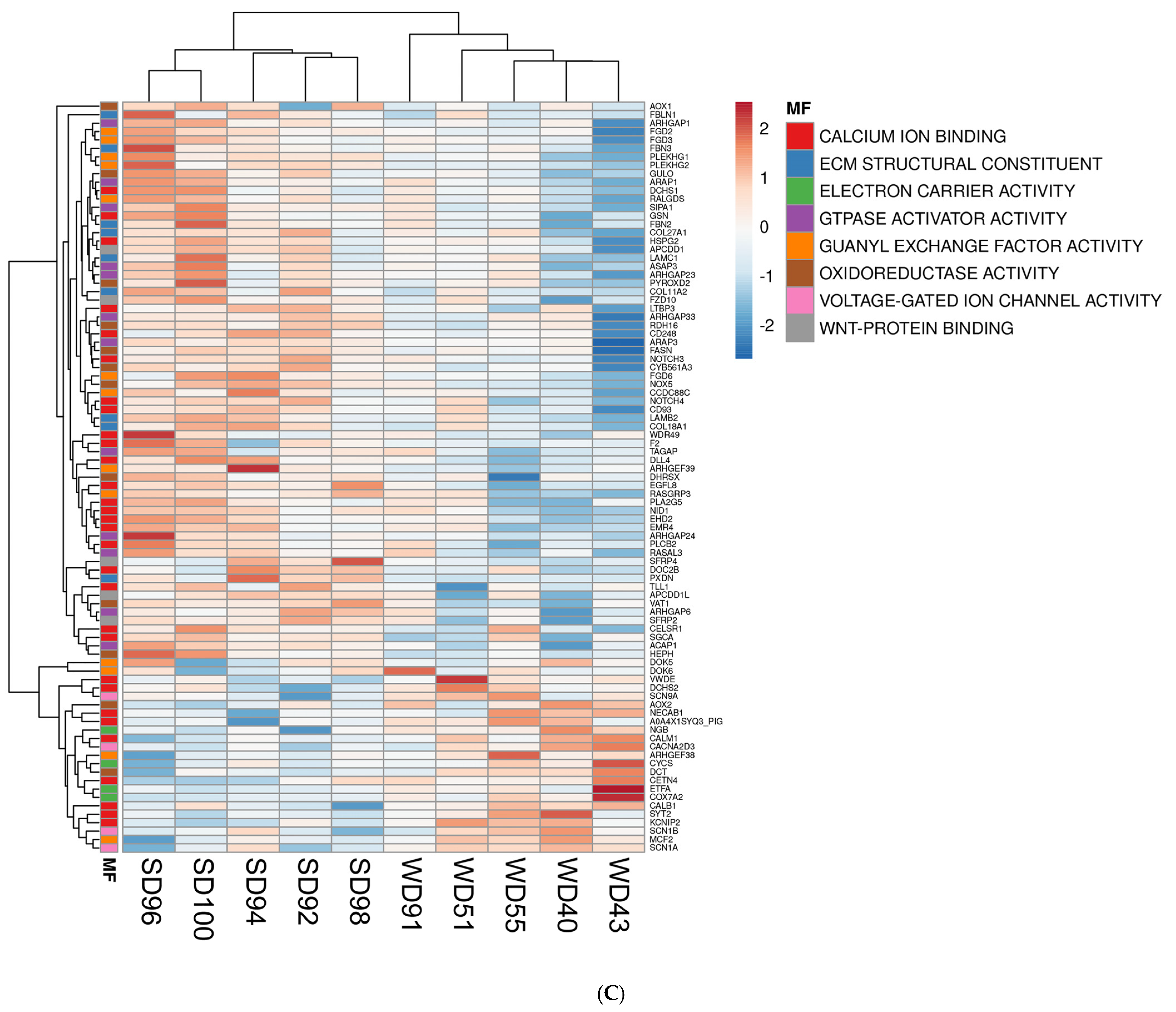
3.2. Western Diet-Fed Pigs Had Dysregulated Cortical Genes Associated with the Wnt/β-Catenin Signaling Pathway, Organization of the Cytoskeleton and Extracellular Matrix, and Mitochondrial Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Procaccini, C.; Santopaolo, M.; Faicchia, D.; Colamatteo, A.; Formisano, L.; de Candia, P.; Galgani, M.; De Rosa, V.; Matarese, G. Role of metabolism in neurodegenerative disorders. Metabolism 2016, 65, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Van Schependom, J.; D’Haeseleer, M. Advances in Neurodegenerative Diseases. J. Clin. Med. 2023, 12, 1709. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Shabani, K.; Hassan, B.A. The brain on time: Links between development and neurodegeneration. Development 2023, 150, dev200397. [Google Scholar] [CrossRef]
- Santos, T.; Fonseca, L.C.; Tedrus, G.; Delbue, J.L. Alzheimer’s disease: Nutritional status and cognitive aspects associated with disease severity. Nutr. Hosp. 2018, 35, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Sliwinska, S.; Jeziorek, M. The role of nutrition in Alzheimer’s disease. Rocz. Panstw. Zakl. Hig. 2021, 72, 29–39. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Dumitrascu, D.I.; Capitanescu, B.; Petcu, E.B.; Surugiu, R.; Fang, W.H.; Dumbrava, D.A. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen. Res. 2020, 15, 394–400. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Schneider, J.; Wilson, R.S. Dietary fats and the risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 194–200. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Cordner, Z.A.; Tamashiro, K.L. Effects of high-fat diet exposure on learning & memory. Physiol. Behav. 2015, 152, 363–371. [Google Scholar] [CrossRef]
- Zeltser, N.; Meyer, I.; Hernandez, G.V.; Trahan, M.J.; Fanter, R.K.; Abo-Ismail, M.; Glanz, H.; Strand, C.R.; Burrin, D.G.; La Frano, M.R.; et al. Neurodegeneration in juvenile Iberian pigs with diet-induced nonalcoholic fatty liver disease. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E592–E606. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.V.; Smith, V.A.; Melnyk, M.; Burd, M.A.; Sprayberry, K.A.; Edwards, M.S.; Peterson, D.G.; Bennet, D.C.; Fanter, R.K.; Columbus, D.A.; et al. Dysregulated FXR-FGF19 signaling and choline metabolism are associated with gut dysbiosis and hyperplasia in a novel pig model of pediatric NASH. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G582–G609. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, J.; Kehar, M.; Ibrahim, S.H.; Hartmann, P.; Sood, S.; Hassan, S.; Ramirez, C.M.; Kohli, R.; Censani, M.; Mauney, E.; et al. Metabolic dysfunction-associated steatotic liver disease (MASLD) in children with obesity: An Obesity Medicine Association (OMA) and expert joint perspective. Obes. Pillars 2025, 14, 100164. [Google Scholar] [CrossRef]
- Fang, Y.L.; Chen, H.; Wang, C.L.; Liang, L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J. Gastroenterol. 2018, 24, 2974–2983. [Google Scholar] [CrossRef]
- Gao, Y. Inflammation and gut microbiota in the alcoholic liver disease. Food Med. Homol. 2024, 1, 9420020. [Google Scholar] [CrossRef]
- Goyal, N.P.; Xanthakos, S.; Schwimmer, J.B. Metabolic dysfunction-associated steatotic liver disease in children. Gut 2025, 74, 669–677. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Zhao, Z.-B.; Yan, E.P.; Lian, Z.-X.; Zhang, W. Complex interplay between the immune system, metabolism, and epigenetic factors in autoimmune liver diseases. Med. Adv. 2023, 1, 97–114. [Google Scholar] [CrossRef]
- Li, M.-Y.; Gu, a.; Li, J.; Tang, N.; Matin, M.; Yang, Y.; Zengin, G.; Atanasov, A.G. Exploring food and medicine homology: Potential implications for cancer treatment innovations. Acta Mater. Medica 2025, 4, 200–206. [Google Scholar] [CrossRef]
- Serbis, A.; Polyzos, S.A.; Paschou, S.A.; Siomou, E.; Kiortsis, D.N. Diet, exercise, and supplements: What is their role in the management of the metabolic dysfunction-associated steatotic liver disease in children? Endocrine 2024, 85, 988–1006. [Google Scholar] [CrossRef]
- Pei, Y.; Goh, G.B. Genetic Risk Factors for Metabolic Dysfunction-Associated Steatotic Liver Disease. Gut Liver 2025, 19, 8–18. [Google Scholar] [CrossRef]
- Weinstein, G.; Zelber-Sagi, S.; Preis, S.R.; Beiser, A.S.; DeCarli, C.; Speliotes, E.K.; Satizabal, C.L.; Vasan, R.S.; Seshadri, S. Association of Nonalcoholic Fatty Liver Disease With Lower Brain Volume in Healthy Middle-aged Adults in the Framingham Study. JAMA Neurol. 2018, 75, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, B.; Markovic, O.; Duric, V.; Filipovic, B. Cognitive Changes and Brain Volume Reduction in Patients with Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2018, 2018, 9638797. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Gottesman, R.F.; Clark, J.M.; Hernaez, R.; Chang, Y.; Kim, C.; Ha, K.H.; Guallar, E.; Lazo, M. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology 2016, 86, 1136–1142. [Google Scholar] [CrossRef]
- Bosoi, C.R.; Vandal, M.; Tournissac, M.; Leclerc, M.; Fanet, H.; Mitchell, P.L.; Verreault, M.; Trottier, J.; Virgili, J.; Tremblay, C.; et al. High-Fat Diet Modulates Hepatic Amyloid beta and Cerebrosterol Metabolism in the Triple Transgenic Mouse Model of Alzheimer’s Disease. Hepatol. Commun. 2021, 5, 446–460. [Google Scholar] [CrossRef]
- Kim, D.G.; Krenz, A.; Toussaint, L.E.; Maurer, K.J.; Robinson, S.A.; Yan, A.; Torres, L.; Bynoe, M.S. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J. Neuroinflammation 2016, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Jakhmola Mani, R.; Dogra, N.; Katare, D.P. The Connection between Chronic Liver Damage and Sporadic Alzheimer’s Disease: Evidence and Insights from a Rat Model. Brain Sci. 2023, 13, 1391. [Google Scholar] [CrossRef]
- Spurlock, M.E.; Gabler, N.K. The development of porcine models of obesity and the metabolic syndrome. J. Nutr. 2008, 138, 397–402. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Lind, N.M.; Moustgaard, A.; Jelsing, J.; Vajta, G.; Cumming, P.; Hansen, A.K. The use of pigs in neuroscience: Modeling brain disorders. Neurosci. Biobehav. Rev. 2007, 31, 728–751. [Google Scholar] [CrossRef]
- Fleming, S.A.; Dilger, R.N. Young pigs exhibit differential exploratory behavior during novelty preference tasks in response to age, sex, and delay. Behav. Brain Res. 2017, 321, 50–60. [Google Scholar] [CrossRef]
- Smith, D.H.; Chen, X.H.; Nonaka, M.; Trojanowski, J.Q.; Lee, V.M.; Saatman, K.E.; Leoni, M.J.; Xu, B.N.; Wolf, J.A.; Meaney, D.F. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J. Neuropathol. Exp. Neurol. 1999, 58, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Manjarin, R.; Dillard, K.; Coffin, M.; Hernandez, G.V.; Smith, V.A.; Noland-Lidell, T.; Gehani, T.R.; Smart, H.J.; Wheeler, K.; Sprayberry, K.A.; et al. Dietary fat composition shapes bile acid metabolism and severity of liver injury in a pig model of pediatric NAFLD. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E187–E206. [Google Scholar] [CrossRef] [PubMed]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Maj, M.A.; Gehani, T.R.; Immoos, C.; Medrano, M.S.; Fanter, R.K.; Strand, C.R.; Glanz, H.; Piccolo, B.D.; Abo-Ismail, M.K.; La Frano, M.R.; et al. Olive- and Coconut-Oil-Enriched Diets Decreased Secondary Bile Acids and Regulated Metabolic and Transcriptomic Markers of Brain Injury in the Frontal Cortexes of NAFLD Pigs. Brain Sci. 2022, 12, 1193. [Google Scholar] [CrossRef]
- Gifford, A.K.; Cloutier, S.; Newberry, R.C. Objects as enrichment: Effects of object exposure time and delay interval on object recognition memory of the domestic pig. Appl. Anim. Behav. Sci. 2007, 107, 206–217. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, S.; Bittencourt, S.A. FastQC: A quality control tool for high throughput sequence data. Babraham Bioinform. 2010. [Google Scholar]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.A.; Burrin, D.G.; Manjarin, R. Decreased FXR Agonism in the Bile Acid Pool Is Associated with Impaired FXR Signaling in a Pig Model of Pediatric NAFLD. Biomedicines 2023, 11, 3303. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, C.; Tian, Y.; Du, X.; Wang, Q.; Zhao, H. Expression and Function of WNT6: From Development to Disease. Front. Cell Dev. Biol. 2020, 8, 558155. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.A.; Khan, M.; V, M.; Kar, D. The Molecular Basis of Wnt/beta-Catenin Signaling Pathways in Neurodegenerative Diseases. Int. J. Cell Biol. 2023, 2023, 9296092. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- Toledo, E.M.; Colombres, M.; Inestrosa, N.C. Wnt signaling in neuroprotection and stem cell differentiation. Prog. Neurobiol. 2008, 86, 281–296. [Google Scholar] [CrossRef]
- Cerpa, W.; Toledo, E.M.; Varela-Nallar, L.; Inestrosa, N.C. The role of Wnt signaling in neuroprotection. Drug News Perspect. 2009, 22, 579–591. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, Z.; Zhao, J.; Su, D.; He, B.; Shi, C.; Shi, Y. Transcriptomic analysis to identify genes associated with hypothalamus vulnerability in aging mice with cognitive decline. Behav. Brain Res. 2024, 465, 114943. [Google Scholar] [CrossRef]
- Bostaille, N.; Gauquier, A.; Twyffels, L.; Vanhollebeke, B. Molecular insights into Adgra2/Gpr124 and Reck intracellular trafficking. Biol. Open 2016, 5, 1874–1881. [Google Scholar] [CrossRef]
- Eubelen, M.; Bostaille, N.; Cabochette, P.; Gauquier, A.; Tebabi, P.; Dumitru, A.C.; Koehler, M.; Gut, P.; Alsteens, D.; Stainier, D.Y.R.; et al. A molecular mechanism for Wnt ligand-specific signaling. Science 2018, 361, eaat1178. [Google Scholar] [CrossRef]
- Riise, J.; Plath, N.; Pakkenberg, B.; Parachikova, A. Aberrant Wnt signaling pathway in medial temporal lobe structures of Alzheimer’s disease. J. Neural Transm. 2015, 122, 1303–1318. [Google Scholar] [CrossRef]
- Guo, G.; Fan, L.; Yan, Y.; Xu, Y.; Deng, Z.; Tian, M.; Geng, Y.; Xia, Z.; Xu, Y. Shared metabolic shifts in endothelial cells in stroke and Alzheimer’s disease revealed by integrated analysis. Sci. Data 2023, 10, 666. [Google Scholar] [CrossRef]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of beta-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- Moon, H.E.; Paek, S.H. Mitochondrial Dysfunction in Parkinson’s Disease. Exp. Neurobiol. 2015, 24, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.G.; Sugandhi, V.V.; Jha, S.K.; Nangare, S.N.; Gupta, G.; Singh, S.K.; Dua, K.; Cho, H.; Hansbro, P.M.; Paudel, K.R. Neurodegenerative disorders: Mechanisms of degeneration and therapeutic approaches with their clinical relevance. Ageing Res. Rev. 2024, 99, 102357. [Google Scholar] [CrossRef]
- Bustamante-Barrientos, F.A.; Luque-Campos, N.; Araya, M.J.; Lara-Barba, E.; de Solminihac, J.; Pradenas, C.; Molina, L.; Herrera-Luna, Y.; Utreras-Mendoza, Y.; Elizondo-Vega, R.; et al. Mitochondrial dysfunction in neurodegenerative disorders: Potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. J. Transl. Med. 2023, 21, 613. [Google Scholar] [CrossRef] [PubMed]
- Muddapu, V.R.; Dharshini, S.A.P.; Chakravarthy, V.S.; Gromiha, M.M. Neurodegenerative Diseases—Is Metabolic Deficiency the Root Cause? Front. Neurosci. 2020, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Berdun, R.; Obis, E.; Mota-Martorell, N.; Bassols, A.; Valent, D.; Serrano, J.C.E.; Martin-Gari, M.; Rodriguez-Palmero, M.; Moreno-Munoz, J.A.; Tibau, J.; et al. High-Fat Diet-Induced Obesity Increases Brain Mitochondrial Complex I and Lipoxidation-Derived Protein Damage. Antioxidants 2024, 13, 161. [Google Scholar] [CrossRef]
- Glaab, E.; Schneider, R. Comparative pathway and network analysis of brain transcriptome changes during adult aging and in Parkinson’s disease. Neurobiol. Dis. 2015, 74, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Pissas, G.; Liakopoulos, V.; Stefanidis, I. Cytochrome c as a Potentially Clinical Useful Marker of Mitochondrial and Cellular Damage. Front. Immunol. 2016, 7, 279. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Al-Abdulla, N.A.; Brambrink, A.M.; Kirsch, J.R.; Sieber, F.E.; Portera-Cailliau, C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res. Bull. 1998, 46, 281–309. [Google Scholar] [CrossRef]
- Kusuma, V.I.; I’tishom, R.; Qurnianingsih, E.; Rejeki, P.S. A Systematical Review of The Effect of Ketogenic Diet on Bcl-2 (B-cell lymphoma-2) Expression as An Apoptosis Marker in Cancer Treatment. Biomolec. Health Sci. J. 2021, 4, 125–130. [Google Scholar] [CrossRef]
- Yang, L.; Wei, M.; Xing, B.; Zhang, C. Extracellular matrix and synapse formation. Biosci. Rep. 2023, 43, BSR20212411. [Google Scholar] [CrossRef]
- Bonneh-Barkay, D.; Wiley, C.A. Brain extracellular matrix in neurodegeneration. Brain Pathol. 2009, 19, 573–585. [Google Scholar] [CrossRef]
- Dent, E.W.; Merriam, E.B.; Hu, X. The dynamic cytoskeleton: Backbone of dendritic spine plasticity. Curr. Opin. Neurobiol. 2011, 21, 175–181. [Google Scholar] [CrossRef]
- Li, M.; Hao, X.; Hu, Z.; Tian, J.; Shi, J.; Ma, D.; Guo, M.; Li, S.; Zuo, C.; Liang, Y.; et al. Microvascular and cellular dysfunctions in Alzheimer’s disease: An integrative analysis perspective. Sci. Rep. 2024, 14, 20944. [Google Scholar] [CrossRef]
- Ozsan McMillan, I.; Li, J.P.; Wang, L. Heparan sulfate proteoglycan in Alzheimer’s disease: Aberrant expression and functions in molecular pathways related to amyloid-beta metabolism. Am. J. Physiol. Cell Physiol. 2023, 324, C893–C909. [Google Scholar] [CrossRef]
- Calabrese, G.; Molzahn, C.; Mayor, T. Protein interaction networks in neurodegenerative diseases: From physiological function to aggregation. J. Biol. Chem. 2022, 298, 102062. [Google Scholar] [CrossRef]
- Chandra, P.K.; Cikic, S.; Rutkai, I.; Guidry, J.J.; Katakam, P.V.G.; Mostany, R.; Busija, D.W. Effects of aging on protein expression in mice brain microvessels: ROS scavengers, mRNA/protein stability, glycolytic enzymes, mitochondrial complexes, and basement membrane components. Geroscience 2022, 44, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Cappello, S.; Gray, M.J.; Badouel, C.; Lange, S.; Einsiedler, M.; Srour, M.; Chitayat, D.; Hamdan, F.F.; Jenkins, Z.A.; Morgan, T.; et al. Mutations in genes encoding the cadherin receptor-ligand pair DCHS1 and FAT4 disrupt cerebral cortical development. Nat. Genet. 2013, 45, 1300–1308. [Google Scholar] [CrossRef]
- Miyashita, A.; Hatsuta, H.; Kikuchi, M.; Nakaya, A.; Saito, Y.; Tsukie, T.; Hara, N.; Ogishima, S.; Kitamura, N.; Akazawa, K.; et al. Genes associated with the progression of neurofibrillary tangles in Alzheimer’s disease. Transl. Psychiatry 2014, 4, e396. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, L.; Chalavi, S.; Swinnen, S.P. Aging and brain plasticity. Aging 2018, 10, 1789–1790. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Gomez, A.G. The influence of dietary factors in central nervous system plasticity and injury recovery. PM&R 2011, 3, S111–S116. [Google Scholar] [CrossRef]
- Mou, Y.; Blok, E.; Barroso, M.; Jansen, P.W.; White, T.; Voortman, T. Dietary patterns, brain morphology and cognitive performance in children: Results from a prospective population-based study. Eur. J. Epidemiol. 2023, 38, 669–687. [Google Scholar] [CrossRef]
- Marzola, P.; Melzer, T.; Pavesi, E.; Gil-Mohapel, J.; Brocardo, P.S. Exploring the Role of Neuroplasticity in Development, Aging, and Neurodegeneration. Brain Sci. 2023, 13, 1610. [Google Scholar] [CrossRef]
- Lathia, J.D.; Mattson, M.P.; Cheng, A. Notch: From neural development to neurological disorders. J. Neurochem. 2008, 107, 1471–1481. [Google Scholar] [CrossRef]
- Salmina, A.B.; Kharitonova, E.V.; Gorina, Y.V.; Teplyashina, E.A.; Malinovskaya, N.A.; Khilazheva, E.D.; Mosyagina, A.I.; Morgun, A.V.; Shuvaev, A.N.; Salmin, V.V.; et al. Blood-Brain Barrier and Neurovascular Unit In Vitro Models for Studying Mitochondria-Driven Molecular Mechanisms of Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 4661. [Google Scholar] [CrossRef]
- Romay, M.C.; Knutsen, R.H.; Ma, F.; Mompeon, A.; Hernandez, G.E.; Salvador, J.; Mirkov, S.; Batra, A.; Sullivan, D.P.; Procissi, D.; et al. Age-related loss of Notch3 underlies brain vascular contractility deficiencies, glymphatic dysfunction, and neurodegeneration in mice. J. Clin. Investig. 2024, 134, e166134. [Google Scholar] [CrossRef]
- Manda, V.K.; Mittapalli, R.K.; Geldenhuys, W.J.; Lockman, P.R. Chronic exposure to nicotine and saquinavir decreases endothelial Notch-4 expression and disrupts blood-brain barrier integrity. J. Neurochem. 2010, 115, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Brunal, A.A.; Clark, K.C.; Studtmann, C.; Stebbins, K.; Higashijima, S.I.; Pan, Y.A. Deficiency in the cell-adhesion molecule dscaml1 impairs hypothalamic CRH neuron development and perturbs normal neuroendocrine stress axis function. Front. Cell Dev. Biol. 2023, 11, 1113675. [Google Scholar] [CrossRef]
- Chang, R.Y.; Etheridge, N.; Nouwens, A.S.; Dodd, P.R. SWATH analysis of the synaptic proteome in Alzheimer’s disease. Neurochem. Int. 2015, 87, 1–12. [Google Scholar] [CrossRef]
- Briguglio, M.; Dell’Osso, B.; Panzica, G.; Malgaroli, A.; Banfi, G.; Zanaboni Dina, C.; Galentino, R.; Porta, M. Dietary Neurotransmitters: A Narrative Review on Current Knowledge. Nutrients 2018, 10, 591. [Google Scholar] [CrossRef]
- Pongrac, J.L.; Slack, P.J.; Innis, S.M. Dietary polyunsaturated fat that is low in (n − 3) and high in (n − 6) fatty acids alters the SNARE protein complex and nitrosylation in rat hippocampus. J. Nutr. 2007, 137, 1852–1856. [Google Scholar] [CrossRef]
- Sevlever, D.; Zou, F.; Ma, L.; Carrasquillo, S.; Crump, M.G.; Culley, O.J.; Hunter, T.A.; Bisceglio, G.D.; Younkin, L.; Allen, M.; et al. Genetically-controlled Vesicle-Associated Membrane Protein 1 expression may contribute to Alzheimer’s pathophysiology and susceptibility. Mol. Neurodegener. 2015, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- O’Day, D.H. Protein Biomarkers Shared by Multiple Neurodegenerative Diseases Are Calmodulin-Binding Proteins Offering Novel and Potentially Universal Therapeutic Targets. J. Clin. Med. 2023, 12, 7045. [Google Scholar] [CrossRef]
- Glavan, G.; Schliebs, R.; Zivin, M. Synaptotagmins in neurodegeneration. Anat. Rec. 2009, 292, 1849–1862. [Google Scholar] [CrossRef]
- Yu, L.; Petyuk, V.A.; Gaiteri, C.; Mostafavi, S.; Young-Pearse, T.; Shah, R.C.; Buchman, A.S.; Schneider, J.A.; Piehowski, P.D.; Sontag, R.L.; et al. Targeted brain proteomics uncover multiple pathways to Alzheimer’s dementia. Ann. Neurol. 2018, 84, 78–88. [Google Scholar] [CrossRef]

| Item | SD | WD |
|---|---|---|
| Whey protein concentrate 1 | 8.5 | 8.9 |
| Fructose 2 | 0 | 12 |
| Dextrose 2 | 6 | 3 |
| Fat Pak 80 3 | 3.2 | 0 |
| Hydrogenated lard 4 | 0 | 3.7 |
| Hydrogenated coconut oil 2 | 0 | 5 |
| Corn oil 5 | 3.2 | 0 |
| Xanthan gum 6 | 0.4 | 0.4 |
| Vitamin premix 7,8 | 0.32 | 0.32 |
| Mineral premix 7,8 | 1.2 | 1.2 |
| Cholesterol 7 | 0 | 0.6 |
| Water | 77.2 | 64.4 |
| Item | SD | WD |
|---|---|---|
| Feed amount, L/kg BW/day | 0.18 | 0.18 |
| Dry matter, g/kg BW/day | 40.8 | 62.6 |
| Crude protein, g/kg BW/day | 12.9 | 13 |
| Metabolizable energy, kcal/kg BW/day | 199.3 | 302.6 |
| Carbohydrates, g/kg BW/day | 12.8 | 29.1 |
| Ether extract, g/kg BW/day | 11.2 | 16.7 |
| Item 1 | SD | WD |
|---|---|---|
| No. pigs (pen) | 8 (4) | 10 (5) |
| Sex (M/F) | 6/2 | 6/4 |
| Liver histology 2 | ||
| Steatosis | 0.13 a ± 0.32 | 3.50 b ± 0.53 |
| Ballooning | 0 ± 0 | 0.30 ± 0.48 |
| Mallory–Denk Bodies | 0 ± 0 | 0.30 ± 0.48 |
| Inflammation | 1.0 ± 0 | 1.33 ± 0.42 |
| Necrosis | 0 d ± 0 | 0.70 e ± 0.58 |
| Ki67+ cells 3 | 6.50 a ± 3.45 | 14.7 b ± 6.85 |
| Composite lesion score | 1.13 d ± 0.35 | 6.00 e ± 0.82 |
| Serum biochemistry | ||
| Alanine aminotransferase, U·L−1 | 19.5 a ± 3.5 | 62.2 b ± 40.4 |
| Aspartate aminotransferase, U·L−1 | 26.9 ± 12.8 | 199.6 ± 149.2 |
| Alkaline phosphatase, U·L−1 | 332.9 ± 59.8 | 391.6 ± 149.2 |
| γ-glutamyl transferase, U·L−1 | 29.1 a ± 5.91 | 56.8 b ± 29.4 |
| Lactate dehydrogenase, U·L−1 | 959.3 a ± 1078.3 | 3114.2 b ± 1492.7 |
| Biological Process (BP) | p-Value |
|---|---|
| Upregulated WD vs. SD | |
| mitochondrial respiratory chain complex I assembly | 0.0000 |
| mitochondrial electron transport, ubiquinol to cytochrome c | 0.0140 |
| vesicle-mediated transport | 0.0150 |
| Downregulated WD vs. SD | |
| cell migration | 0.0012 |
| canonical Wnt signaling pathway | 0.0013 |
| axon guidance | 0.0024 |
| signal transduction | 0.0038 |
| regulation of GTPase activity | 0.0055 |
| regulation of cell migration | 0.0130 |
| ceramide biosynthetic process | 0.0150 |
| small GTPase mediated signal transduction | 0.0250 |
| cytoskeleton organization | 0.0270 |
| extracellular matrix organization | 0.0320 |
| Cellular Component (CC) | p-value |
| Upregulated WD vs. SD | |
| mitochondrial respiratory chain complex I | 0.0000 |
| perikaryon | 0.0011 |
| mitochondrial inner membrane | 0.0020 |
| respiratory chain | 0.0031 |
| mitochondrion | 0.0045 |
| mitochondrial nucleoid | 0.0080 |
| voltage-gated sodium channel complex | 0.0150 |
| Downregulated WD vs. SD | |
| cell surface | 0.0000 |
| basement membrane | 0.0001 |
| focal adhesion | 0.0003 |
| beta-catenin-TCF complex | 0.0004 |
| integral component of plasma membrane | 0.0075 |
| extracellular matrix | 0.0077 |
| adherens junction | 0.0230 |
| cell junction | 0.0250 |
| Molecular Function (MF) | p-value |
| Upregulated WD vs. SD | |
| voltage-gated ion channel activity | 0.0039 |
| oxygen binding | 0.0260 |
| electron carrier activity | 0.0300 |
| Downregulated WD vs. SD | |
| extracellular matrix structural constituent | 0.0000 |
| GTPase activator activity | 0.0001 |
| calcium ion binding | 0.0001 |
| guanyl-nucleotide exchange factor activity | 0.0002 |
| Wnt-protein binding | 0.0011 |
| oxidoreductase activity | 0.0045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahon, K.; Abo-Ismail, M.; Auten, E.; Manjarin, R.; Maj, M. Transcriptomic Changes in the Frontal Cortex of Juvenile Pigs with Diet-Induced Metabolic Dysfunction-Associated Liver Disease. Biomedicines 2025, 13, 1567. https://doi.org/10.3390/biomedicines13071567
Mahon K, Abo-Ismail M, Auten E, Manjarin R, Maj M. Transcriptomic Changes in the Frontal Cortex of Juvenile Pigs with Diet-Induced Metabolic Dysfunction-Associated Liver Disease. Biomedicines. 2025; 13(7):1567. https://doi.org/10.3390/biomedicines13071567
Chicago/Turabian StyleMahon, Kyle, Mohammed Abo-Ismail, Emily Auten, Rodrigo Manjarin, and Magdalena Maj. 2025. "Transcriptomic Changes in the Frontal Cortex of Juvenile Pigs with Diet-Induced Metabolic Dysfunction-Associated Liver Disease" Biomedicines 13, no. 7: 1567. https://doi.org/10.3390/biomedicines13071567
APA StyleMahon, K., Abo-Ismail, M., Auten, E., Manjarin, R., & Maj, M. (2025). Transcriptomic Changes in the Frontal Cortex of Juvenile Pigs with Diet-Induced Metabolic Dysfunction-Associated Liver Disease. Biomedicines, 13(7), 1567. https://doi.org/10.3390/biomedicines13071567






