Abstract
Congenital heart diseases (CHDs) are usually defined as structural anomalies of the heart and great arteries, present since birth, that are due to embryological maldevelopment, with overt or potential dysfunction. Nowadays, most of the patients with CHD in adulthood (age > 18 years) had been operated on with success in infancy or childhood and undergo periodical screening. Pathology and nosology of CHDs are herein treated with special attention to adulthood according to the involved cardiac structures (aorta, valves, coronary arteries, myocardium, great arteries, conduction system). Moreover, the purpose is to postulate, in the era of molecular medicine, that genetically determined defects are also congenital cardiac disorders, with or without structural abnormality, and should be defined CHDs as well since their molecular background is material and present since conception.
1. Introduction
Traditionally, congenital heart diseases (CHDs) are defined as structural anomalies of the heart and great arteries, present at birth, that are due to embryological maldevelopment, with evident or potential dysfunction [1,2,3,4,5,6,7,8,9,10,11,12,13].
Nowadays, almost all CHDs may be repaired with success in infancy and childhood, requiring periodic check-up. Actually, they represent the majority of patients with CHD in adulthood (>18 years old), who may achieve a life expectancy almost equal to subjects born with normal hearts [11]. Moreover, many structural CHDs may escape diagnosis at birth and remain silent until adulthood. Genetically determined cardiac diseases, whether structural or functional, should be considered CHDs as well.
We approach the topic on the basis of the involved cardiac structures: coronary arteries, great arteries, myocardium, valves, and conduction system.
2. Coronary Arteries
The origin of an epicardial coronary artery from the pulmonary artery is usually lethal at birth, incompatible with life, and exceptionally seen in people growing up [14].
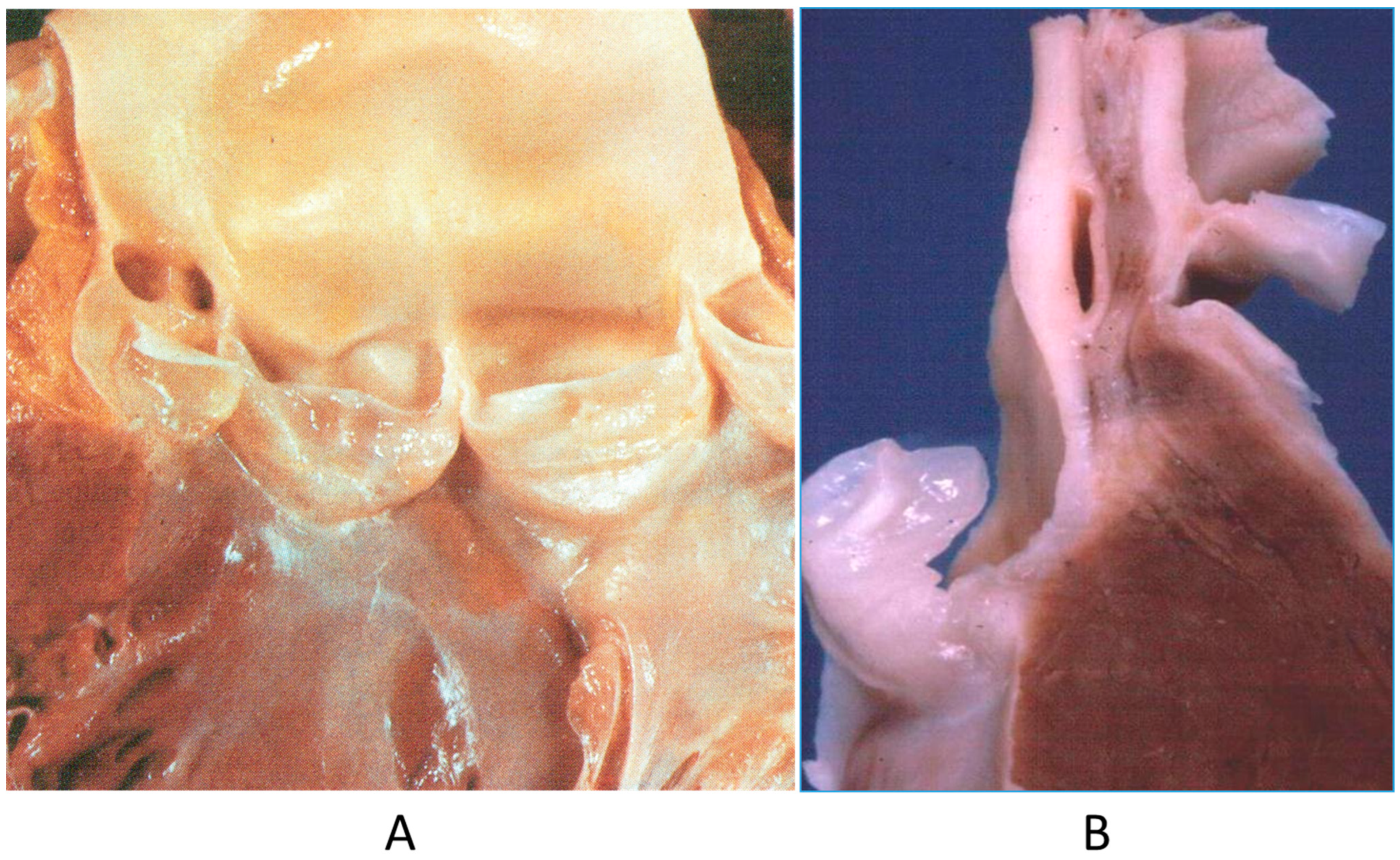
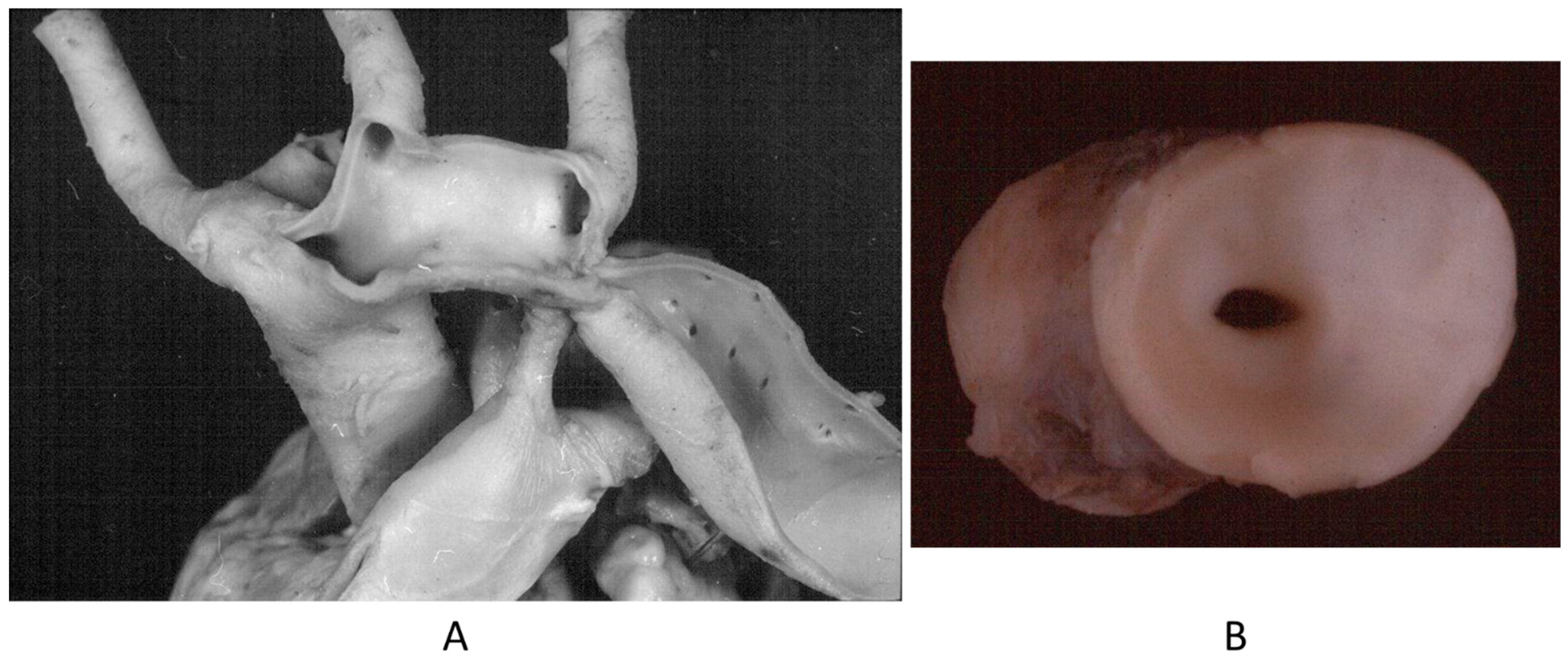
The origin of a coronary artery from the wrong aortic sinus (Figure 1A) is one of the most frequent causes of sudden death in the young, particularly in grown-up athletes (>18 years old) [15], precipitated by a strong effort. It is at risk of myocardial ischemia, because the first tract runs between the aorta and pulmonary artery, with the lumen flattened during systolic blood ejection. Moreover, the initial course may be intramural of the aortic wall (Figure 1B). High take-off from the aorta and the retroaortic course of the circumflex artery arising from the right coronary artery may also be life threatening, even late in adulthood, because of myocardial ischemia [15].
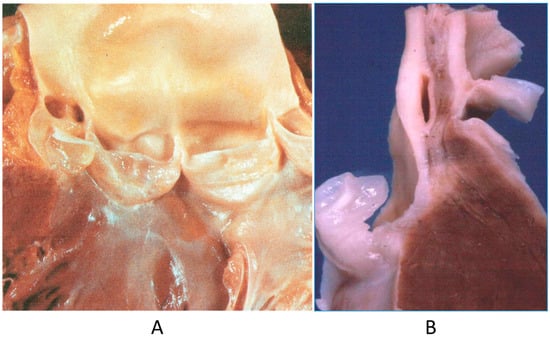
Figure 1.
(A) Both coronary arteries take the origin from the anterior left sinus of Valsalva, from a 22-year-old male who died suddenly during a soccer game. (B) The intramural aortic course of the anomalous artery [15].
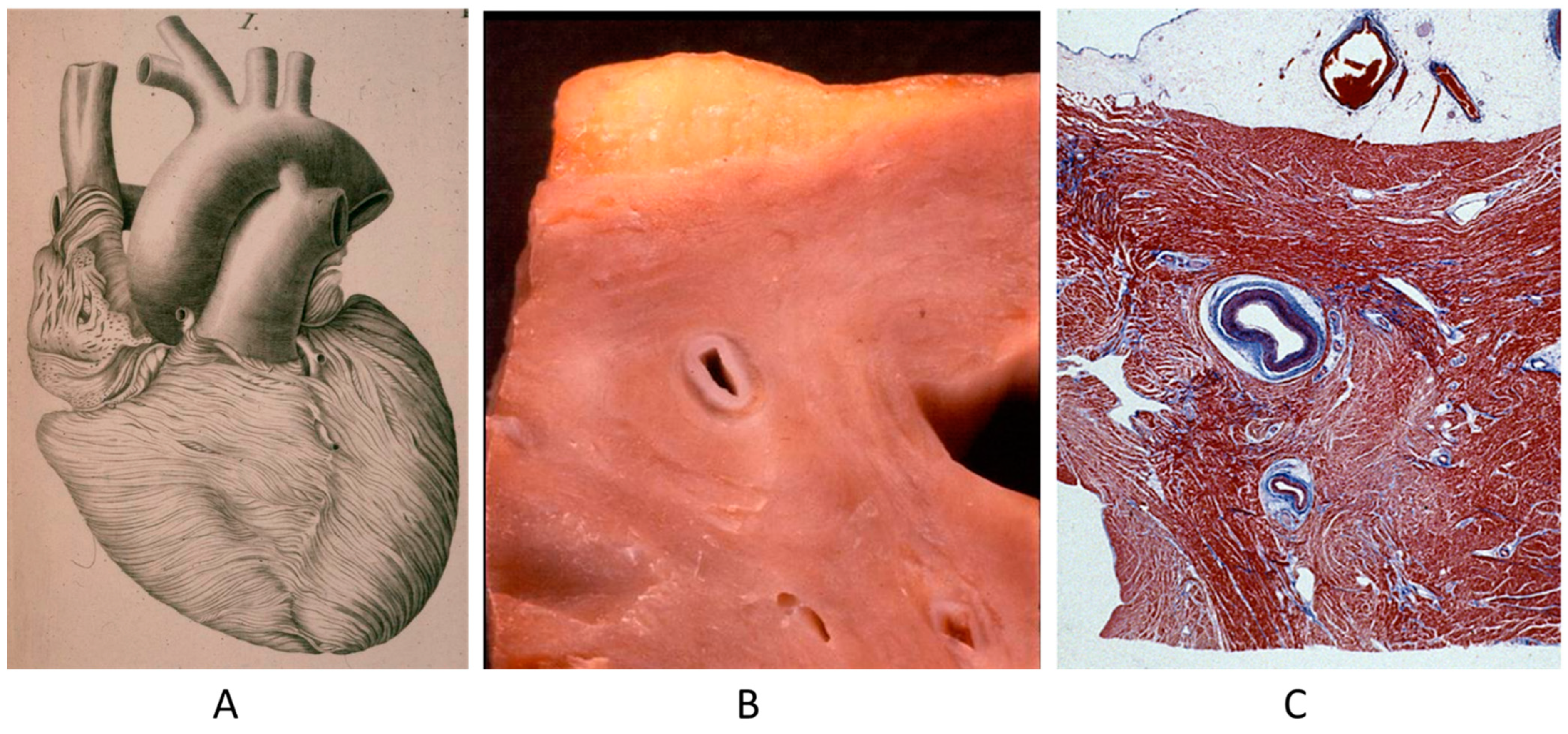
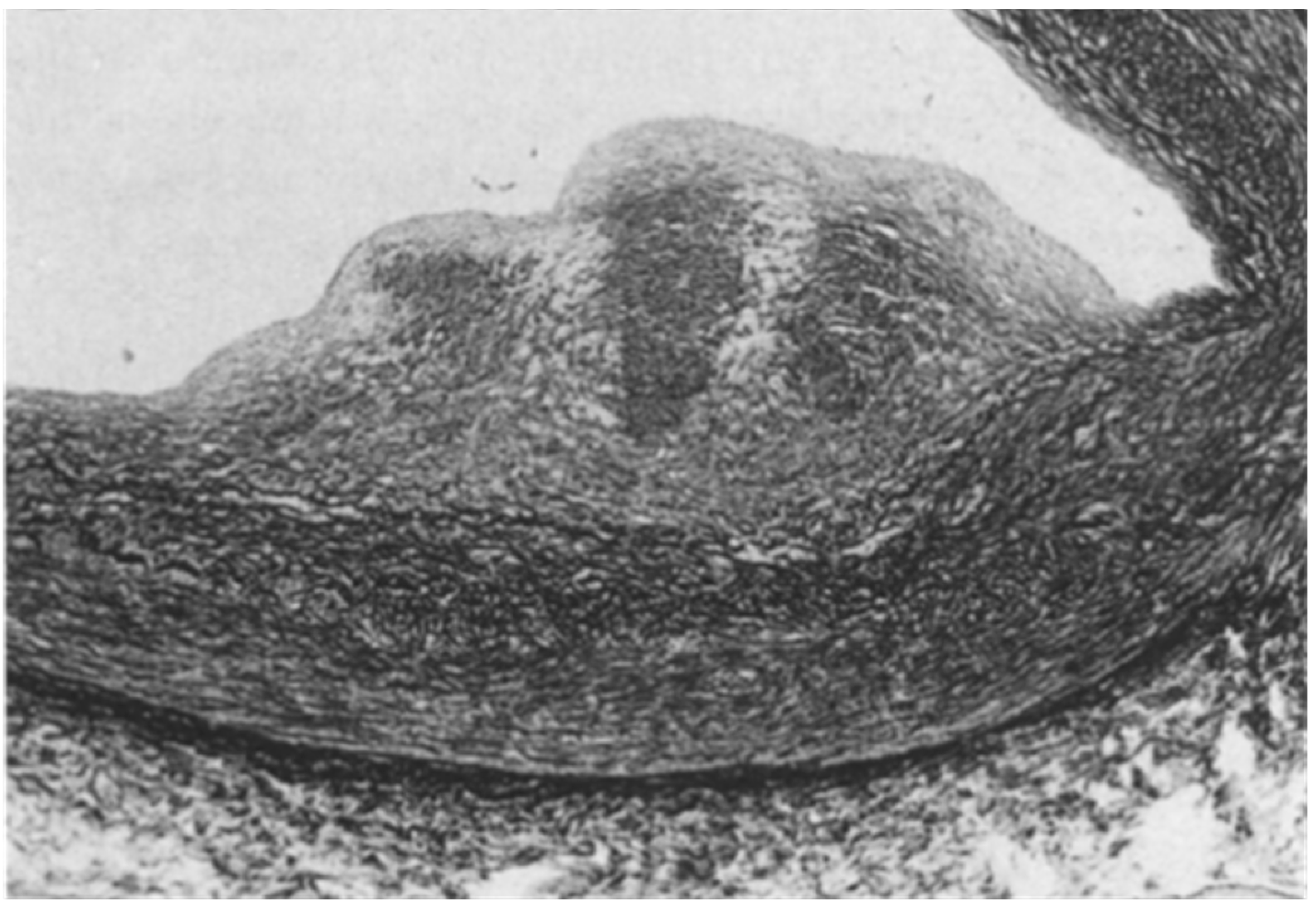
A controversial issue is the course of the descending coronary artery within the myocardium (Figure 2). It is so frequent as to be considered a variant of normal. The myocardium, covering the coronary segment, is known as a “bridge” (Figure 2A). It is a risky abnormality when the course is intramural, long and deep and when surrounded by a sleeve of myocardium prone to spasm (Figure 2C) [15]. Concealed coronary atherosclerosis may appear in childhood and become worrisome at early adulthood (Figure 3) [15].
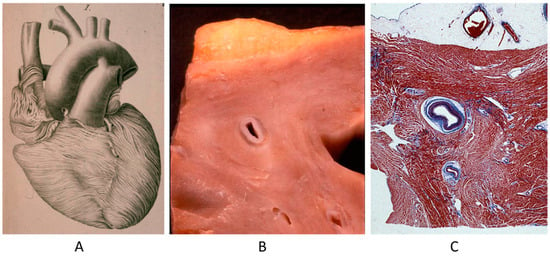
Figure 2.
Anomalous intramural myocardial course of the descending coronary artery. (A) Myocardial bridge. (B) Intramural course. (C) Sleeve of myocardium around the intramural coronary artery [15].
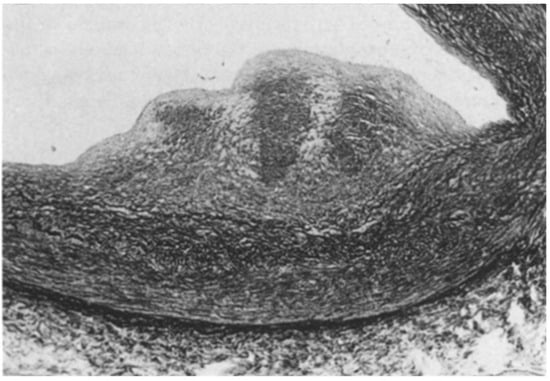
Figure 3.
Coronary artery atherosclerotic plaque in a 19-year-old boy.
3. Aorta and Pulmonary Artery
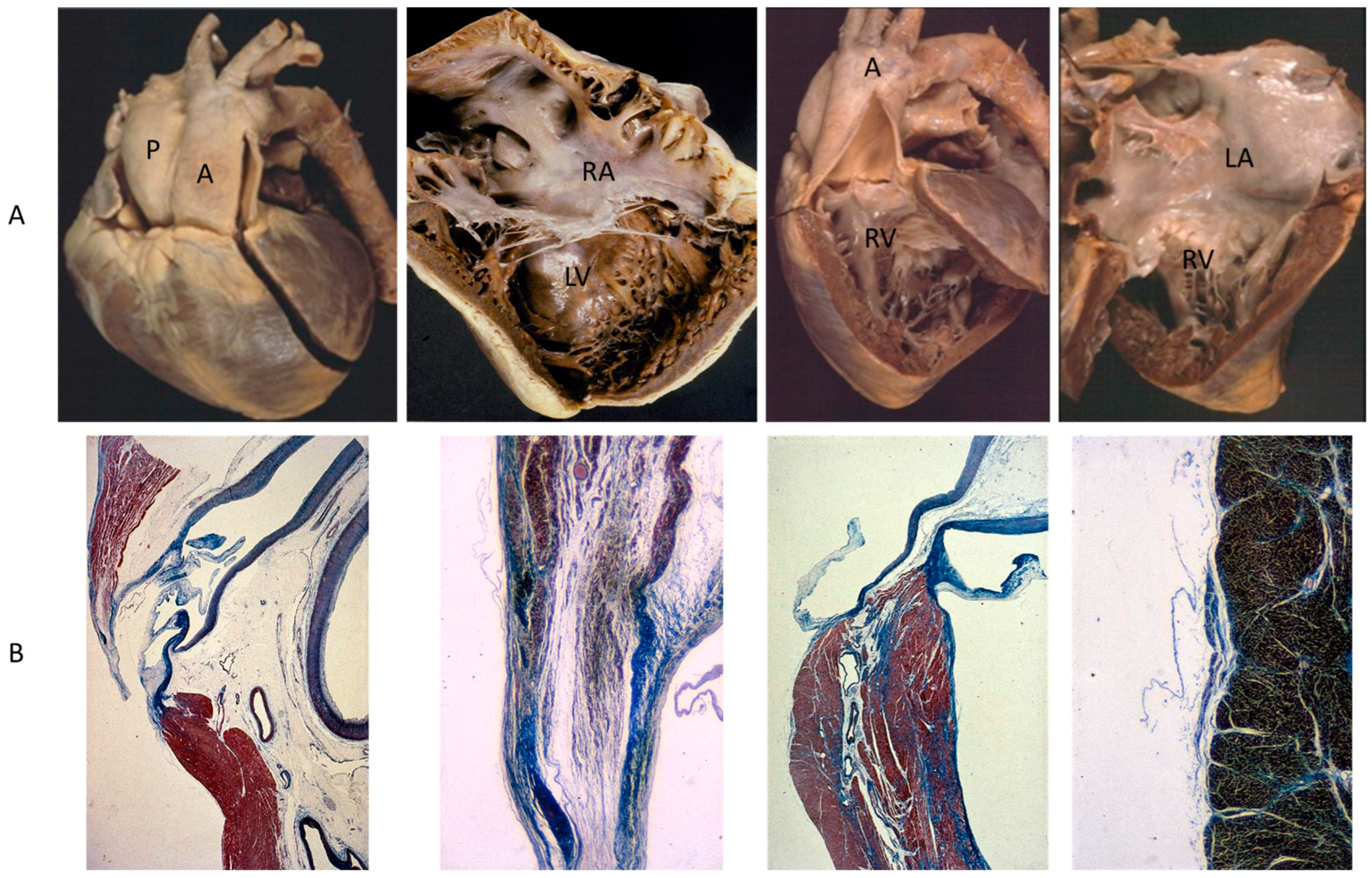
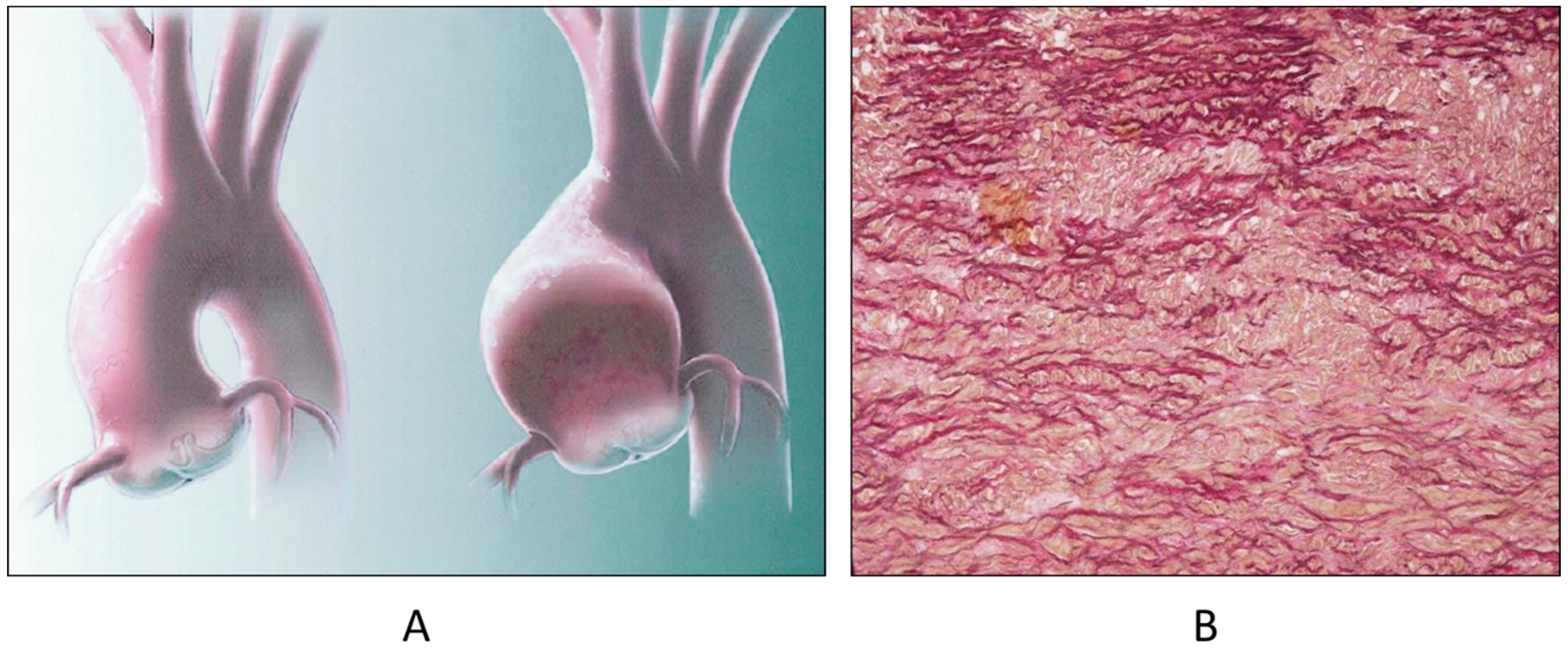
A rare CHD, reaching even the elderly, is corrected transposition of the great arteries (Figure 4A). The term “corrected” means “physiologically corrected” by atrioventricular and ventriculoarterial discordance in sequence, ensuring normal physiology of the blood stream (systemic and pulmonary circulations in series and not parallel, as in complete transposition) [16]. In this CHD, the AV conduction is located anteriorly with progressive fibrotic degeneration of the His bundle, putting the patient at risk of sudden death by AV block [17] (Figure 4B). Oddly enough, complete transposition may also exceptionally reach adulthood when associated with pulmonary stenosis and a ventricular septal defect.
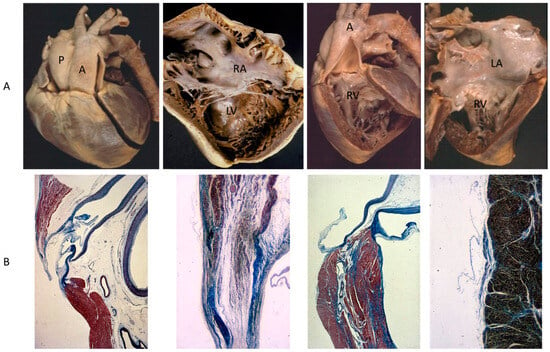
Figure 4.
(A) Corrected transposition with atrioventricular and ventricular arterial discordance. (B) The AV node is anteriorly located and the His bundle interrupted by fibrosis. From a 43-year-old female with sudden death by AV block. A = aorta; LA = morphologically left atrium; LV = morphologically left ventricle; P = pulmonary artery; RV = morphologically right ventricle, RA= morphologically right atrium.
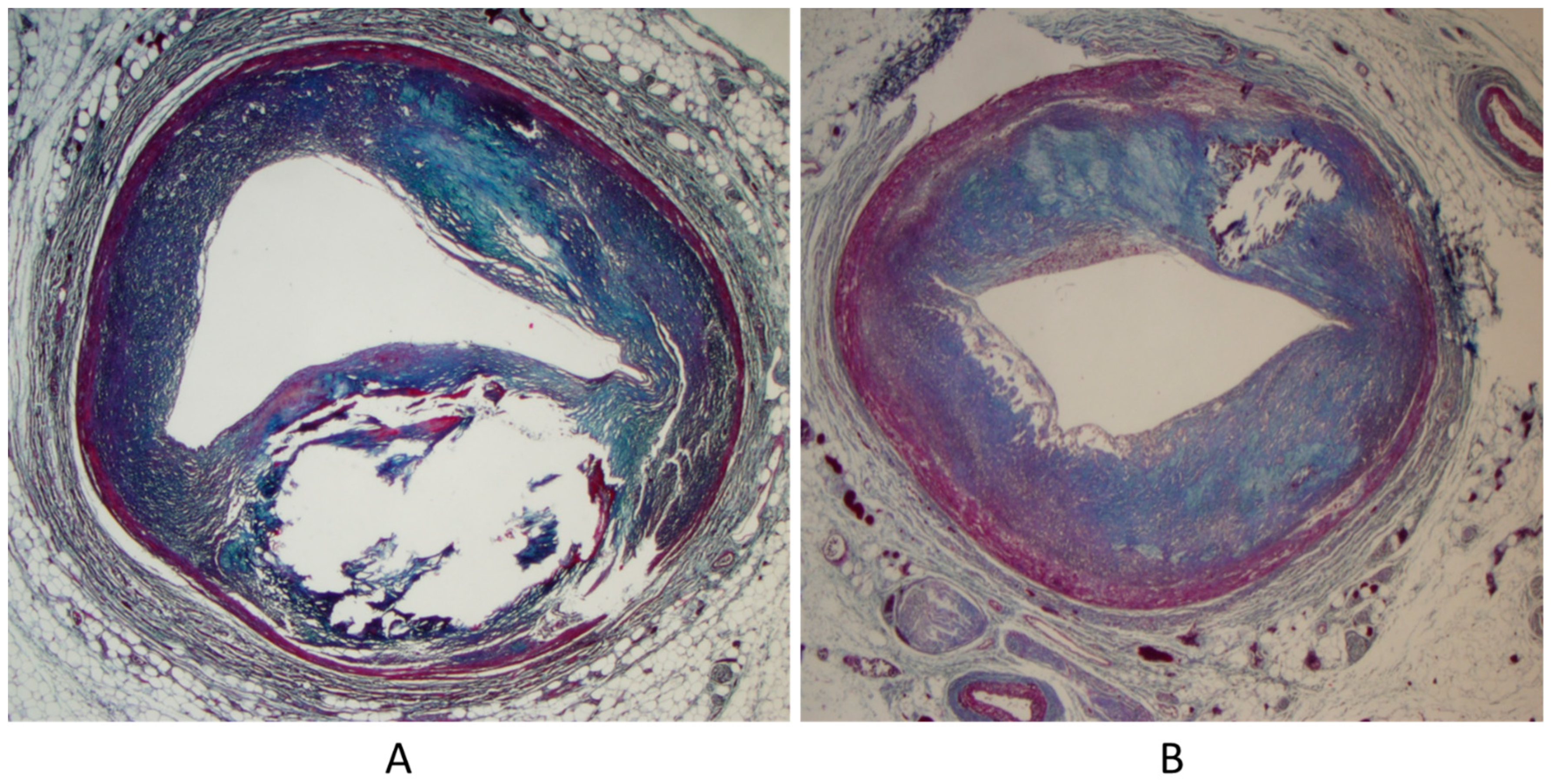
Another paucisymptomatic CHD is coarctation of the aortic arch (Figure 5), with ischemia of inferior limbs. It is at risk of lethal complications by aortic dissection because of proximal arterial hypertension and obstructive coronary atherosclerosis (Figure 6), which may trigger myocardial ischemia, myocardial infarction, and even sudden death. Moreover, acquired aneurysms of cerebral arteries in the Willis circuit can undergo rupture and fatal subarachnoid hemorrhage.
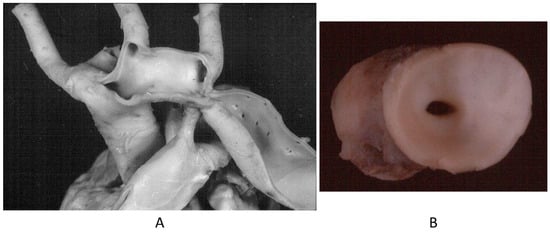
Figure 5.
Aortic coarctation (A,B) in adulthood.
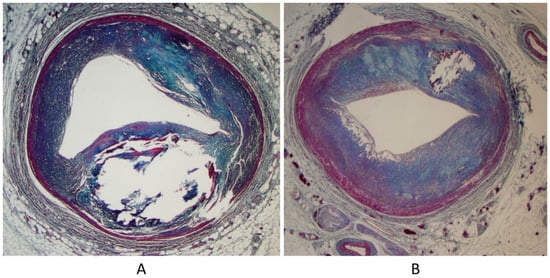
Figure 6.
Sudden death in aortic coarctation by obstructive coronary atherosclerosis (A,B).
4. Valves
The bicuspid aortic valve (BAV), the most frequent CHD, is not an innocent malformation, because of the occurrence of progressive dystrophic calcification with early aortic stenosis (Figure 7) and incompetence by dilatation of the sinusal ascending aorta, ascribable to aortopathy by Erdheim’s medionecrosis (Figure 8), at risk of dissection in the grown-up patient (Figure 9). The BAV syndrome may be inherited, requiring screening of the family for primary prevention [18]. Even the pulmonary valve may show an anomalous number of cusps, late observed in the elderly (Figure 10).
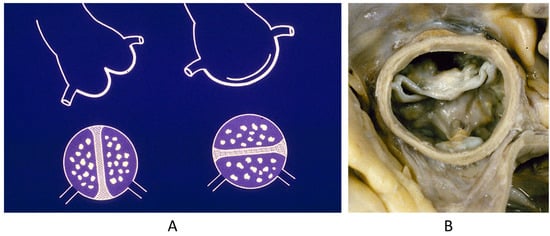
Figure 7.
Bicuspid aortic valve (A) with severe calcific stenosis (B) in a 65-year-old man [18].
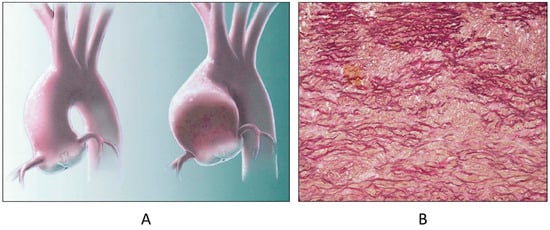
Figure 8.
Aortopathy associated with bicuspid valve with dilatation of sinusal ascending aorta (A) and elastic lamellae disruption in the aortic tunica media (B) [18].
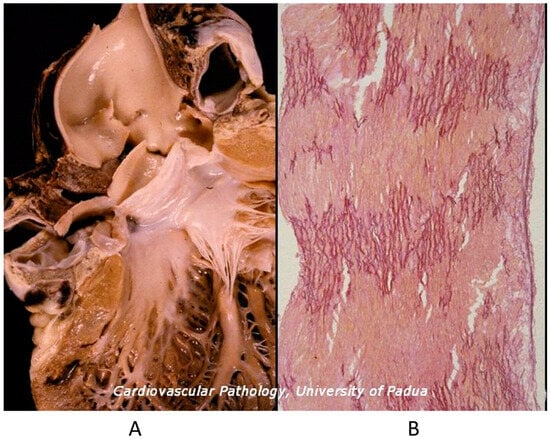
Figure 9.
Fatal dissecting aneurysm of the ascending aorta in bicuspid aortic valve (A) with disappearance of elastic lamellae in the tunica media (B) [18].
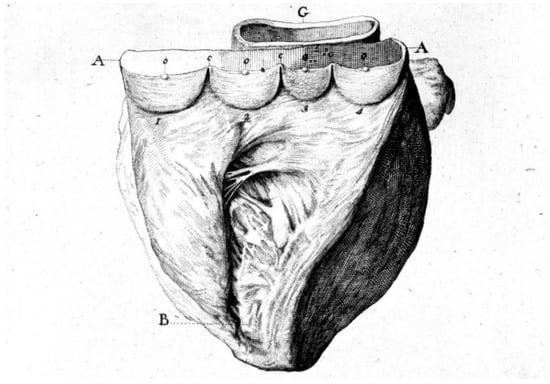
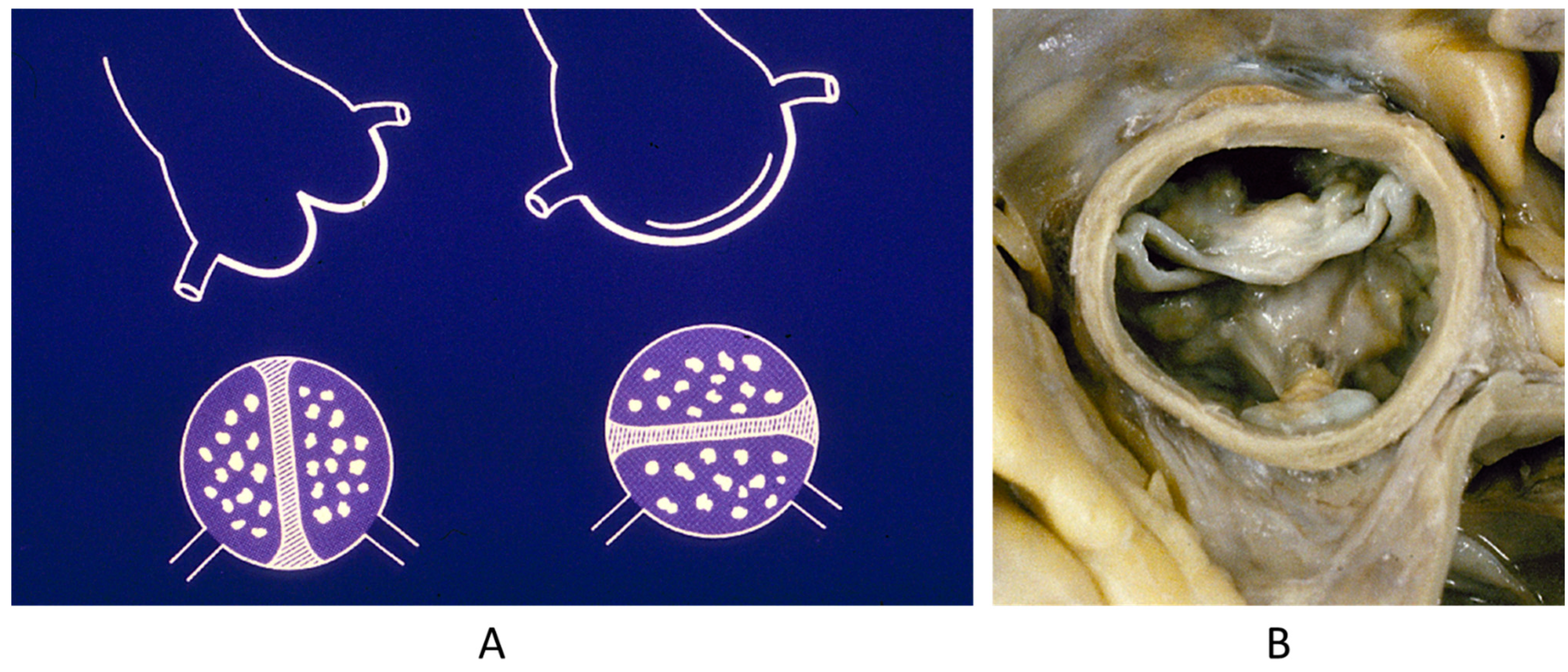
Figure 10.
A quadricuspid pulmonary valve incidentally observed in a 70-year-old patient. A = quadricuspid pulmonary valve, B = right ventricle, C = Aorta.
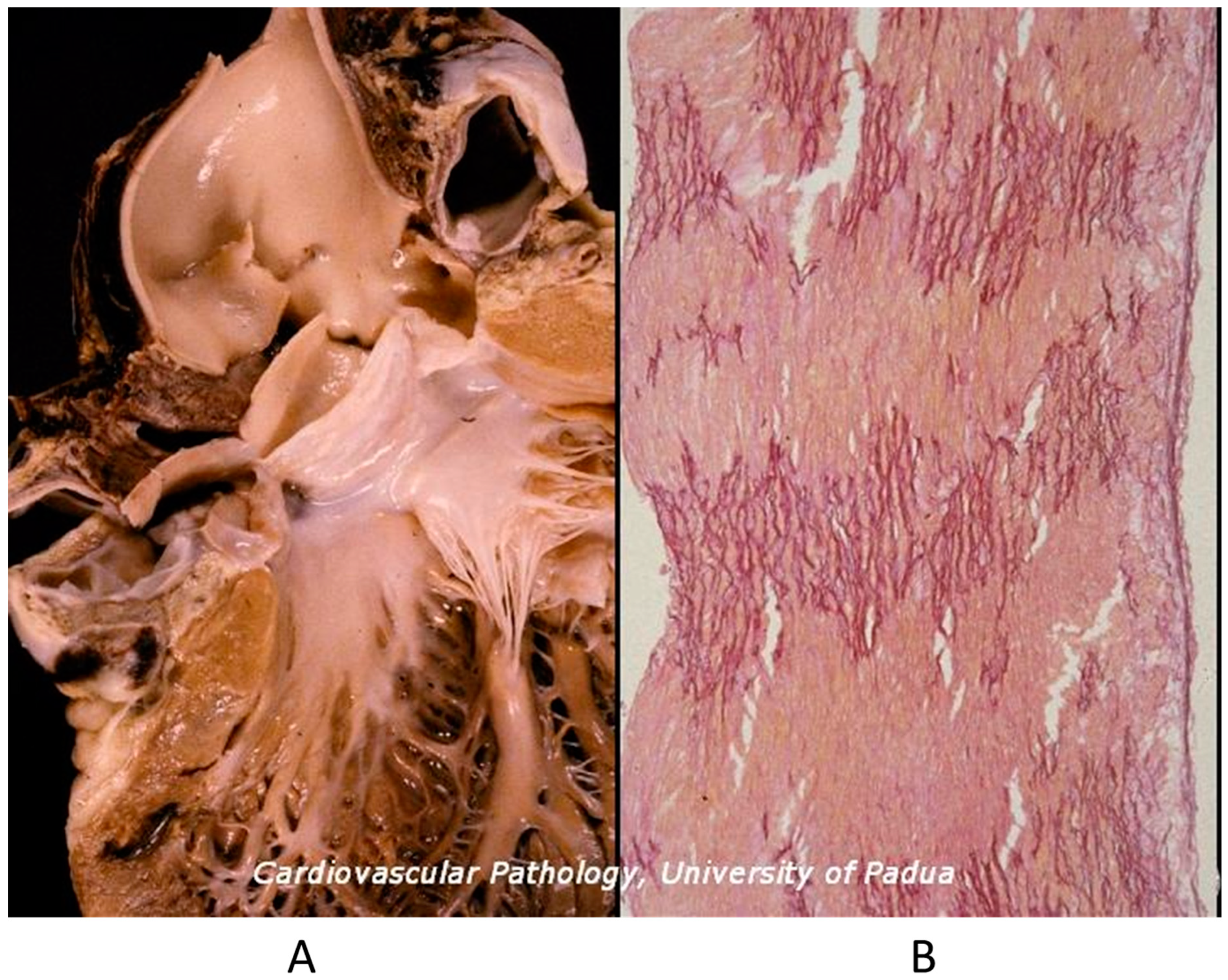
Mitral valve prolapse is a CHD because of the disjunction of the atrial and ventricular myocardium at the annulus insertion (Figure 11) [19]. In this setting, the billions of ventricular systoles which close the valve orifice during life account for traumatic injury of the leaflets with myxoid degeneration, mitral prolapse, and incompetence, as well as fibrosis of the papillary muscles and ventricular myocardium, which is an arrhythmic substrate placing the patient at risk of sudden death [19].
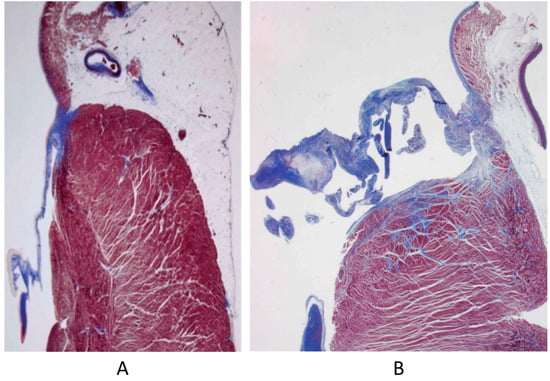
Figure 11.
Normal mitral annulus (A). Mitral annulus with annular disjunction (B) [19].
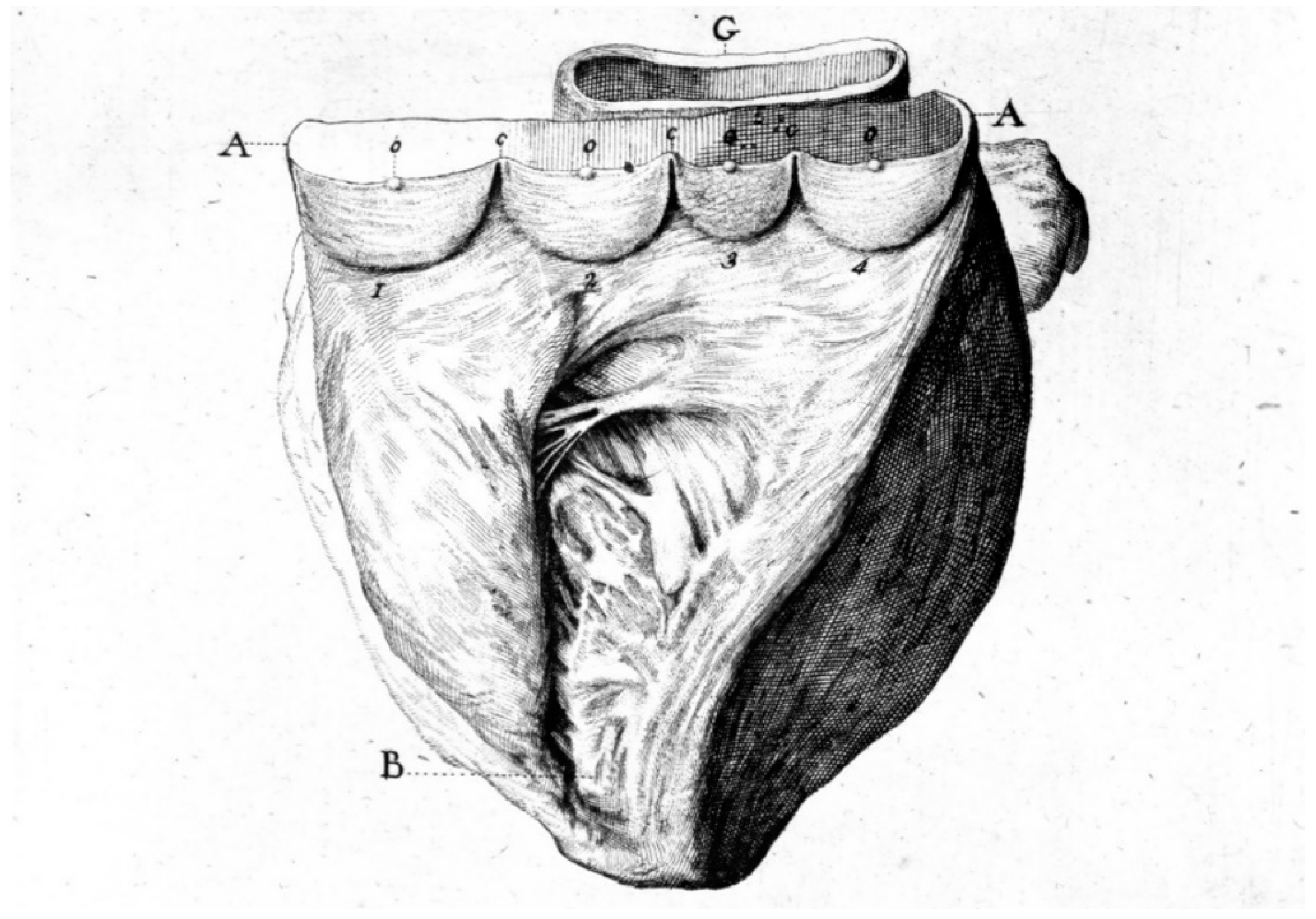
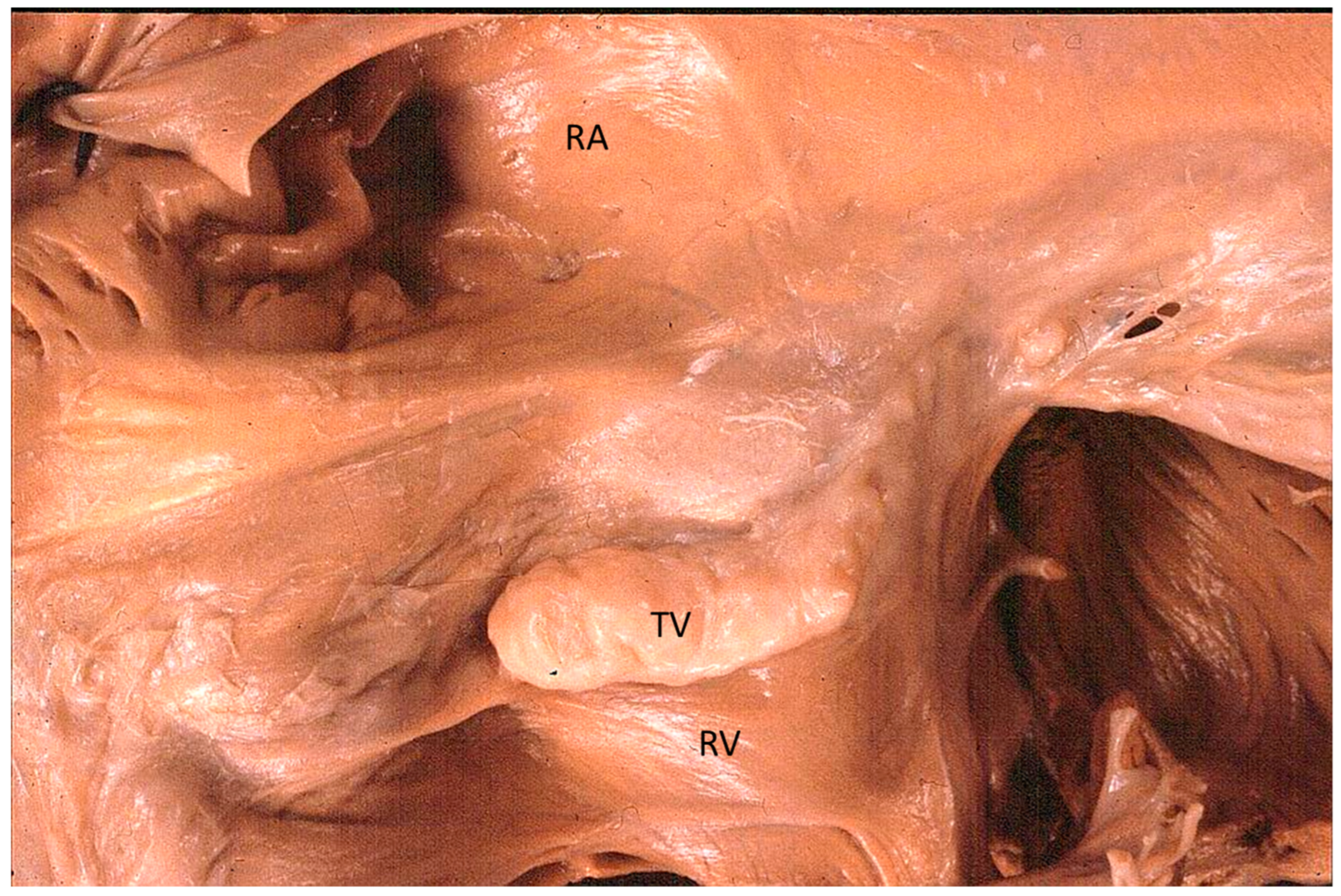
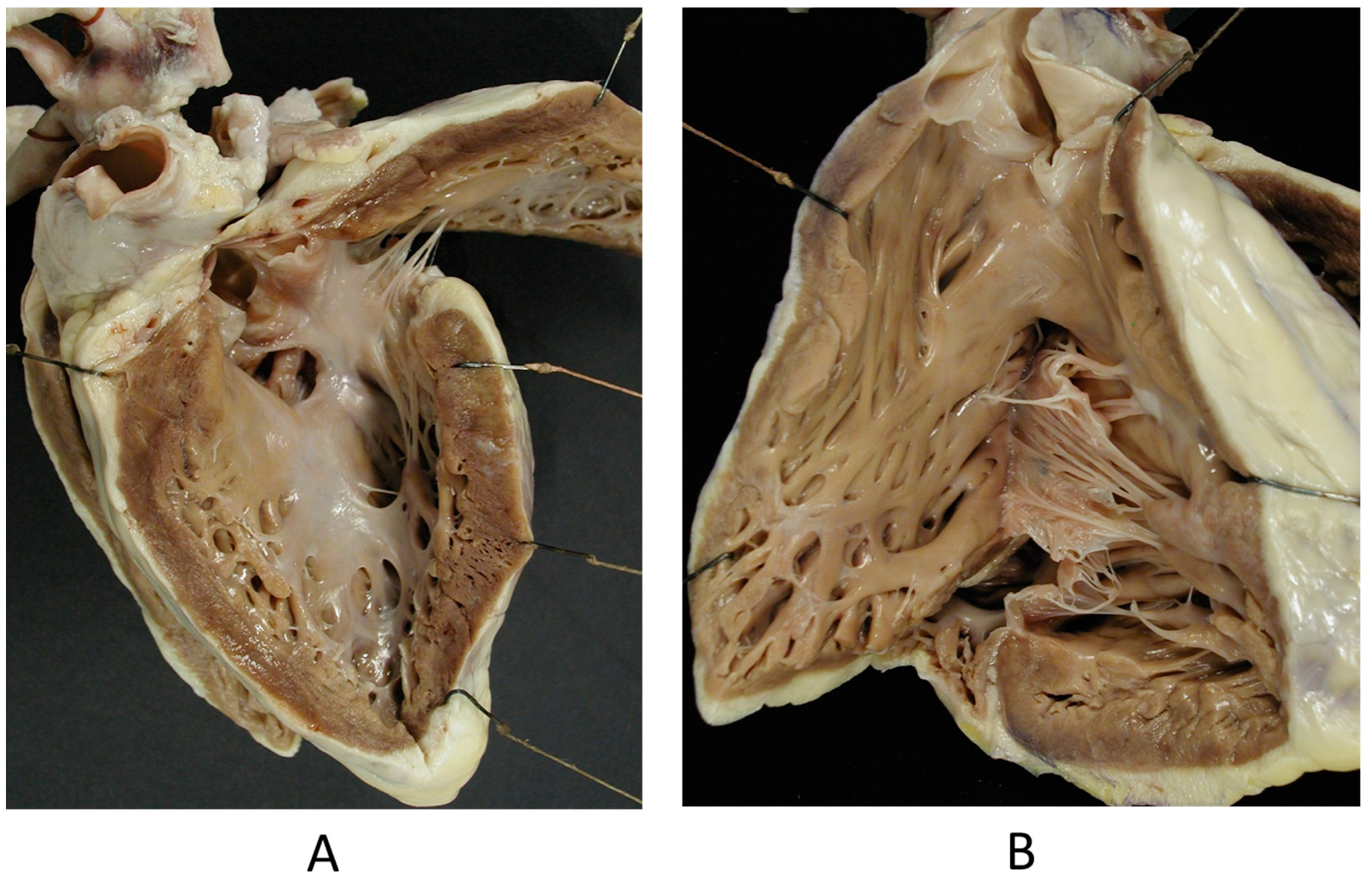
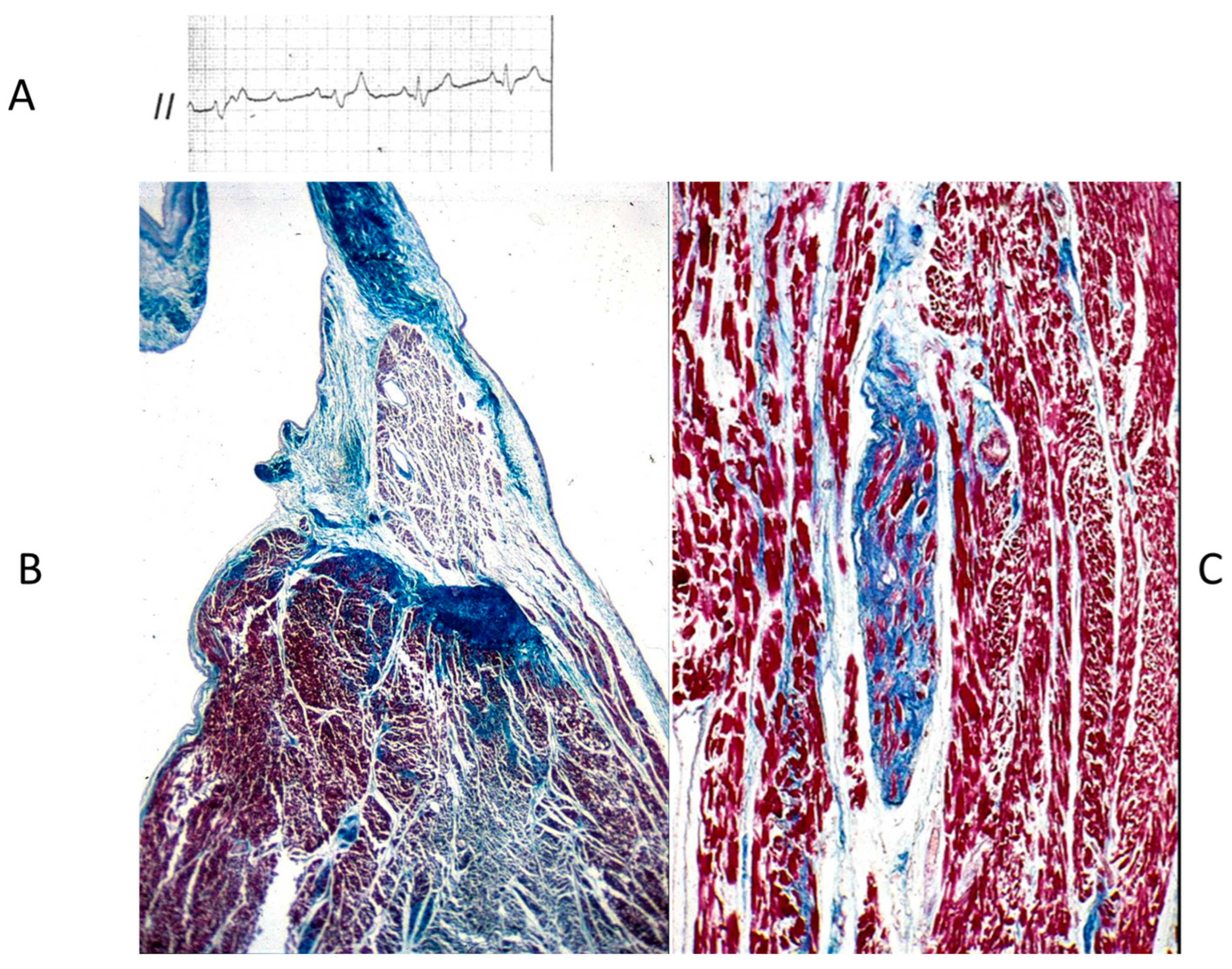
The Ebstein anomaly [20,21,22], with lowering of the leaflets and incompetence of the tricuspid valve (Figure 12 and Figure 13), is associated with myocardial dystrophy of the right ventricle free wall (Figure 14) and with ventricular preexcitation (Figure 15).
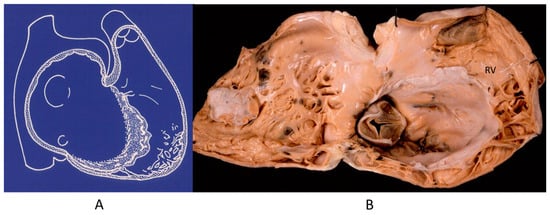
Figure 12.
Ebstein anomaly with downward displacement of the tricuspid valve leaflets (A). Note a porcine bioprosthetic valve in place of the tricuspid valve (B) [9]. RV = right ventricle [20].
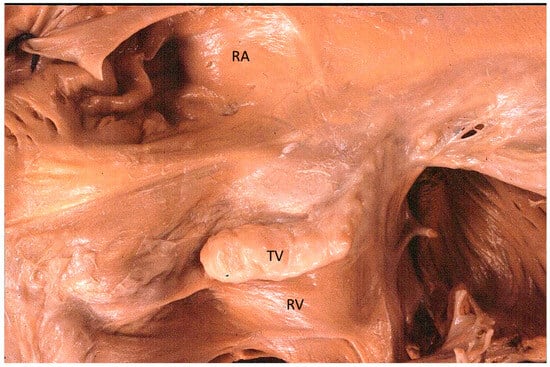
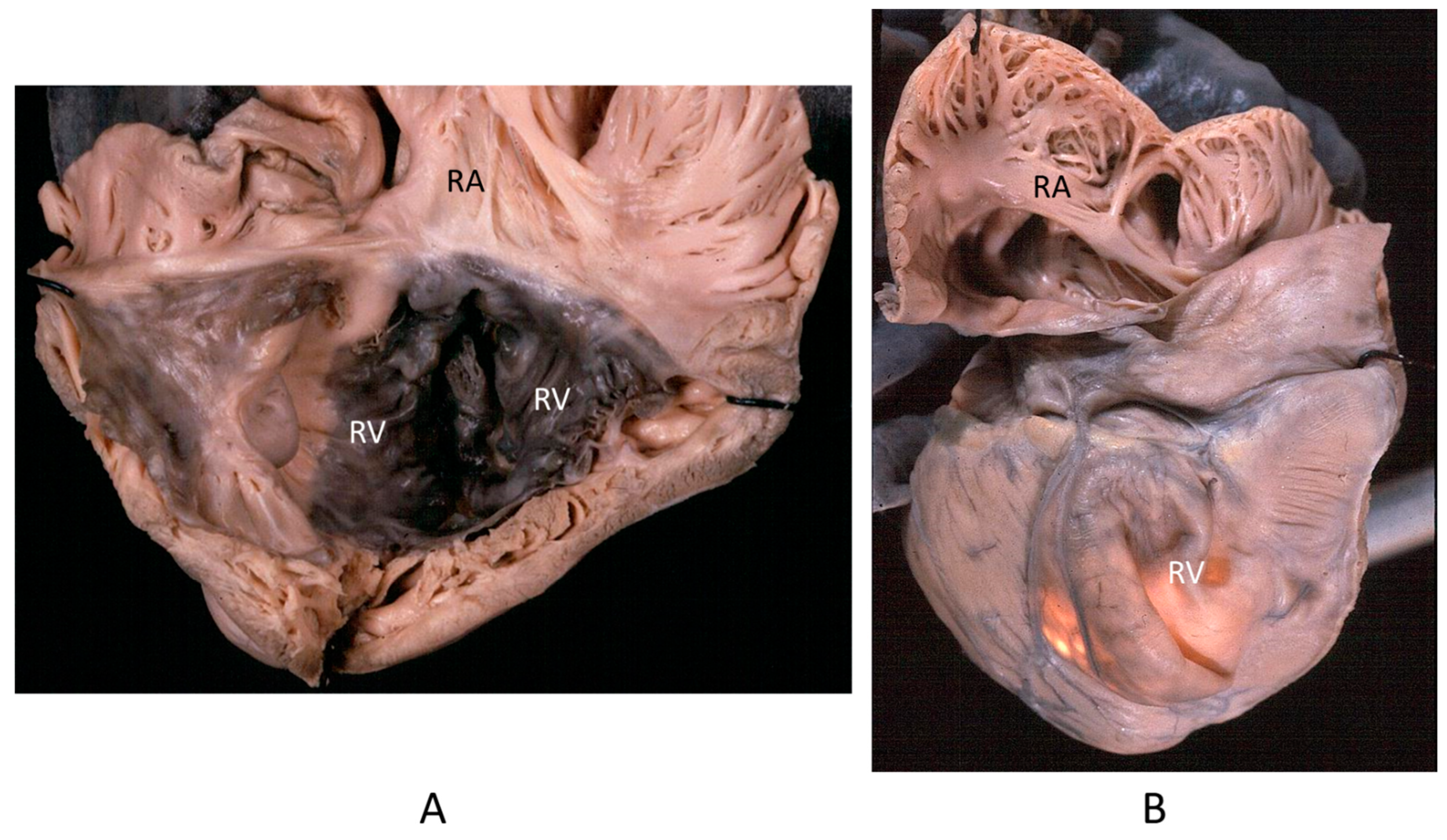
Figure 13.
Other examples of the Ebstein anomaly, with the septal leaflet of the tricuspid valve downward displaced in an adult woman [20]. RA = right atrium; RV = right ventricle; TV = tricuspid valve [20].
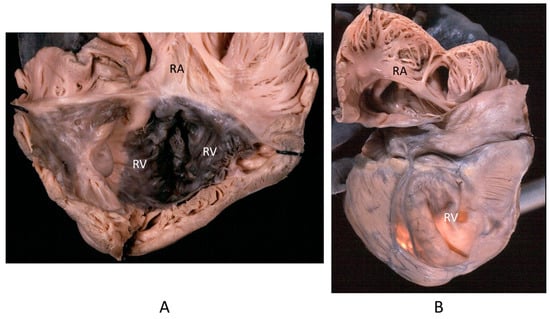
Figure 14.
Aneurysm by dystrophy of the right ventricular wall (B) in an adult patient with Ebstein anomaly of the tricuspid valve (A). RA = right atrium; RV = right ventricle.
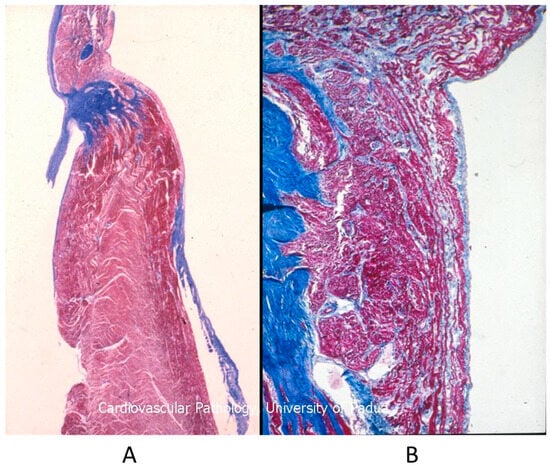
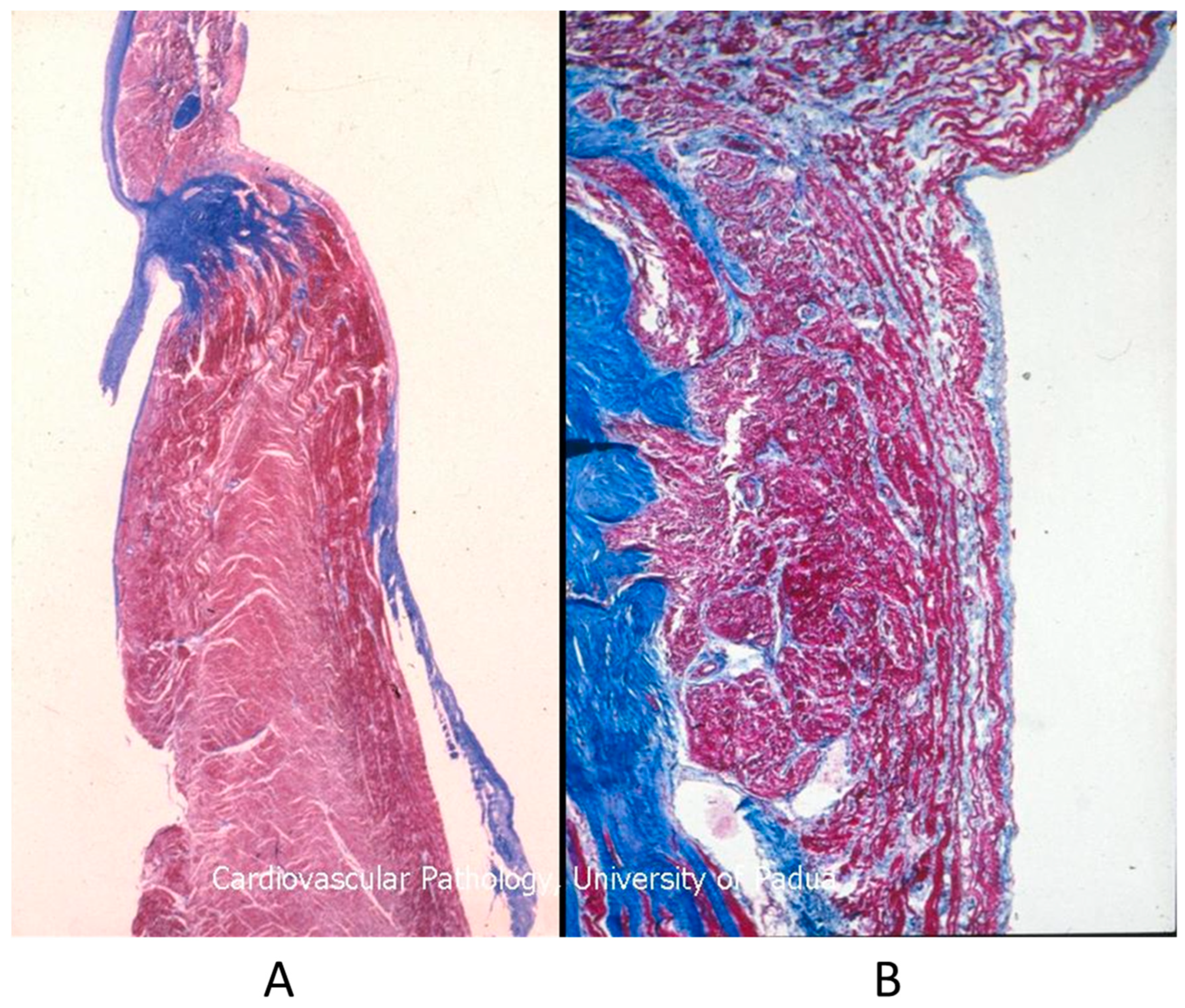
Figure 15.
Histology of Ebstein anomaly with displaced septal leaflet of the tricuspid valve (A) and Kent septal fascicle (B) in a young person who died suddenly [20].
5. Myocardium
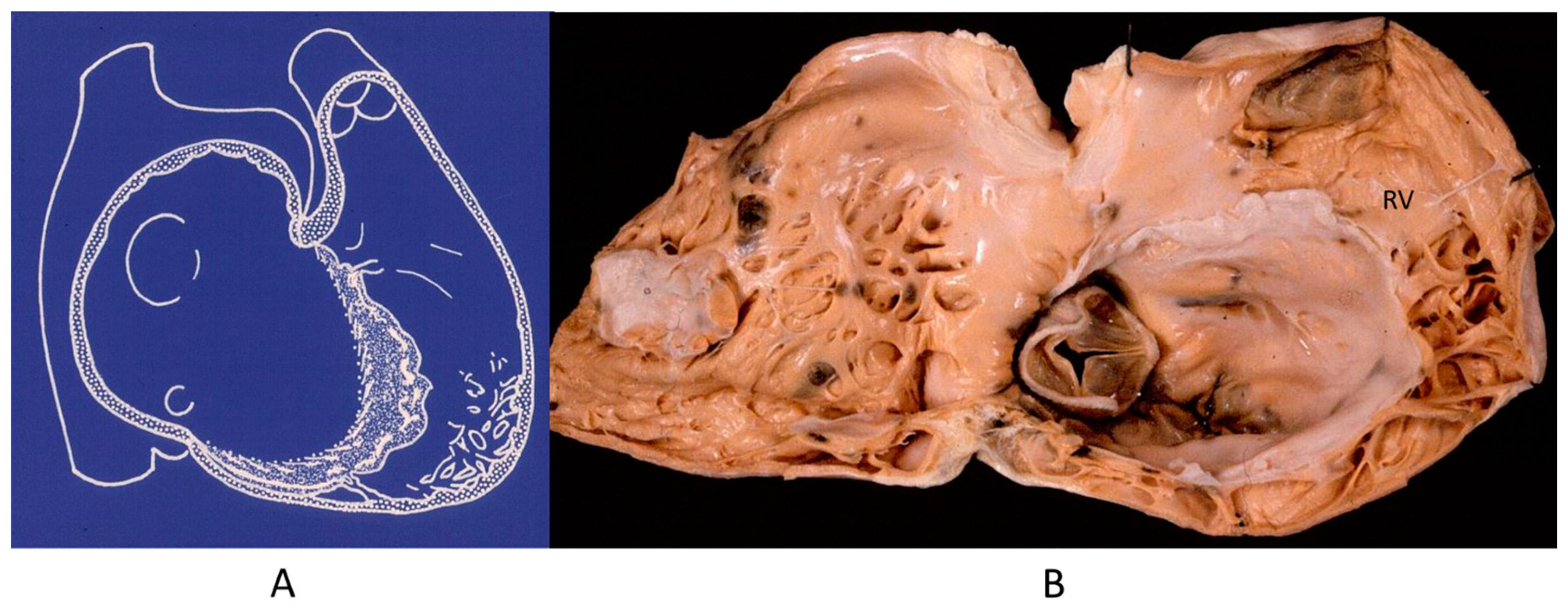
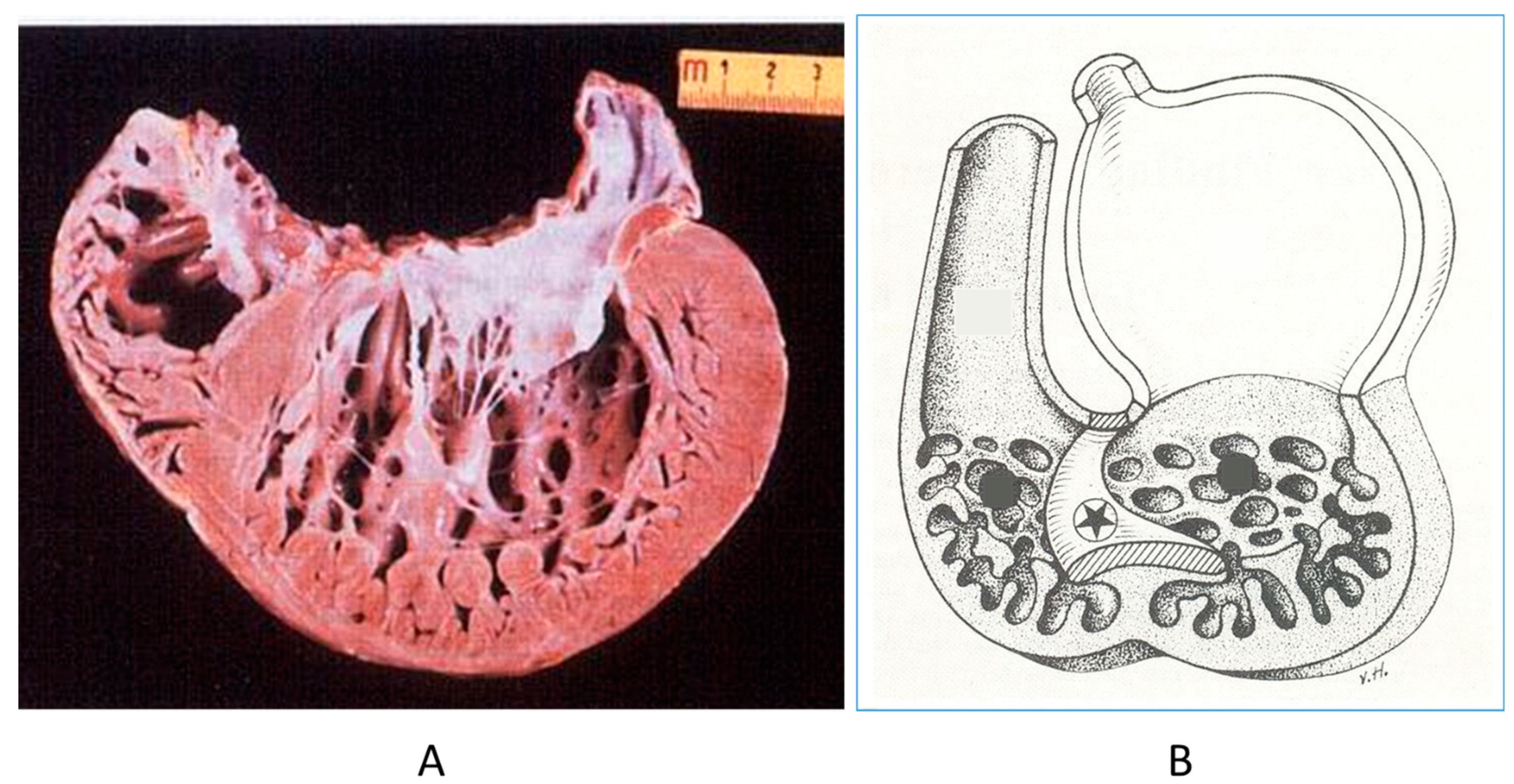
The noncompacted left ventricle (Figure 16) is a CHD due to embryological failure of ventricular myocardium compaction. It accounts for progressive ventricular dilatation and severe congestive heart failure, mimicking dilated cardiomyopathy because of the scarcity of contractile myocardium. The fissures in between the trabeculae are so deep that the endocardium may reach the epicardium. Moreover, the trabeculae are so big as to be erroneously interpreted as mural thrombi of a dilated cardiomyopathy by 2D echo.
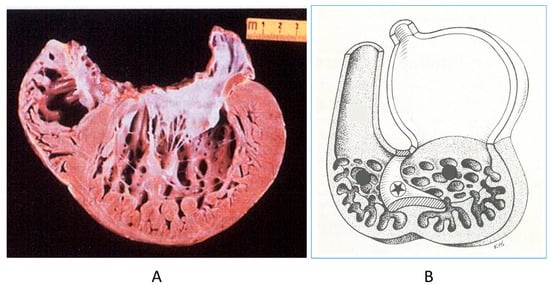
Figure 16.
A gross view of a heart specimen with biventricular noncompaction (A) and drawing of noncompacted ventricles (B).
6. Septal Defects
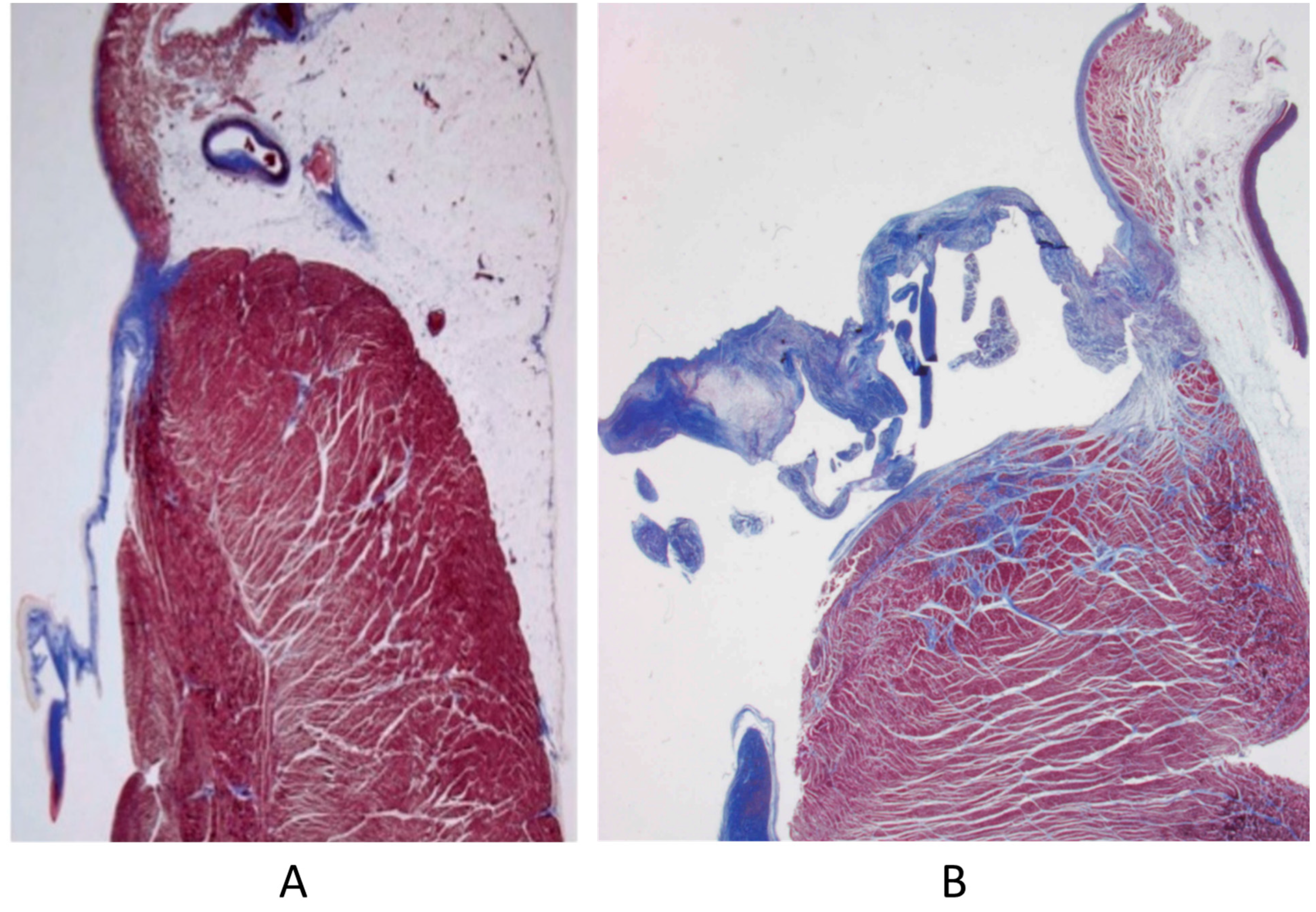
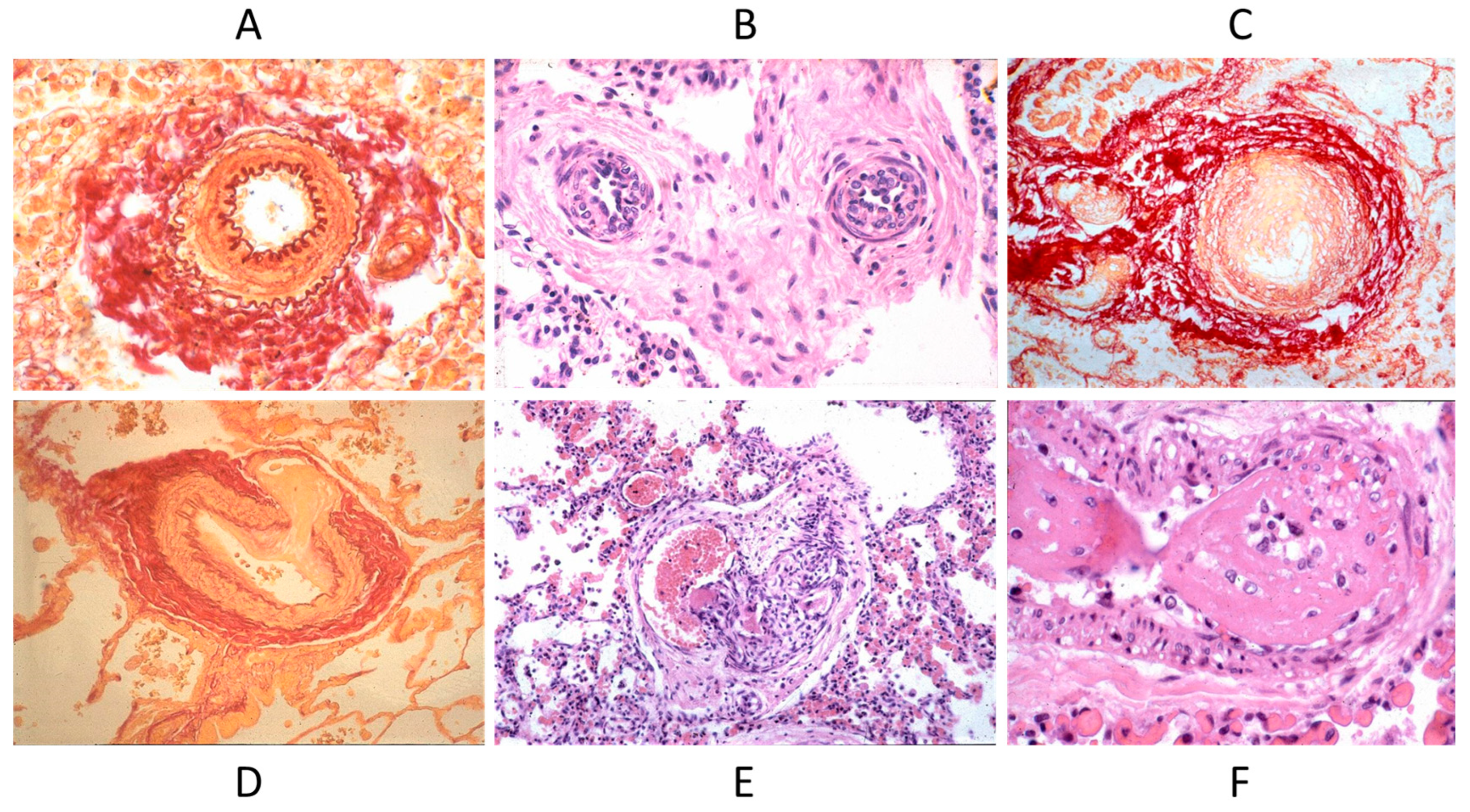
Septal defects with a left to right shunt, like an atrial septal defect, ventricular septal defect, AV canal, truncus arteriosus, aorto-pulmonary window, and patent ductus arteriosus, may persist until adulthood if not diagnosed and repaired early by surgery or interventional cardiology. They can be complicated with pulmonary hypertension by pulmonary vascular diseases (Figure 17), with the appearance of cyanosis because of an inverted shunt from right to left, as in Eisenmenger syndrome (Figure 18) [23,24].
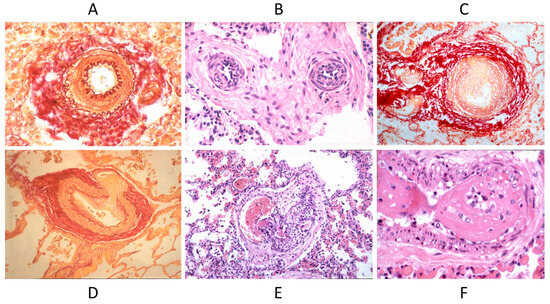
Figure 17.
Pulmonary vascular disease in Eisenmenger syndrome with obstructed lumen (A–C) and aneurysm (D), glomerular-like proliferation (E), and fibrinoid necrosis (F).
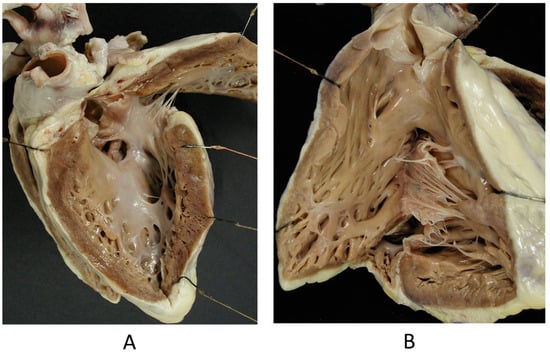
Figure 18.
Eisenmenger complex in 20-year-old boy with biventricular origin of the aorta (A), without pulmonary stenosis (B).
7. Conduction System
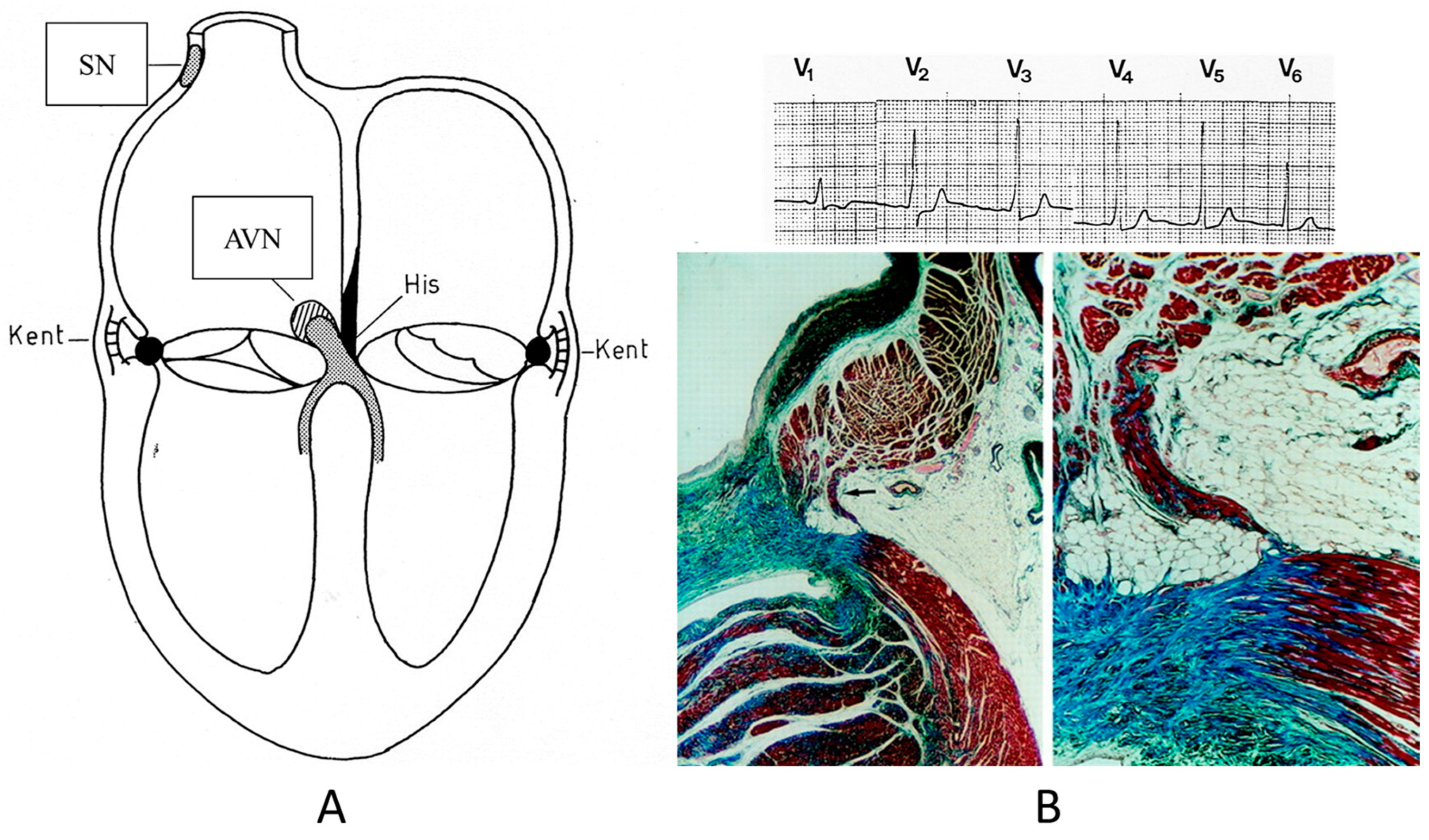
Concerning CHDs of the conduction system in adulthood, ventricular preexcitation (VPE) represents the smallest malformation, consisting of a fascicle of ordinary myocardium (1–2 mm thick) known as the Kent fascicle. It is usually located in the lateral rings (Figure 19A). It connects the atria to the ventricles (Figure 19B) and is bare of the decremental properties of the Tawarian System, which the opposite, as it is located in the central AV septum and consists of specialized conducting tissue endowed with decremental properties.
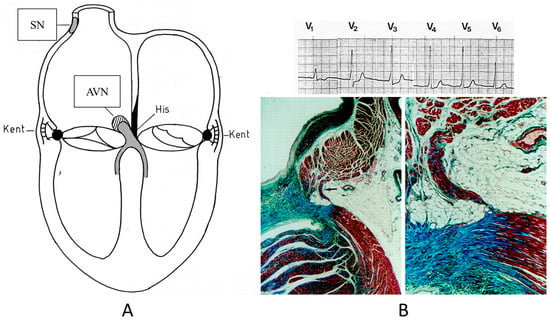
Figure 19.
Schematic representation of ventricular preexcitation with Kent fascicle in the lateral atrio-ventricular rings (A). Histology of Kent fascicle and electrocardiogram with delta wave (B). SN = sinus node, AVN = atrio-ventricular node.
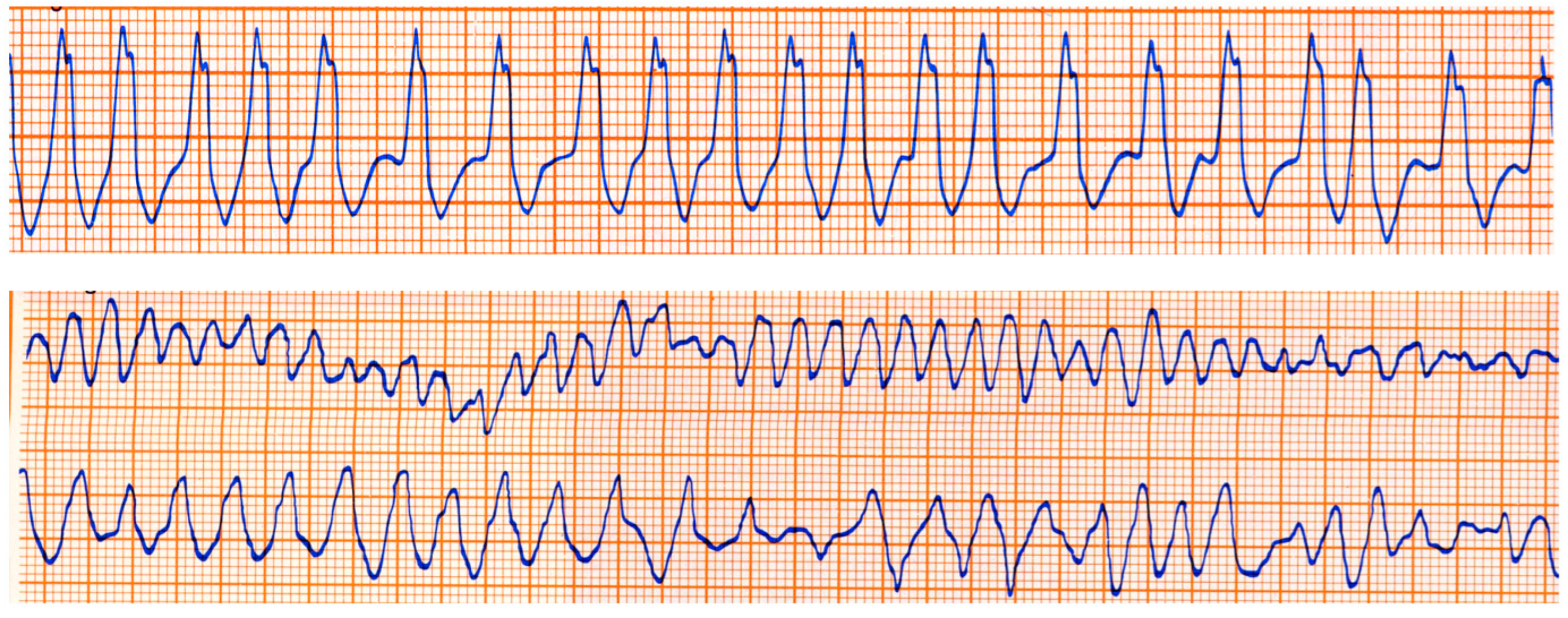
The Kent fascicle accounts not only for VPE but also for atrial reentry with palpitations by supraventricular tachycardia. In the case of an atrial fibrillation burst (usually about 500 beats per minute), because of the absence of a slowing-down of the electrical impulse, a step one-to-one transmission to the ventricles may trigger a lethal ventricular fibrillation (Figure 20).
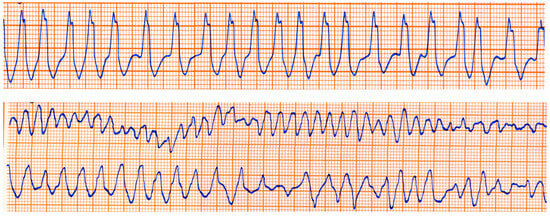
Figure 20.
Sudden death in Wolff Parkinson White syndrome: atrial fibrillation turns into ventricular fibrillation.
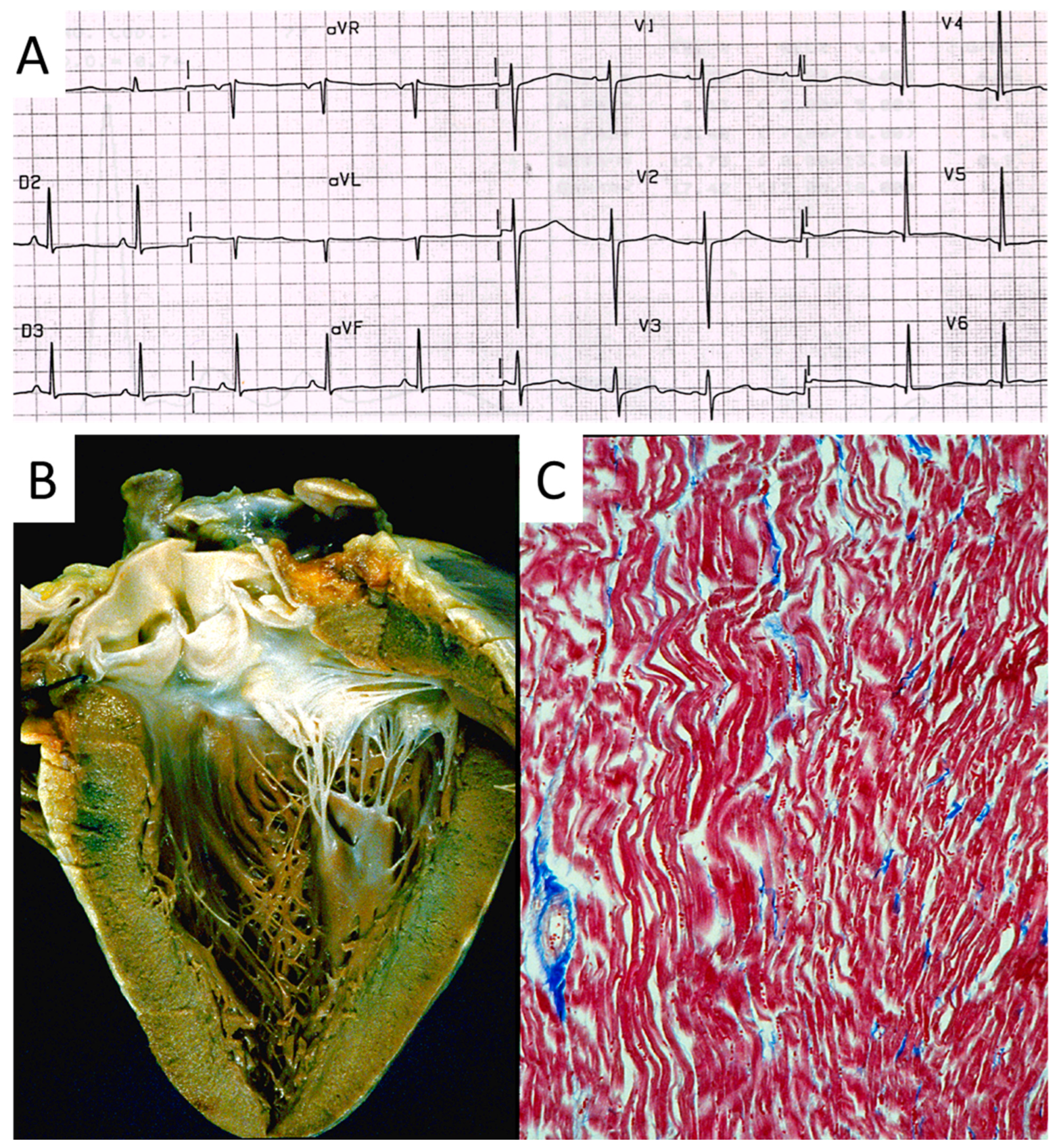
Also, progressive AV block (Figure 21), usually manifesting in in elderly patients, may be genetically determined due to a mutation of the SN5 (Sodium channel five) gene.
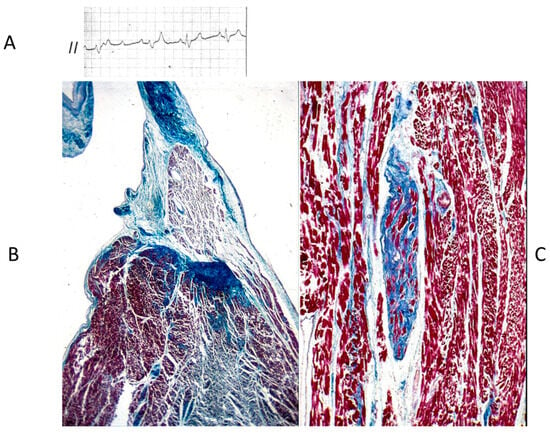
Figure 21.
Electrocardiogram with AV block (A). At histology, bifurcation of His bundle with fibrous interruption of the left bundle branch (B) and the right bundle branch (C) were observed [15].
8. Should Genetically Determined Cardiovascular Diseases Be Considered Congenital Heart Diseases? A Nosological Problem
Different from most of the so-called CHDs (90% due to embryological maldevelopment) are cardiac and vascular diseases that are genetically determined in the code of life.
The genetic defect of channelopathies, like simple missense mutation, lies in the double helix of the DNA and is usually transmitted at the time of conception but may manifest phenotypically with growing up. Should channelopathies be termed CHDs? Should genetically determined cardiomyopathies (like hypertrophic, dilated, restrictive, and arrhythmogenic ones) be considered CHDs as well? Why not?
Fibroelastosis is the consequence of a viral infection (mumps) during fetal life. Is it a CHD?
As for birth defects, Wikipedia states, “A birth defect is an abnormal condition that is present at birth, regardless of its cause. [...] Birth defects are divided into two main types: structural disorders, in which problems are seen with the shape of a body part, and functional disorders in which problems exist with how a body part works. Some birth defects include both structural and functional disorders”. Congenital functional disorders may be genetically determined and as such they are CHDs.
Genetic defects of DNA may manifest after infancy or childhood. Is a wrong molecular sequence of DNA (even a small missense mutation) enough to be called a CHD? Are babies with a channelopathies-like long QT affected by a CHD? (See Figure 22). The substrate of channelopathies is molecular and not anatomic–structural, as seen earlier in the definition of CHDs. However, molecular genes code wrong amino acids and proteins, which are material rather than dysfunctional.
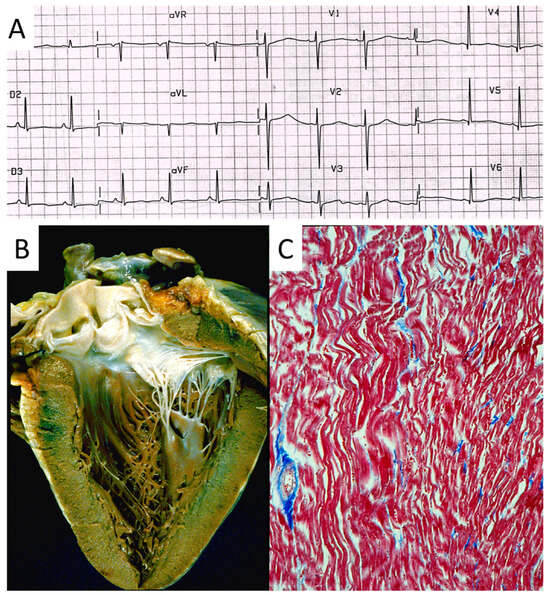
Figure 22.
Sudden death in a 20-year-old young adult with long QT in an electrocardiogram image (A). The heart is normal in gross (B) and histology (C) views [15].
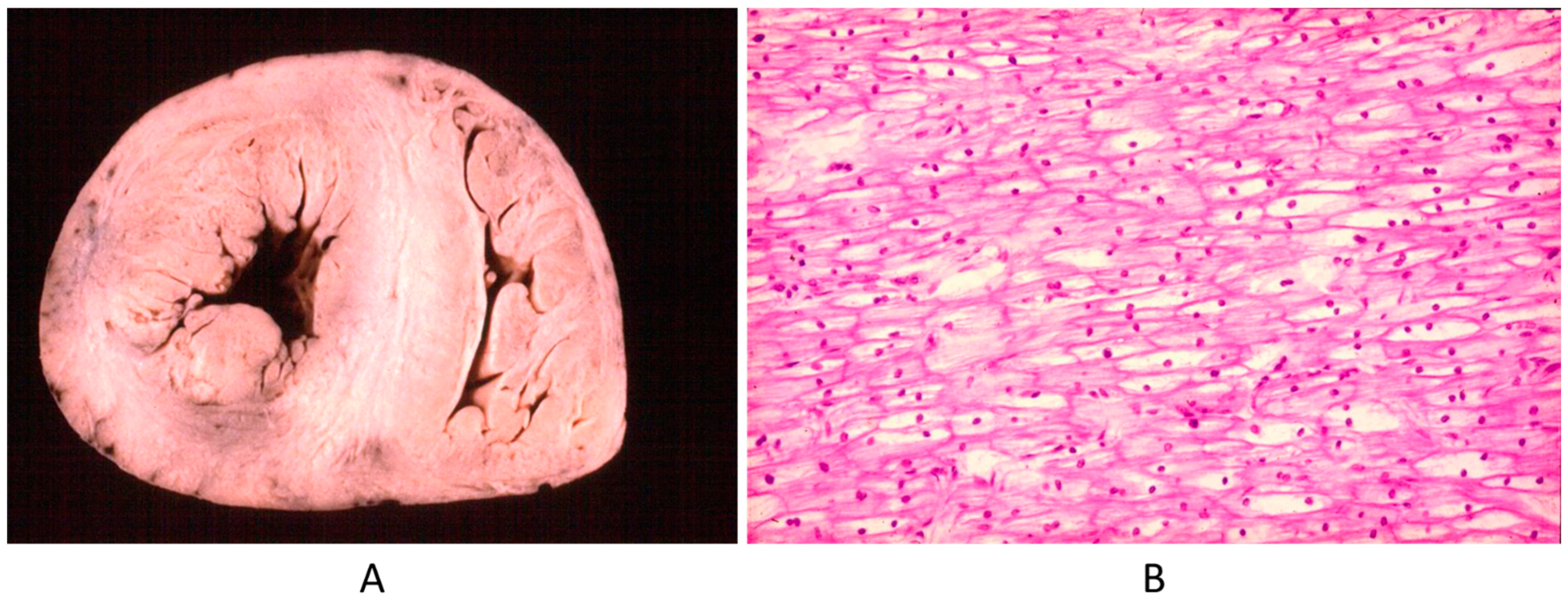
Another example is Pompe disease (glycogenosis with a big heart at birth by intracellular glycogen storage by gene defect) (Figure 23). Why is this not a CHD? It is clearly a structural defect present at birth, in agreement with the actual CHD definition.
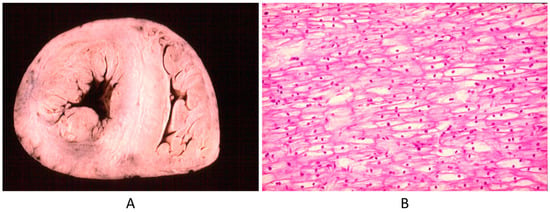
Figure 23.
Big heart (A) by glycogenosis at histology (B) (Pompe disease) in an infant.
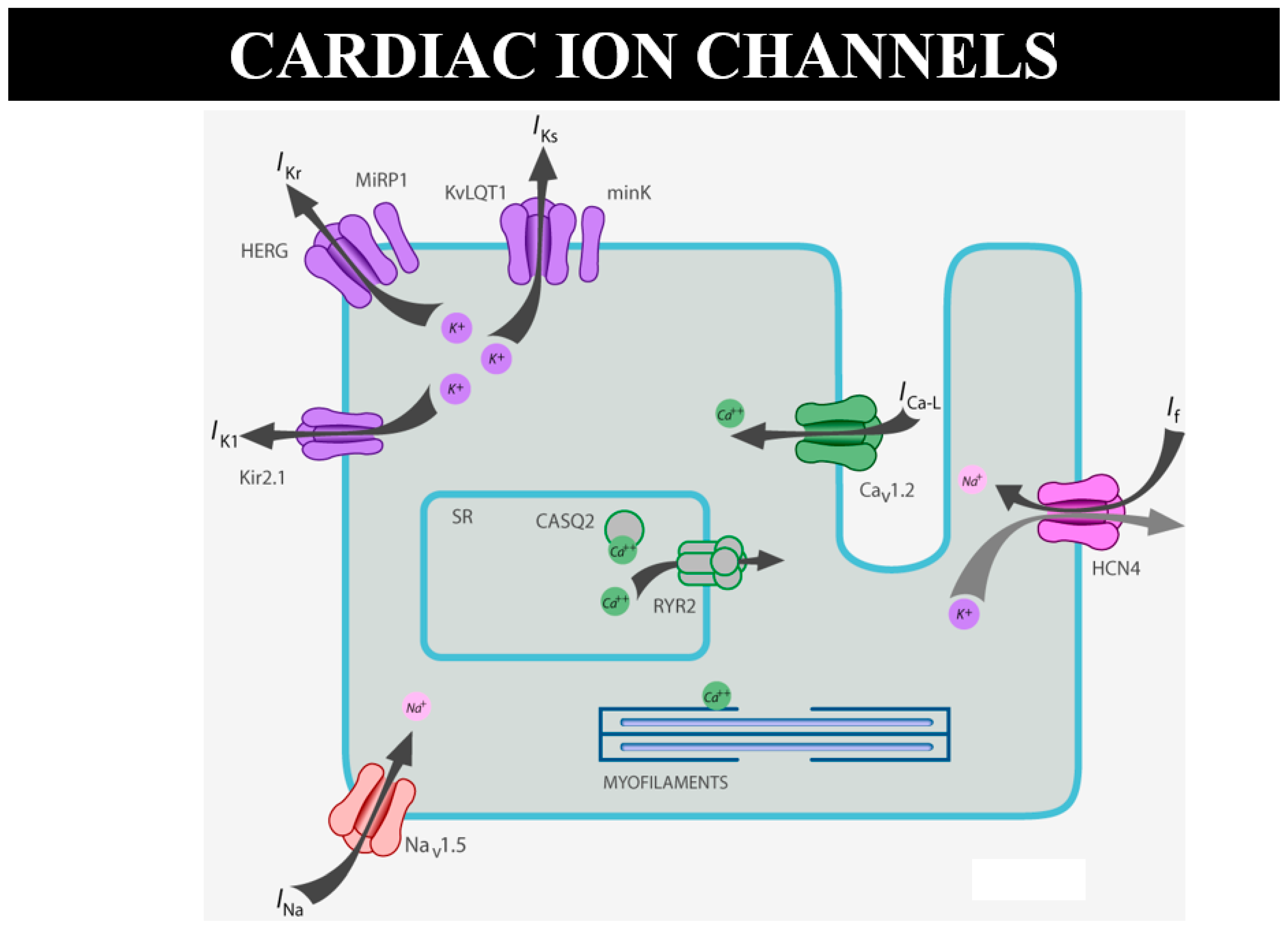
As for sudden death in the young, there are many cases of “mors sine materia”, namely, with an apparently normal heart without a structural defect. For instance, molecular investigation finds the cause of sudden death in catecholaminergic tachycardia, with mutation of ryanodine [15] or calsequestrin genes, devoted to the electromechanical association of Ca++ release by the smooth reticulum (Figure 24). A missense mutation can cause sudden arrhythmic death without an anatomical substrate [15]. Why is this not considered sudden death due CHD?
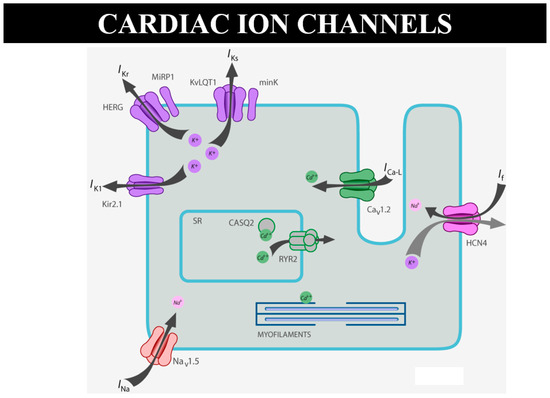
Figure 24.
Schematic representation of ion channels and ryanodine receptor, the latter in charge of Ca++ release from the smooth reticulum for electromechanical coupling.
9. Conclusions
CHDs are usually defined as structural anomalies of the heart. The success of cardiac surgery is improving longevity and the burden of adult CHDs. There are several cardiac disorders that are molecular, with dysfunction of the electrical activity of the heart.
They deserve to be considered congenital heart disease even if the defect is molecular, at the level of the code of life.
Funding
This study was supported by the Cardio-Cerebro-Vascular Pathology Registry, Veneto Region, Venice and by ARCA Foundation, Padua, Italy.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
2D = two-dimensional; A = aorta; AV = atrioventricular; BAV = bicuspid aortic valve; CHD = congenital heart disease; CS = conduction system; DNA = Deoxyribonucleic Acid; LA = morphologically left atrium; LV = morphologically left ventricle; P = pulmonary artery; RA = right atrium; RV = right ventricle; TV = tricuspid valve; VPE = ventricular preexcitation.
References
- Baumgartner, H.; Bonhoeffer, P.; De Groot, N.M.; de Haan, F.; Deanfield, J.E.; Galie, N.; Gatzoulis, M.A.; Gohlke-Baerwolf, C.; Kaemmerer, H.; Kilner, P.; et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur. Heart J. 2010, 31, 2915–2957. [Google Scholar] [CrossRef] [PubMed]
- Dellborg, M.; Giang, K.W.; Eriksson, P.; Liden, H.; Fedchenko, M.; Ahnfelt, A.; Rosengren, A.; Mandalenakis, Z. Adults With Congenital Heart Disease: Trends in Event-Free Survival Past Middle Age. Circulation 2023, 147, 930–938. [Google Scholar] [PubMed]
- Khairy, P.; Ionescu-Ittu, R.; Mackie, A.S.; Abrahamowicz, M.; Pilote, L.; Marelli, A.J. Changing mortality in congenital heart disease. J. Am. Coll. Cardiol. 2010, 56, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, M.; Webb, G. Adult with Congenital Heart Disease: A Growing Population. In Diagnosis and Management of Adult Congenital Heart Disease; Gatzoulis, M., Webb, G., Daubeney, P., Eds.; Elsevier: Phyladelphia, PA, USA, 2011. [Google Scholar] [CrossRef]
- Perloff, J.K.; Warnes, C.A. Challenges posed by adults with repaired congenital heart disease. Circulation 2001, 103, 2637–2643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Webb, G.; Mulder, B.J.; Aboulhosn, J.; Daniels, C.J.; Elizari, M.A.; Hong, G.; Horlick, E.; Landzberg, M.J.; Marelli, A.J.; O'Donnell, C.P.; et al. The care of adults with congenital heart disease across the globe: Current assessment and future perspective: A position statement from the International Society for Adult Congenital Heart Disease (ISACHD). Int. J. Cardiol. 2015, 195, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.B.; Foster, E.; Kuehl, K.; Alpert, J.; Brabeck, S.; Crumb, S.; Davidson, W.R., Jr.; Earing, M.G.; Ghoshhajra, B.B.; Karamlou, T.; et al. Congenital heart disease in the older adult: A scientific statement from the American heart association. Circulation 2015, 131, 1884–1931. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019, 73, e81–e192. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.K.; Broberg, C.S.; Book, W.M.; Cecchin, F.; Chen, J.M.; Dimopoulos, K.; Everitt, M.D.; Gatzoulis, M.; Harris, L.; Hsu, D.T.; et al. Chronic heart failure in congenital heart Disease: A Scientific Statement from the American Heart Association. Circulation 2016, 133, 770–801. [Google Scholar] [CrossRef] [PubMed]
- Warnes, C.A.; Williams, R.G.; Bashore, T.M.; Child, J.; Connolly, H.M.; Dearani, J.A.; Del Nido, P.; Fasules, J.W.; Graham, T.P., Jr.; Hijazi, Z.M.; et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 2008, 52, e1–e121. [Google Scholar] [CrossRef]
- Angelini, A.; di Gioia, C.; Doran, H.; Fedrigo, M.; Henriques de Gouveia, R.; Ho, S.Y.; Leone, O.; Sheppard, M.N.; Thiene, G.; Dimopoulos, K.; et al. Autopsy in adults with congenital heart disease (ACHD). Virchows Arch. 2020, 476, 797–820. [Google Scholar] [PubMed]
- Diller, G.P.; Kempny, A.; Alonso-Gonzalez, R.; Swan, L.; Uebing, A.; Li, W.; Babu-Narayan, S.; Wort, S.J.; Dimopoulos, K.; Gatzoulis, M.A. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow-up at a large tertiary Centre. Circulation 2015, 132, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C. (Ed.) Cardiovascular Clinics; Brest, A.N. (Editor-in-Chief); Congenital Heart Disease in Adults; F.A. Davis Company: Philadelphia, PA, USA, 1979. [Google Scholar]
- Tomanek, R.; Angelini, P. Embryology of coronary arteries and anatomy/pathophysiology of coronary anomalies. A comprehensive update. Int. J. Cardiol. 2019, 281, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Thiene, G.; Corrado, D.; Basso, C. (Eds.) Sudden Cardiac Death in the Young and Athletes; Springer: Milano, Italy, 2016; ISBN 978-88-470-5775-3. [Google Scholar]
- Connelly, M.S.; Liu, P.P.; Williams, W.G.; Webb, G.D.; Robertson, P.; McLaughlin, P.R. Congenitally corrected transposition of the great arteries in the adult: Functional status and complications. J. Am. Coll. Cardiol. 1996, 27, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Baruteau, A.; Abrams, D.J.; Ho, S.Y.; Thambo, J.B.; McLeod, C.J.; Shah, M.J. Cardiac conduction system in congenitally corrected transposition of the great arteries and its clinical relevance. J. Am. Heart Assoc. 2017, 6, e007759. [Google Scholar] [CrossRef] [PubMed]
- Thiene, G.; Rizzo, S.; Basso, C. Bicuspid aortic valve: The most frequent and not so benign congenital heart disease. Cardiovasc. Pathol. 2024, 70, 107604. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thiene, G.; Basso, C.; Rizzo, S.; Della Barbera, M.; Valente, M.; Bortolotti, U. (Eds.) Pathology of Cardiac Valve Disease Surgical and Interventional Anatomy; Springer: Cham, Switzerland, 2023; ISBN 978-3-031-35497-7. [Google Scholar]
- Barbara, D.W.; Edwards, W.D.; Connolly, H.M.; Dearani, J.A. Surgical pathology of 104 tricuspid valves (2000-2005) with classic right-sided Ebstein’s malformation. Cardiovasc. Pathol. 2008, 17, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Luu, Q.; Choudhary, P.; Jackson, D.; Canniffe, C.; McGuire, M.; Chard, R.; Celermajer, D.S. Ebstein’s anomaly in those surviving to adult life—A single Centre experience. Hear. Lung Circ. 2015, 24, 996–1001. [Google Scholar] [CrossRef]
- Dimopoulos, K.; Wort, S.J.; Gatzoulis, M.A. Pulmonary hypertension related to congenital heart disease: A call for action. Eur. Heart J. 2014, 35, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).