The Elevation and Impact of Peripheral Bile Acids in Chronic Lymphocytic Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Murine Plasma Metabolomics
2.2. Human Plasma Metabolomics
2.3. Colorimetric Detection of Total Bile Acids
2.4. Reagents
2.5. Primary Human Cells
2.6. MTS Cytotoxicity Assay
2.7. Co-Cultures
2.8. Flow Cytometry
2.9. Statistics
3. Results
3.1. Metabolomic Analysis of Human and Murine Plasma
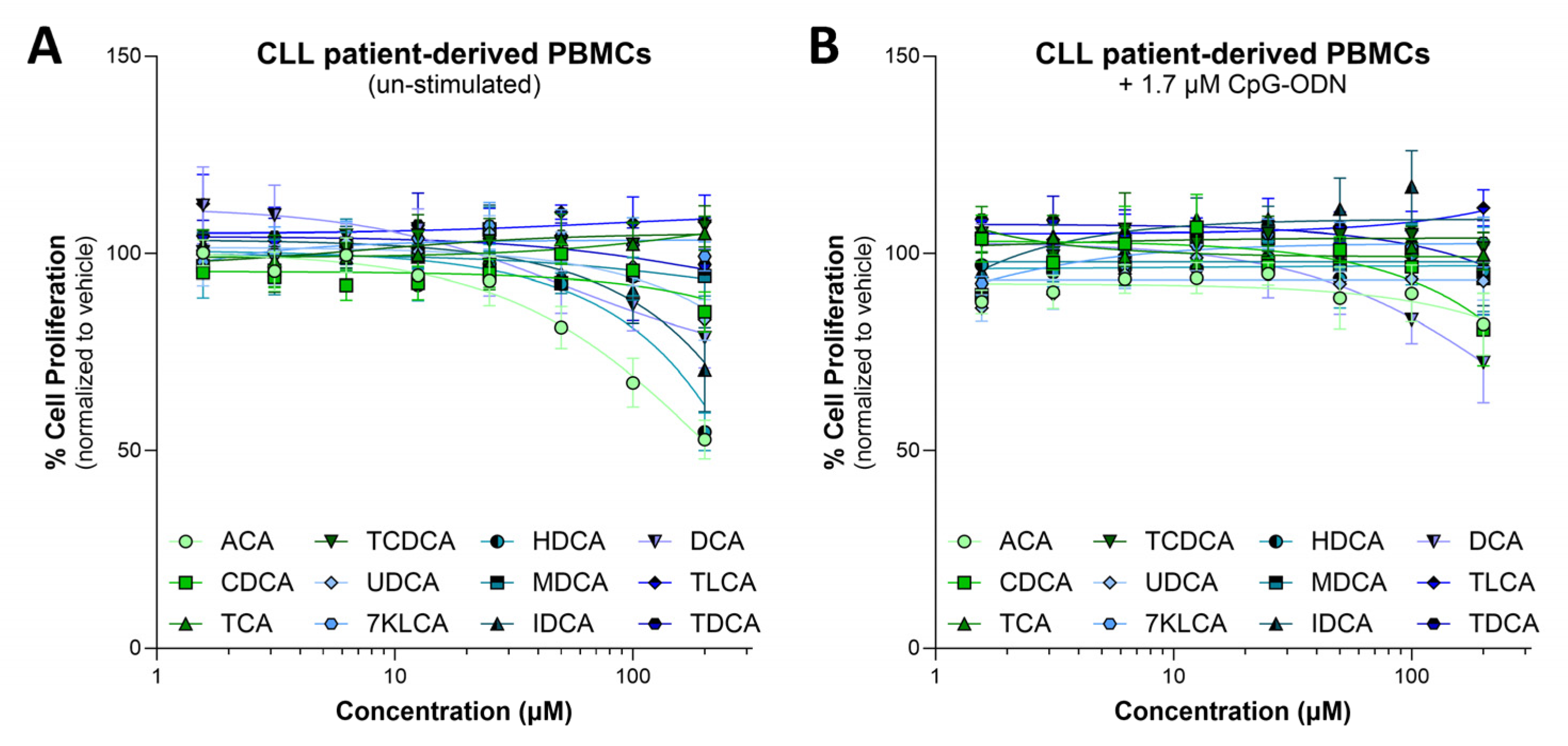
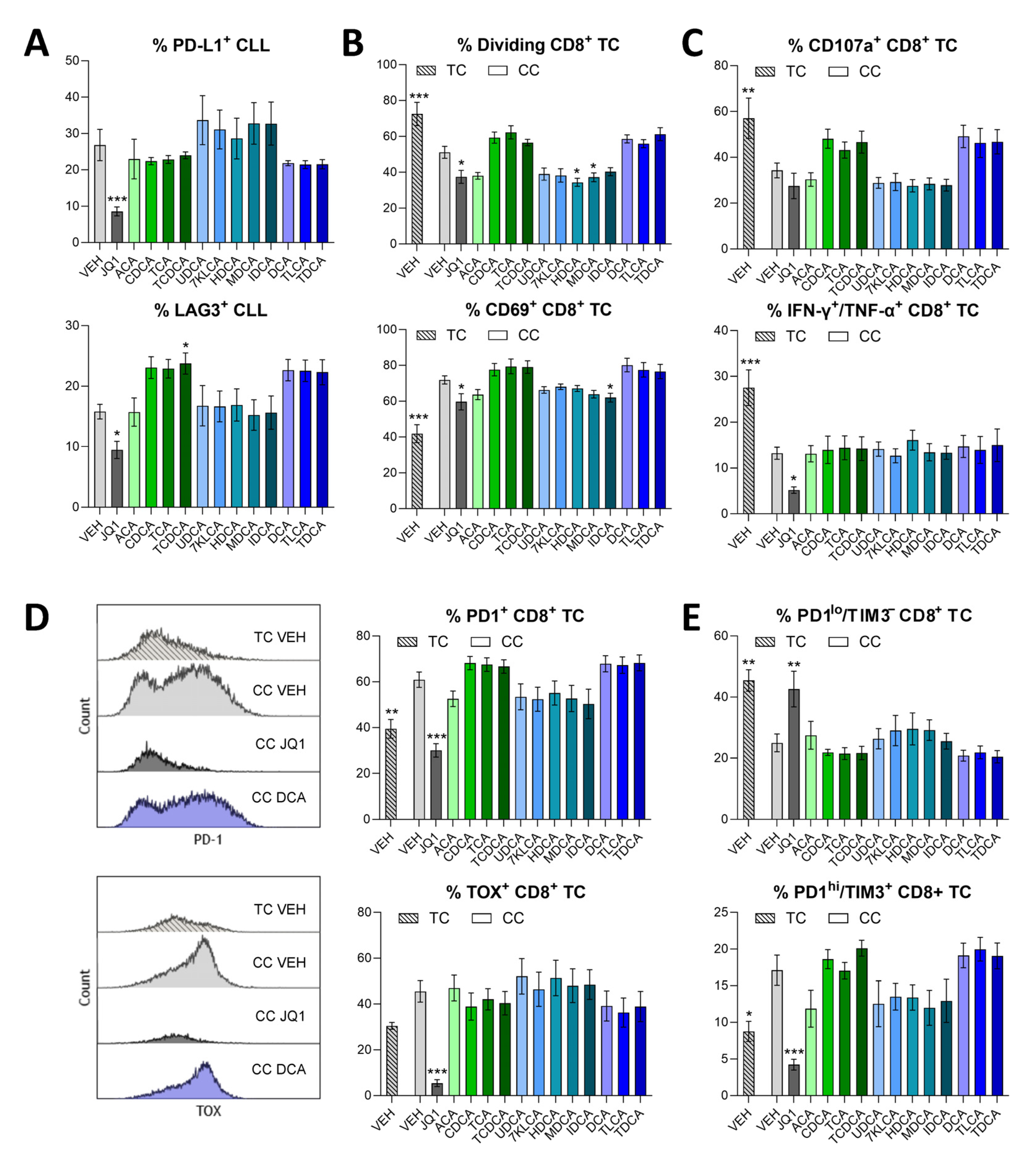
3.2. Cytotoxic and Immunodulatory Action of Bile Acids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kipps, T.J.; Stevenson, F.K.; Wu, C.J.; Croce, C.M.; Packham, G.; Wierda, W.G.; O’Brien, S.; Gribben, J.; Rai, K. Chronic lymphocytic leukaemia. Nat. Rev. Dis. Primers 2017, 3, 16096. [Google Scholar]
- Hallek, M. Chronic Lymphocytic Leukemia: 2025 Update on the Epidemiology, Pathogenesis, Diagnosis, and Therapy. Am. J. Hematol. 2025, 100, 450–480. [Google Scholar] [PubMed]
- Arruga, F.; Gyau, B.B.; Iannello, A.; Vitale, N.; Vaisitti, T.; Deaglio, S. Immune Response Dysfunction in Chronic Lymphocytic Leukemia: Dissecting Molecular Mechanisms and Microenvironmental Conditions. Int. J. Mol. Sci. 2020, 21, 1825. [Google Scholar] [CrossRef] [PubMed]
- Vom Stein, A.F.; Hallek, M.; Nguyen, P.H. Role of the tumor microenvironment in CLL pathogenesis. Semin. Hematol. 2024, 61, 142–154. [Google Scholar] [CrossRef] [PubMed]
- di Gregorio, M.C.; Cautela, J.; Galantini, L. Physiology and Physical Chemistry of Bile Acids. Int. J. Mol. Sci. 2021, 22, 1780. [Google Scholar] [CrossRef]
- Poland, J.C.; Flynn, C.R. Bile Acids, Their Receptors, and the Gut Microbiota. Physiology 2021, 36, 235–245. [Google Scholar]
- Fiamoncini, J.; Rist, M.J.; Frommherz, L.; Giesbertz, P.; Pfrang, B.; Kremer, W.; Huber, F.; Kastenmuller, G.; Skurk, T.; Hauner, H.; et al. Dynamics and determinants of human plasma bile acid profiles during dietary challenges. Front. Nutr. 2022, 9, 932937. [Google Scholar]
- Lin, S.; Wang, S.; Wang, P.; Tang, C.; Wang, Z.; Chen, L.; Luo, G.; Chen, H.; Liu, Y.; Feng, B.; et al. Bile acids and their receptors in regulation of gut health and diseases. Prog. Lipid Res. 2023, 89, 101210. [Google Scholar]
- Banerjee, P.; Kumaravel, S.; Roy, S.; Gaddam, N.; Odeh, J.; Bayless, K.J.; Glaser, S.; Chakraborty, S. Conjugated Bile Acids Promote Lymphangiogenesis by Modulation of the Reactive Oxygen Species-p90RSK-Vascular Endothelial Growth Factor Receptor 3 Pathway. Cells 2023, 12, 526. [Google Scholar] [CrossRef]
- Proungvitaya, S.; Sombattheera, S.; Boonsiri, P.; Limpaiboon, T.; Wongkham, S.; Wongkham, C.; Titapun, A.; Proungvitaya, T. Diagnostic value of serum bile acid composition patterns and serum glycocholic acid levels in cholangiocarcinoma. Oncol. Lett. 2017, 14, 4943–4948. [Google Scholar]
- Rezen, T.; Rozman, D.; Kovacs, T.; Kovacs, P.; Sipos, A.; Bai, P.; Miko, E. The role of bile acids in carcinogenesis. Cell Mol. Life Sci. 2022, 79, 243. [Google Scholar] [PubMed]
- Wang, M.; Lou, E.; Xue, Z. The role of bile acid in intestinal metaplasia. Front. Physiol. 2023, 14, 1115250. [Google Scholar]
- Lee, C.K.; Jeong, S.H.; Jang, C.; Bae, H.; Kim, Y.H.; Park, I.; Kim, S.K.; Koh, G.Y. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 2019, 363, 644–649. [Google Scholar] [PubMed]
- Campbell, C.; McKenney, P.T.; Konstantinovsky, D.; Isaeva, O.I.; Schizas, M.; Verter, J.; Mai, C.; Jin, W.B.; Guo, C.J.; Violante, S.; et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020, 581, 475–479. [Google Scholar]
- Cong, J.; Liu, P.; Han, Z.; Ying, W.; Li, C.; Yang, Y.; Wang, S.; Yang, J.; Cao, F.; Shen, J.; et al. Bile acids modified by the intestinal microbiota promote colorectal cancer growth by suppressing CD8(+) T cell effector functions. Immunity 2024, 57, 876–889.e811. [Google Scholar] [CrossRef]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature 2019, 576, 143–148. [Google Scholar]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar]
- Carra, G.; Nicoli, P.; Lingua, M.F.; Maffeo, B.; Cartella, A.; Circosta, P.; Brancaccio, M.; Parvis, G.; Gaidano, V.; Guerrasio, A.; et al. Inhibition of bromodomain and extra-terminal proteins increases sensitivity to venetoclax in chronic lymphocytic leukaemia. J. Cell. Mol. Med. 2020, 24, 1650–1657. [Google Scholar]
- Kong, W.; Dimitri, A.; Wang, W.; Jung, I.Y.; Ott, C.J.; Fasolino, M.; Wang, Y.; Kulikovskaya, I.; Gupta, M.; Yoder, T.; et al. BET bromodomain protein inhibition reverses chimeric antigen receptor extinction and reinvigorates exhausted T cells in chronic lymphocytic leukemia. J. Clin. Investig. 2021, 131, e145459. [Google Scholar]
- Smith, A.L.; Eiken, A.P.; Skupa, S.A.; Moore, D.Y.; Umeta, L.T.; Smith, L.M.; Lyden, E.R.; D’Angelo, C.R.; Kallam, A.; Vose, J.M.; et al. A Novel Triple-Action Inhibitor Targeting B-Cell Receptor Signaling and BRD4 Demonstrates Preclinical Activity in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2022, 23, 6712. [Google Scholar] [CrossRef]
- Ramsay, A.G.; Johnson, A.J.; Lee, A.M.; Gorgun, G.; Le Dieu, R.; Blum, W.; Byrd, J.C.; Gribben, J.G. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J. Clin. Investig. 2008, 118, 2427–2437. [Google Scholar] [PubMed]
- Smith, A.L.; Skupa, S.A.; Eiken, A.P.; Reznicek, T.E.; Schmitz, E.; Williams, N.; Moore, D.Y.; D’Angelo, C.R.; Kallam, A.; Lunning, M.A.; et al. BET inhibition reforms the immune microenvironment and alleviates T cell dysfunction in chronic lymphocytic leukemia. JCI Insight 2024, 9, e177054. [Google Scholar] [PubMed]
- Gorgun, G.; Ramsay, A.G.; Holderried, T.A.; Zahrieh, D.; Le Dieu, R.; Liu, F.; Quackenbush, J.; Croce, C.M.; Gribben, J.G. E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of chronic lymphocytic leukemia-induced T-cell dysfunction. Proc. Natl. Acad. Sci. USA 2009, 106, 6250–6255. [Google Scholar] [PubMed]
- Johnson, A.J.; Lucas, D.M.; Muthusamy, N.; Smith, L.L.; Edwards, R.B.; De Lay, M.D.; Croce, C.M.; Grever, M.R.; Byrd, J.C. Characterization of the TCL-1 transgenic mouse as a preclinical drug development tool for human chronic lymphocytic leukemia. Blood 2006, 108, 1334–1338. [Google Scholar]
- Faitova, T.; Coelho, M.; Da Cunha-Bang, C.; Ozturk, S.; Kartal, E.; Bork, P.; Seiffert, M.; Niemann, C.U. The diversity of the microbiome impacts chronic lymphocytic leukemia development in mice and humans. Haematologica 2024, 109, 3237–3250. [Google Scholar]
- Kreiniz, N.; Beyar Katz, O.; Polliack, A.; Tadmor, T. The Clinical Spectrum of Hepatic Manifestations in Chronic Lymphocytic Leukemia. Clin. Lymphoma Myeloma Leuk. 2017, 17, 863–869. [Google Scholar]
- Decker, T.; Schneller, F.; Sparwasser, T.; Tretter, T.; Lipford, G.B.; Wagner, H.; Peschel, C. Immunostimulatory CpG-oligonucleotides cause proliferation, cytokine production, and an immunogenic phenotype in chronic lymphocytic leukemia B cells. Blood 2000, 95, 999–1006. [Google Scholar]
- Franco, F.; Jaccard, A.; Romero, P.; Yu, Y.R.; Ho, P.C. Metabolic and epigenetic regulation of T-cell exhaustion. Nat. Metab. 2020, 2, 1001–1012. [Google Scholar]
- Thurgood, L.A.; Dwyer, E.S.; Lower, K.M.; Chataway, T.K.; Kuss, B.J. Altered expression of metabolic pathways in CLL detected by unlabelled quantitative mass spectrometry analysis. Br. J. Haematol. 2019, 185, 65–78. [Google Scholar]
- Piszcz, J.; Armitage, E.G.; Ferrarini, A.; Ruperez, F.J.; Kulczynska, A.; Bolkun, L.; Kloczko, J.; Kretowski, A.; Urbanowicz, A.; Ciborowski, M.; et al. To treat or not to treat: Metabolomics reveals biomarkers for treatment indication in chronic lymphocytic leukaemia patients. Oncotarget 2016, 7, 22324–22338. [Google Scholar]
- Nguyen Van Long, F.; Valcourt-Gendron, D.; Caron, P.; Rouleau, M.; Villeneuve, L.; Simonyan, D.; Le, T.; Sergerie, R.; Laverdiere, I.; Vanura, K.; et al. Untargeted metabolomics identifies metabolic dysregulation of sphingolipids associated with aggressive chronic lymphocytic leukaemia and poor survival. Clin. Transl. Med. 2023, 13, e1442. [Google Scholar]
- Turk, A.; Ceh, E.; Calin, G.A.; Kunej, T. Multiple omics levels of chronic lymphocytic leukemia. Cell Death Discov. 2024, 10, 293. [Google Scholar] [PubMed]
- Faitova, T.; Svanberg, R.; Da Cunha-Bang, C.; Ilett, E.E.; Jorgensen, M.; Noguera-Julian, M.; Paredes, R.; MacPherson, C.R.; Niemann, C.U. The gut microbiome in patients with chronic lymphocytic leukemia. Haematologica 2022, 107, 2238–2243. [Google Scholar] [PubMed]
- White, A.M.; Best, O.G.; Hotinski, A.K.; Kuss, B.J.; Thurgood, L.A. The Role of Cholesterol in Chronic Lymphocytic Leukemia Development and Pathogenesis. Metabolites 2023, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.F.; Peters, F.S.; Camerini, E.; Cretenet, G.; Rietveld, J.; Schomakers, B.V.; van Weeghel, M.; Hahn, N.; Verberk, S.G.S.; Van den Bossche, J.; et al. Cholesterol homeostasis and lipid raft dynamics at the basis of tumor-induced immune dysfunction in chronic lymphocytic leukemia. Cell Mol. Immunol. 2025. [Google Scholar] [CrossRef]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Riches, J.C.; Gribben, J.G. Immunomodulation and immune reconstitution in chronic lymphocytic leukemia. Semin. Hematol. 2014, 51, 228–234. [Google Scholar]
- Girisa, S.; Henamayee, S.; Parama, D.; Rana, V.; Dutta, U.; Kunnumakkara, A.B. Targeting Farnesoid X receptor (FXR) for developing novel therapeutics against cancer. Mol. Biomed. 2021, 2, 21. [Google Scholar]
- Kalaany, N.Y.; Mangelsdorf, D.J. LXRS and FXR: The yin and yang of cholesterol and fat metabolism. Annu. Rev. Physiol. 2006, 68, 159–191. [Google Scholar]
- Wang, Y.D.; Chen, W.D.; Moore, D.D.; Huang, W. FXR: A metabolic regulator and cell protector. Cell Res. 2008, 18, 1087–1095. [Google Scholar]
- Farhana, L.; Nangia-Makker, P.; Arbit, E.; Shango, K.; Sarkar, S.; Mahmud, H.; Hadden, T.; Yu, Y.; Majumdar, A.P. Bile acid: A potential inducer of colon cancer stem cells. Stem Cell Res. Ther. 2016, 7, 181. [Google Scholar] [PubMed]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [PubMed]
- You, W.; Li, L.; Sun, D.; Liu, X.; Xia, Z.; Xue, S.; Chen, B.; Qin, H.; Ai, J.; Jiang, H. Farnesoid X Receptor Constructs an Immunosuppressive Microenvironment and Sensitizes FXR(high)PD-L1(low) NSCLC to Anti-PD-1 Immunotherapy. Cancer Immunol. Res. 2019, 7, 990–1000. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, A.L.; Ridout, A.; Skupa, S.A.; Martinez-Rico, R.; Drengler, E.M.; Mohamed, E.; D’Angelo, C.R.; El-Gamal, D. The Elevation and Impact of Peripheral Bile Acids in Chronic Lymphocytic Leukemia. Biomedicines 2025, 13, 874. https://doi.org/10.3390/biomedicines13040874
Smith AL, Ridout A, Skupa SA, Martinez-Rico R, Drengler EM, Mohamed E, D’Angelo CR, El-Gamal D. The Elevation and Impact of Peripheral Bile Acids in Chronic Lymphocytic Leukemia. Biomedicines. 2025; 13(4):874. https://doi.org/10.3390/biomedicines13040874
Chicago/Turabian StyleSmith, Audrey L., Abigail Ridout, Sydney A. Skupa, Rolando Martinez-Rico, Erin M. Drengler, Eslam Mohamed, Christopher R. D’Angelo, and Dalia El-Gamal. 2025. "The Elevation and Impact of Peripheral Bile Acids in Chronic Lymphocytic Leukemia" Biomedicines 13, no. 4: 874. https://doi.org/10.3390/biomedicines13040874
APA StyleSmith, A. L., Ridout, A., Skupa, S. A., Martinez-Rico, R., Drengler, E. M., Mohamed, E., D’Angelo, C. R., & El-Gamal, D. (2025). The Elevation and Impact of Peripheral Bile Acids in Chronic Lymphocytic Leukemia. Biomedicines, 13(4), 874. https://doi.org/10.3390/biomedicines13040874




