Antimicrobial Efficacy and Biocompatibility of a Denture Cleanser Containing Paeonia lactiflora Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations and Sample Size Determination
2.2. Preparation of a DC-PL
2.3. Formation of Oral Microcosm Biofilms
2.4. Treatment of Oral Microcosm Biofilms
2.5. Evaluation of the Antimicrobial Activity of DC-PL
2.5.1. Analysis of Red Fluorescence Intensity
2.5.2. Measurement of Aciduric Bacterial Counts
2.6. Biocompatibility of DC-PL: Oral Mucosal Irritation Test
2.7. Statistical Analysis
3. Results
3.1. Red Fluorescence Intensity
3.2. Aciduric Bacterial Counts
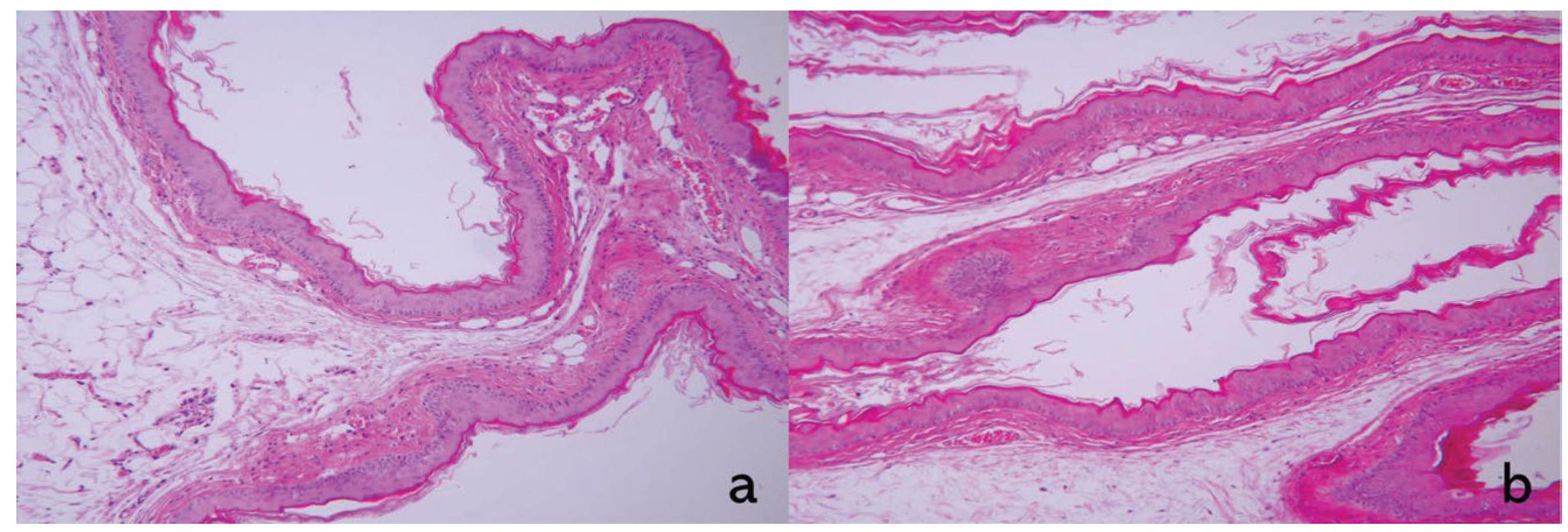
3.3. Oral Mucosal Irritation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DC-PL | denture cleanser containing Paeonia lactiflora extract |
| DW | distilled water |
| RatioR/G | ratio of red to green fluorescence intensity |
| PL | Paeonia lactiflora |
| ANOVA | analysis of variance |
| PBS | phosphate-buffered saline |
References
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Wu, T.; McLean, J.; Edlund, A.; Young, Y.; He, X.; Lv, H.; Zhou, X.; Shi, W.; Li, H. The denture-associated oral microbiome in health and stomatitis. mSphere 2016, 1, 00215-16. [Google Scholar] [CrossRef] [PubMed]
- Beikler, T.; Flemmig, T.F. Oral biofilm-associated diseases: Trends and implications for quality of life, systemic health and expenditures. Periodontol. 2000 2011, 55, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Vaidya, R.Y.; Hegde, V.; Sherigar, P.; Prabhu, N. Exploring microbial interactions with denture resin surface and implications for plant based plaque control strategies: A narrative review. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 110. [Google Scholar] [CrossRef]
- Monteiro, D.R.; de Souza Batista, V.E.; Caldeirão, A.C.M.; Jacinto, R.d.C.; Pessan, J.P. Oral prosthetic microbiology: Aspects related to the oral microbiome, surface properties, and strategies for controlling biofilms. Biofouling 2021, 37, 353–371. [Google Scholar] [CrossRef]
- Redfern, J.; Tosheva, L.; Malic, S.; Butcher, M.; Ramage, G.; Verran, J. The denture microbiome in health and disease: An exploration of a unique community. Lett. Appl. Microbiol. 2022, 75, 195–209. [Google Scholar] [CrossRef]
- O’Donnell, L.E.; Smith, K.; Williams, C.; Nile, C.J.; Lappin, D.F.; Bradshaw, D.; Lambert, M.; Robertson, D.P.; Bagg, J.; Hannah, V. Dentures are a reservoir for respiratory pathogens. J. Prosthodont. 2016, 25, 99–104. [Google Scholar] [CrossRef]
- Coulthwaite, L.; Verran, J. Potential pathogenic aspects of denture plaque. Br. J. Biomed. Sci. 2007, 64, 180–189. [Google Scholar] [CrossRef]
- Bello-Corral, L.; Alves-Gomes, L.; Fernández-Fernández, J.A.; Fernández-García, D.; Casado-Verdejo, I.; Sánchez-Valdeón, L. Implications of gut and oral microbiota in neuroinflammatory responses in Alzheimer’s disease. Life Sci. 2023, 333, 122132. [Google Scholar] [CrossRef]
- Shay, K. Denture hygiene: A review and update. J. Contemp. Dent. Pract. 2000, 1, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Rimondini, L.; Cochis, A.; Varoni, E.; Azzimonti, B.; Carrassi, A. Biofilm formation on implants and prosthetic dental materials. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Cham, Switzerland, 2016; pp. 991–1027. [Google Scholar] [CrossRef]
- Bergman, B.; Carlsson, G.E. Clinical long-term study of complete denture wearers. J. Prosthet. Dent. 1985, 53, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Huang, F.-M.; Tai, K.-W.; Chou, M.-Y. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg. Oral Med. Oral Pathol. Oral. Radiol. Endod. 2001, 92, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Osterberg, R.E.; Bierbower, G.W.; Seabaugh, V.M.; Porter, W.K., Jr.; Lowther, D.; Hoheisel, C.A. Potential biological hazards of commercially available cleansers for dentures. J. Toxicol. Environ. Health Part A Curr. Issues 1977, 3, 969–977. [Google Scholar] [CrossRef]
- Anuradha, K.; Sarada, J.; Aparna, Y.; Anju, S. Antimicrobial Agents Induced Microbiome Dysbiosis Its Impact on Immune System and Metabolic Health. In Human Microbiome in Health, Disease, and Therapy; Springer: Singapore, 2023; pp. 81–95. [Google Scholar] [CrossRef]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef]
- Milho, C.; Silva, J.; Guimarães, R.; Ferreira, I.C.; Barros, L.; Alves, M.J. Antimicrobials from medicinal plants: An emergent strategy to control oral biofilms. Appl. Sci. 2021, 11, 4020. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, Y.; Ji, M.; Li, Y.; Zhu, H.; Yan, Y.; Fu, D.; Zou, L.; Ren, B. Natural products from traditional medicine as promising agents targeting at different stages of oral biofilm development. Front. Microbiol. 2022, 13, 955459. [Google Scholar] [CrossRef]
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Kim, C.Y.; Kim, H.J.; Park, J.H.; Ahn, M.-J. Differences in the chemical profiles and biological activities of Paeonia lactiflora and Paeonia obovata. J. Med. Food. 2015, 18, 224–232. [Google Scholar] [CrossRef]
- Lim, J.-W.; Kang, M.-K.; Kim, H.-E. Antibacterial Effects of Paeonia lactiflora Extracton Oral Microcosm Biofilms. Appl. Sci. 2024, 14, 11290. [Google Scholar] [CrossRef]
- Brożek, R.; Rogalewicz, R.; Koczorowski, R.; Voelkel, A. The influence of denture cleansers on the release of organic compounds from soft lining materials. J. Environ. Monit. 2008, 10, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-R.; Kang, M.-K. Evaluation of cytotoxic and antibacterial effect of methanolicextract of Paeonia lactiflora. Medicina 2022, 58, 1272. [Google Scholar] [CrossRef]
- Jo, A.; Kim, H.-E. Antibacterial Effects of Black Cumin Seed Oil on Oral MicrocosmBiofilms. Microorganisms 2024, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; de Josselin de Jong, E.; Kim, B.I. Detection of dental plaque and its potential pathogenicity using quantitative light-induced fluorescence. J. Biophotonics 2019, 12, e201800414. [Google Scholar] [CrossRef]
- ISO 10993-10:2021; Biological Evaluation of Medical Devices—Part 10: Tests for Skin Sensitization. International Organization for Standardization: Geneva, Switzerland, 2021.
- Kaypetch, R.; Rudrakanjana, P.; Tua-Ngam, P.; Tosrisawatkasem, O.; Thairat, S.; Tonput, P.; Tantivitayakul, P. Effects of two novel denture cleansers on multispecies microbial biofilms, stain removal and the denture surface: An in vitro study. BMC Oral Health 2023, 23, 852. [Google Scholar] [CrossRef]
- de Lucena-Ferreira, S.C.; Ricomini-Filho, A.P.; Da Silva, W.J.; Cury, J.A.; Del Bel Cury, A.A. Influence of daily immersion in denture cleanser on multispecies biofilm. Clin. Oral Investig. 2014, 18, 2179–2185. [Google Scholar] [CrossRef]
- Salli, K.M.; Ouwehand, A.C. The use of in vitro model systems to study dental biofilms associated with caries: A short review. J. Oral Microbiol. 2015, 7, 26149. [Google Scholar] [CrossRef]
- Bradshaw, D.; McKee, A.; Marsh, P. Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. J. Dent. Res. 1989, 68, 1298–1302. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. Caries ecology revisited: Microbial dynamics and the caries process. Caries Res. 2008, 42, 409–418. [Google Scholar] [CrossRef]
- Salman, M.; Saleem, S. Effect of different denture cleanser solutions on some mechanical and physical properties of nylon and acrylic denture base materials. J. Baghdad Coll. Dent. 2011, 23, 19–23. [Google Scholar]
- Elrahim, R.A.A.; Shown, A.; Abdellah, M.; Abualsaud, R.; Helal, M.A. Impact of different chemical denture cleansers on the properties of digitally fabricated denture base resin materials. J. Prosthodont. 2024, 33, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ochi, N.; Yamane, H.; Honda, Y.; Takigawa, N. Accidental aspiration of denture cleanser tablets caused severe mucosal edema in upper airway. Clin. Respir. J. 2018, 12, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Choi, Y.-R.; Kang, M.-K. Physical Properties, Antimicrobial Efficacy, and Biocompatibility of Denture Base Resins Coated with Natural Peony Extract. Kor. J. Mater. Res. 2023, 33, 47–53. [Google Scholar] [CrossRef]
- Choi, Y.-R.; Bae, S.-S.; Kang, M.-K. Antibacterial effect of tea tree ingredient for denture cleaners. J. Dent. Hyg. Sci. 2016, 16, 409–414. [Google Scholar] [CrossRef][Green Version]
- Lee, M.-J.; Yang, S.-Y.; Kang, M.-K. Biological, Antifungal, and Physical Efficacy of a Denture Cleanser Formulated with Cnidium officinale Extracts. Biomedicines 2024, 12, 2029. [Google Scholar] [CrossRef]
- Tsuji, M.; Ueda, T.; Sawaki, K.; Kawaguchi, M.; Sakurai, K. Biocompatibility of a titanium dioxide-coating method for denture base acrylic resin. Gerodontology 2016, 33, 539–544. [Google Scholar] [CrossRef]
- Huttenhower, C.L.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Takahashi, N. Oral microbiome metabolism: From “who are they?” to “what are they doing?”. J. Dent. Res. 2015, 94, 1628–1637. [Google Scholar] [CrossRef]
- Viana, C.S.; Maske, T.T.; Signori, C.; van de Sande, F.H.; de Oliveira, E.F.; Cenci, M.S. Influence of caries activity and number of saliva donors: Mineral and microbiological responses in a microcosm biofilm model. J. Appl. Oral Sci. 2021, 29, e20200778. [Google Scholar]
- Signori, C.; van de Sande, F.H.; Maske, T.T.; de Oliveira, E.F.; Cenci, M.S. Influence of the inoculum source on the cariogenicity of in vitro microcosm biofilms. Caries Res. 2016, 50, 97–103. [Google Scholar] [CrossRef]
- Işeri, U.; Uludamar, A.; Ozkan, Y.K. Effectiveness of different cleaning agents on the adherence of Candida albicans to acrylic denture base resin. Gerodontology 2011, 28, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Martorano-Fernandes, L.; Rodrigues, N.C.; Bezerra, N.V.F.; de Souza Borges, M.H.; Cavalcanti, Y.W.; de Almeida, L.d.F.D. Cinnamaldehyde and α-terpineol as an alternative for using as denture cleansers: Antifungal activity and acrylic resin color stability. Res. Soc. Dev. 2021, 10, e28010313512. [Google Scholar] [CrossRef]
- Khan, M.A.; Dhaded, S.; Joshi, S. Commercial and plant extract denture cleansers in prevention of Candida albicans growth on soft denture reliner: In vitro study. J. Clin. Diagn. Res. 2016, 10, ZC42. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.M.; Badaro, M.M.; Arruda, C.N.F.d.; Leite, V.M.F.; Silva, C.H.L.d.; Watanabe, E.; Oliveira, V.d.C.; Paranhos, H.d.F.O. Antimicrobial activity of complete denture cleanser solutions based on sodium hypochlorite and Ricinus communis–a randomized clinical study. J. Appl. Oral Sci. 2015, 23, 637–642. [Google Scholar] [CrossRef]
- Bowden, G. The microbial ecology of dental caries. Microb. Ecol. Health Dis. 2000, 12, 138–148. [Google Scholar] [CrossRef]


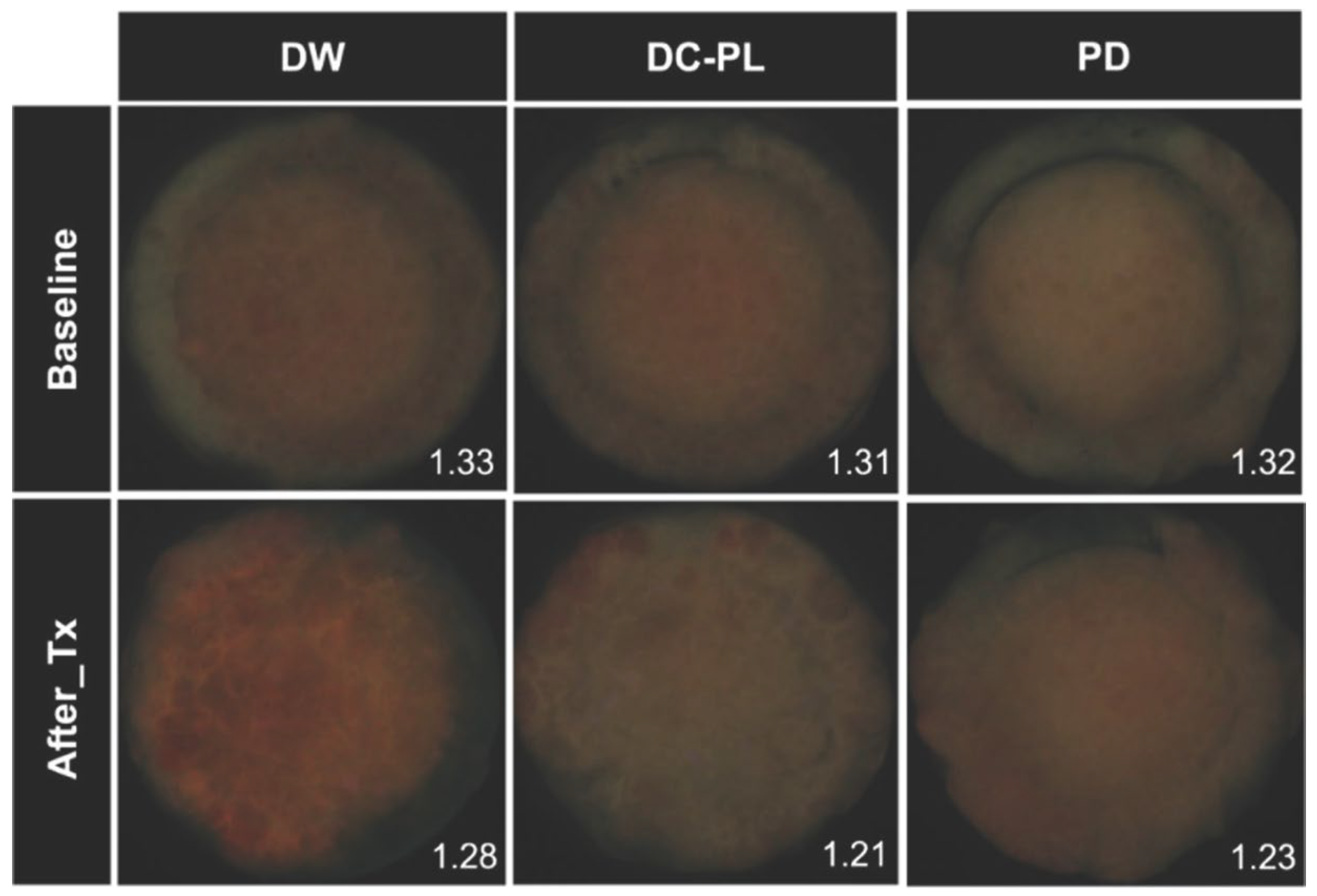
| Treatment | n | RatioR/G | |
|---|---|---|---|
| Baseline | After treatment | ||
| Distilled water | 14 | 1.33 (0.07) a | 1.28 (0.07) a |
| Denture cleanser containing Paeonia lactiflora extract | 14 | 1.30 (0.03) a | 1.21 (0.06) b |
| Polident® | 14 | 1.32 (0.07) a | 1.23 (0.07) b |
| p-values † | 0.407 | 0.026 | |
| Treatment | n | logCFU/mL |
|---|---|---|
| Distilled water | 14 | 5.98 (0.33) a |
| Denture cleanser containing Paeonia lactiflora extract | 14 | 5.50 (0.65) b |
| Polident® | 14 | 5.73 (0.46) b |
| p-value † | 0.048 | |
| Reaction | Control | DC-PL | ||||
|---|---|---|---|---|---|---|
| Hamster 1 | Hamster 2 | Hamster 3 | Hamster 1 | Hamster 2 | Hamster 3 | |
| Epithelium | 0 | 0 | 0 | 0 | 0 | 0 |
| Leucocyte infiltration | 1 | 1 | 1 | 1 | 1 | 1 |
| Vascular congestion | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 1 | 1 | 1 | 0 | 0 |
| Total | 1 | 2 | 2 | 2 | 1 | 1 |
| Mean | 1.7 | 1.3 | ||||
| Irritation index | 0 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-W.; Lee, J.; Kang, M.-K.; Kim, H.-E. Antimicrobial Efficacy and Biocompatibility of a Denture Cleanser Containing Paeonia lactiflora Extract. Biomedicines 2025, 13, 869. https://doi.org/10.3390/biomedicines13040869
Lim J-W, Lee J, Kang M-K, Kim H-E. Antimicrobial Efficacy and Biocompatibility of a Denture Cleanser Containing Paeonia lactiflora Extract. Biomedicines. 2025; 13(4):869. https://doi.org/10.3390/biomedicines13040869
Chicago/Turabian StyleLim, Ji-Won, Jiyeon Lee, Min-Kyung Kang, and Hee-Eun Kim. 2025. "Antimicrobial Efficacy and Biocompatibility of a Denture Cleanser Containing Paeonia lactiflora Extract" Biomedicines 13, no. 4: 869. https://doi.org/10.3390/biomedicines13040869
APA StyleLim, J.-W., Lee, J., Kang, M.-K., & Kim, H.-E. (2025). Antimicrobial Efficacy and Biocompatibility of a Denture Cleanser Containing Paeonia lactiflora Extract. Biomedicines, 13(4), 869. https://doi.org/10.3390/biomedicines13040869





