Biphasic Effects of Blue Light Irradiation on Different Drug-Resistant Bacterium and Exploration of Its Mechanism
Abstract
1. Introduction
2. Materials and Methods
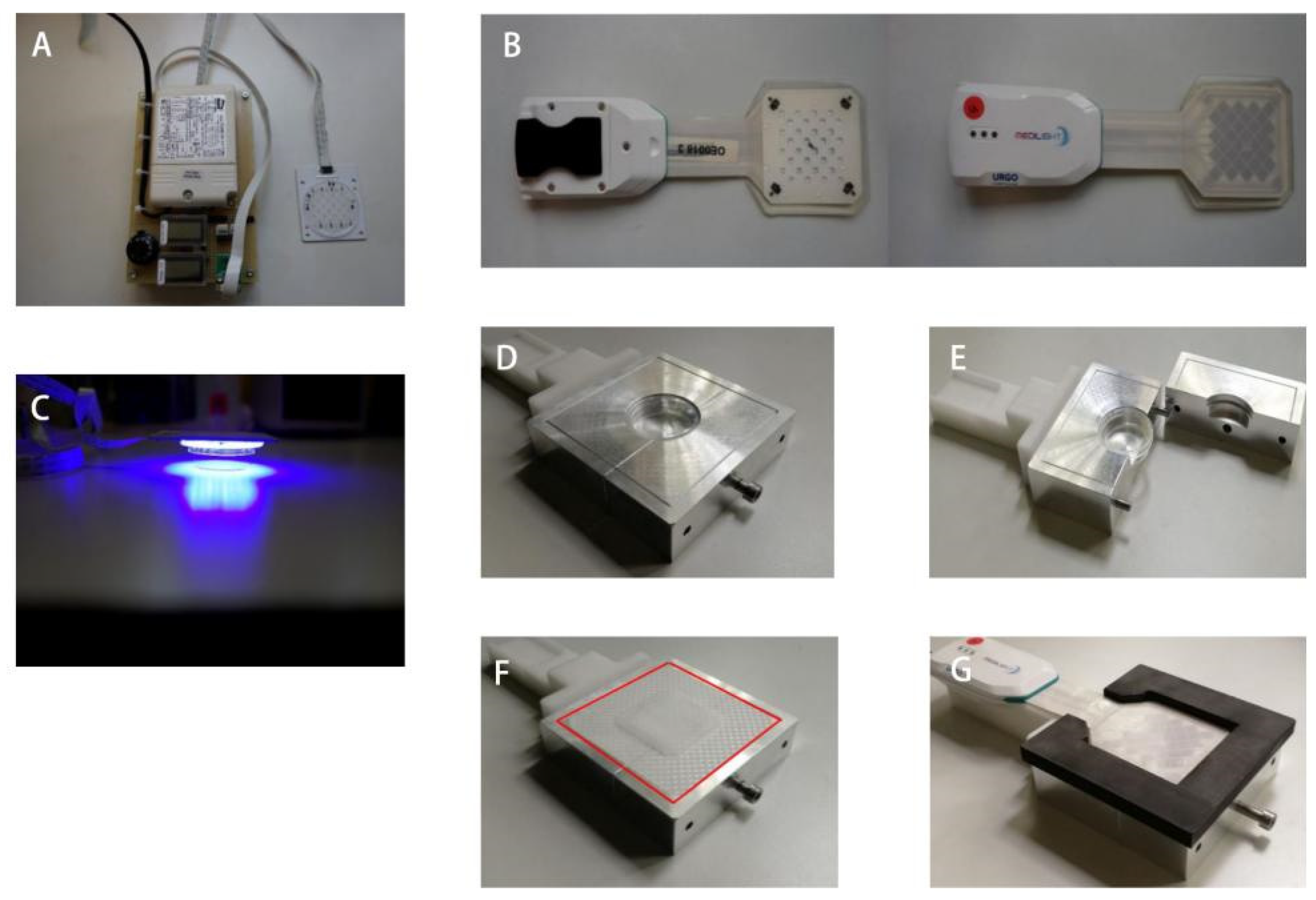
2.1. Device
2.2. Different Blue Light Irradiation Mode
2.3. Bacteria Strain and Culture
2.4. Blue Light Irradiation (BLI) of Bacterial Suspension
2.5. Blue Light Irradiation (BLI) on Infected Implants with 23 mW/cm2
2.6. Oxidative Stress of E. coli After Blue Light Irradiation (BLI)
2.7. Vitality Determination with ATP Assay
2.8. RNA Isolation and Quality Control
2.9. Bioinformatic Analysis of GeneChip
2.10. Statistical Analysis
3. Results
3.1. Different Power Densities of BLI Showed Distinct Suppression Effects Against Bacteria
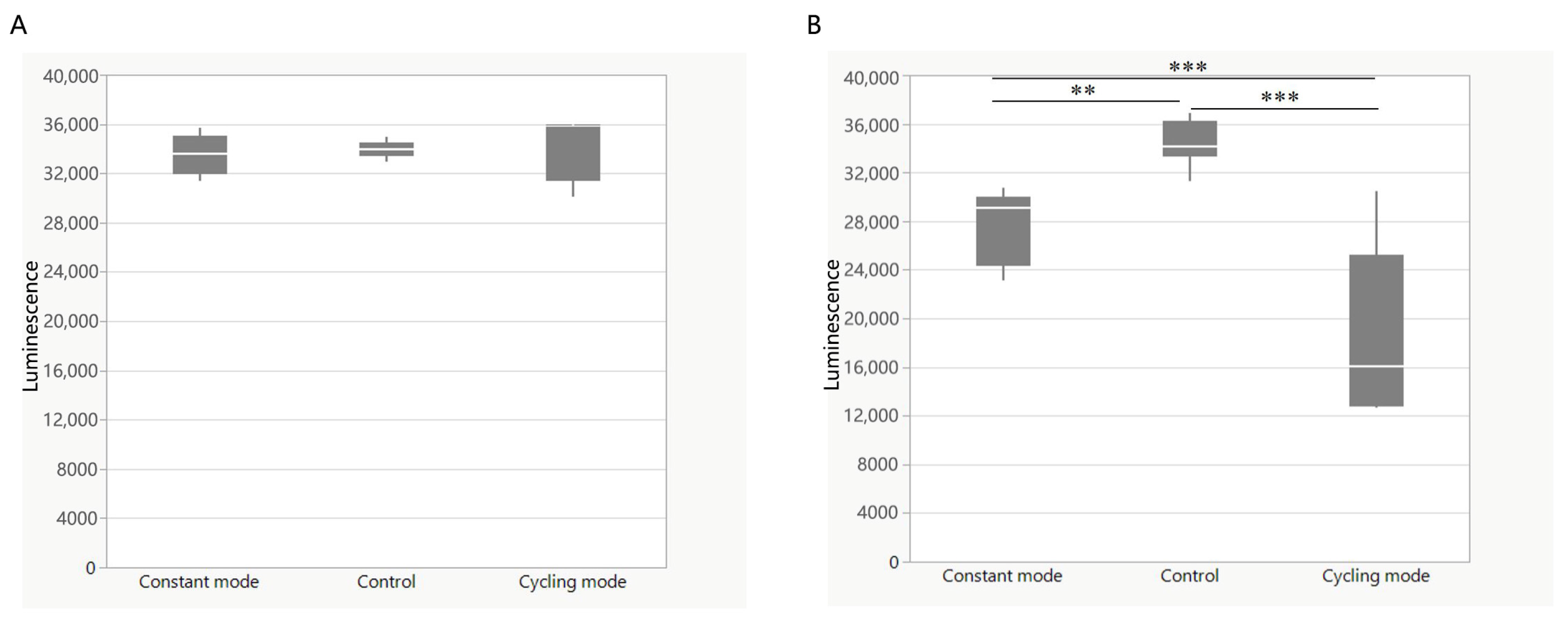
3.2. Cycling Irradiation Mode Showed a Better Bactericidal Effect
3.3. The Bactericidal Use of BLI on Different Kinds of Implants
3.4. Change of ROS Level After BLI
3.5. Change in ATP Level After BLI
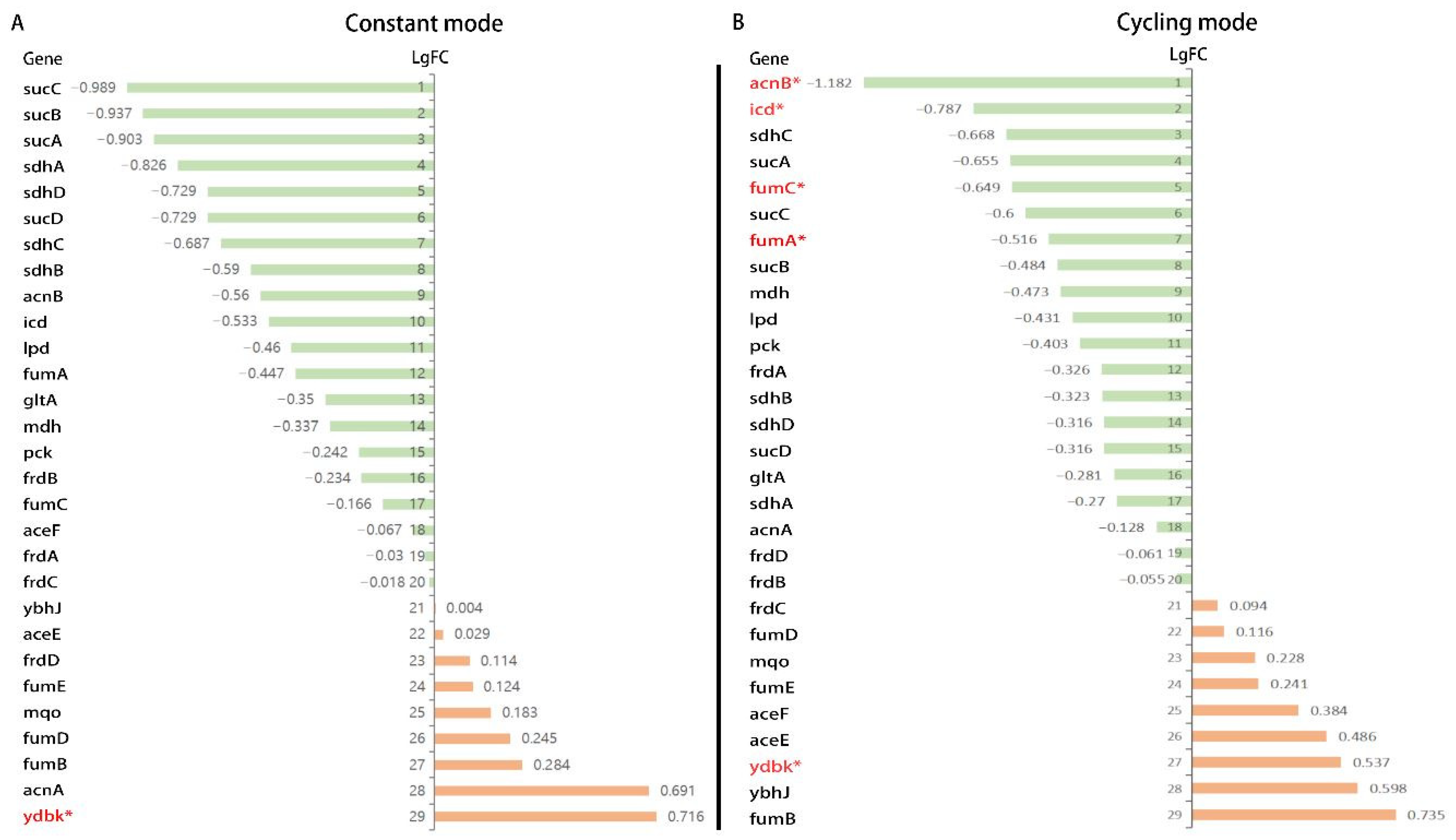
3.6. Gene Expression Analysis from RNA-Sequencing
4. Discussion
4.1. Medium-Irradiance and Cycling Mode BLI Could Have an Improved Bactericidal Effect
4.2. BLI May Also Be a Promising Method for Sterilizing Implants
4.3. The Mechanism of BLI on the Inhibition of Bacterial Growth
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Blue Light Irradiation | BLI |
| Colony-Forming Units | CFU |
| Constant mode | Cst |
| Cycling mode | Cys |
| Escherichia coli | E. coli |
| Helicobacter pylori | H. pylori |
| Gene Set Enrichment Analysis | GSEA |
| Klebsiella pneumoniae | K. pneumoniae |
| Normalized Enrichment Scores | NES |
| Pseudomonas aeruginosa | P. aeruginosa |
| Staphylococcus aureus | S. aureus |
| Ultraviolet Light | UV light |
| Kyoto Encyclopedia of Genes and Genomes | KEGG |
| Phosphate Buffered Saline | PBS |
| URGO |
References
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance-A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. Bacterial spectrum colonizing chronic leg ulcers: A 10-year comparison from a German wound care center. J. Dtsch. Dermatol. Ges. 2014, 12, 1121–1127. [Google Scholar]
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.A.; Kishony, R.; Kreiswirth, B.N.; Kutter, E.; et al. Tackling antibiotic resistance. Nat. Rev. Microbiol. 2011, 9, 894–896. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.; Gupta, S.; Maclean, M.; Ramakrishnan, P.; Anderson, J.; MacGregor, S.; Meek, R.; Grant, M. 405 nm Light exposure of osteoblasts and inactivation of bacterial isolates from arthroplasty patients: Potential for new disinfection applications? Eur. Cell Mater. 2013, 25, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Wheeland, R.G.; Koreck, A. Safety and Effectiveness of a New Blue Light Device for the Self-treatment of Mild-to-moderate Acne. J. Clin. Aesthet. Dermatol. 2012, 5, 25–31. [Google Scholar]
- Megraud, F.; Lamouliatte, H. Review article: The treatment of refractory Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2003, 17, 1333–1343. [Google Scholar] [CrossRef]
- Ganz, R.A.; Veveiros, B.S.J.; Ahmad, A.; Ahmadi, A.; Khalil, A.; Tolkoff, M.S.J.; Nishioka, N.S.; Hamblin, M.R. Helicobacter pylori in patients can be killed by visible light. Lasers Surg. Med. 2005, 36, 260–265. [Google Scholar] [CrossRef]
- Kan, K.; Mu, Y.; Bouschbacher, M.; Sticht, C.; Kuch, N.; Sigl, M.; Rahbari, N.; Gretz, N.; Pallavi, P.; Keese, M. Biphasic Effects of Blue Light Irradiation on Human Umbilical Vein Endothelial Cells. Biomedicines 2021, 9, 829. [Google Scholar] [CrossRef]
- Grzelak, A.; Rychlik, B.; Bartosz, G. Light-dependent generation of reactive oxygen species in cell culture media. Free Radic. Biol. Med. 2001, 30, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Natoli, R.; Rutar, M.; Lu, Y.-Z.; Chu-Tan, J.A.; Chen, Y.; Saxena, K.; Madigan, M.; Valter, K.; Provis, J.M. The Role of Pyruvate in Protecting 661W Photoreceptor-Like Cells Against Light-Induced Cell Death. Curr. Eye Res. 2016, 41, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Mukherjee, M. Reactive oxygen species and uspA overexpession: An alternative bacterial response toward selection and maintenance of multidrug resistance in clinical isolates of uropathogenic E. coli. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Dutta, D. Bicyclomycin generates ROS and blocks cell division in Escherichia coli. PLoS ONE 2024, 19, e293858. [Google Scholar] [CrossRef] [PubMed]
- Hoenes, K.; Bauer, R.; Spellerberg, B.; Hessling, M. Microbial Photoinactivation by Visible Light Results in Limited Loss of Membrane Integrity. Antibiotics 2021, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Cloud, J.L.; Shutt, C.; Aldous, W.; Woods, G. Evaluation of a modified gen-probe amplified direct test for detection of Mycobacterium tuberculosis complex organisms in cerebrospinal fluid. J. Clin. Microbiol. 2004, 42, 5341–5344. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, B.; Johnson, B.J.; Rubin, R.A.; Malanoski, A.P.; Ligler, F.S. Iron chelation by cranberry juice and its impact on Escherichia coli growth. Biofactors 2011, 37, 121–130. [Google Scholar] [CrossRef]
- Guan, N.; Shin, H.-D.; Long, L.; Azadi, P.; Chen, R. TCA cycle-powered synthesis of fucosylated oligosaccharides. Glycobiology 2018, 28, 468–473. [Google Scholar] [CrossRef]
- Lu, M.; Wang, S.; Wang, T.; Hu, S.; Bhayana, B.; Ishii, M.; Kong, Y.; Cai, Y.; Dai, T.; Cui, W.; et al. Bacteria-specific phototoxic reactions triggered by blue light and phytochemical carvacrol. Sci. Transl. Med. 2021, 13, eaba3571. [Google Scholar] [CrossRef]
- Bonatti, S.; Hochman, B.; Tucci-Viegas, V.M.; Furtado, F.; Pinfildi, C.E.; Pedro, A.C.; Ferreira, L.M. In vitro effect of 470 nm LED (Light Emitting Diode) in keloid fibroblasts. Acta Cir. Bras. 2011, 26, 25–30. [Google Scholar] [PubMed]
- Yoshida, A.; Yoshino, F.; Makita, T.; Maehata, Y.; Higashi, K.; Miyamoto, C.; Wada-Takahashi, S.; Takahashi, S.-S.; Takahashi, O.; Lee, M.C.-I. Reactive oxygen species production in mitochondria of human gingival fibroblast induced by blue light irradiation. J. Photochem. Photobiol. B 2013, 129, 1–5. [Google Scholar] [CrossRef]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl. Environ. Microbiol. 2009, 75, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dai, T.; Hamblin, M.R. Antimicrobial photodynamic inactivation and photodynamic therapy for infections. Methods Mol. Biol. 2010, 635, 155–173. [Google Scholar] [PubMed]
- Hoenes, K.; Bauer, R.; Meurle, T.; Spellerberg, B.; Hessling, M. Inactivation Effect of Violet and Blue Light on ESKAPE Pathogens and Closely Related Non-pathogenic Bacterial Species—A Promising Tool Against Antibiotic-Sensitive and Antibiotic-Resistant Microorganisms. Front. Microbiol. 2020, 11, 612367. [Google Scholar] [CrossRef]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.B.; Maclean, M.; Given, M.J.; Wilson, M.P.; Judd, M.D.; Timoshkin, I.V.; MacGregor, S.J. Efficacy of Pulsed 405-nm Light-Emitting Diodes for Antimicrobial Photodynamic Inactivation: Effects of Intensity, Frequency, and Duty Cycle. Photomed. Laser Surg. 2017, 35, 150–156. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Bumah, V.V.; Castel, C.; Castel, D.; Enwemeka, C.S. Pulsed 450 nm blue light significantly inactivates Propionibacterium acnes more than continuous wave blue light. J. Photochem. Photobiol. B 2020, 202, 111719. [Google Scholar] [CrossRef]
- Bowman, C.; Bumah, V.V.; Niesman, I.R.; Cortez, P.; Enwemeka, C.S. Structural membrane changes induced by pulsed blue light on methicillin-resistant Staphylococcus aureus (MRSA). J. Photochem. Photobiol. B 2021, 216, 112150. [Google Scholar] [CrossRef] [PubMed]
- Bumah, V.V.; Cortez, P.M.; Morrow, B.N.; Rojas, P.; Bowman, C.R.; Masson-Meyers, D.S.; Enwemeka, C.S. Blue light absorbing pigment in Streptococcus agalactiae does not potentiate the antimicrobial effect of pulsed 450 nm light. J. Photochem. Photobiol. B 2021, 216, 112149. [Google Scholar] [CrossRef]
- Soltes, G.D.; Barth, M.H.; Roehm, J.O. Preventing complications of central venous catheterization. N. Engl. J. Med. 2003, 348, 2684–2686. [Google Scholar] [PubMed]
- Azizi, B.; Budimir, A.; Bago, I.; Mehmeti, B.; Jakovljević, S.; Kelmendi, J.; Stanko, A.P.; Gabrić, D. Antimicrobial efficacy of photodynamic therapy and light-activated disinfection on contaminated zirconia implants: An in vitro study. Photodiagnosis Photodyn. Ther. 2018, 21, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Nazarian, A.; Tam, J.; Farinelli, W.; Korupolu, S.; Drake, L.; Isaacson, B.; Pasquina, P.; Williams, D. An antimicrobial blue light device to manage infection at the skin-implant interface of percutaneous osseointegrated implants. PLoS ONE 2023, 18, e290347. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkawa, H.; Kaneko, T.; Fukuzawa, H.; Tabata, S.; Kaplan, A.; Ogawa, T. Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: Genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc. Natl. Acad. Sci. USA 2001, 98, 11789–11794. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, N.; Keeler, W.J.; Nandakumar, K.; Leung, K.T. The bactericidal effect of ultraviolet and visible light on Escherichia coli. Biotechnol. Bioeng. 2008, 99, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Zaffina, S.; Camisa, V.; Lembo, M.; Vinci, M.R.; Tucci, M.G.; Borra, M.; Napolitano, A.; Cannatà, V. Accidental exposure to UV radiation produced by germicidal lamp: Case report and risk assessment. Photochem. Photobiol. 2012, 88, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Kleinpenning, M.M.; Smits, T.; Frunt, M.H.A.; van Erp, P.E.J.; van de Kerkhof, P.C.M.; Gerritsen, R.M.J.P. Clinical and histological effects of blue light on normal skin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 16–21. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Grant, M.H. Cytotoxic responses to 405 nm light exposure in mammalian and bacterial cells: Involvement of reactive oxygen species. Toxicol. In Vitro 2016, 33, 54–62. [Google Scholar] [CrossRef]
- Galbis-Martínez, M.; Padmanabhan, S.; Murillo, F.J.; Elías-Arnanz, M. CarF mediates signaling by singlet oxygen, generated via photoexcited protoporphyrin IX, in Myxococcus xanthus light-induced carotenogenesis. J. Bacteriol. 2012, 194, 1427–1436. [Google Scholar] [CrossRef]
- Bumah, V.V.; Morrow, B.N.; Cortez, P.M.; Bowman, C.R.; Rojas, P.; Masson-Meyers, D.S.; Suprapto, J.; Tong, W.G.; Enwemeka, C.S. The importance of porphyrins in blue light suppression of Streptococcus agalactiae. J. Photochem. Photobiol. B 2020, 212, 111996. [Google Scholar] [CrossRef]
- Lu, M.; Wong, K.I.; Li, X.; Wang, F.; Wei, L.; Wang, S.; Wu, M.X. Oregano Oil and Harmless Blue Light to Synergistically Inactivate Multidrug-Resistant Pseudomonas aeruginosa. Front. Microbiol. 2022, 13, 810746. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Mempin, R.; Tran, H.; Chen, C.; Gong, H.; Ho, K.K.; Lu, S. Release of extracellular ATP by bacteria during growth. BMC Microbiol. 2013, 13, 301. [Google Scholar] [CrossRef]
- Kwong, W.K.; Zheng, H.; Moran, N.A. Convergent evolution of a modified, acetate-driven TCA cycle in bacteria. Nat. Microbiol. 2017, 2, 17067. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.P.; Chandel, N.S. Mitochondria as Signaling Organelles Control Mammalian Stem Cell Fate. Cell Stem Cell 2021, 28, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Zhao, C.; Li, G.; Wang, H.; Li, T.; Yan, P.; Wei, S. Blue LED light induces cytotoxicity via ROS production and mitochondrial damage in bovine subcutaneous preadipocytes. Environ. Pollut. 2023, 322, 121195. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Klapczynski, A.; Kuch, N.; Arpino, F.; Simon-Keller, K.; De La Torre, C.; Sticht, C.; van Abeelen, F.A.; Oversluizen, G.; Gretz, N. Gene expression profiling reveals aryl hydrocarbon receptor as a possible target for photobiomodulation when using blue light. Sci. Rep. 2016, 6, 33847. [Google Scholar] [CrossRef]
- Nakayama, E.; Kushibiki, T.; Mayumi, Y.; Azuma, R.; Ishihara, M.; Kiyosawa, T. Blue Laser Irradiation Decreases the ATP Level in Mouse Skin and Increases the Production of Superoxide Anion and Hypochlorous Acid in Mouse Fibroblasts. Biology 2022, 11, 301. [Google Scholar] [CrossRef]
- Tsuchiya, T. Oxidative phosphorylation in right-side-out membrane vesicles from Escherichia coli. J. Biol. Chem. 1976, 251, 5315–5320. [Google Scholar] [CrossRef] [PubMed]
- Wessels, H.J.; de Almeida, N.M.; Kartal, B.; Keltjens, J.T. Bacterial Electron Transfer Chains Primed by Proteomics. Adv. Microb. Physiol. 2016, 68, 219–352. [Google Scholar] [PubMed]
- Leelanarathiwat, K.; Katsuta, Y.; Katsuragi, H.; Watanabe, F. Antibacterial activity of blue high-power light-emitting diode-activated flavin mononucleotide against Staphylococcus aureus biofilm on a sandblasted and etched surface. Photodiagnosis Photodyn. Ther. 2020, 31, 101855. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Godfrey, R.; Nazarian, A.; Tam, J.; Drake, L.; Isaacson, B.; Pasquina, P.; Williams, D. Antimicrobial blue light as a biofilm management therapy at the skin-implant interface in an ex vivo percutaneous osseointegrated implant model. J. Orthop. Res. 2023, 41, 2046–2054. [Google Scholar] [PubMed]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updates 2012, 15, 223–236. [Google Scholar] [CrossRef]
- Shany-Kdoshim, S.; Polak, D.; Houri-Haddad, Y.; Feuerstein, O. Killing mechanism of bacteria within multi-species biofilm by blue light. J. Oral Microbiol. 2019, 11, 1628577. [Google Scholar]






| Strain | DSM No. |
|---|---|
| Escherichia coli (K12) | DSM 18039 |
| Pseudomonas aeruginosa | DSM 1128 |
| Staphylococcus aureus | DSM 799 |
| Klebsiella pneumoniae | DSM 789 |
| Constant Mode vs. Control | Cycling Mode vs. Control | |
|---|---|---|
| Total genes screened by microarray | 10,208 | |
| Significantly upregulated genes | 380 (7.48%) | 734 (13.27%) |
| Significantly downregulated genes | 318 (6.20%) | 857 (18.32%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Y.; Shen, Y.; Gretz, N.; Bouschbacher, M.; Miethke, T.; Keese, M. Biphasic Effects of Blue Light Irradiation on Different Drug-Resistant Bacterium and Exploration of Its Mechanism. Biomedicines 2025, 13, 868. https://doi.org/10.3390/biomedicines13040868
Mu Y, Shen Y, Gretz N, Bouschbacher M, Miethke T, Keese M. Biphasic Effects of Blue Light Irradiation on Different Drug-Resistant Bacterium and Exploration of Its Mechanism. Biomedicines. 2025; 13(4):868. https://doi.org/10.3390/biomedicines13040868
Chicago/Turabian StyleMu, Yifei, Yilin Shen, Norbert Gretz, Marielle Bouschbacher, Thomas Miethke, and Michael Keese. 2025. "Biphasic Effects of Blue Light Irradiation on Different Drug-Resistant Bacterium and Exploration of Its Mechanism" Biomedicines 13, no. 4: 868. https://doi.org/10.3390/biomedicines13040868
APA StyleMu, Y., Shen, Y., Gretz, N., Bouschbacher, M., Miethke, T., & Keese, M. (2025). Biphasic Effects of Blue Light Irradiation on Different Drug-Resistant Bacterium and Exploration of Its Mechanism. Biomedicines, 13(4), 868. https://doi.org/10.3390/biomedicines13040868






