Novel mRNA-Engineered Fully Human CAR-T Cells Targeting AXL in Solid Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Ex Vivo Expansion of T Cells
2.3. CAR Construct and mRNA Preparation
2.4. Generation of mfhAXL CAR-T Cells Through Electroporation
2.5. Cytotoxicity Analysis of CAR-T Cells
2.6. Cytokine Secretion Assay by ELISA
2.7. Flow Cytometric Analysis
2.8. In Vivo Animal Experiment
2.9. Statistical Analyses
3. Results
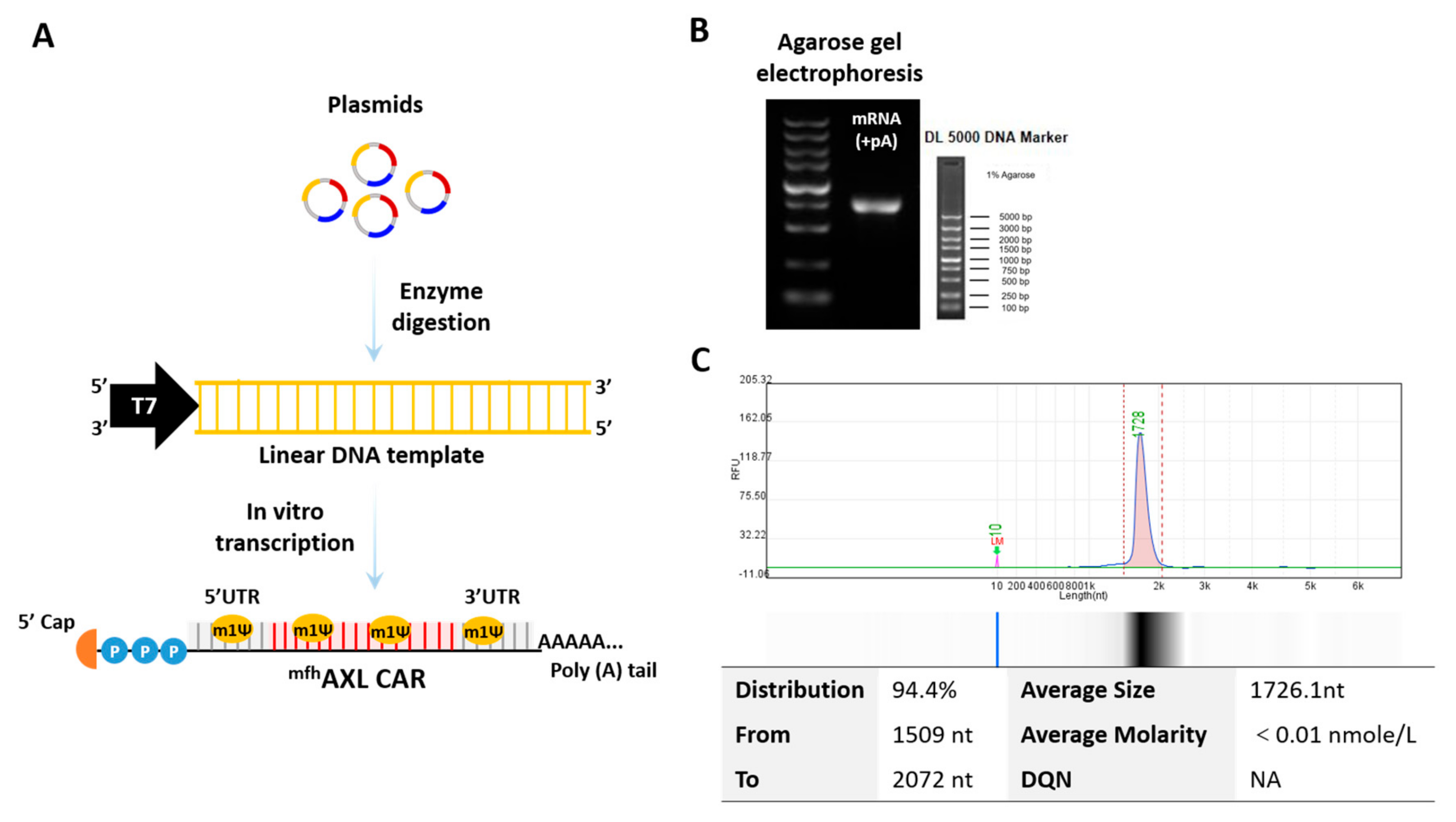
3.1. Features of mRNA Encoding mfhAXL CAR
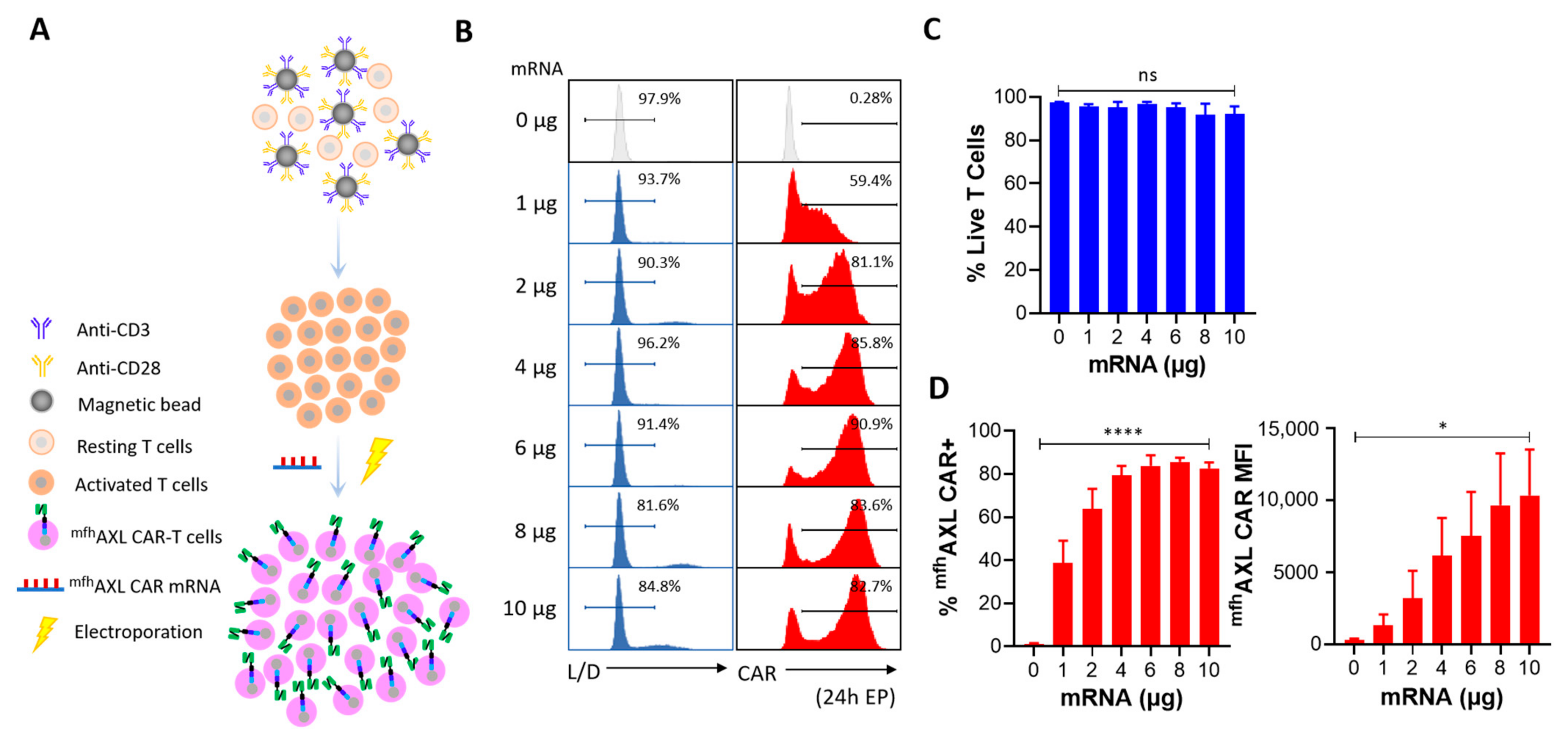
3.2. High Performance of mfhAXL CAR mRNA Delivery Using Electroporation
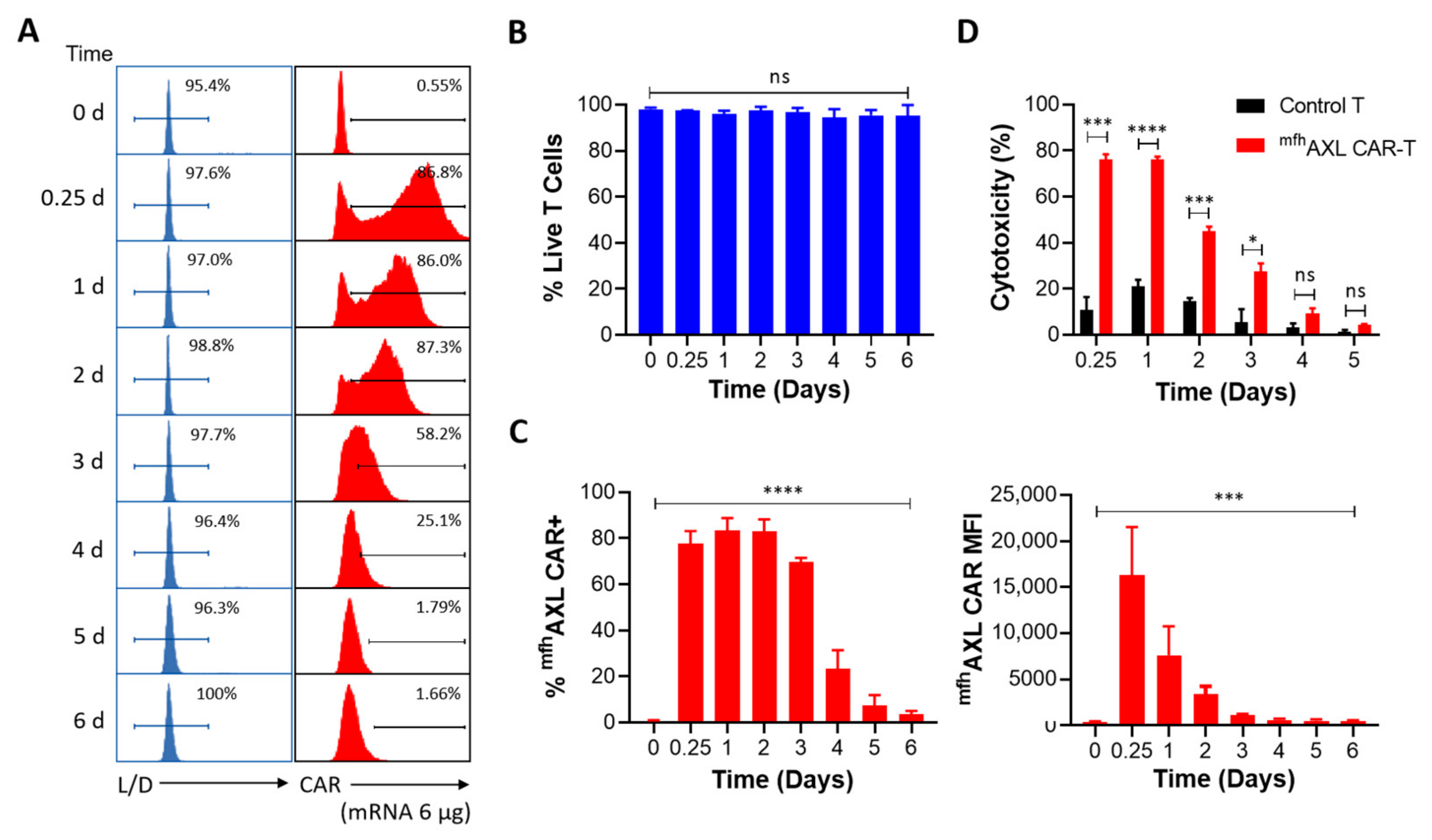
3.3. The Expression and Functional Dynamics of mfhAXL CAR-T Cells
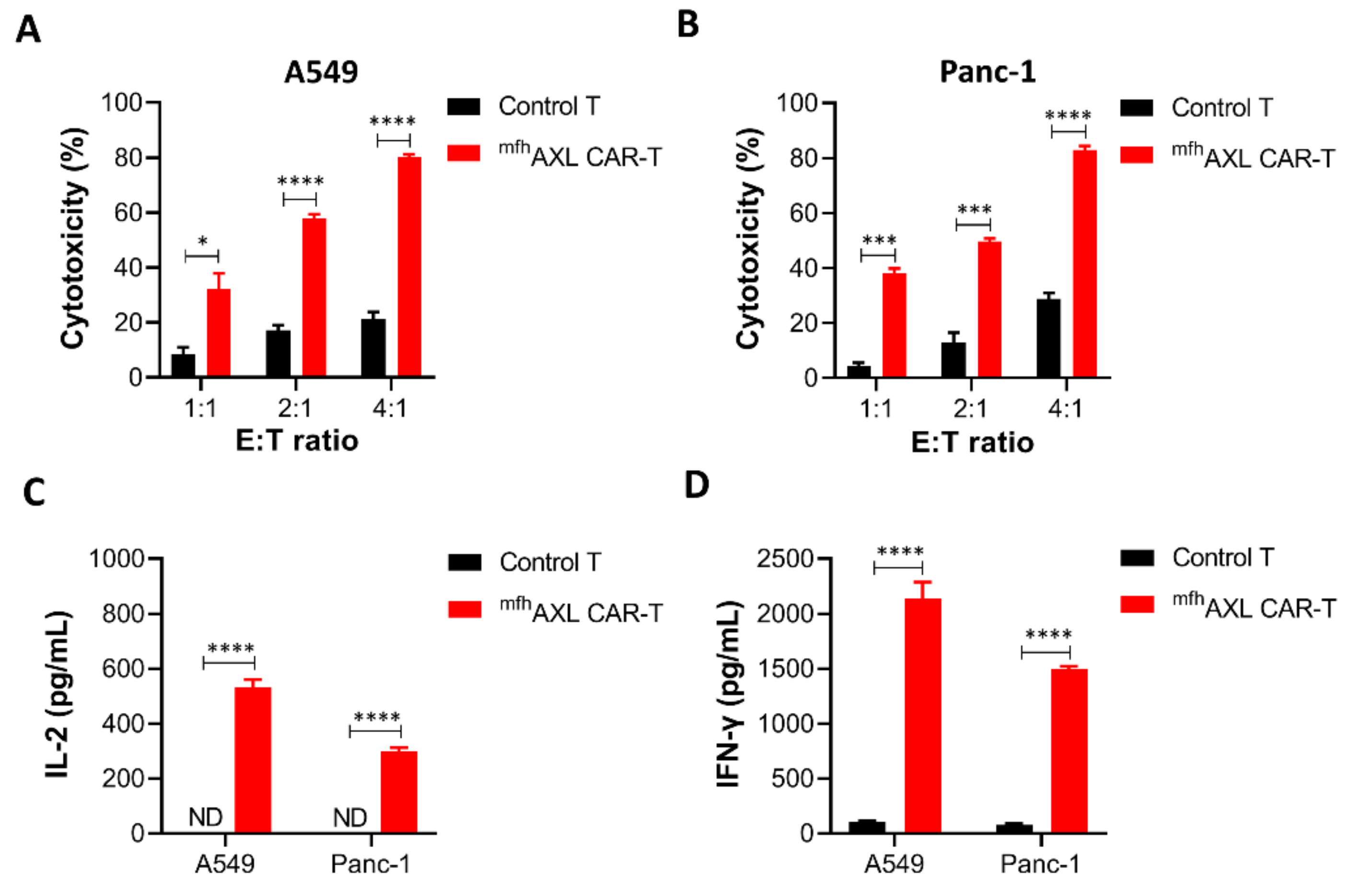
3.4. mfhAXL CAR-T Cells Exhibit Cytotoxic Activity Against Various Cancer Cell Lines and Demonstrate Specific Cytokines Secretion
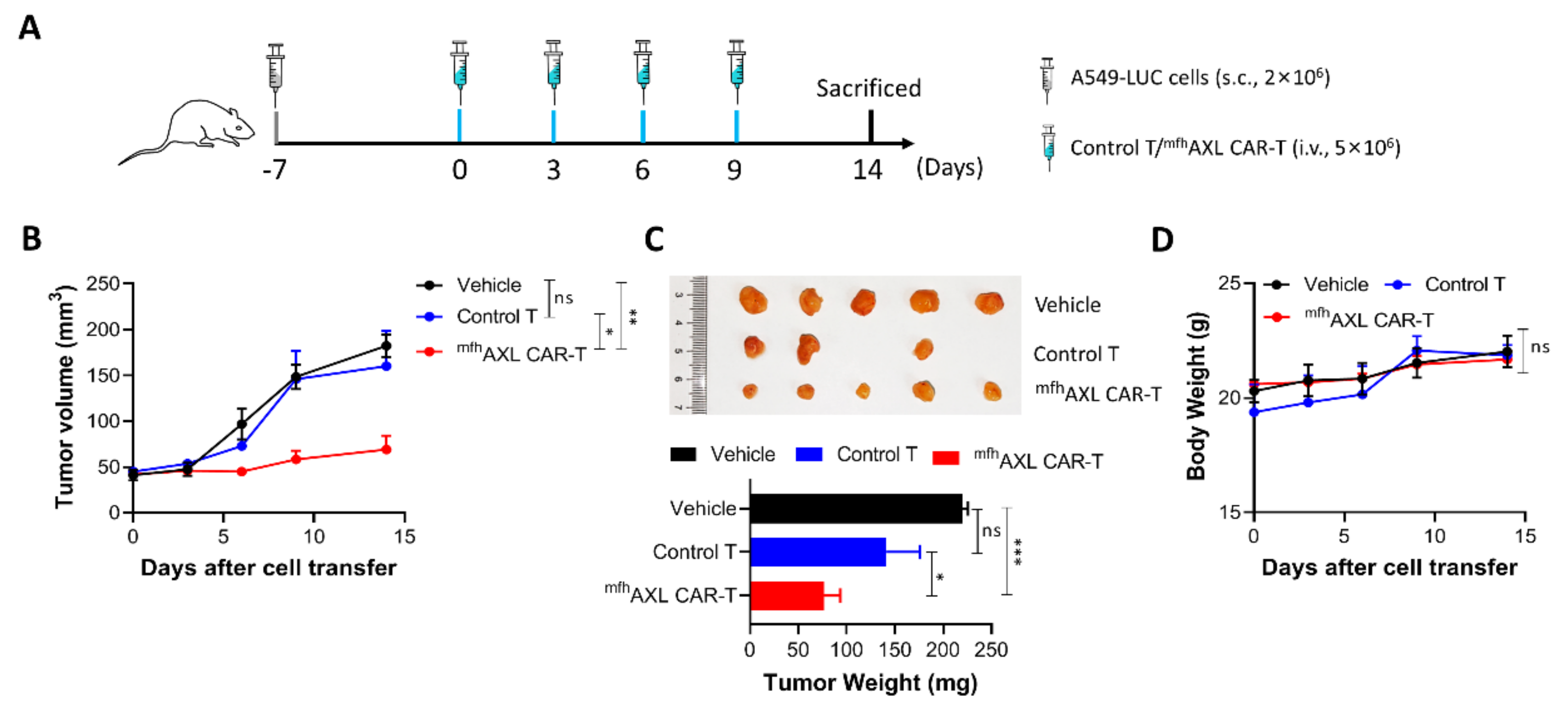
3.5. mfhAXL CAR-T Cells Exhibit Potent In Vivo Anti-Tumor Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2022. J. Natl. Cancer. Cent. 2024, 4, 47–53. [Google Scholar] [PubMed]
- Filin, I.Y.; Solovyeva, V.V.; Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A. Current Trends in Cancer Immunotherapy. Biomedicines 2020, 8, 621. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, G. The journey of CAR-T therapy in hematological malignancies. Mol. Cancer 2022, 21, 1–15. [Google Scholar] [CrossRef]
- Linger, R.M.; Keating, A.K.; Earp, H.S.; Graham, D.K. TAM receptor tyrosine kinases: Biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv. Cancer Res. 2008, 100, 35–83. [Google Scholar]
- Ruan, G.-X.; Kazlauskas, A. Axl is essential for VEGF-A-dependent activation of PI3K/Akt. EMBO J. 2012, 31, 1692–1703. [Google Scholar] [CrossRef]
- Demarchi, F.; Verardo, R.; Varnum, B.; Brancolini, C.; Schneider, C. Gas6 anti-apoptotic signaling requires NF-kappa B activation. J. Biol. Chem. 2001, 276, 31738–31744. [Google Scholar]
- Namba, K.; Shien, K.; Takahashi, Y.; Torigoe, H.; Sato, H.; Yoshioka, T.; Takeda, T.; Kurihara, E.; Ogoshi, Y.; Yamamoto, H.; et al. Activation of AXL as a Preclinical Acquired Resistance Mechanism Against Osimertinib Treatment in EGFR-Mutant Non–Small Cell Lung Cancer Cells. Mol. Cancer Res. 2019, 17, 499–507. [Google Scholar] [CrossRef]
- Goyette, M.A.; Côté, J.F. AXL Receptor Tyrosine Kinase as a Promising Therapeutic Target Directing Multiple Aspects of Cancer Progression and Metastasis. Cancers 2022, 14, 466. [Google Scholar] [CrossRef]
- Osipov, A.; Murphy, A.; Zheng, L. From Immune Checkpoints to Vaccines: The Past, Present and Future of Cancer Immunotherapy. Adv. Cancer. Res. 2019, 143, 63–144. [Google Scholar]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef]
- Loeuillard, E.; Yang, J.; Buckarma, E.; Wang, J.; Liu, Y.; Conboy, C.; Pavelko, K.D.; Li, Y.; O’brien, D.; Wang, C.; et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J. Clin. Investig. 2020, 130, 5380–5396. [Google Scholar] [CrossRef] [PubMed]
- Holtzhausen, A.; Harris, W.; Ubil, E.; Hunter, D.M.; Zhao, J.; Zhang, Y.; Zhang, D.; Liu, Q.; Wang, X.; Graham, D.K.; et al. TAM Family Receptor Kinase Inhibition Reverses MDSC-Mediated Suppression and Augments Anti-PD-1 Therapy in Melanoma. Cancer Immunol. Res. 2019, 7, 1672–1686. [Google Scholar] [PubMed]
- Nokihara, H.; Nishio, M.; Yamamoto, N.; Fujiwara, Y.; Horinouchi, H.; Kanda, S.; Horiike, A.; Ohyanagi, F.; Yanagitani, N.; Nguyen, L.; et al. Phase 1 Study of Cabozantinib in Japanese Patients with Expansion Cohorts in Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2019, 20, e317–e328. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Drappatz, J.; de Groot, J.; Prados, M.D.; Reardon, D.A.; Schiff, D.; Chamberlain, M.; Mikkelsen, T.; Desjardins, A.; Ping, J.; et al. Phase II study of cabozantinib in patients with progressive glioblastoma: Subset analysis of patients with prior antiangiogenic therapy. Neuro. Oncol. 2018, 20, 259–267. [Google Scholar]
- Onken, J.; Torka, R.; Korsing, S.; Radke, J.; Krementeskaia, I.; Nieminen, M.; Bai, X.; Ullrich, A.; Heppner, F.; Vajkoczy, P. Inhibiting receptor tyrosine kinase AXL with small molecule inhibitor BMS-777607 reduces glioblastoma growth, migration, and invasion in vitro and in vivo. Oncotarget 2016, 7, 9876–9889. [Google Scholar] [CrossRef]
- Ye, X.; Li, Y.; Stawicki, S.; Couto, S.; Eastham-Anderson, J.; Kallop, D.; Weimer, R.; Wu, Y.; Pei, L. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene 2010, 29, 5254–5264. [Google Scholar]
- Wei, J.; Sun, H.; Zhang, A.; Wu, X.; Li, Y.; Liu, J.; Duan, Y.; Xiao, F.; Wang, H.; Lv, M.; et al. A novel AXL chimeric antigen receptor endows T cells with anti-tumor effects against triple negative breast cancers. Cell. Immunol. 2018, 331, 49–58. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Liu, W.; Li, X. Engineered IL-7 Receptor Enhances the Therapeutic Effect of AXL-CAR-T Cells on Tri-ple-Negative Breast Cancer. Biomed. Res. Int. 2020, 2020, 4795171. [Google Scholar]
- Cao, B.; Liu, M.; Wang, L.; Zhu, K.; Cai, M.; Chen, X.; Feng, Y.; Yang, S.; Fu, S.; Zhi, C.; et al. Remodelling of tumour microenvironment by microwave ablation potentiates immunotherapy of AXL-specific CAR T cells against non-small cell lung cancer. Nat. Commun. 2022, 13, 6203. [Google Scholar]
- Fan, J.; Yu, Y.; Yan, L.; Yuan, Y.; Sun, B.; Yang, D.; Liu, N.; Guo, J.; Zhang, J.; Zhao, X. GAS6-based CAR-T cells exhibit potent antitumor activity against pancreatic cancer. J. Hematol. Oncol. 2023, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Chiappella, A.; Casadei, B.; Bramanti, S.; Ljevar, S.; Chiusolo, P.; Di Rocco, A.; Tisi, M.C.; Barbui, A.M.; Fa-rina, M.; et al. Secondary primary malignancies after CD-19 directed CAR-T-cell therapy in lymphomas: A report from the Italian CART-SIE study. Br. J. Haematol. 2024, 205, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Berg, P.; Ruppert-Seipp, G.; Müller, S.; Maurer, G.D.; Hartmann, J.; Holtick, U.; Buchholz, C.J.; Funk, M.B. CAR T-cell-associated secondary malignancies challenge current pharmacovigilance concepts. EMBO Mol. Med. 2025, 17, 211–218. [Google Scholar] [CrossRef]
- Cao, J.; Wang, G.; Cheng, H.; Wei, C.; Qi, K.; Sang, W.; Zhenyu, L.; Shi, M.; Li, H.; Qiao, J.; et al. Potent anti-leukemia activities of humanized CD19-targeted Chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am. J. Hematol. 2018, 93, 851–858. [Google Scholar] [CrossRef]
- Guo, J.; He, S.; Zhu, Y.; Yu, W.; Yang, D.; Zhao, X. Humanized CD30-Targeted Chimeric Antigen Receptor T Cells Exhibit Potent Preclinical Activity Against Hodgkin’s Lymphoma Cells. Front. Cell Dev. Biol. 2022, 9, 775599. [Google Scholar] [CrossRef]
- Wu, S.; Luo, Q.; Li, F.; Zhang, S.; Zhang, C.; Liu, J.; Shao, B.; Hong, Y.; Tan, T.; Dong, X.; et al. Development of novel humanized CD19/BAFFR bicistronic chimeric antigen receptor T cells with potent antitumor activity against B-cell lineage neoplasms. Br. J. Haematol. 2024, 205, 1361–1373. [Google Scholar]
- Song, F.; Hu, Y.; Zhang, Y.; Zhang, M.; Yang, T.; Wu, W.; Huang, S.; Xu, H.; Chang, A.H.; Huang, H.; et al. Safety and efficacy of autologous and allogeneic humanized CD19-targeted CAR-T cell therapy for patients with relapsed/refractory B-ALL. J. Immunother. Cancer 2023, 11, e005701. [Google Scholar]
- Metanat, Y.; Viktor, P.; Amajd, A.; Kaur, I.; Hamed, A.M.; Al-Abadi, N.K.A.; Alwan, N.H.; Chaitanya, M.; Lakshmaiya, N.; Ghildiyal, P.; et al. The paths toward non-viral CAR-T cell manufacturing: A comprehensive review of state-of-the-art methods. Life Sci. 2024, 348, 122683. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Ding, X.; Liao, Q. Antibody targeting AXL protein and antigen binding fragment thereof, and preparation method thereof and application thereof. European patent EP 4357365 A1, 16 June 2024. [Google Scholar]
- Bai, S.; Yang, T.; Zhu, C.; Feng, M.; Zhang, L.; Zhang, Z.; Wang, X.; Yu, R.; Pan, X.; Zhao, C.; et al. A single vaccination of nucleoside-modified Rabies mRNA vaccine induces prolonged highly protective immune responses in mice. Front. Immunol. 2023, 13, 1099991. [Google Scholar] [CrossRef]
- Khan, S.H.; Choi, Y.; Veena, M.; Lee, J.K.; Shin, D.S. Advances in CAR T cell therapy: Antigen selection, modifications, and current trials for solid tumors. Front. Immunol. 2025, 15, 1489827. [Google Scholar] [CrossRef]
- Maalej, K.M.; Merhi, M.; Inchakalody, V.P.; Mestiri, S.; Alam, M.; Maccalli, C.; Cherif, H.; Uddin, S.; Steinhoff, M.; Marincola, F.M.; et al. CAR-cell therapy in the era of solid tumor treatment: Current challenges and emerging therapeutic advances. Mol. Cancer 2023, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Miliotou, A.N.; Papadopoulou, L.C. In Vitro-Transcribed (IVT)-mRNA CAR Therapy Development. Methods Mol. Biol. 2020, 2086, 87–117. [Google Scholar] [PubMed]
- Rajan, T.S.; Gugliandolo, A.; Bramanti, P.; Mazzon, E. In Vitro-Transcribed mRNA Chimeric Antigen Receptor T Cell (IVT mRNA CAR T) Therapy in Hematologic and Solid Tumor Management: A Preclinical Update. Int. J. Mol. Sci. 2020, 21, 6514. [Google Scholar] [CrossRef] [PubMed]
- Kitte, R.; Rabel, M.; Geczy, R.; Park, S.; Fricke, S.; Koehl, U.; Tretbar, U.S. Lipid nanoparticles outperform electroporation in mRNA-based CAR T cell engineering. Mol. Ther. Methods. Clin. Dev. 2023, 31, 101139. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, C.; Zhang, Y.; Fan, Y.; Zhang, J.; Lin, X.; Guo, T.; Gu, Y.; Wu, J.; Gao, J.; et al. CD19/CD20 dual-targeted chimeric antigen receptor-engineered natural killer cells exhibit improved cytotoxicity against acute lymphoblastic leukemia. J. Transl. Med. 2024, 22, 274. [Google Scholar]
- Birkholz, K.; Hombach, A.; Krug, C.; Reuter, S.; Kershaw, M.; Kämpgen, E.; Schuler, G.; Abken, H.; Schaft, N.; Dörrie, J. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4+ and CD8+ T cells for use in the adoptive immunotherapy of cancer. Gene Ther. 2009, 16, 596–604. [Google Scholar] [CrossRef]
- Shah, P.D.; Huang, A.C.; Xu, X.; Orlowski, R.; Amaravadi, R.K.; Schuchter, L.M.; Zhang, P.; Tchou, J.; Matlawski, T.; Cervini, A.; et al. Phase I Trial of Autologous RNA-electroporated cMET-directed CAR T Cells Administered Intravenously in Patients with Melanoma and Breast Carcinoma. Cancer. Res. Commun. 2023, 3, 821–829. [Google Scholar]
- Beatty, G.L.; O’hara, M.H.; Lacey, S.F.; Torigian, D.A.; Nazimuddin, F.; Chen, F.; Kulikovskaya, I.M.; Soulen, M.C.; McGarvey, M.; Nelson, A.M.; et al. Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells Against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology 2018, 155, 29–32. [Google Scholar] [CrossRef]
- Campillo-Davo, D.; De Laere, M.; Roex, G.; Versteven, M.; Flumens, D.; Berneman, Z.N.; Van Tendeloo, V.F.I.; Anguille, S.; Lion, E. The Ins and Outs of Messenger RNA Electroporation for Physical Gene Delivery in Immune Cell-Based Therapy. Pharmaceutics 2021, 13, 396. [Google Scholar] [CrossRef]
- Ng, Y.-Y.; Tay, J.C.; Li, Z.; Wang, J.; Zhu, J.; Wang, S. T Cells Expressing NKG2D CAR with a DAP12 Signaling Domain Stimulate Lower Cytokine Production While Effective in Tumor Eradication. Mol. Ther. 2020, 29, 75–85. [Google Scholar] [CrossRef]
- Pennell, C.A.; Campbell, H.; Storlie, M.D.; Bolivar-Wagers, S.; Osborn, M.J.; Refaeli, Y.; Jensen, M.; Viaud, S.; Young, T.S.; Blazar, B.R. Human CD19-specific switchable CAR T-cells are efficacious as constitutively active CAR T-cells but cause less morbidity in a mouse model of human CD19+ malignancy. J. Immunother. Cancer 2022, 10, e005934. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, I.; Lasarte-Cia, A.; Martín-Otal, C.; Casares, N.; Navarro, F.; Gorraiz, M.; Sarrión, P.; Hervas-Stubbs, S.; Jordana, L.; Rodriguez-Madoz, J.R.; et al. Tethered IL15-IL15Rα augments antitumor activity of CD19 CAR-T cells but displays long-term toxicity in an immunocompetent lymphoma mouse model. J. Immunother. Cancer 2024, 12, e008572. [Google Scholar] [CrossRef] [PubMed]
- Meister, H.; Look, T.; Roth, P.; Pascolo, S.; Sahin, U.; Lee, S.; Hale, B.D.; Snijder, B.; Regli, L.; Ravi, V.M.; et al. Multi-functional mRNA-Based CAR T Cells Display Promising Antitumor Activity Against Glioblastoma. Clin. Cancer Res. 2022, 28, 4747–4756. [Google Scholar] [PubMed]
- Hamilton, A.G.; Swingle, K.L.; Joseph, R.A.; Mai, D.; Gong, N.; Billingsley, M.M.; Alameh, M.; Weissman, D.; Sheppard, N.C.; June, C.H.; et al. Ionizable Lipid Nanoparticles with Integrated Immune Checkpoint Inhibition for mRNA CAR T Cell Engineering. Adv. Health Mater. 2023, 12, e2301515. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, B.; Wang, M.; Bai, S.; Li, N.; Fan, Z.; Peng, Y.; Han, M.; Zeng, C.; Lu, H.; Qi, L.; et al. Novel mRNA-Engineered Fully Human CAR-T Cells Targeting AXL in Solid Tumors. Biomedicines 2025, 13, 844. https://doi.org/10.3390/biomedicines13040844
Zou B, Wang M, Bai S, Li N, Fan Z, Peng Y, Han M, Zeng C, Lu H, Qi L, et al. Novel mRNA-Engineered Fully Human CAR-T Cells Targeting AXL in Solid Tumors. Biomedicines. 2025; 13(4):844. https://doi.org/10.3390/biomedicines13040844
Chicago/Turabian StyleZou, Bo, Mengge Wang, Shimeng Bai, Ning Li, Zhongyi Fan, Yuanzheng Peng, Mingshu Han, Chen Zeng, Hongzhou Lu, Lin Qi, and et al. 2025. "Novel mRNA-Engineered Fully Human CAR-T Cells Targeting AXL in Solid Tumors" Biomedicines 13, no. 4: 844. https://doi.org/10.3390/biomedicines13040844
APA StyleZou, B., Wang, M., Bai, S., Li, N., Fan, Z., Peng, Y., Han, M., Zeng, C., Lu, H., Qi, L., Zhang, X., Tan, X., & Liao, Q. (2025). Novel mRNA-Engineered Fully Human CAR-T Cells Targeting AXL in Solid Tumors. Biomedicines, 13(4), 844. https://doi.org/10.3390/biomedicines13040844






