Metabolic and Structural Consequences of GM3 Synthase Deficiency: Insights from an HEK293-T Knockout Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antibodies
2.3. Cell Culture
2.4. Cell Culture Starvation
2.5. Labeling of Cell Sphingolipids with [1-3H]-Sphingosine
2.6. Protein Content Determination
2.7. Protein Analysis
2.8. Isolation of Detergent-Resistant Membrane (DRM) Fractions
2.9. Analysis of the Endogenous Lipid Patterns
Sialidase Treatment
2.10. Analysis of the Radioactive Lipid Patterns
2.11. Evaluation of Enzymatic Activities in Cell Lysates
2.12. Evaluation of Enzymatic Activity on the Surface of Living Cells
2.13. Immunofluoresce Analysis
2.14. ATP Conent Analysis
2.15. Statistical Analysis
3. Results
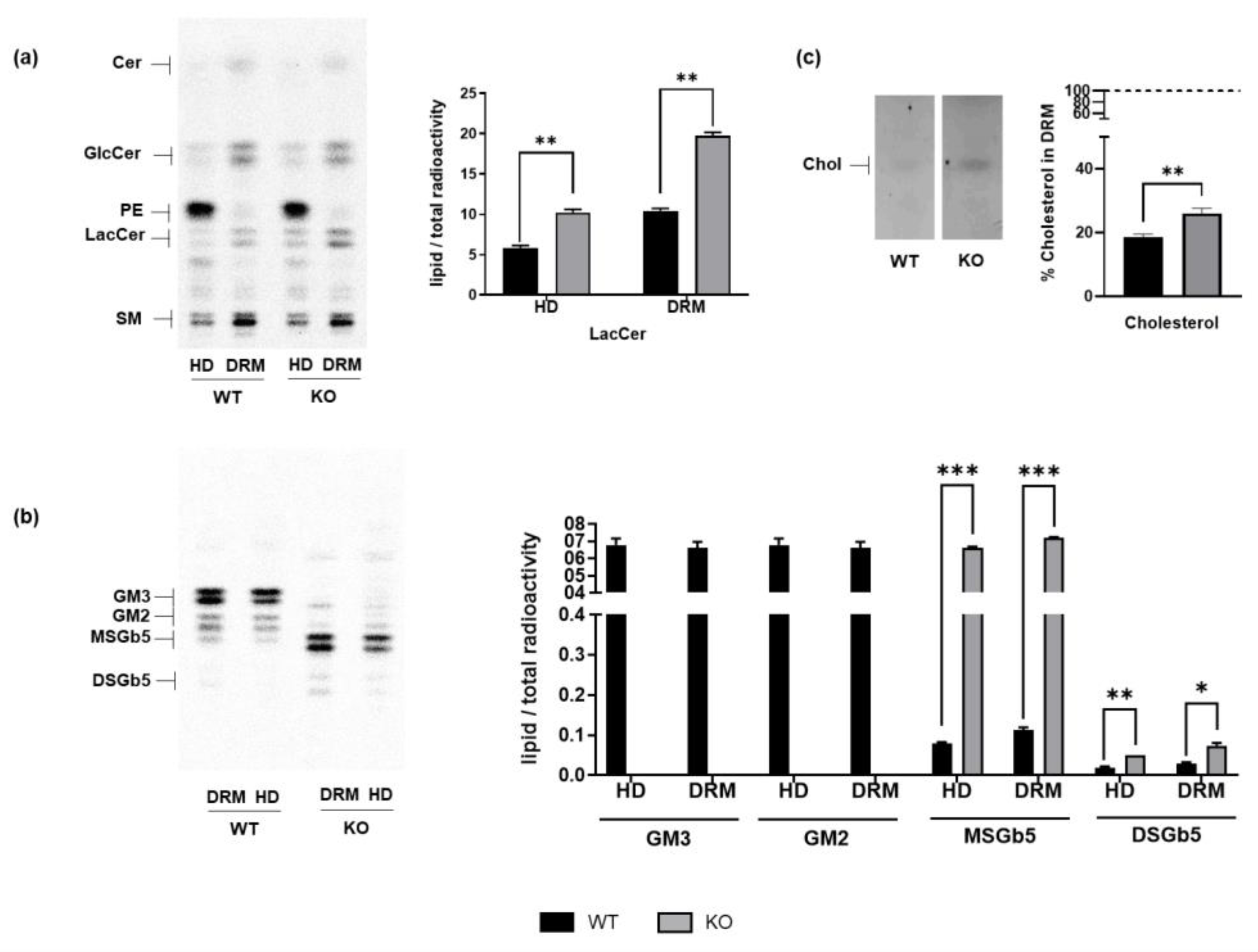
3.1. GM3SD Induces Changes in Lipid Composition
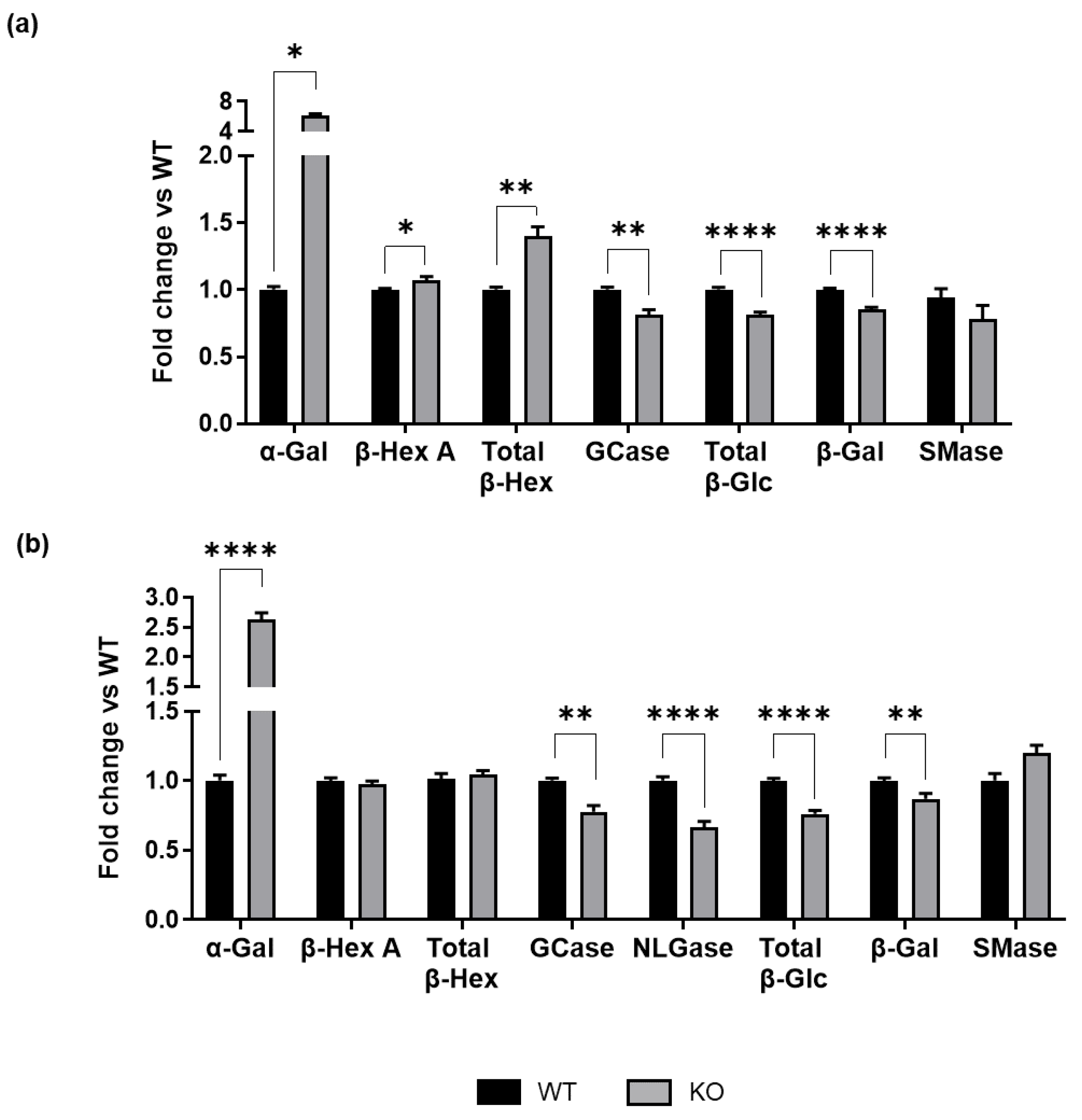
3.2. GM3SD Determines Alterations in Glycosphingolipid Glycohydrolase Activity
3.3. GM3SD Prompts a Secondary Alteration in the Lysosomal Compartment
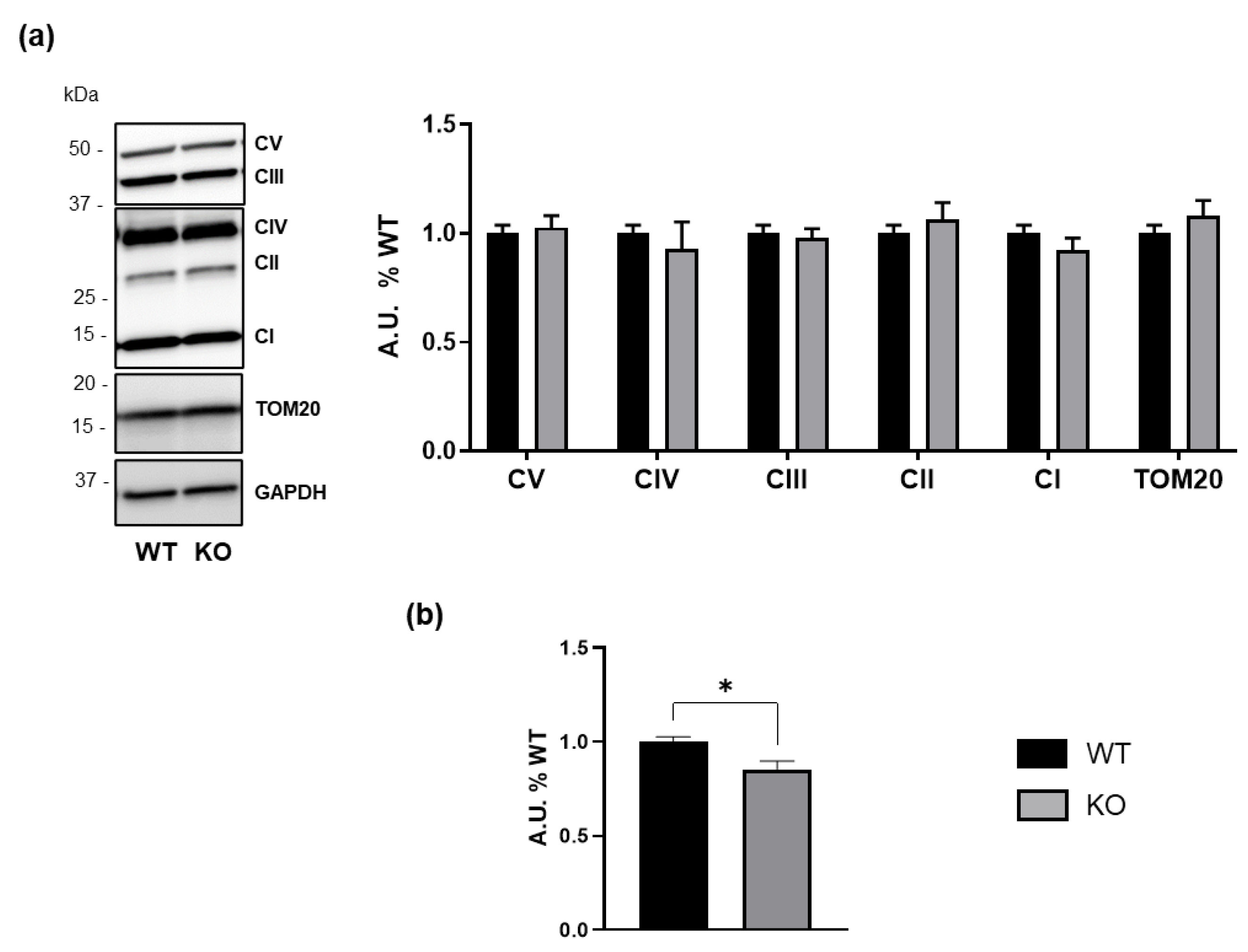
3.4. GM3SD Induces Bioenergetic Deficiency
4. Discussion
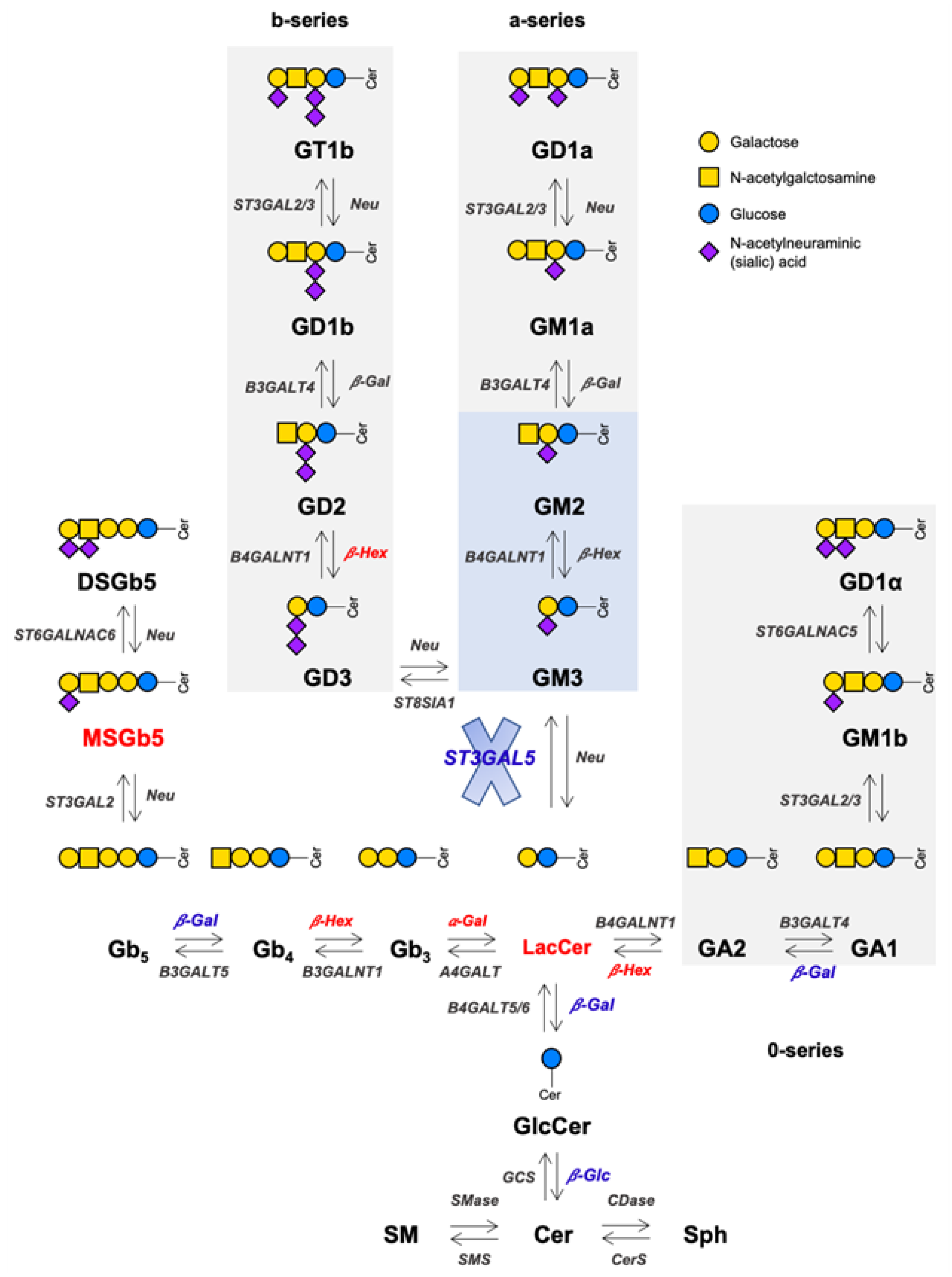
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| The glycosphingolipid nomenclature is in accordance with IUPAC-IUBB recommendations [50]. | |
| α-Gal | Alpha-galactosidase |
| ATP | Adenosine triphosphate |
| A.U. | Arbitrary unit |
| β-Gal | Beta-galactosidase |
| β-Glc | Beta-glucosidase |
| β-Hex | Beta-hexosaminidase |
| β-Man | Beta-mannosidase |
| BSA | Bovine serum albumin |
| DMEM | Dulbecco’s modified eagles’ medium |
| d.p.m. | Disintegrations per minute |
| DSGb5 | Neu5Acα3Galβ3(NeuAcα6)GalNacβ3Galα4Galβ1GlcCer, disialyl-Gb5 globoside, disialyl-globopentaosylceramide |
| DRM | Detergent-resistant membrane |
| EDTA | Ethylenediaminetetraacetic acid |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GA2 | GalNacβ4Galβ4GlcCer |
| GA1 | Galβ3GalNacβ4Galβ4GlcCer |
| Gb3 | Galα4Galβ1GlcCer, Gb3 globoside, globotriaosylceramide |
| Gb4 | GalNacβ3Galα4Galβ1GlcCer, Gb4 globoside, globotetraosylceramide |
| Gb5 | Galβ3GalNacβ3Galα4Galβ1GlcCer, Gb5 globoside |
| Gb3 | Galα4Galβ1GlcCer, Gb3 globoside |
| GCase | Beta-glucocerebrosidase |
| GD1a | Neu5Acα3Galβ3GalNAcβ4(Neu5Acα3)Galβ4GlcCer, GD1a ganglioside |
| GD1b | Galβ3GalNAcβ4(Neu5Acα8Neu5Acα3)Galβ4GlcCer, GD1b ganglioside |
| GD1α | Neu5Acα3Galβ3(Neu5Acα6)GalNAcβ4Galβ4GlcCer, GD1 alpha ganglioside |
| GD2 | GalNAcβ4(Neu5Acα8Neu5Acα3)Galβ4GlcCer, GD2 ganglioside |
| GD3 | Neu5Acα8(Neu5Acα3)Galβ4GlcCer, GD3 ganglioside |
| GlcCer | Glucosylceramide |
| GM1a | Galβ3GalNAcβ4(Neu5Acα3)Galβ4GlcCer, GM1a ganglioside |
| GM1b | Neu5Acα3Galβ3GalNAcβ4Galβ4GlcCer, GM1b ganglioside |
| GM2 | GalNAcβ4(Neu5Acα3)Galβ4GlcCer, GM2 ganglioside |
| GM3 | Neu5Acα3Galβ4GlcCer, GM3 ganglioside |
| GM3S | GM3 synthase |
| GM3SD | GM3 Synthase Deficiency |
| GT1b | Neu5Acα3Galβ3GalNAcβ4(Neu5Acα8Neu5Acα3)Galβ4GlcCer, GT1b ganglioside |
| HMUB-PC | 6-hexadecanoylamino 4-MU-phosphoryl-choline |
| HD | High-density fractions |
| HEK293-T | Human embryonic kidney 293-T |
| HPTLC | High-performance thin-layer chromatography |
| IntDen | Integrated density |
| KO | Knockout |
| LC3 | Microtubule-associated proteins 1A/1B light chain 3A |
| LacCer | Galβ4GlcCer, lactosylceramide |
| Lamp-1 | Lysosomal associated membrane protein 1 |
| LC3 | Microtubule-associated proteins 1A/1B light chain 3A |
| MSGb5 | Neu5Acα3Galβ3GalNacβ3Galα4Galβ1GlcCer, monosialyl-globoside Gb5, monosialyl-globopentaosylceramide |
| MUB-α-Gal | 4-methylumbelliferyl α-D-galactopyranoside |
| MUB-β-Gal | 4-methylumbelliferyl β-D-galactopyranoside |
| MUB-β-Glc | 4-methylumbelliferyl β-D-glucopyranoside |
| MUB-β-Man | 4-methylumbelliferyl-β-D-mannopyranoside |
| MUG | 4-methylumbelliferyl N-acetyl-β-D-glucosaminide |
| MUGS | 4-methylumbelliferyl N-acetyl-β-D-glucosaminide-6-sulfate |
| NaCl | Sodium chloride |
| Na3VO4 | Sodium orthovanadate |
| NLGase | Non-lysosomal glucosylcerebrosidase |
| Oxphos | Oxidative phosphorylation |
| PBS | Phosphate-buffered saline |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PI | Phosphatidylinositol |
| PM | Plasma membrane |
| PMSF | Phenylmethanesulfonyl fluoride |
| Prp | Prion protein |
| PS | Phosphatidylserine |
| PVDF | Polyvinylidene difluoride |
| p62 | Ubiquitin-binding protein p62 |
| RRID | Research resource identifier |
| SDS | Sodium dodecyl sulfate |
| SM | Sphingomyelin |
| SEM | Standard error of the mean |
| SMAse | Sphingomyelinase |
| TBS-T | Tris-buffered saline containing 0.1% tween-20 |
| TOM20 | Translocase of outer mitochondrial membrane 20 |
| TX-100 | Triton X-100 |
| WB | Western blotting |
| WT | Wild type |
References
- Ledeen, R.; Wu, G. Gangliosides of the Nervous System. Methods Mol. Biol. 2018, 1804, 19–55. [Google Scholar] [CrossRef] [PubMed]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef]
- Ohmi, Y.; Ohkawa, Y.; Yamauchi, Y.; Tajima, O.; Furukawa, K.; Furukawa, K. Essential roles of gangliosides in the formation and maintenance of membrane microdomains in brain tissues. Neurochem. Res. 2012, 37, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef]
- D’Angelo, G.; Capasso, S.; Sticco, L.; Russo, D. Glycosphingolipids: Synthesis and functions. FEBS J. 2013, 280, 6338–6353. [Google Scholar] [CrossRef]
- Sandhoff, R.; Sandhoff, K. Emerging concepts of ganglioside metabolism. FEBS Lett. 2018, 592, 3835–3864. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Yu, R.K.; Nakatani, Y.; Yanagisawa, M. The role of glycosphingolipid metabolism in the developing brain. J. Lipid Res. 2009, 50, S440–S445. [Google Scholar] [CrossRef]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef]
- Li, T.A.; Schnaar, R.L. Congenital Disorders of Ganglioside Biosynthesis. Prog. Mol. Biol. Transl. Sci. 2018, 156, 63–82. [Google Scholar] [CrossRef]
- Simpson, M.A.; Cross, H.; Proukakis, C.; Priestman, D.A.; Neville, D.C.; Reinkensmeier, G.; Wang, H.; Wiznitzer, M.; Gurtz, K.; Verganelaki, A.; et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat. Genet. 2004, 36, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Fragaki, K.; Ait-El-Mkadem, S.; Chaussenot, A.; Gire, C.; Mengual, R.; Bonesso, L.; Beneteau, M.; Ricci, J.E.; Desquiret-Dumas, V.; Procaccio, V.; et al. Refractory epilepsy and mitochondrial dysfunction due to GM3 synthase deficiency. Eur. J. Hum. Genet. 2013, 21, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Yoo, Y.; Lim, B.C.; Kim, K.J.; Song, J.; Choi, M.; Chae, J.H. GM3 synthase deficiency due to ST3GAL5 variants in two Korean female siblings: Masquerading as Rett syndrome-like phenotype. Am. J. Med. Genet. A 2016, 170, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Heide, S.; Jacquemont, M.L.; Cheillan, D.; Renouil, M.; Tallot, M.; Schwartz, C.E.; Miquel, J.; Bintner, M.; Rodriguez, D.; Darcel, F.; et al. GM3 synthase deficiency in non-Amish patients. Genet. Med. 2022, 24, 492–498. [Google Scholar] [CrossRef]
- Boccuto, L.; Aoki, K.; Flanagan-Steet, H.; Chen, C.F.; Fan, X.; Bartel, F.; Petukh, M.; Pittman, A.; Saul, R.; Chaubey, A.; et al. A mutation in a ganglioside biosynthetic enzyme, ST3GAL5, results in salt & pepper syndrome, a neurocutaneous disorder with altered glycolipid and glycoprotein glycosylation. Hum. Mol. Genet. 2014, 23, 418–433. [Google Scholar] [CrossRef]
- Bowser, L.E.; Young, M.; Wenger, O.K.; Ammous, Z.; Brigatti, K.W.; Carson, V.J.; Moser, T.; Deline, J.; Aoki, K.; Morlet, T.; et al. Recessive GM3 synthase deficiency: Natural history, biochemistry, and therapeutic frontier. Mol. Genet. Metab. 2019, 126, 475–488. [Google Scholar] [CrossRef]
- Wang, H.; Wang, A.; Wang, D.; Bright, A.; Sency, V.; Zhou, A.; Xin, B. Early growth and development impairments in patients with ganglioside GM3 synthase deficiency. Clin. Genet. 2016, 89, 625–629. [Google Scholar] [CrossRef]
- Inamori, K.; Inokuchi, J. Ganglioside GM3 Synthase Deficiency in Mouse Models and Human Patients. Int. J. Mol. Sci. 2022, 23, 5368. [Google Scholar] [CrossRef]
- Shaw, G.; Morse, S.; Ararat, M.; Graham, F.L. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002, 16, 869–871. [Google Scholar] [CrossRef]
- Nihei, W.; Nagafuku, M.; Hayamizu, H.; Odagiri, Y.; Tamura, Y.; Kikuchi, Y.; Veillon, L.; Kanoh, H.; Inamori, K.I.; Arai, K.; et al. NPC1L1-dependent intestinal cholesterol absorption requires ganglioside GM3 in membrane microdomains. J. Lipid Res. 2018, 59, 2181–2187. [Google Scholar] [CrossRef]
- Schiumarini, D.; Loberto, N.; Mancini, G.; Bassi, R.; Giussani, P.; Chiricozzi, E.; Samarani, M.; Munari, S.; Tamanini, A.; Cabrini, G.; et al. Evidence for the Involvement of Lipid Rafts and Plasma Membrane Sphingolipid Hydrolases in Pseudomonas aeruginosa Infection of Cystic Fibrosis Bronchial Epithelial Cells. Mediators Inflamm. 2017, 2017, 1730245. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Ciampa, M.G.; Brasile, G.; Compostella, F.; Prinetti, A.; Nakayama, H.; Ekyalongo, R.C.; Iwabuchi, K.; Sonnino, S.; Mauri, L. Direct interaction, instrumental for signaling processes, between LacCer and Lyn in the lipid rafts of neutrophil-like cells. J. Lipid Res. 2015, 56, 129–141. [Google Scholar] [CrossRef]
- Valsecchi, M.; Cazzetta, V.; Oriolo, F.; Lan, X.; Piazza, R.; Saleem, M.A.; Singhal, P.C.; Mavilio, D.; Mikulak, J.; Aureli, M. APOL1 polymorphism modulates sphingolipid profile of human podocytes. Glycoconj. J. 2020, 37, 729–744. [Google Scholar] [CrossRef]
- Prinetti, A.; Prioni, S.; Chiricozzi, E.; Schuchman, E.H.; Chigorno, V.; Sonnino, S. Secondary alterations of sphingolipid metabolism in lysosomal storage diseases. Neurochem. Res. 2011, 36, 1654–1668. [Google Scholar] [CrossRef]
- Samarani, M.; Loberto, N.; Solda, G.; Straniero, L.; Asselta, R.; Duga, S.; Lunghi, G.; Zucca, F.A.; Mauri, L.; Ciampa, M.G.; et al. A lysosome-plasma membrane-sphingolipid axis linking lysosomal storage to cell growth arrest. FASEB J. 2018, 32, 5685–5702. [Google Scholar] [CrossRef]
- Aureli, M.; Loberto, N.; Lanteri, P.; Chigorno, V.; Prinetti, A.; Sonnino, S. Cell surface sphingolipid glycohydrolases in neuronal differentiation and aging in culture. J. Neurochem. 2011, 116, 891–899. [Google Scholar] [CrossRef]
- Loberto, N.; Lunghi, G.; Schiumarini, D.; Samarani, M.; Chiricozzi, E.; Aureli, M. Methods for Assay of Ganglioside Catabolic Enzymes. Methods Mol. Biol. 2018, 1804, 383–400. [Google Scholar] [CrossRef]
- Fazzari, M.; Audano, M.; Lunghi, G.; Di Biase, E.; Loberto, N.; Mauri, L.; Mitro, N.; Sonnino, S.; Chiricozzi, E. The oligosaccharide portion of ganglioside GM1 regulates mitochondrial function in neuroblastoma cells. Glycoconj. J. 2020, 37, 293–306. [Google Scholar] [CrossRef]
- Audano, M.; Pedretti, S.; Cermenati, G.; Brioschi, E.; Diaferia, G.R.; Ghisletti, S.; Cuomo, A.; Bonaldi, T.; Salerno, F.; Mora, M.; et al. Zc3h10 is a novel mitochondrial regulator. EMBO Rep. 2018, 19, e45531. [Google Scholar] [CrossRef]
- Dookwah, M.; Wagner, S.K.; Ishihara, M.; Yu, S.H.; Ulrichs, H.; Kulik, M.J.; Zeltner, N.; Dalton, S.; Strauss, K.A.; Aoki, K.; et al. Neural-specific alterations in glycosphingolipid biosynthesis and cell signaling associated with two human ganglioside GM3 synthase deficiency variants. Hum. Mol. Genet. 2023, 32, 3323–3341. [Google Scholar] [CrossRef] [PubMed]
- Aureli, M.; Bassi, R.; Loberto, N.; Regis, S.; Prinetti, A.; Chigorno, V.; Aerts, J.M.; Boot, R.G.; Filocamo, M.; Sonnino, S. Cell surface associated glycohydrolases in normal and Gaucher disease fibroblasts. J. Inherit. Metab. Dis. 2012, 35, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hashiramoto, A.; Haluzik, M.; Mizukami, H.; Beck, S.; Norton, A.; Kono, M.; Tsuji, S.; Daniotti, J.L.; Werth, N.; et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc. Natl. Acad. Sci. USA 2003, 100, 3445–3449. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Wiznitzer, M.; Epifano, O.; Ladisch, S. Ganglioside depletion and EGF responses of human GM3 synthase-deficient fibroblasts. Glycobiology 2008, 18, 593–601. [Google Scholar] [CrossRef]
- Russo, D.; Della Ragione, F.; Rizzo, R.; Sugiyama, E.; Scalabri, F.; Hori, K.; Capasso, S.; Sticco, L.; Fioriniello, S.; De Gregorio, R.; et al. Glycosphingolipid metabolic reprogramming drives neural differentiation. EMBO J. 2018, 37, e97674. [Google Scholar] [CrossRef]
- Chatterjee, S.; Balram, A.; Li, W. Convergence: Lactosylceramide-Centric Signaling Pathways Induce Inflammation, Oxidative Stress, and Other Phenotypic Outcomes. Int. J. Mol. Sci. 2021, 22, 1816. [Google Scholar] [CrossRef]
- Roseman, S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem. Phys. Lipids 1970, 5, 270–297. [Google Scholar] [CrossRef]
- Preti, A.; Fiorilli, A.; Lombardo, A.; Caimi, L.; Tettamanti, G. Occurrence of sialyltransferase activity in the synaptosomal membranes prepared from calf brain cortex. J. Neurochem. 1980, 35, 281–296. [Google Scholar] [CrossRef]
- Chakraborty, S.; Doktorova, M.; Molugu, T.R.; Heberle, F.A.; Scott, H.L.; Dzikovski, B.; Nagao, M.; Stingaciu, L.R.; Standaert, R.F.; Barrera, F.N.; et al. How cholesterol stiffens unsaturated lipid membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 21896–21905. [Google Scholar] [CrossRef]
- Lunghi, G.; Carsana, E.V.; Loberto, N.; Cioccarelli, L.; Prioni, S.; Mauri, L.; Bassi, R.; Duga, S.; Straniero, L.; Asselta, R.; et al. beta-Glucocerebrosidase Deficiency Activates an Aberrant Lysosome-Plasma Membrane Axis Responsible for the Onset of Neurodegeneration. Cells 2022, 11, 2343. [Google Scholar] [CrossRef]
- Cooper, A.; Wraith, J.E.; Savage, W.J.; Thornley, M.; Noronha, M.J. beta-mannosidase deficiency in a female infant with epileptic encephalopathy. J. Inherit. Metab. Dis. 1991, 14, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Bodas, M.; Min, T.; Vij, N. Lactosylceramide-accumulation in lipid-rafts mediate aberrant-autophagy, inflammation and apoptosis in cigarette smoke induced emphysema. Apoptosis 2015, 20, 725–739. [Google Scholar] [CrossRef]
- Bharathi, S.S.; Zhang, B.B.; Paul, E.; Zhang, Y.; Schmidt, A.V.; Fowler, B.; Wu, Y.; Tiemeyer, M.; Inamori, K.I.; Inokuchi, J.I.; et al. GM3 synthase deficiency increases brain glucose metabolism in mice. Mol. Genet. Metab. 2022, 137, 342–348. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Colell, A.; Paris, R.; Fernandez-Checa, J.C. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 2000, 14, 847–858. [Google Scholar] [CrossRef]
- Indellicato, R.; Parini, R.; Domenighini, R.; Malagolini, N.; Iascone, M.; Gasperini, S.; Masera, N.; dall’Olio, F.; Trinchera, M. Total loss of GM3 synthase activity by a normally processed enzyme in a novel variant and in all ST3GAL5 variants reported to cause a distinct congenital disorder of glycosylation. Glycobiology 2019, 29, 229–241. [Google Scholar] [CrossRef]
- Inokuchi, J.I.; Go, S.; Suzuki, A.; Nakagawasai, O.; Odaira-Satoh, T.; Veillon, L.; Nitta, T.; McJarrow, P.; Kanoh, H.; Inamori, K.I.; et al. Dietary gangliosides rescue GM3 synthase deficiency outcomes in mice accompanied by neurogenesis in the hippocampus. Front. Neurosci. 2024, 18, 1387221. [Google Scholar] [CrossRef]
- Chester, M.A. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids--recommendations 1997. Eur. J. Biochem. 1998, 257, 293–298. [Google Scholar] [CrossRef]
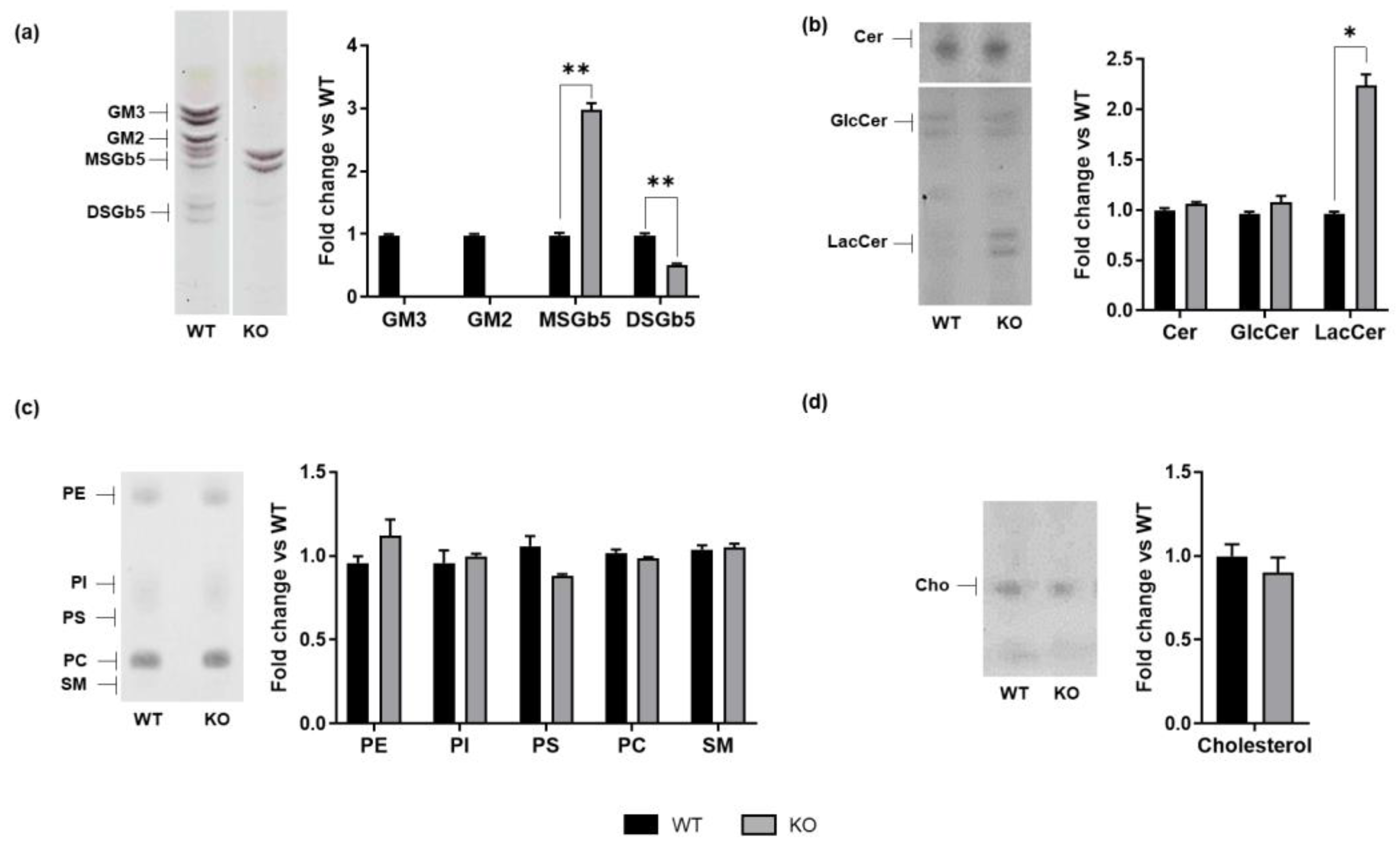


| Lipids | WT | ST3GAL5 KO |
|---|---|---|
| GM3 | 0.72 ± 0.03 | ND |
| GM2 | 0.50 ± 0.01 | ND |
| MSGb5 | 0.29 ± 0.01 | 0.85 ± 0.01 ** |
| DSGb5 | 0.12 ± 0.01 | 0.06 ± 0.01 ** |
| Cholesterol | 29.53 ± 3.79 | 26.88 ± 4.18 |
| Ceramide | 1.89 ± 0.06 | 1.99 ± 0.09 |
| LacCer | 0.64 ± 0.13 | 1.43 ± 0.29 * |
| GlcCer | 0.93 ± 0.11 | 0.99 ± 0.11 |
| PE | 21.47 ± 2.90 | 23.63 ± 2.68 |
| PI | 10.80 ± 1.48 | 10.75 ± 1.39 |
| PS | 11.02 ± 1.23 | 9.70 ± 1.02 |
| PC | 40.50 ± 3.10 | 39.98 ± 2.95 |
| Sphingomyelin | 5.31 ± 0.95 | 5.55 ± 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiricozzi, E.; Lunghi, G.; Valsecchi, M.; Carsana, E.V.; Bassi, R.; Di Biase, E.; Dobi, D.; Ciampa, M.G.; Mauri, L.; Aureli, M.; et al. Metabolic and Structural Consequences of GM3 Synthase Deficiency: Insights from an HEK293-T Knockout Model. Biomedicines 2025, 13, 843. https://doi.org/10.3390/biomedicines13040843
Chiricozzi E, Lunghi G, Valsecchi M, Carsana EV, Bassi R, Di Biase E, Dobi D, Ciampa MG, Mauri L, Aureli M, et al. Metabolic and Structural Consequences of GM3 Synthase Deficiency: Insights from an HEK293-T Knockout Model. Biomedicines. 2025; 13(4):843. https://doi.org/10.3390/biomedicines13040843
Chicago/Turabian StyleChiricozzi, Elena, Giulia Lunghi, Manuela Valsecchi, Emma Veronica Carsana, Rosaria Bassi, Erika Di Biase, Dorina Dobi, Maria Grazia Ciampa, Laura Mauri, Massimo Aureli, and et al. 2025. "Metabolic and Structural Consequences of GM3 Synthase Deficiency: Insights from an HEK293-T Knockout Model" Biomedicines 13, no. 4: 843. https://doi.org/10.3390/biomedicines13040843
APA StyleChiricozzi, E., Lunghi, G., Valsecchi, M., Carsana, E. V., Bassi, R., Di Biase, E., Dobi, D., Ciampa, M. G., Mauri, L., Aureli, M., Inamori, K.-i., Inokuchi, J.-i., Sonnino, S., & Fazzari, M. (2025). Metabolic and Structural Consequences of GM3 Synthase Deficiency: Insights from an HEK293-T Knockout Model. Biomedicines, 13(4), 843. https://doi.org/10.3390/biomedicines13040843










