Synthesis and Evaluation of Isosteviol Derivatives: Promising Anticancer Therapies for Colon Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
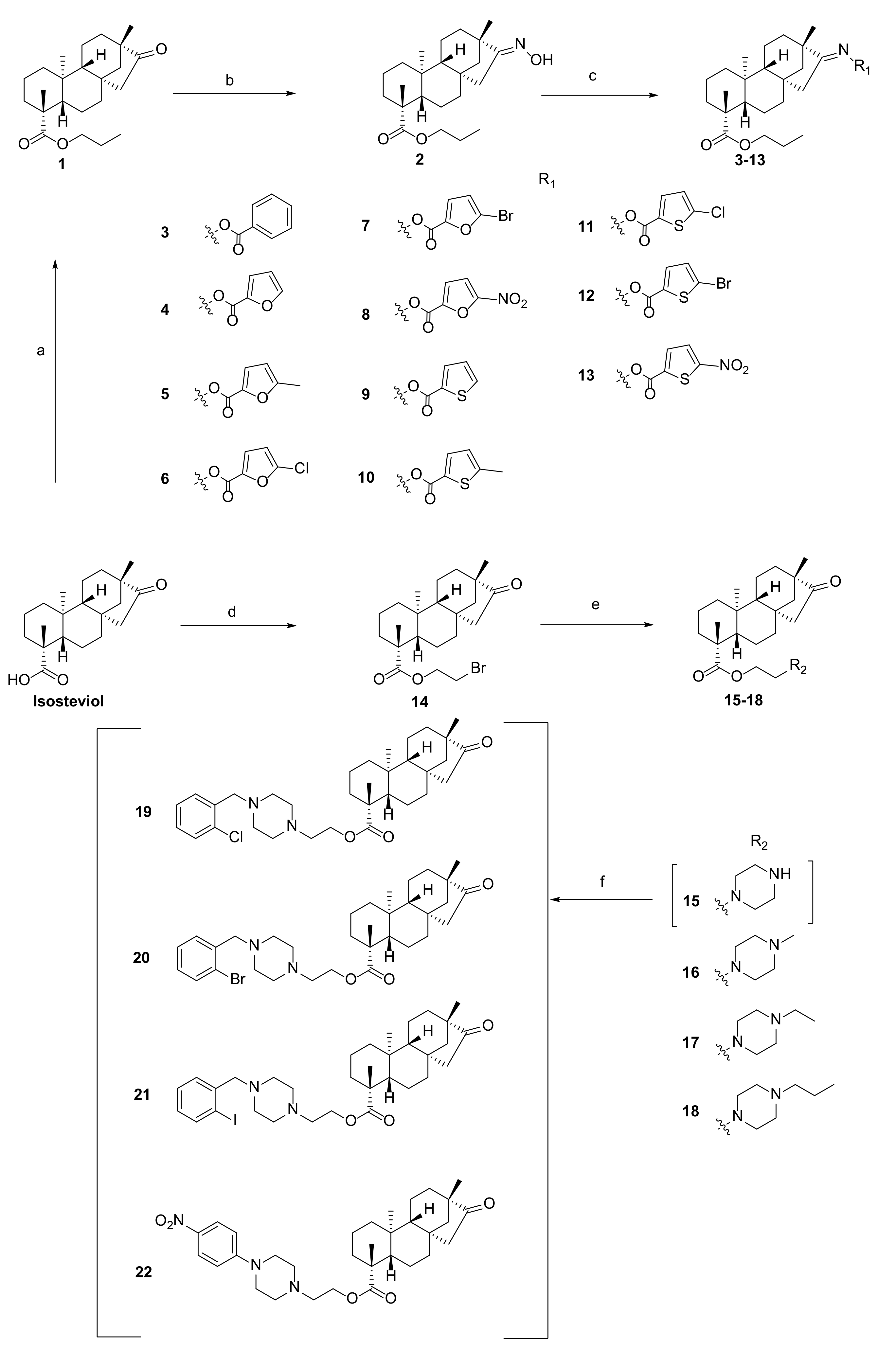
2.2. Synthesis
2.2.1. General Procedure for Preparation of Derivative 1
2.2.2. General Procedure for Preparation of Derivative 2
2.2.3. General Procedure for Preparation of Derivative 3
2.2.4. General Procedure for Preparation of Derivatives 4–13
2.2.5. General Procedure for Preparation of Derivative 14
2.2.6. General Procedure for Preparation of Derivatives 15–18
2.2.7. General Procedure for Preparation of Derivatives 19–22
2.3. Biological Evaluation and Pharmacological Mechanisms
2.3.1. Sample Pretreatment
2.3.2. Cell Culture and Treatments
2.3.3. Antiproliferative Activity In Vitro [23]
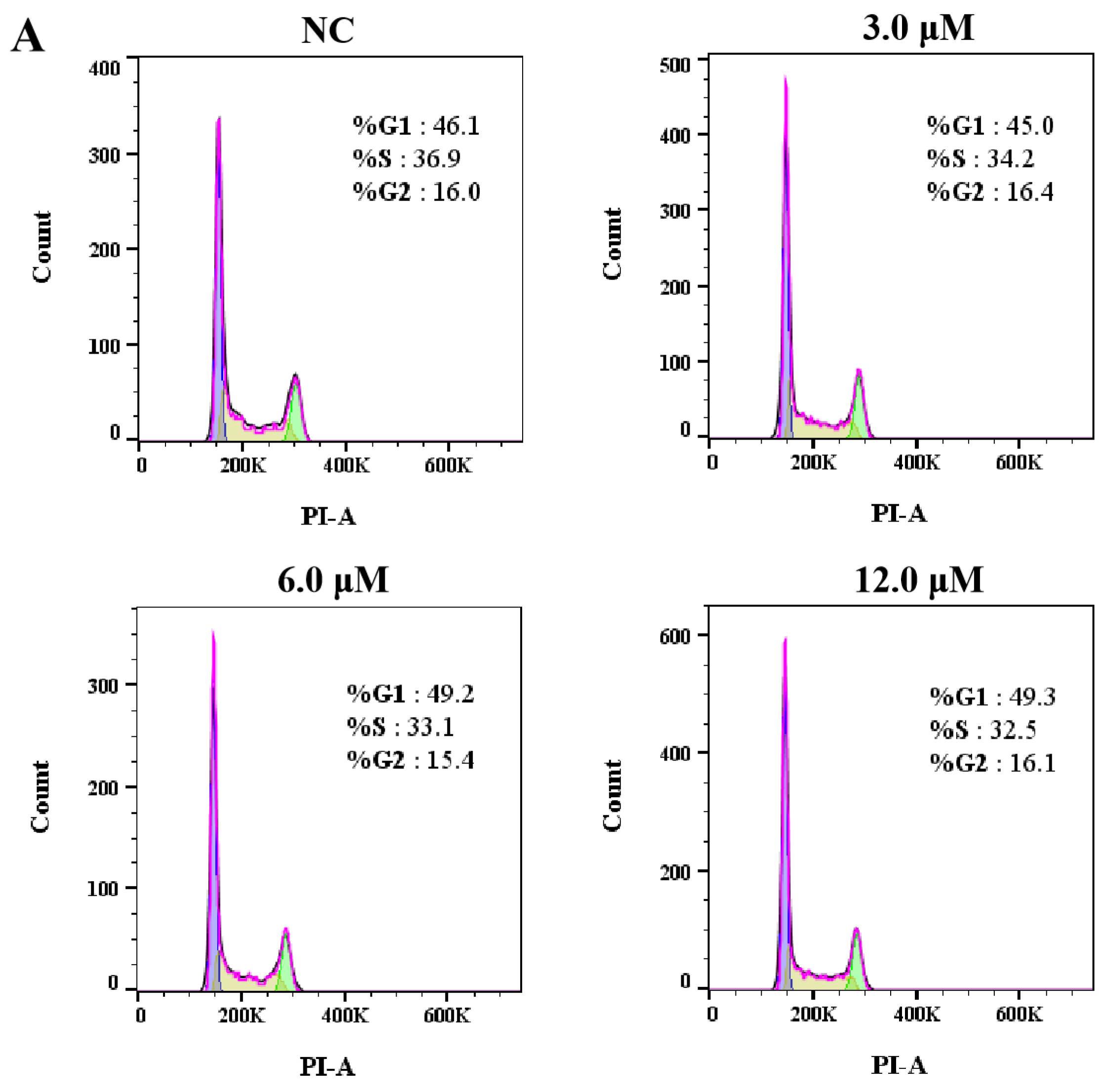
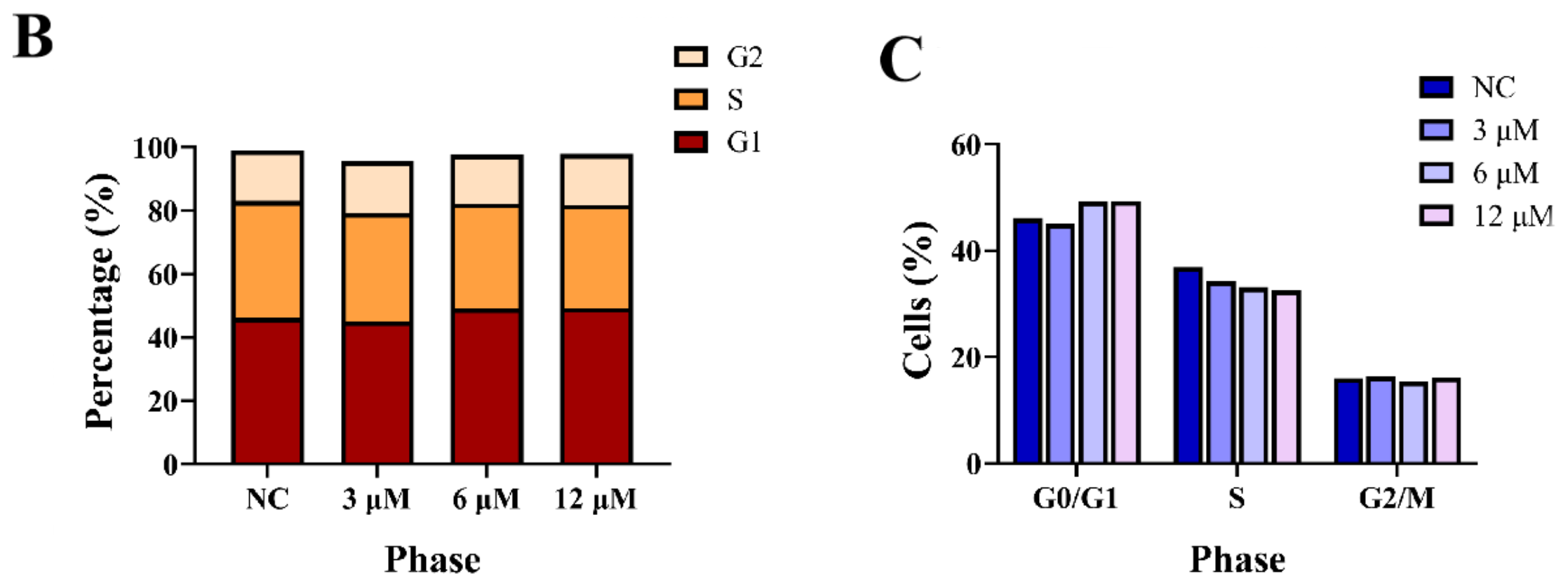
2.3.4. Cell Cycle Assay [24]
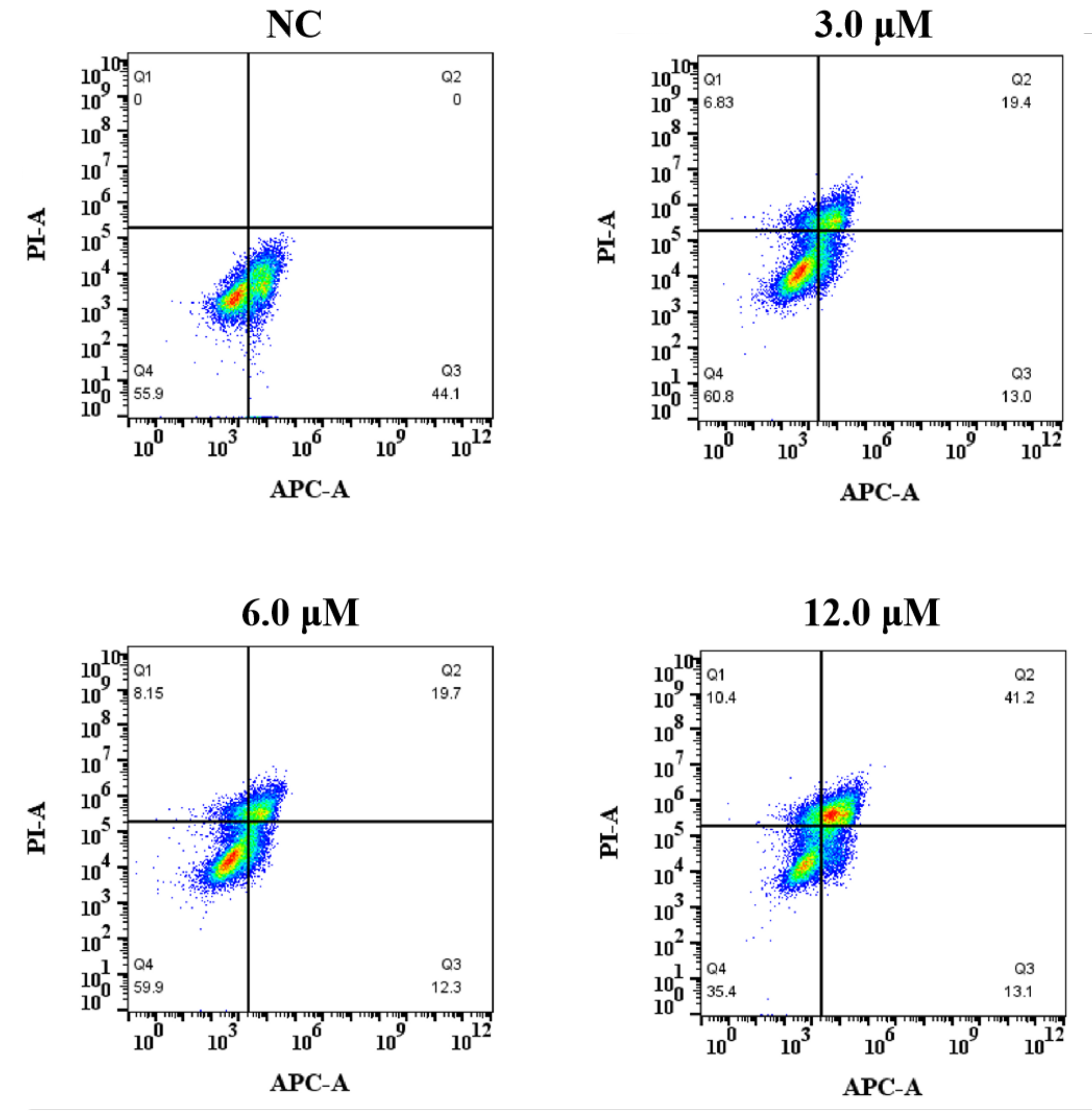
2.3.5. Cell Apoptosis Assay [24]
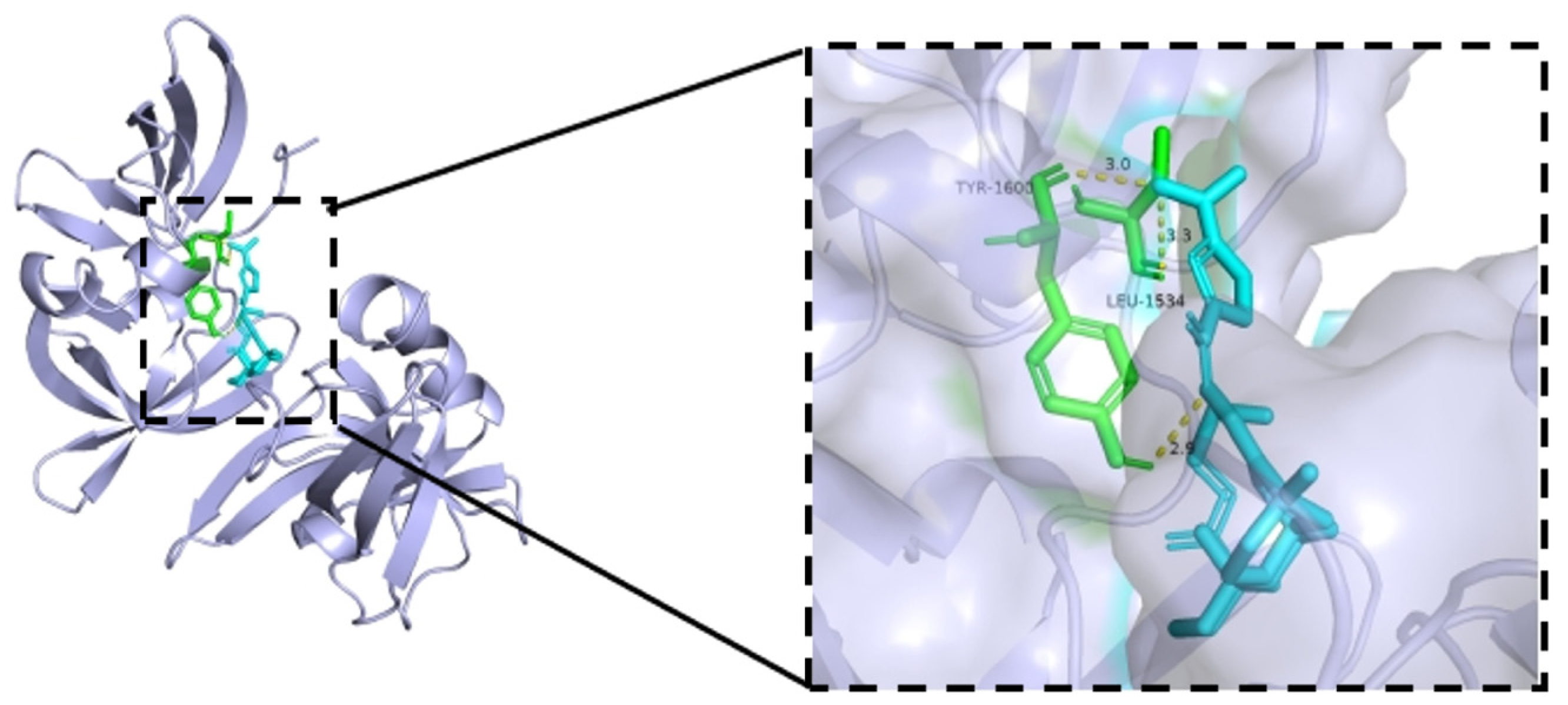
2.3.6. Molecular Docking Studies [25]
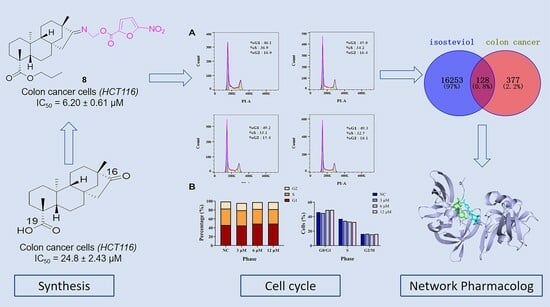
3. Results and Discussion
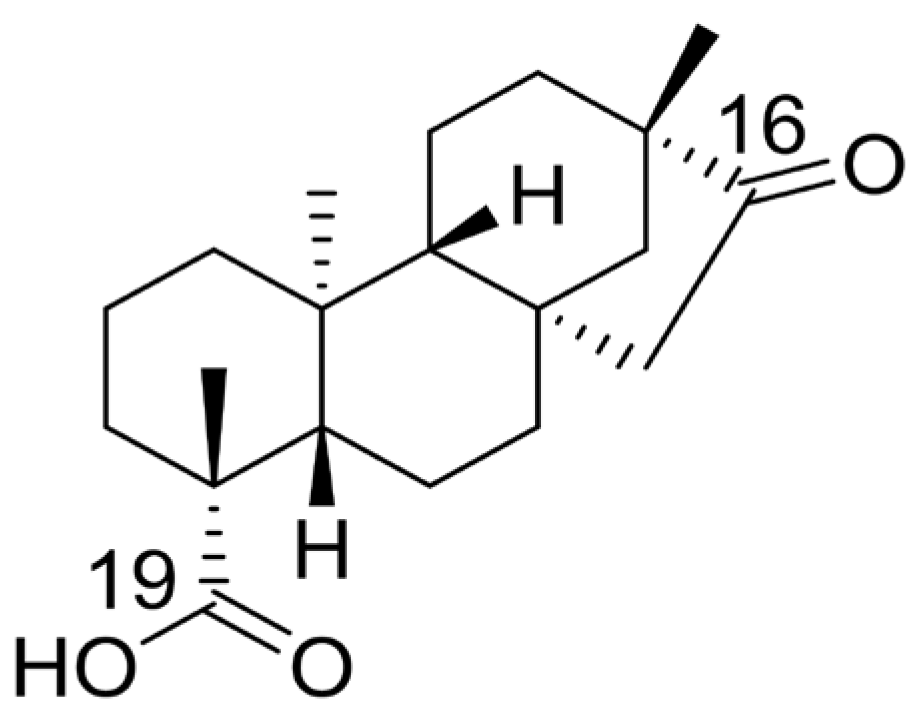
3.1. Chemistry
3.2. Biological Evaluation
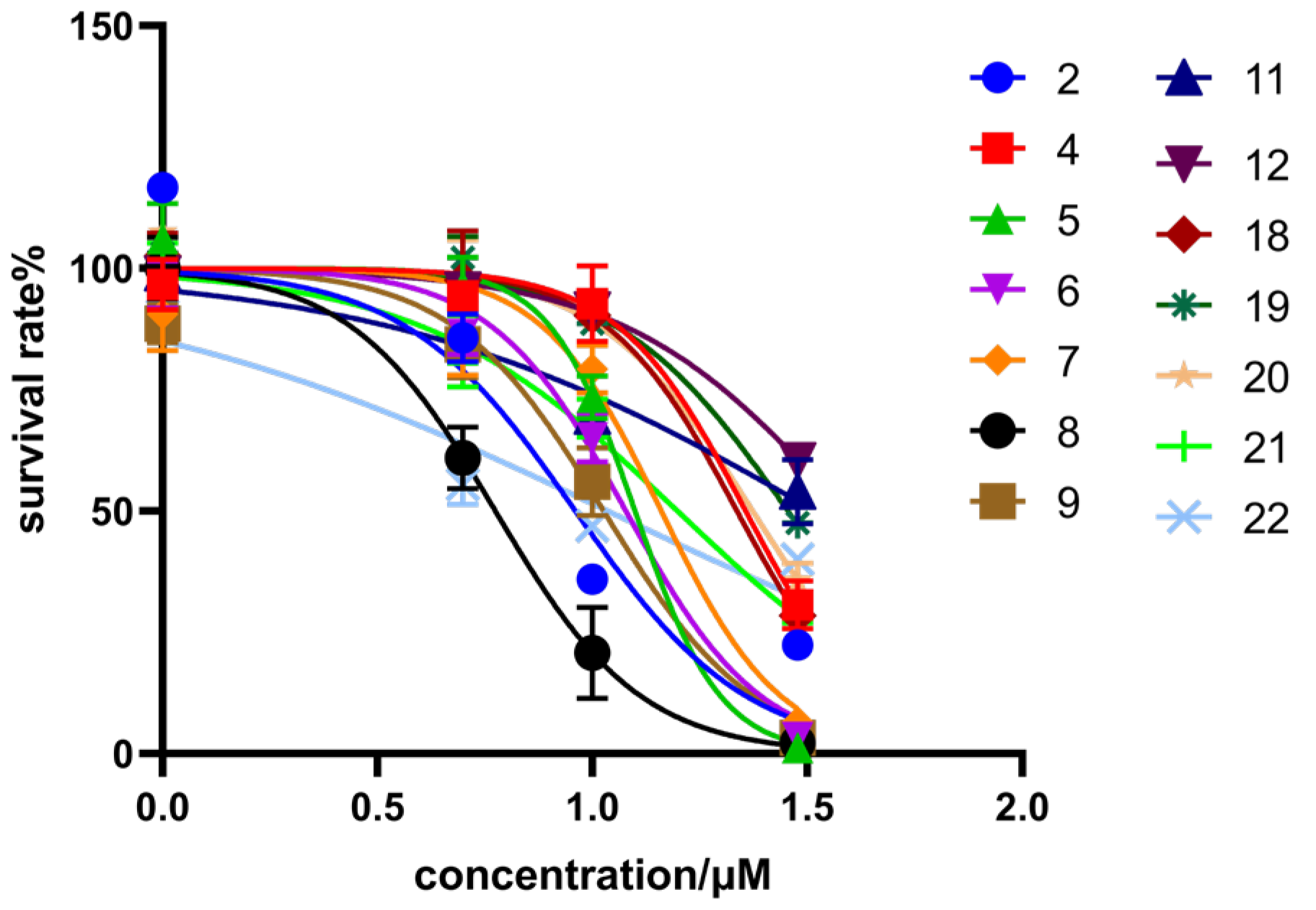
3.2.1. HCT116 Inhibitory Activity and Structure–Activity Relationship
3.2.2. Effects of Derivative 8 on Cell Cycle Distribution
3.2.3. The Effect of Derivative 8 on Apoptosis
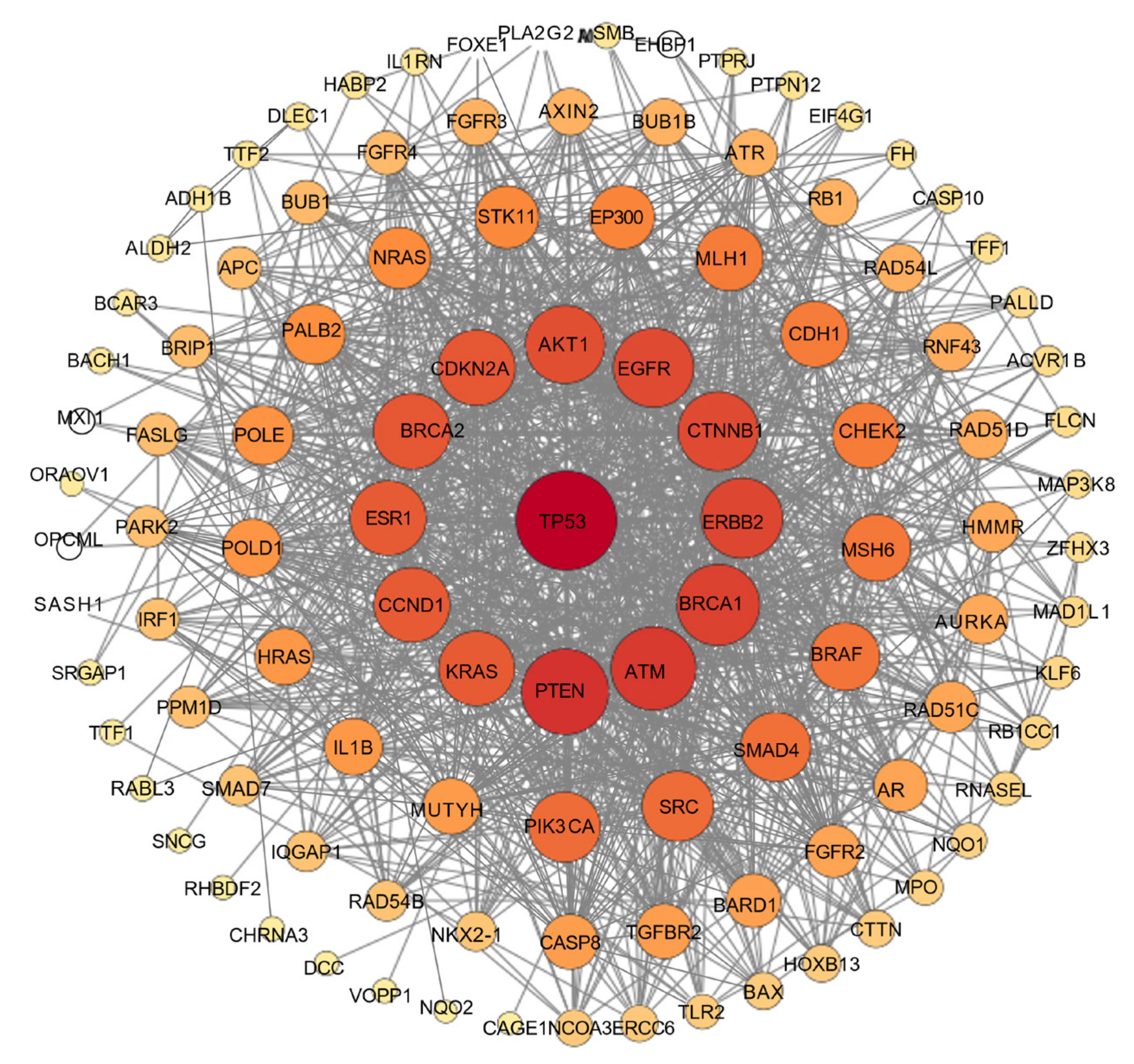
3.3. Network Pharmacology
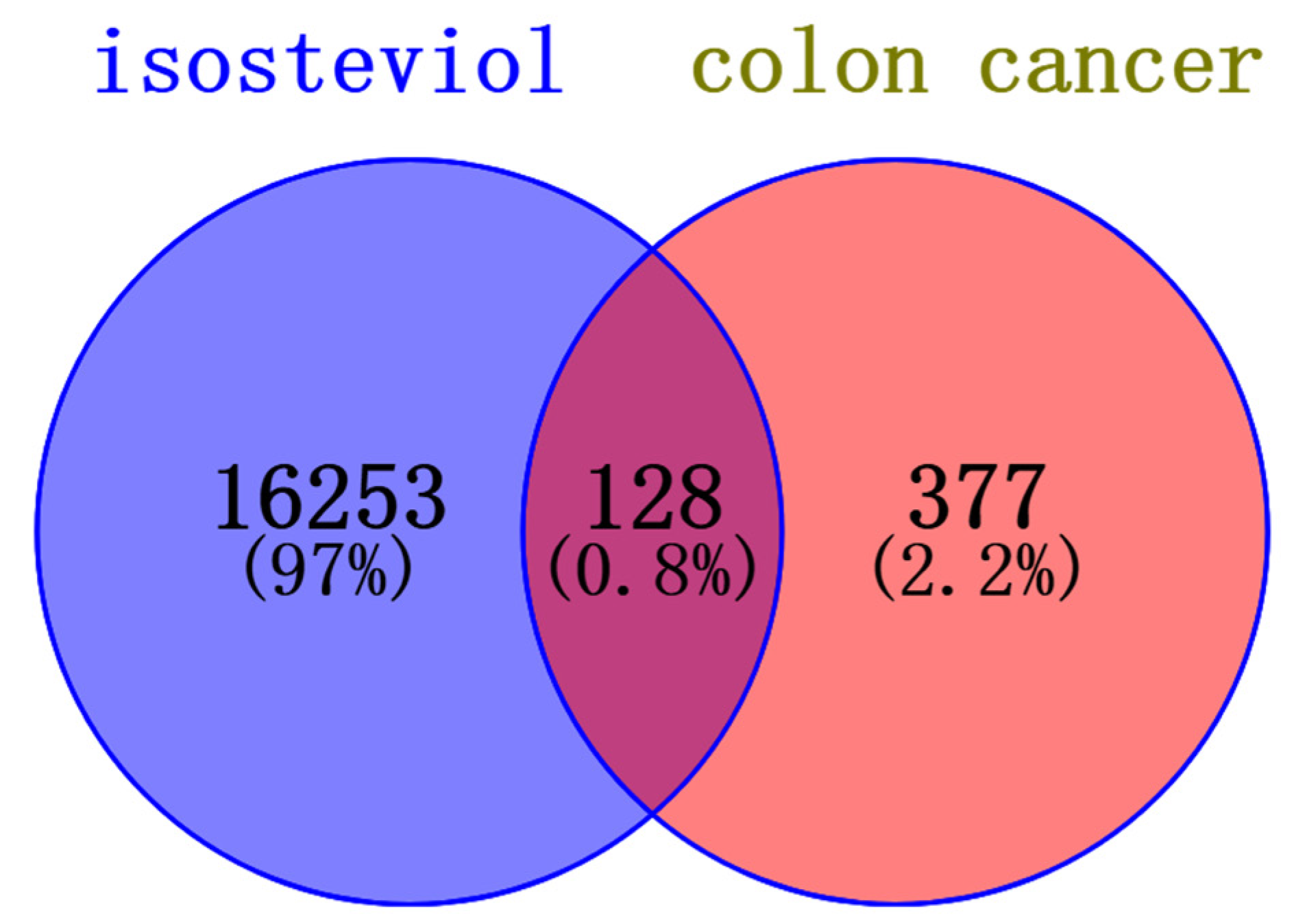
3.3.1. Characterization of Potential Protein Targets of the Natural Product Isosteviol
3.3.2. Compound–Target–Disease Network
3.3.3. PPI Network
3.3.4. Molecular Docking Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- John Kenneth, M.; Tsai, H.C.; Fang, C.Y.; Hussain, B.; Chiu, Y.; Hsu, B. Diet-mediated gut microbial community modulation and signature metabolites as potential biomarkers for early diagnosis, prognosis, prevention and stage-specific treatment of colorectal cancer. J. Adv. Res. 2023, 52, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liu, S.; He, J.; Li, H.; Liu, X.; Hu, Z.; Wang, X.; Wu, Z.; Xu, G.; Liu, W.; et al. A novel piperine derivative hjj_3_5 inhibits colorectal cancer progression by modulating emt signaling pathways. Biochem. Biophys. Res. Commun. 2025, 12, 151323. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Shi, Z.; Mahdavian, Y.; Mahdigholizad, S.; Irani, P.; Karimian, M.; Abbasi, N.; Ghaneialvar, H.; Zangeneh, A.; Zangeneh, M.M. Cu immobilized on chitosan-modified iron oxide magnetic nanoparticles: Preparation, characterization and investigation of its anti-lung cancer effects. Arab. J. Chem. 2021, 14, 103224. [Google Scholar] [CrossRef]
- Cremolini, C.; Schirripa, M.; Antoniotti, C.; Moretto, R.; Salvatore, L.; Masi, G.; Falcone, A.; Loupakis, F. First-line chemotherapy for mcrc—A review and evidence-based algorithm. Nat. Rev. Clin. Oncol. 2015, 12, 607–619. [Google Scholar] [CrossRef]
- Vassilev, Z.P.; Fan, X.; Xu, J.; Ostojic, H.; Barzi, A. Use of folfoxiri plus bevacizumab and subsequent therapies in metastatic colorectal cancer: An age-stratified analysis. Clin. Color. Cancer 2024, 23, 258–271. [Google Scholar] [CrossRef]
- Aranda, E.; Viéitez, J.M.; Gómez-España, A.; Calle, S.G.; Salud-Salvia, A.; Graña, B.; Garcia-Alfonso, P.; Rivera, F.; Quintero-Aldana, G.A.; Reina-Zoilo, J.J.; et al. Folfoxiri plus bevacizumab versus folfox plus bevacizumab for patients with metastatic colorectal cancer and ≥3 circulating tumour cells: The randomised phase iii visnÚ-1 trial. ESMO Open 2020, 5, e000944. [Google Scholar] [CrossRef]
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial therapy with folfoxiri and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 2014, 371, 1609–1618. [Google Scholar] [CrossRef]
- Ao, J.; Lai, C.; Wu, X.; Chen, Z.; Yang, W.; Qiu, L.; Li, X.; Cao, R. Design, synthesis and biological evaluation of novel β-carbolines as antitumor agents via targeting autophagy in colorectal cancer. Eur. J. Med. Chem. 2025, 283, 117145. [Google Scholar] [CrossRef]
- Ao, J.; Zeng, F.; Wang, L.; Qiu, L.; Cao, R.; Li, X. Design, synthesis and pharmacological evaluation of β-carboline derivatives as potential antitumor agent via targeting autophagy. Eur. J. Med. Chem. 2023, 246, 114955. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Li, L.; Feng, L.; Wang, Y.; Wang, Z.; Tan, N. Ra-xii, a bicyclic hexapeptidic glucoside isolated from rubia yunnanensis diels, exerts antitumor activity by inhibiting protective autophagy and activating akt-mtor pathway in colorectal cancer cells. J. Ethnopharmacol. 2021, 266, 113438. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Hamasaki, Y.; Tokuda, H.; Ukiya, M.; Kimura, Y.; Nishino, H. Microbial transformation of isosteviol and inhibitory effects on Epstein-Barr virus activation of the transformation products. J. Nat. Prod. 2004, 67, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ma, Z.; Wang, J.; Milne, R.W.; Xu, D.; Davey, A.K.; Evans, A.M. Isosteviol reduces plasma glucose levels in the intravenous glucose tolerance test in zucker diabetic fatty rats. Diabetes Obe. Metab. 2007, 9, 597–599. [Google Scholar] [CrossRef]
- Olas, B. Stevia rebaudiana bertoni and its secondary metabolites: Their effects on cardiovascular risk factors. Nutrition 2022, 99–100, 111655. [Google Scholar] [CrossRef]
- Yasukawa, K.; Kitanaka, S.; Seo, S. Inhibitory effect of stevioside on tumor promotion by 12-o-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Biol. Pharm. Bull. 2002, 25, 1488–1490. [Google Scholar] [CrossRef]
- Zhang, S.J.; Xu, D.Y. Effects of isosteviol against myocardium injury induced by global ischemia-reperfusion in isolated guinea pig hearts. Chin. J. Pharmacol. Toxicol. 2004, 18, 194–198. [Google Scholar] [CrossRef]
- Chan, K.; Fujioka, Q.; Nakano, T. T neotripterifordin, a novel antihiv principle from Tripterygium wilfordii: Isolation and structural elucidation. Bioorg. Med. Chem. 1995, 3, 1345–1348. [Google Scholar] [CrossRef]
- Zhu, S.L.; Wu, Y.; Liu, C.J.; Wei, C.Y.; Tao, J.C.; Liu, H.M. Design and stereoselective synthesis of novel isosteviol-fused pyrazolines and pyrazoles as potential anticancer agents. Eur. J. Med. Chem. 2013, 65, 70–82. [Google Scholar] [CrossRef]
- Zhu, S.L.; Wu, Y.; Liu, C.J.; Wei, C.Y.; Tao, J.C.; Liu, H.M. Synthesis and in vitro cytotoxic activity evaluation of novel heterocycle bridged carbothioamide type isosteviol derivatives as antitumor agents. Bioorg. Med. Chem. Lett. 2013, 23, 1343–1346. [Google Scholar] [CrossRef]
- Al-Jumaili, M.H.A.; Hamad, A.A.; Hashem, H.E.; Hussein, A.D.; Muhaidi, M.J.; Ahmed, M.A.; Albnaa, A.H.A.; Siddique, F.; Bakr, E.A. Comprehensive review on the bis–heterocyclic compounds and their anticancer efficacy. J. Mol. Struct. 2023, 1271, 133970. [Google Scholar] [CrossRef]
- Kumar, A.; Kaushal, A.; Verma, P.K.; Gupta, M.K.; Chandra, G.; Kumar, U.; Yadav, A.K.; Kumar, D. An insight into recent developments in imidazole based heterocyclic compounds as anticancer agents: Synthesis, sars, and mechanism of actions. Eur. J. Med. Chem. 2024, 280, 116896. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, S.A.C.; Salvador, J.A.R.; Cort’es, R.; Cascante, M. Design, synthesis and biological evaluation of novel C-29 carbamate celastrol derivatives as potent and selective cytotoxic compounds. Eur. J. Med. Chem. 2017, 139, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Y.; Li, X.P.; Tan, H.S.; Yu, C.H.; Zhang, J.H.; Shen, Z.W. Studies on the total synthesis of hirtellanine a: Regioselective synthesis of benzopyran. Eur. J. Org. Chem. 2013, 7, 1356–1366. [Google Scholar] [CrossRef]
- Sreelekshmi, P.K.; Pooja, S.K.; Vidya, N.; Sinosh, S.; Thejaswini, V. Integrative investigation of flavonoids targeting YBX1 protein–protein interaction network in breast cancer: From computational analysis to experimental validation. Mol. Biotechnol. 2024, 20, 1–21. [Google Scholar] [CrossRef]
- Luan, T.; Cao, L.H.; Deng, H.; Shen, Q.K.; Tian, Y.S.; Quan, Z.S. Design and synthesis of C-19 isosteviol derivatives as potent and highly selective antiproliferative agents. Molecules 2018, 24, 121. [Google Scholar] [CrossRef]
- Hu, J.; Li, Z.; Rao, B.; Thafar, M.A.; Arif, M. Improving protein-protein interaction prediction using protein language model and protein network features. Anal. Biochem. 2024, 693, 115550. [Google Scholar] [CrossRef]
- Khalil, A.M.; Fayek, N.M.; Sabry, O.M.; ElZalabani, S.M.; Mohamed, A.F.; El-Askary, H.I. Carob seeds as a source of bioactive flavonoid derivatives: Isolation, network pharmacology-guided anti-cancer activity, and HPLC standardization. Chem. Biodivers. 2024, 22, 1–11. [Google Scholar] [CrossRef]
- Clasen, K.; Ballin, N.; Schütz, L.; Bonzheim, I.; Kelemen, O.; Orth, M.; Gani, C.; Rieb, O.; Ossowski, S.; Niyazi, M.; et al. Tumor sequencing before and after neoadjuvant chemoradiotherapy in locally advanced rectal cancer: Genetic tumor characterization and clinical outcome. Clin. Transl. Radiat. Oncol. 2025, 50, 100894. [Google Scholar] [CrossRef]
- The, J.; Hong, Z.; Dong, A.; Headey, S.; Gunzburg, M.; Doak, B.; James, L.I.; Bountra, C.; Arrowsmith, C.H.; Edwards, A.M.; et al. Tudor Domain of Tumor Suppressor P53BP1 with MFP-6008; Structural Genomics Consortium (SGC): Toronto, ON, Canada, 2023. [Google Scholar] [CrossRef]


 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | IC50 (μM) 1 | |
| HCT116 | HepG2 | |||
| Isosteviol | H | O | 24.8 ± 2.43 | >100 |
| 2 |  | NOH | 7.31 ± 1.12 | >100 |
| 3 |  | 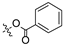 | >30 | 74.43 ± 0.95 |
| 4 |  |  | 22.75 ± 0.86 | >100 |
| 5 |  |  | 15.52 ± 0.82 | >100 |
| 6 |  |  | 14.47 ± 0.71 | >100 |
| 7 |  |  | 11.97 ± 0.91 | >100 |
| 8 |  |  | 6.20 ± 0.61 | 39.84 ± 0.43 |
| 9 |  |  | 10.62 ± 1.21 | 74.25 ± 1.53 |
| 10 |  |  | >30 | >100 |
| 11 |  | 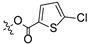 | 10.44 ± 0.84 | >100 |
| 12 |  | 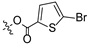 | 10.00 ± 0.65 | >100 |
| 13 |  | 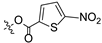 | >30 | >100 |
| 15 |  | O | >30 | >100 |
| 16 |  | O | >30 | >100 |
| 17 | 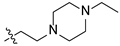 | O | >30 | >100 |
| 18 | 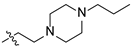 | O | 21.69 ± 0.35 | >100 |
| 19 | 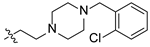 | O | 28.50 ± 0.16 | >100 |
| 20 |  | O | 23.61 ± 0.63 | >100 |
| 21 |  | O | 15.77 ± 0.69 | >100 |
| 22 | 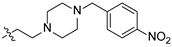 | O | 10.85 ± 0.26 | >100 |
| DOX 2 | 0.16 ± 0.002 | 0.003 ± 0.004 | ||
| Derivative 8 | Apoptosis (%) | Necrosis (%) | ||
|---|---|---|---|---|
| Early | Late | Total (%) | ||
| NC | 44.1 | 0 | 44.1 | 0 |
| 3.0 μM | 13.0 | 19.4 | 32.4 | 6.83 |
| 6.0 μM | 12.3 | 19.7 | 32.0 | 8.15 |
| 12.0 μM | 13.1 | 41.2 | 54.3 | 10.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhu, F.; Ding, Y.; Xing, L.; Wang, E.; Fang, Y.; Sheng, R.; Tu, Q.; Guo, R. Synthesis and Evaluation of Isosteviol Derivatives: Promising Anticancer Therapies for Colon Cancer. Biomedicines 2025, 13, 793. https://doi.org/10.3390/biomedicines13040793
Chen Y, Zhu F, Ding Y, Xing L, Wang E, Fang Y, Sheng R, Tu Q, Guo R. Synthesis and Evaluation of Isosteviol Derivatives: Promising Anticancer Therapies for Colon Cancer. Biomedicines. 2025; 13(4):793. https://doi.org/10.3390/biomedicines13040793
Chicago/Turabian StyleChen, Yecang, Feifei Zhu, Yuxin Ding, Lin Xing, Enxiao Wang, Yixiang Fang, Ruilong Sheng, Qidong Tu, and Ruihua Guo. 2025. "Synthesis and Evaluation of Isosteviol Derivatives: Promising Anticancer Therapies for Colon Cancer" Biomedicines 13, no. 4: 793. https://doi.org/10.3390/biomedicines13040793
APA StyleChen, Y., Zhu, F., Ding, Y., Xing, L., Wang, E., Fang, Y., Sheng, R., Tu, Q., & Guo, R. (2025). Synthesis and Evaluation of Isosteviol Derivatives: Promising Anticancer Therapies for Colon Cancer. Biomedicines, 13(4), 793. https://doi.org/10.3390/biomedicines13040793