Effects of Subanesthetic Intravenous Ketamine Infusion on Stress Hormones and Synaptic Density in Rats with Mild Closed-Head Injury
Abstract
1. Introduction
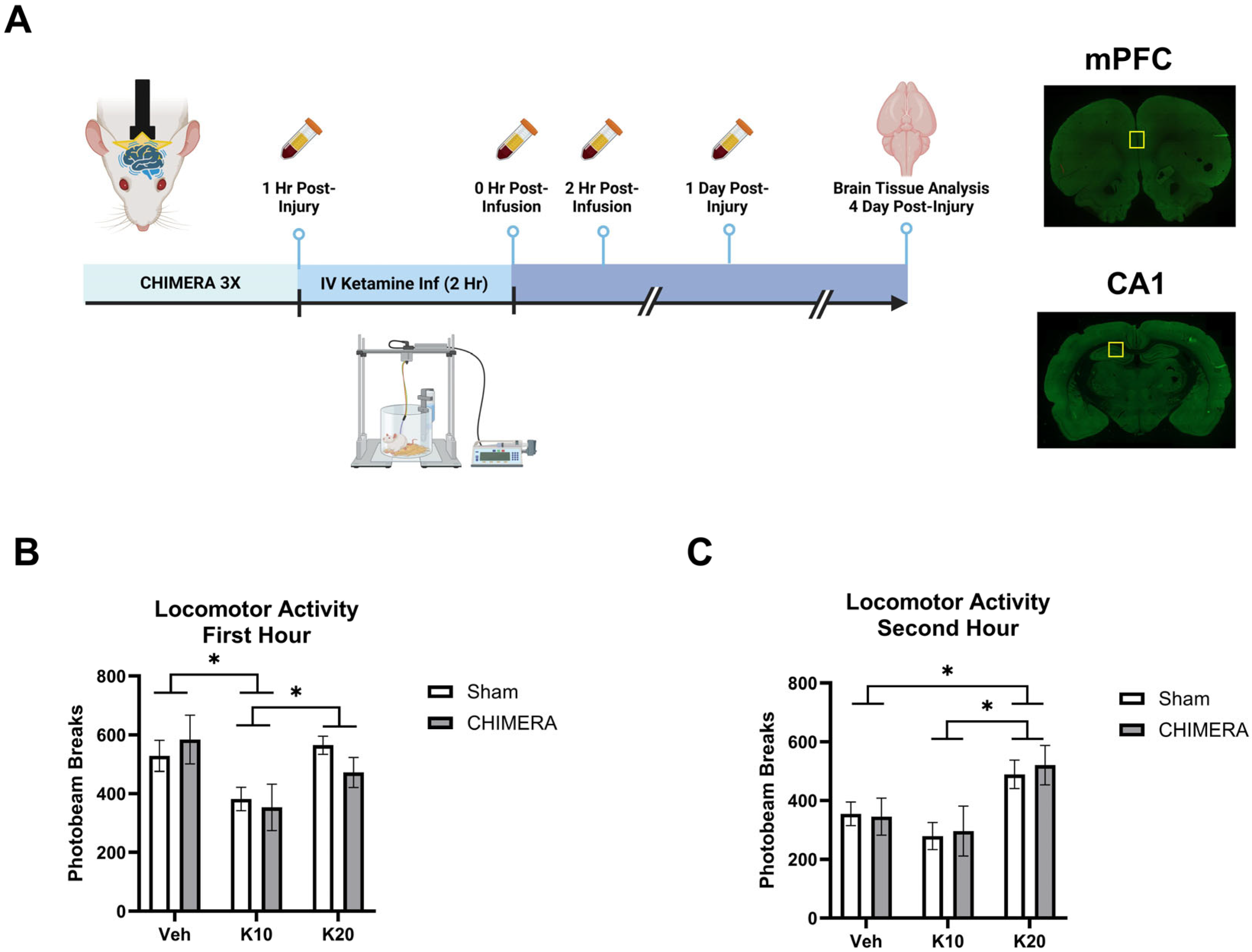
2. Methods
2.1. Animals
2.2. CHIMERA
2.3. IV Ketamine Infusion
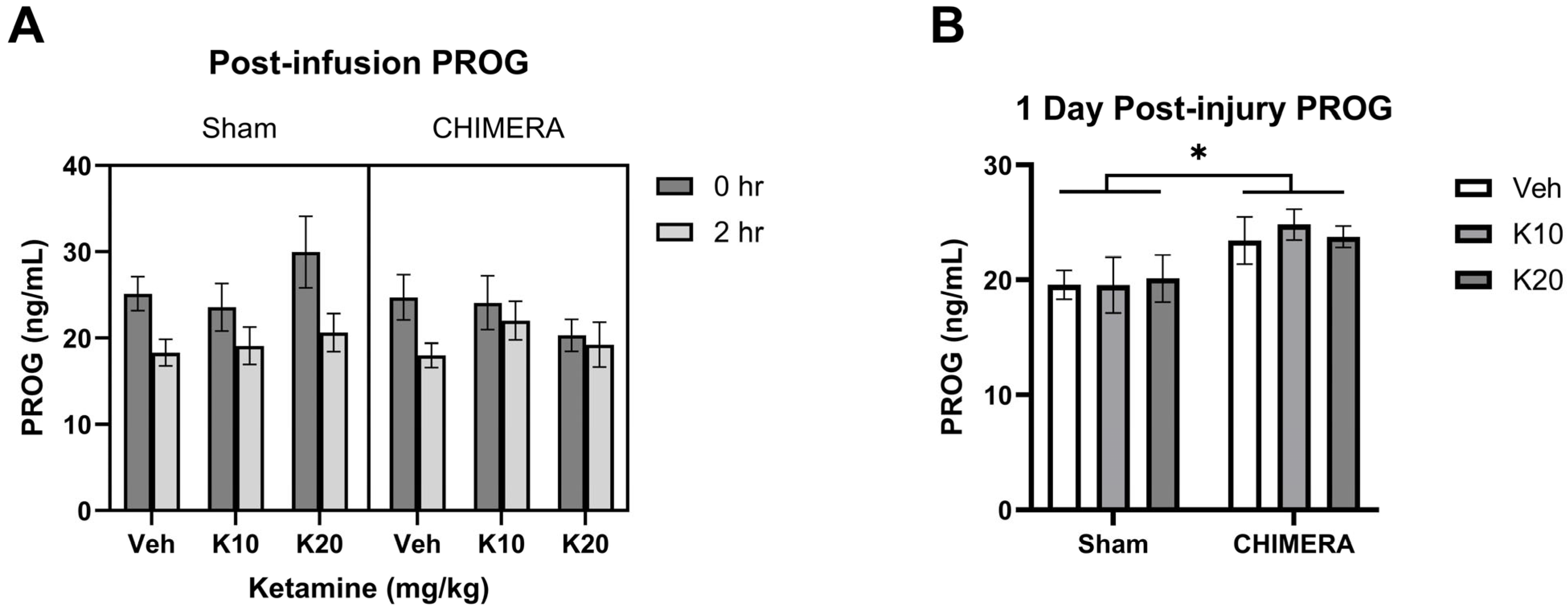
2.4. CORT and PROG ELISA
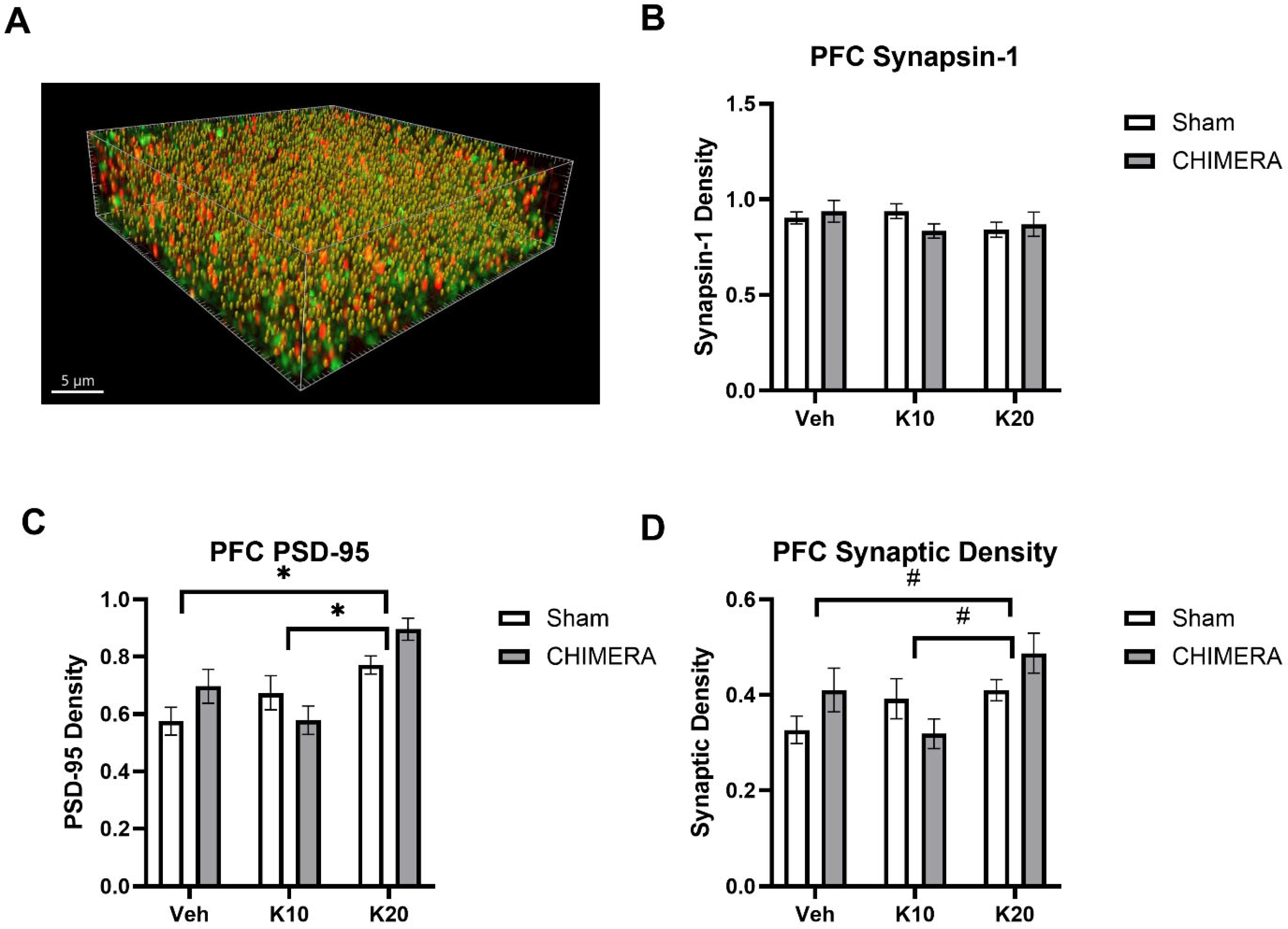
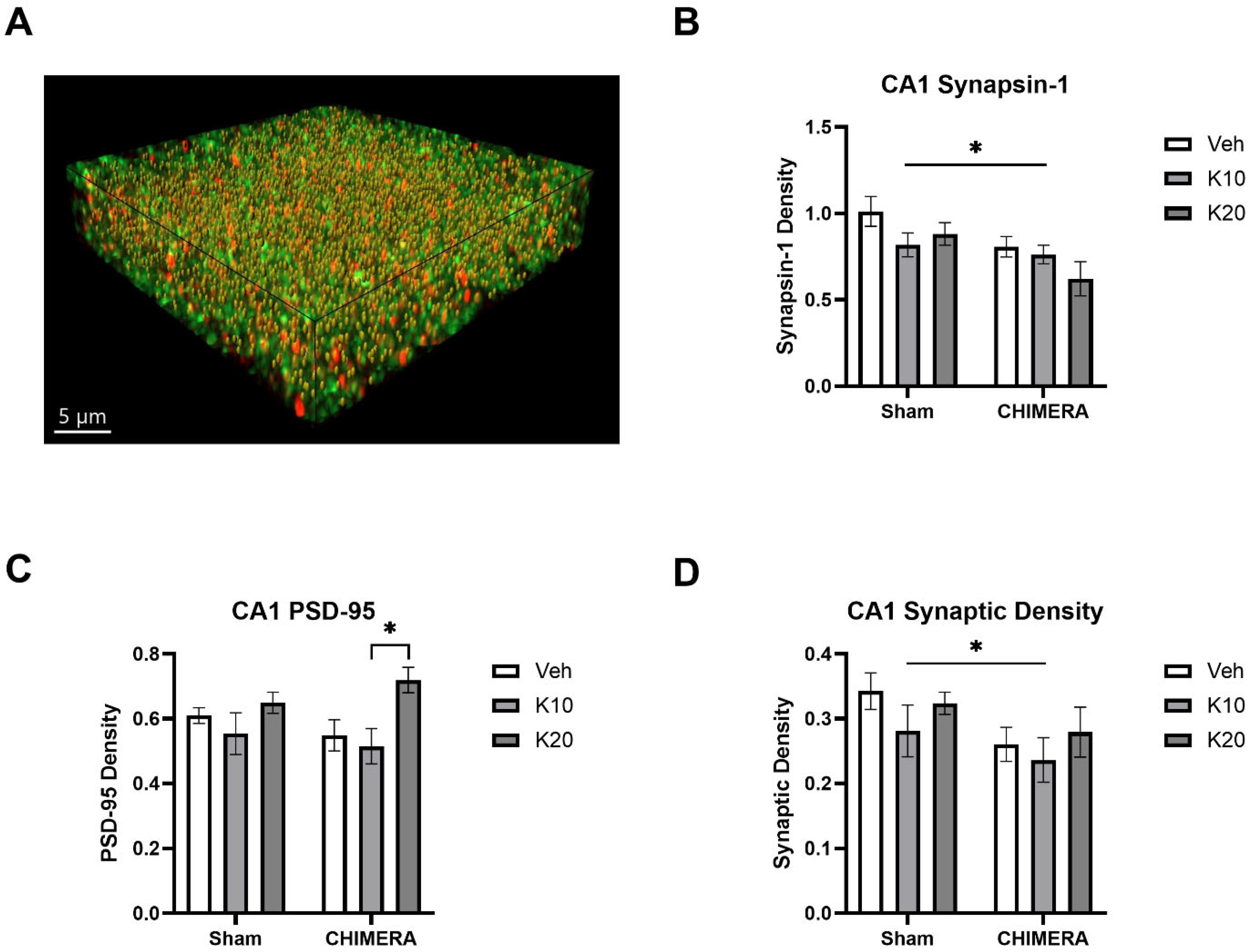
2.5. Immunohistochemistry
2.6. SEQUIN
2.7. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamjoom, A.A.B.; Rhodes, J.; Andrews, P.J.D.; Grant, S.G.N. The synapse in traumatic brain injury. Brain 2021, 144, 18–31. [Google Scholar] [PubMed]
- Kaplan, G.B.; Leite-Morris, K.A.; Wang, L.; Rumbika, K.K.; Heinrichs, S.C.; Zeng, X.; Wu, L.; Arena, D.T.; Teng, Y.D. Pathophysiological Bases of Comorbidity: Traumatic Brain Injury and Post-Traumatic Stress Disorder. J. Neurotrauma 2018, 35, 210–225. [Google Scholar] [PubMed]
- Cassidy, J.D.; Carroll, L.J.; Peloso, P.M.; Borg, J.; von Holst, H.; Holm, L.; Kraus, J.; Coronado, V.G.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004, 36 (Suppl. S43), 28–60. [Google Scholar]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 2017, 12, e0174847. [Google Scholar]
- Ryan, L.M.; Warden, D.L. Post concussion syndrome. Int. Rev. Psychiatry 2003, 15, 310–316. [Google Scholar]
- Spencer, H.F.; Boese, M.; Berman, R.Y.; Radford, K.D.; Choi, K.H. Effects of a Subanesthetic Ketamine Infusion on Inflammatory and Behavioral Outcomes after Closed Head Injury in Rats. Bioengineering 2023, 10, 941. [Google Scholar] [CrossRef]
- Xu, X.; Cowan, M.; Beraldo, F.; Schranz, A.; McCunn, P.; Geremia, N.; Brown, Z.; Patel, M.; Nygard, K.L.; Khazaee, R.; et al. Repetitive mild traumatic brain injury in mice triggers a slowly developing cascade of long-term and persistent behavioral deficits and pathological changes. Acta Neuropathol. Commun. 2021, 9, 60. [Google Scholar]
- Babb, J.A.; Zuberer, A.; Heinrichs, S.; Rumbika, K.K.; Alfiler, L.; Lakis, G.A.; Leite-Morris, K.A.; Kaplan, G.B. Disturbances in fear extinction learning after mild traumatic brain injury in mice are accompanied by alterations in dendritic plasticity in the medial prefrontal cortex and basolateral nucleus of the amygdala. Brain Res. Bull. 2023, 198, 15–26. [Google Scholar]
- Eikermann, M.; Grosse-Sundrup, M.; Zaremba, S.; Henry, M.E.; Bittner, E.A.; Hoffmann, U.; Chamberlin, N.L. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology 2012, 116, 35–46. [Google Scholar]
- Reede, K.; Bartholomew, R.; Nielsen, D.; Ahmeti, M.; Zreik, K. Ketamine in Trauma: A Literature Review and Administration Guidelines. Cureus 2023, 15, e48099. [Google Scholar]
- Zietlow, J.; Berns, K.; Jenkins, D.; Zietlow, S. Prehospital Use of Ketamine: Effectiveness in Critically Ill and Injured Patients. Mil. Med. 2019, 184 (Suppl. S1), 542–544. [Google Scholar] [PubMed]
- Spencer, H.F.; Berman, R.Y.; Boese, M.; Zhang, M.; Kim, S.Y.; Radford, K.D.; Choi, K.H. Effects of an intravenous ketamine infusion on inflammatory cytokine levels in male and female Sprague-Dawley rats. J. Neuroinflamm. 2022, 19, 75. [Google Scholar]
- Sala, N.; Paoli, C.; Bonifacino, T.; Mingardi, J.; Schiavon, E.; La Via, L.; Milanese, M.; Tornese, P.; Datusalia, A.K.; Rosa, J.; et al. Acute Ketamine Facilitates Fear Memory Extinction in a Rat Model of PTSD Along With Restoring Glutamatergic Alterations and Dendritic Atrophy in the Prefrontal Cortex. Front. Pharmacol. 2022, 13, 759626. [Google Scholar]
- Liu, W.X.; Wang, J.; Xie, Z.M.; Xu, N.; Zhang, G.F.; Jia, M.; Zhou, Z.Q.; Hashimoto, K.; Yang, J.J. Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology 2016, 233, 405–415. [Google Scholar]
- Moda-Sava, R.N.; Murdock, M.H.; Parekh, P.K.; Fetcho, R.N.; Huang, B.S.; Huynh, T.N.; Witztum, J.; Shaver, D.C.; Rosenthal, D.L.; Alway, E.J.; et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019, 364, eaat8078. [Google Scholar]
- Wang, C.Q.; Ye, Y.; Chen, F.; Han, W.C.; Sun, J.M.; Lu, X.; Guo, R.; Cao, K.; Zheng, M.J.; Liao, L.C. Posttraumatic administration of a sub-anesthetic dose of ketamine exerts neuroprotection via attenuating inflammation and autophagy. Neuroscience 2017, 343, 30–38. [Google Scholar]
- Fraga, D.B.; Camargo, A.; Olescowicz, G.; Padilha, D.A.; Mina, F.; Budni, J.; Brocardo, P.S.; Rodrigues, A.L.S. Ketamine, but not fluoxetine, rapidly rescues corticosterone-induced impairments on glucocorticoid receptor and dendritic branching in the hippocampus of mice. Metab. Brain Dis. 2021, 36, 2223–2233. [Google Scholar]
- Hueston, C.M.; Deak, T. On the time course, generality, and regulation of plasma progesterone release in male rats by stress exposure. Endocrinology 2014, 155, 3527–3537. [Google Scholar]
- Spencer, R.L.; Deak, T. A users guide to HPA axis research. Physiol. Behav. 2017, 178, 43–65. [Google Scholar]
- Temizkan, S.; Kelestimur, F. A clinical and pathophysiological approach to traumatic brain injury-induced pituitary dysfunction. Pituitary 2019, 22, 220–228. [Google Scholar]
- Tapp, Z.M.; Godbout, J.P.; Kokiko-Cochran, O.N. A Tilted Axis: Maladaptive Inflammation and HPA Axis Dysfunction Contribute to Consequences of TBI. Front. Neurol. 2019, 10, 345. [Google Scholar]
- Rothman, M.S.; Arciniegas, D.B.; Filley, C.M.; Wierman, M.E. The neuroendocrine effects of traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 363–372. [Google Scholar] [PubMed]
- Cernak, I.; Savic, V.J.; Lazarov, A.; Joksimovic, M.; Markovic, S. Neuroendocrine responses following graded traumatic brain injury in male adults. Brain Inj. 1999, 13, 1005–1015. [Google Scholar] [PubMed]
- Mountney, A.; Boutte, A.M.; Cartagena, C.M.; Flerlage, W.F.; Johnson, W.D.; Rho, C.; Lu, X.C.; Yarnell, A.; Marcsisin, S.; Sousa, J.; et al. Functional and Molecular Correlates after Single and Repeated Rat Closed-Head Concussion: Indices of Vulnerability after Brain Injury. J. Neurotrauma 2017, 34, 2768–2789. [Google Scholar]
- Wei, J.; Xiao, G.M. The neuroprotective effects of progesterone on traumatic brain injury: Current status and future prospects. Acta Pharmacol. Sin. 2013, 34, 1485–1490. [Google Scholar]
- Santarsieri, M.; Niyonkuru, C.; McCullough, E.H.; Dobos, J.A.; Dixon, C.E.; Berga, S.L.; Wagner, A.K. Cerebrospinal fluid cortisol and progesterone profiles and outcomes prognostication after severe traumatic brain injury. J. Neurotrauma 2014, 31, 699–712. [Google Scholar]
- Lopez-Rodriguez, A.B.; Acaz-Fonseca, E.; Spezzano, R.; Giatti, S.; Caruso, D.; Viveros, M.P.; Melcangi, R.C.; Garcia-Segura, L.M. Profiling Neuroactive Steroid Levels After Traumatic Brain Injury in Male Mice. Endocrinology 2016, 157, 3983–3993. [Google Scholar]
- Lopez-Rodriguez, A.B.; Acaz-Fonseca, E.; Giatti, S.; Caruso, D.; Viveros, M.P.; Melcangi, R.C.; Garcia-Segura, L.M. Correlation of brain levels of progesterone and dehydroepiandrosterone with neurological recovery after traumatic brain injury in female mice. Psychoneuroendocrinology 2015, 56, 1–11. [Google Scholar]
- Zanza, C.; Piccolella, F.; Racca, F.; Romenskaya, T.; Longhitano, Y.; Franceschi, F.; Savioli, G.; Bertozzi, G.; De Simone, S.; Cipolloni, L.; et al. Ketamine in Acute Brain Injury: Current Opinion Following Cerebral Circulation and Electrical Activity. Healthcare 2022, 10, 566. [Google Scholar] [CrossRef]
- Tran, K.P.; Nguyen, Q.; Truong, X.N.; Le, V.; Le, V.P.; Mai, N.; Husum, H.; Losvik, O.K. A comparison of ketamine and morphine analgesia in prehospital trauma care: A cluster randomized clinical trial in rural Quang Tri province, Vietnam. Prehospital Emerg. Care 2014, 18, 257–264. [Google Scholar]
- Khalili-Mahani, N.; Martini, C.H.; Olofsen, E.; Dahan, A.; Niesters, M. Effect of subanaesthetic ketamine on plasma and saliva cortisol secretion. Br. J. Anaesth 2015, 115, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Khalili-Mahani, N.; Niesters, M.; van Osch, M.J.; Oitzl, M.; Veer, I.; de Rooij, M.; van Gerven, J.; van Buchem, M.A.; Beckmann, C.F.; Rombouts, S.A.; et al. Ketamine interactions with biomarkers of stress: A randomized placebo-controlled repeated measures resting-state fMRI and PCASL pilot study in healthy men. Neuroimage 2015, 108, 396–409. [Google Scholar] [CrossRef] [PubMed]
- van Berckel, B.N.; Oranje, B.; van Ree, J.M.; Verbaten, M.N.; Kahn, R.S. The effects of low dose ketamine on sensory gating, neuroendocrine secretion and behavior in healthy human subjects. Psychopharmacology 1998, 137, 271–281. [Google Scholar] [CrossRef]
- Radford, C.K.D.; Park, T.Y.; Osborne-Smith, L.; Choi, K.H. Effects of Subanesthetic Intravenous Ketamine Infusion on Corticosterone and Brain-Derived Neurotrophic Factor in the Plasma of Male Sprague-Dawley Rats. AANA J. 2018, 86, 393–400. [Google Scholar]
- Radford, K.D.; Berman, R.Y.; Jaiswal, S.; Kim, S.Y.; Zhang, M.; Spencer, H.F.; Choi, K.H. Enhanced Fear Memories and Altered Brain Glucose Metabolism ((18)F-FDG-PET) following Subanesthetic Intravenous Ketamine Infusion in Female Sprague-Dawley Rats. Int. J. Mol. Sci. 2022, 23, 1922. [Google Scholar] [CrossRef]
- Radford, K.D.; Spencer, H.F.; Zhang, M.; Berman, R.Y.; Girasek, Q.L.; Choi, K.H. Association between intravenous ketamine-induced stress hormone levels and long-term fear memory renewal in Sprague-Dawley rats. Behav. Brain Res. 2020, 378, 112259. [Google Scholar] [CrossRef]
- Reitz, S.J.; Sauerbeck, A.D.; Kummer, T.T. SEQUIN: An imaging and analysis platform for quantification and characterization of synaptic structures in mouse. STAR Protoc. 2021, 2, 100268. [Google Scholar] [CrossRef]
- Sauerbeck, A.D.; Gangolli, M.; Reitz, S.J.; Salyards, M.H.; Kim, S.H.; Hemingway, C.; Gratuze, M.; Makkapati, T.; Kerschensteiner, M.; Holtzman, D.M.; et al. SEQUIN Multiscale Imaging of Mammalian Central Synapses Reveals Loss of Synaptic Connectivity Resulting from Diffuse Traumatic Brain Injury. Neuron 2020, 107, 257–273.E5. [Google Scholar] [CrossRef]
- Radford, K.D.; Park, T.Y.; Jaiswal, S.; Pan, H.; Knutsen, A.; Zhang, M.; Driscoll, M.; Osborne-Smith, L.A.; Dardzinski, B.J.; Choi, K.H. Enhanced fear memories and brain glucose metabolism ((18)F-FDG-PET) following sub-anesthetic intravenous ketamine infusion in Sprague-Dawley rats. Transl. Psychiatry 2018, 8, 263. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C.R. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- McCullers, D.L.; Sullivan, P.G.; Scheff, S.W.; Herman, J.P. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 2002, 947, 41–49. [Google Scholar] [CrossRef]
- Rowe, R.K.; Ortiz, J.B.; Thomas, T.C. Mild and Moderate Traumatic Brain Injury and Repeated Stress Affect Corticosterone in the Rat. Neurotrauma Rep. 2020, 1, 113–124. [Google Scholar] [PubMed]
- Wang, W.; Liu, L.; Yang, X.; Gao, H.; Tang, Q.K.; Yin, L.Y.; Yin, X.Y.; Hao, J.R.; Geng, D.Q.; Gao, C. Ketamine improved depressive-like behaviors via hippocampal glucocorticoid receptor in chronic stress induced- susceptible mice. Behav. Brain Res. 2019, 364, 75–84. [Google Scholar] [PubMed]
- Birnie, M.T.; Eapen, A.V.; Kershaw, Y.M.; Lodge, D.; Collingridge, G.L.; Conway-Campbell, B.L.; Lightman, S.L. Time of day influences stress hormone response to ketamine. J. Neuroendocrinol. 2022, 34, e13194. [Google Scholar] [CrossRef]
- Danan, D.; Matar, M.A.; Kaplan, Z.; Zohar, J.; Cohen, H. Blunted basal corticosterone pulsatility predicts post-exposure susceptibility to PTSD phenotype in rats. Psychoneuroendocrinology 2018, 87, 35–42. [Google Scholar]
- Morris, M.C.; Compas, B.E.; Garber, J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clin. Psychol. Rev. 2012, 32, 301–315. [Google Scholar]
- Zuckerman, A.; Ram, O.; Ifergane, G.; Matar, M.A.; Kaplan, Z.; Hoffman, J.R.; Sadot, O.; Cohen, H. Role of Endogenous and Exogenous Corticosterone on Behavioral and Cognitive Responses to Low-Pressure Blast Wave Exposure. J. Neurotrauma 2019, 36, 380–394. [Google Scholar]
- Kinlein, S.A.; Phillips, D.J.; Keller, C.R.; Karatsoreos, I.N. Role of corticosterone in altered neurobehavioral responses to acute stress in a model of compromised hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology 2019, 102, 248–255. [Google Scholar]
- Villegas, E.; Hartsock, M.J.; Aben, B.; Lenahan, K.N.; Hernandez, T.D.; Spencer, R.L. Association between Altered Cortisol Profiles and Neurobehavioral Impairment after Mild Traumatic Brain Injury in College Students. J. Neurotrauma 2022, 39, 809–820. [Google Scholar]
- Zanos, P.; Gould, T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar]
- Moghaddam, B.; Adams, B.; Verma, A.; Daly, D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997, 17, 2921–2927. [Google Scholar]
- Aleksandrova, L.R.; Phillips, A.G. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends. Pharmacol. Sci. 2021, 42, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.H.; Abdallah, C.G.; Sanacora, G.; Charney, D.S.; Duman, R.S. Ketamine: A Paradigm Shift for Depression Research and Treatment. Neuron 2019, 101, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lee, B.; Liu, R.J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.Y.; Aghajanian, G.; Duman, R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef]
- Li, N.; Liu, R.J.; Dwyer, J.M.; Banasr, M.; Lee, B.; Son, H.; Li, X.Y.; Aghajanian, G.; Duman, R.S. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry 2011, 69, 754–761. [Google Scholar] [CrossRef]
- Sarkar, A.; Kabbaj, M. Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biol. Psychiatry 2016, 80, 448–456. [Google Scholar] [CrossRef]
- Girgenti, M.J.; Ghosal, S.; LoPresto, D.; Taylor, J.R.; Duman, R.S. Ketamine accelerates fear extinction via mTORC1 signaling. Neurobiol. Dis. 2017, 100, 1–8. [Google Scholar] [CrossRef]
- Zhou, Y.; Milham, M.P.; Lui, Y.W.; Miles, L.; Reaume, J.; Sodickson, D.K.; Grossman, R.I.; Ge, Y. Default-mode network disruption in mild traumatic brain injury. Radiology 2012, 265, 882–892. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Averill, L.A.; Collins, K.A.; Geha, P.; Schwartz, J.; Averill, C.; DeWilde, K.E.; Wong, E.; Anticevic, A.; Tang, C.Y.; et al. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology 2017, 42, 1210–1219. [Google Scholar] [CrossRef]
- Thelen, C.; Flaherty, E.; Saurine, J.; Sens, J.; Mohamed, S.; Pitychoutis, P.M. Sex Differences in the Temporal Neuromolecular and Synaptogenic Effects of the Rapid-acting Antidepressant Drug Ketamine in the Mouse Brain. Neuroscience 2019, 398, 182–192. [Google Scholar] [CrossRef]
- Acabchuk, R.; Briggs, D.I.; Angoa-Perez, M.; Powers, M.; Wolferz, R., Jr.; Soloway, M.; Stern, M.; Talbot, L.R.; Kuhn, D.M.; Conover, J.C. Repeated mild traumatic brain injury causes focal response in lateral septum and hippocampus. Concussion 2016, 1, CNC13. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Domel, A.G.; Dennis, E.L.; Georgiadis, M.; Liu, Y.; Raymond, S.J.; Grant, G.; Kleiven, S.; Camarillo, D.; et al. The Presence of the Temporal Horn Exacerbates the Vulnerability of Hippocampus During Head Impacts. Front. Bioeng. Biotechnol. 2022, 10, 754344. [Google Scholar]
- Geddes, D.M.; LaPlaca, M.C.; Cargill, R.S., 2nd. Susceptibility of hippocampal neurons to mechanically induced injury. Exp. Neurol. 2003, 184, 420–427. [Google Scholar] [PubMed]
- Zhang, M.; Radford, K.D.; Driscoll, M.; Purnomo, S.; Kim, J.; Choi, K.H. Effects of subanesthetic intravenous ketamine infusion on neuroplasticity-related proteins in the prefrontal cortex, amygdala, and hippocampus of Sprague-Dawley rats. IBRO Rep. 2019, 6, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Spencer, H.F.; Berman, R.Y.; Radford, K.D.; Choi, K.H. Effects of subanesthetic intravenous ketamine infusion on neuroplasticity-related proteins in male and female Sprague-Dawley rats. IBRO Neurosci. Rep. 2021, 11, 42–51. [Google Scholar]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free. Radic. Biol. Med. 2008, 45, 443–452. [Google Scholar]
- Phoumthipphavong, V.; Barthas, F.; Hassett, S.; Kwan, A.C. Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex. eNeuro 2016, 3, ENEURO.0133-15.2016. [Google Scholar] [CrossRef]
- Knott, G.W.; Holtmaat, A.; Wilbrecht, L.; Welker, E.; Svoboda, K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat. Neurosci. 2006, 9, 1117–1124. [Google Scholar]
- Wu, H.; Savalia, N.K.; Kwan, A.C. Ketamine for a Boost of Neural Plasticity: How, but Also When? Biol. Psychiatry 2021, 89, 1030–1032. [Google Scholar] [CrossRef]
- Hannon, M.J.; Crowley, R.K.; Behan, L.A.; O’Sullivan, E.P.; O’Brien, M.M.; Sherlock, M.; Rawluk, D.; O’Dwyer, R.; Tormey, W.; Thompson, C.J. Acute glucocorticoid deficiency and diabetes insipidus are common after acute traumatic brain injury and predict mortality. J. Clin. Endocrinol. Metab. 2013, 98, 3229–3237. [Google Scholar]
- Olivecrona, Z.; Dahlqvist, P.; Koskinen, L.O. Acute neuro-endocrine profile and prediction of outcome after severe brain injury. Scand. J. Trauma. Resusc. Emerg. Med. 2013, 21, 33. [Google Scholar]
- Cohan, P.; Wang, C.; McArthur, D.L.; Cook, S.W.; Dusick, J.R.; Armin, B.; Swerdloff, R.; Vespa, P.; Muizelaar, J.P.; Cryer, H.G.; et al. Acute secondary adrenal insufficiency after traumatic brain injury: A prospective study. Crit. Care Med. 2005, 33, 2358–2366. [Google Scholar]
- Radford, K.D.; Berman, R.Y.; Zhang, M.; Wu, T.J.; Choi, K.H. Sex-related differences in intravenous ketamine effects on dissociative stereotypy and antinociception in male and female rats. Pharmacol. Biochem. Behav. 2020, 199, 173042. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boese, M.; Berman, R.; Spencer, H.; Rujan, O.; Metz, E.; Radford, K.; Choi, K. Effects of Subanesthetic Intravenous Ketamine Infusion on Stress Hormones and Synaptic Density in Rats with Mild Closed-Head Injury. Biomedicines 2025, 13, 787. https://doi.org/10.3390/biomedicines13040787
Boese M, Berman R, Spencer H, Rujan O, Metz E, Radford K, Choi K. Effects of Subanesthetic Intravenous Ketamine Infusion on Stress Hormones and Synaptic Density in Rats with Mild Closed-Head Injury. Biomedicines. 2025; 13(4):787. https://doi.org/10.3390/biomedicines13040787
Chicago/Turabian StyleBoese, Martin, Rina Berman, Haley Spencer, Oana Rujan, Ellie Metz, Kennett Radford, and Kwang Choi. 2025. "Effects of Subanesthetic Intravenous Ketamine Infusion on Stress Hormones and Synaptic Density in Rats with Mild Closed-Head Injury" Biomedicines 13, no. 4: 787. https://doi.org/10.3390/biomedicines13040787
APA StyleBoese, M., Berman, R., Spencer, H., Rujan, O., Metz, E., Radford, K., & Choi, K. (2025). Effects of Subanesthetic Intravenous Ketamine Infusion on Stress Hormones and Synaptic Density in Rats with Mild Closed-Head Injury. Biomedicines, 13(4), 787. https://doi.org/10.3390/biomedicines13040787





