Prevention of Peripherally Inserted Central Catheter (PICC)-Associated Vein Thrombosis in Cancer: A Narrative Review
Abstract
1. Introduction
2. Methods
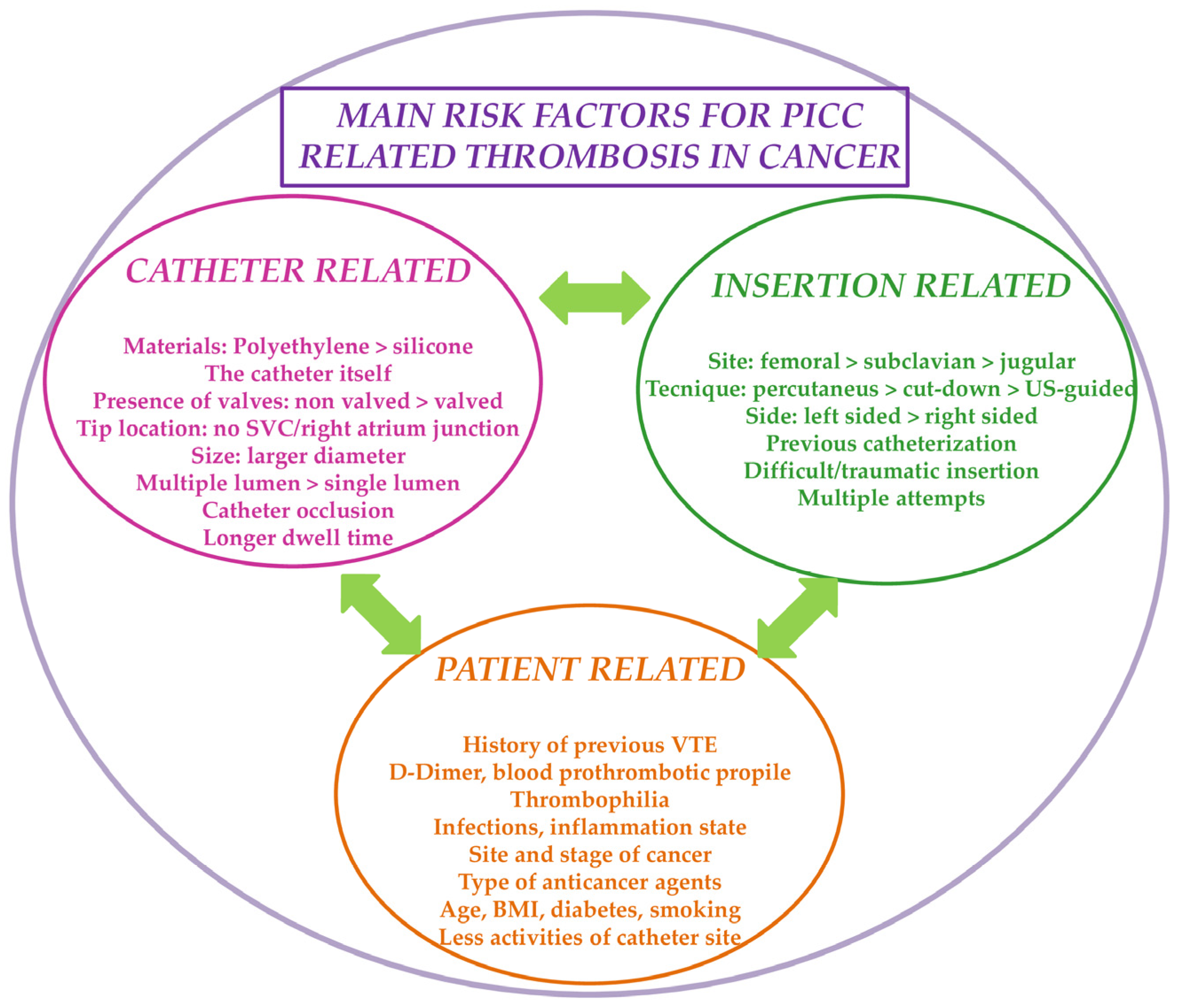
3. Risk Factors for the Development of VTE in Cancer Patients with PICC
4. Risk Assessment Models for PICC-Related Thrombosis in Patients with Cancer
| Risk Assessment Model | Variables | Specific for Patients with Cancer | Specific for Patients with PICC |
|---|---|---|---|
| Khorana risk score [39,42] | Cancer site, platelet count, hemoglobin, erythropoiesis-stimulating agents, leukocyte count, BMI | Yes | No |
| Michigan risk score [40,41,42,50,51] | History of VTE, multi-lumen PICC, active cancer, presence of another CVC, leukocyte count | No | Yes |
| Caprini risk score [43,44,50,51] | Multiple variables including age, cancer, surgery, medical diseases, thrombophilia, female specific health issues, CVC | No | No |
| Padua risk score [45,50,51] | Active cancer, previous VTE, reduced mobility, thrombophilia, recent trauma/surgery, age, heart/respiratory failure, acute myocardial infarction/stroke, acute infection/rheumatologic disorder, obesity, hormonal treatment | No | No |
| Autar DVT scale [46,50] | Age, mobility, trauma, medical diseases, BMI, female specific health issues, surgery | No | No |
| Seeley score [47,50] | Medical diseases | No | Yes |
| Wells score [48,50] | Clinical symptoms for DVT, previous DVT/PE, immobility/surgery, no alternative diagnosis, heart rate > 100 beats/minute, cancer, hemoptysis | No | No |
| Revised Geneva score [49,50,51] | Age, previous DVT/PE, surgery/fracture, active cancer, symptoms, clinical signs | No | No |
5. Thromboprophylaxis in Cancer Patients with PICC
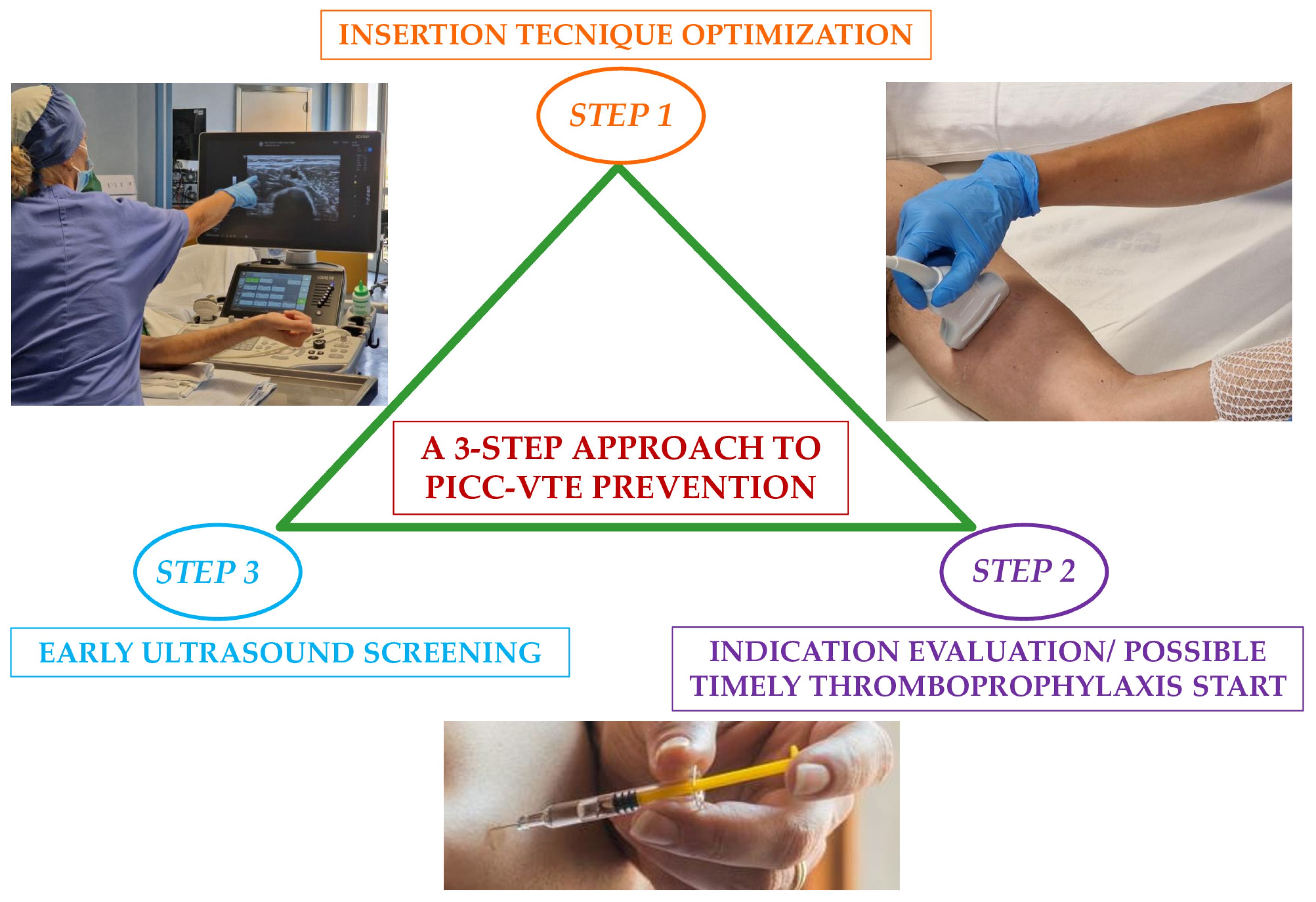
6. Ameliorating PICC Insertion Strategies
7. The Novel PICC-PORT Lines
8. “Head-to-Head” Major Guidelines Comparison
9. Optimal Suggested Management Approach for the Prevention of PICC-VTE in Cancer
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khorana, A.A.; Palaia, J.; Rosenblatt, L.; Pisupati, R.; Huang, N.; Nguyen, C.; Barron, J.; Gallagher, K.; Bond, T.C. Venous thromboembolism incidence and risk factors associated with immune checkpoint inhibitors among patients with advanced non-small cell lung cancer. Immunother. Cancer 2023, 11, e006072. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Pabinger, I.; Cohen, A.T. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb. Haemost. 2017, 117, 219–230. [Google Scholar] [CrossRef]
- Gai, M.; He, W. Clinical Value of Coagulation Index Changes in Early Diagnosis and Nursing Intervention for PICC-Related Venous Thrombosis in Tumor Patients. Contrast. Media Mol. Imaging 2022, 2022, 7579225. [Google Scholar] [CrossRef]
- Madabhavi, I.; Patel, A.; Sarkar, M.; Kataria, P.; Kadakol, N.; Anand, A. A study of the use of peripherally inserted central catheters in cancer patients: A single-center experience. J. Vasc. Nurs. 2018, 36, 149–156. [Google Scholar] [CrossRef]
- Taxbro, K.; Hammarskjöld, F.; Thelin, B.; Lewin, F.; Hagman, H.; Hanberger, H.; Berg, S. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: An open-label, randomised, two-centre trial. Br. J. Anaesth. 2019, 122, 734–741. [Google Scholar] [CrossRef]
- Taglialatela, I.; Mariani, L.; Dotti, K.F.; Di Vico, L.; Pisanu, M.N.; Facchinetti, C.; De Braud, F.; Ferrari, L.A.M. Central venous catheters-related-thrombosis and risk factors in oncological patients: A retrospective evaluation of recent risk scores. Tumori 2023, 109, 363–369. [Google Scholar] [CrossRef]
- Kim, H.J.; Yun, J.; Kim, H.J.; Kim, K.H.; Kim, S.H.; Lee, S.C.; Bae, S.B.; Kim, C.K.; Lee, N.S.; Lee, K.T.; et al. Safety and effectiveness of central venous catheterization in patients with cancer: Prospective observational study. J. Korean Med. Sci. 2010, 25, 1748–1753. [Google Scholar] [PubMed]
- Elias, A.; Debourdeau, P.; Espitia, O.; Sevestre, M.A.; Girard, P.; Mahé, I.; Sanchez, O.; INNOVTE CAT Working Group. Central venous catheter associated upper extremity deep vein thrombosis in cancer patients: Diagnosis and therapeutic management. Arch. Cardiovasc. Dis. 2024, 117, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Dominikus, H.; Veronika, W.; Mair Maximilian, J.; Martina, S.; Pavla, K.; Christoph, K.; Christian, K.; Christian, L.; Rupert, B.; Christoph, M. Complication Rates of Peripherally Inserted Central Catheters in Oncologic Versus Non-Oncologic Patients. Semin. Oncol. Nurs. 2024, 40, 151681. [Google Scholar] [CrossRef]
- Evans, R.S.; Sharp, J.H.; Linford, L.H.; Lloyd, J.F.; Woller, S.C.; Stevens, S.M.; Elliott, C.G.; Tripp, J.S.; Jones, S.S.; Lindell, K. Weaver Reduction of peripherally inserted central catheter-associated DVT. Chest 2013, 143, 627–633. [Google Scholar] [CrossRef]
- Weitz, J.I.; Haas, S.; Ageno, W.; Goldhaber, S.Z.; Turpie, A.G.G.; Goto, S.; Angchaisuksiri, P.; Nielsen, J.D.; Kayani, G.; Farjat, A.E.; et al. Cancer associated thrombosis in everyday practice: Perspectives from GARFIELD-VTE. J. Thromb. Thrombolysis 2020, 50, 267–277. [Google Scholar] [PubMed]
- Liu, B.; Wu, Z.; Lin, C.; Li, L.; Kuang, X. Applicability of TIVAP versus PICC in non-hematological malignancies patients: A meta-analysis and systematic review. PLoS ONE 2021, 16, e0255473. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Soh, K.L.; Ying, Y.; Liu, Y.; Huang, X.; Huang, J. Risk of VTE associated with PORTs and PICCs in cancer patients: A systematic review and meta-analysis. Thromb. Res. 2022, 213, 34–42. [Google Scholar] [PubMed]
- Bertoletti, L.; Madridano, O.; Jiménez, D.; Muriel, A.; Bikdeli, B.; Ay, C.; Trujillo-Santos, J.; Bosevski, M.; Sigüenza, P.; Monreal, M. Cancer-Associated Thrombosis: Trends in Clinical Features, Treatment, and Outcomes From 2001 to 2020. JACC CardioOncol. 2023, 5, 758–772. [Google Scholar] [CrossRef]
- Rieger, M.J.; Schenkel, X.; Dedic, I.; Brunn, T.; Gnannt, R.; Hofmann, M.; de Rougemont, O.; Stolz, S.M.; Rösler, W.; Studt, J.-D.; et al. Complication rates of peripherally inserted central catheters vs implanted ports in patients receiving systemic anticancer therapy: A retrospective cohort study. Int. J. Cancer 2023, 153, 1397–1405. [Google Scholar]
- Moss, J.G.; Wu, O.; Bodenham, A.R.; Agarwal, R.; Menne, T.F.; Jones, B.L.; Heggie, R.; Hill, S.; Dixon-Hughes, J.; Soulis, E.; et al. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): A randomised controlled trial. Lancet 2021, 398, 403–415. [Google Scholar]
- Fioretti, A.M.; Leopizzi, T.; Puzzovivo, A.; Giotta, F.; Lorusso, V.; Luzzi, G.; Oliva, S. Edoxaban: Front-line treatment for brachiocephalic vein thrombosis in primitive mediastinal seminoma: A case report and literature review. Medicine 2022, 101, e29429. [Google Scholar]
- Mitbander, U.B.; Geer, M.J.; Taxbro, K.; Horowitz, J.; Zhang, Q.; O’Malley, M.E.; Ramnath, N.; Chopra, V. Patterns of use and outcomes of peripherally inserted central catheters in hospitalized patients with solid tumors: A multicenter study. Cancer 2022, 128, 3681–3690. [Google Scholar] [CrossRef]
- Sánchez Cánovas, M.; García Torralba, E.; Blaya Boluda, N.; Sánchez Saura, A.; Puche Palao, G.; Sánchez Fuentes, A.; Montesinos, L.M.; Ganga, C.P.; Tomas, L.G.; Jiménez, J.B.; et al. Thrombosis and infections associated with PICC in onco-hematological patients, what is their relevance? Clin. Transl. Oncol. 2024, 26, 3226–3235. [Google Scholar] [CrossRef]
- Li, A.; Brandt, W.; Brown, C.; Wang, T.F.; Ikesaka, R.; Delluc, A.; Wells, P.; Carrier, M. Efficacy and safety of primary thromboprophylaxis for the prevention of venous thromboembolism in patients with cancer and a central venous catheter: A systematic review and meta-analysis. Thromb. Res. 2021, 208, 58–65. [Google Scholar]
- Debourdeau, P.; Farge, D.; Beckers, M.; Baglin, C.; Bauersachs, R.M.; Brenner, B.; Brilhante, D.; Falanga, A.; Gerotzafias, G.T.; Haim, N.; et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J. Thromb. Haemost. 2013, 11, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.F.; Kou, R.; Carrier, M.; Delluc, A. Management of catheter-related upper extremity deep vein thrombosis in patients with cancer: A systematic review and meta-analysis. J. Thromb. Haemost. 2024, 22, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Kamphuisen, P.W. Epidemiology and prevention of catheter-related thrombosis in patients with cancer. J. Thromb. Haemost. 2012, 10, 1491–1499. [Google Scholar] [CrossRef]
- Saber, W.; Moua, T.; Williams, E.C.; Verso, M.; Agnelli, G.; Couban, S.; Young, A.; De Cicco, M.; Biffi, R.; van Rooden, C.J.; et al. Risk factors for catheter-related thrombosis (CRT) in cancer patients: A patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J. Thromb. Haemost. 2011, 9, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Levine, M.N.; Butler, G.; Webb, C.; Costantini, L.; Gu, C.; Julian, J.A. Incidence, risk factors, and outcomes of catheter-related thrombosis in adult patients with cancer. J. Clin. Oncol. 2006, 24, 1404–1408. [Google Scholar] [CrossRef]
- Chopra, V.; Anand, S.; Hickner, A.; Buist, M.; Rogers, M.A.; Saint, S.; Flanders, S.A. Risk of venous thromboembolism associated with peripherally inserted central catheters: A systematic review and meta-analysis. Lancet 2013, 382, 311–325. [Google Scholar] [CrossRef]
- Yi, X.L.; Chen, J.; Li, J.; Feng, L.; Wang, Y.; Zhu, J.A.; Shen, E.; Hu, B. Risk factors associated with PICC-related upper extremity venous thrombosis in cancer patients. J. Clin. Nurs. 2014, 23, 837–843. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Wei, L.; Chen, W.; Ma, X.; Song, L. Peripherally inserted central catheter thrombosis incidence and risk factors in cancer patients: A double-center prospective investigation. Ther. Clin. Risk. Manag. 2015, 11, 153–160. [Google Scholar]
- Simonetti, G.; Bersani, A.; Tramacere, I.; Lusignani, M.; Gaviani, P.; Silvani, A. The role of body mass index in the development of thromboembolic events among cancer patients with PICCs: A systematic review. J. Vasc. Nurs. 2022, 40, 11–16. [Google Scholar] [CrossRef]
- Ellis, M.L.; Okano, S.; McCann, A.; McDowall, A.; Van Kuilenburg, R.; McCarthy, A.L.; Joubert, W.; Harper, J.; Jones, M.; Mollee, P. Catheter-related thrombosis incidence and risk factors in adult cancer patients with central venous access devices. Intern. Med. J. 2020, 50, 1475–1482. [Google Scholar] [CrossRef]
- Zhai, R.; Chen, X.; Wang, G.; Xu, J.; Yang, Y. Predictive Value of Red Cell Distribution Width in the Diagnosis of Peripherally Inserted Central Catheter (PICC)-Related Thrombosis Among Cancer Patients. Int. J. Gen. Med. 2023, 16, 359–365. [Google Scholar] [CrossRef]
- Meng, F.; Fan, S.; Guo, L.; Jia, Z.; Chang, H.; Liu, F. Incidence and risk factors of PICC-related thrombosis in breast cancer: A meta-analysis. Jpn. J. Clin. Oncol. 2024, 54, 863–872. [Google Scholar] [CrossRef]
- Wang, P.; He, L.; Yuan, Q.; Lu, J.; Ji, Q.; Peng, A.; Liu, W.W. Risk factors for peripherally inserted central catheter-related venous thrombosis in adult patients with cancer. Thromb. J. 2024, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Verso, M.; Agnelli, G.; Kamphuisen, P.W.; Ageno, W.; Bazzan, M.; Lazzaro, A.; Paoletti, F.; Paciaroni, M.; Mosca, S.; Bertoglio, S. Risk factors for upper limb deep vein thrombosis associated with the use of central vein catheter in cancer patients. Intern. Emerg. Med. 2008, 3, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, S.; Faccini, B.; Lalli, L.; Cafiero, F.; Bruzzi, P. Peripherally inserted central catheters (PICCs) in cancer patients under chemotherapy: A prospective study on the incidence of complications and overall failures. J. Surg. Oncol. 2016, 113, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Al-Asadi, O.; Almusarhed, M.; Eldeeb, H. Predictive risk factors of venous thromboembolism (VTE) associated with peripherally inserted central catheters (PICC) in ambulant solid cancer patients: Retrospective single Centre cohort study. Thromb. J. 2019, 17, 2. [Google Scholar] [CrossRef]
- Li, N.; Huang, J.; Feng, Y.; Yan, H.; Min, S.; Chen, X. Association Between Systemic Immune Inflammation Indexes and DVT in Patients with Malignancy Requiring PICC Insertion. Biol. Res. Nurs. 2024, 26, 518–525. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, G.; Chen, P.; Gui, Z. Comparative Predictive Modeling for PICC Line Complications in Oncology: A Retrospective Study. Br. J. Hosp. Med. 2024, 85, 1–15. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Chopra, V.; Kaatz, S.; Conlon, A.; Paje, D.; Grant, P.J.; Rogers, M.A.M.; Bernstein, S.J.; Saint, S.; Flanders, S.A. The Michigan Risk Score to predict peripherally inserted central catheter-associated thrombosis. J. Thromb. Haemost. 2017, 15, 1951–1962. [Google Scholar] [CrossRef]
- Kang, J.; Sun, W.; Li, H.; Ma, E.L.; Chen, W. Validation of Michigan risk score and D-dimer to predict peripherally inserted central catheter-related thrombosis: A study of 206,132 catheter days. J. Vasc. Access. 2022, 23, 764–769. [Google Scholar] [PubMed]
- Yuen, H.L.A.; Zhao, J.; Tran, H.; Chunilal, S.D. Development of a risk score to predict peripherally inserted central catheter thrombosis in active cancer. Intern. Med. J. 2022, 52, 1733–1740. [Google Scholar] [PubMed]
- Caprini, J.A. Thrombosis risk assessment as a guide to quality patient care. Dis. Mon. 2005, 51, 70–78. [Google Scholar] [PubMed]
- Lin, Y.; Zeng, Z.; Lin, R.; Zheng, J.; Liu, S.; Gao, X. The Caprini thrombosis risk model predicts the risk of peripherally inserted central catheter-related upper extremity venous thrombosis in patients with cancer. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 1151–1158. [Google Scholar]
- Barbar, S.; Noventa, F.; Rossetto, V.; Ferrari, A.; Brandolin, B.; Perlati, M.; De Bon, E.; Tormene, D.; Pagnan, A.; Prandoni, P. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua Prediction Score. J. Thromb. Haemost. 2010, 8, 2450–2457. [Google Scholar]
- Autar, R. The management of deep vein thrombosis: The Autar DVT risk assessment scale re-visited. J. Orthop. Nurs. 2003, 7, 114–124. [Google Scholar]
- Seeley, M.; Santiago, M.; Shott, S. Prediction tool for thrombi associated with peripherally inserted central catheters. J. Infus. Nurs. 2007, 30, 286. [Google Scholar]
- Wells, P.S.; Anderson, D.R.; Rodger, M.; Ginsberg, J.S.; Kearon, C.; Gent, M.; Turpie, A.G.; Bormanis, J.; Weitz, J.; Chamberlain, M.; et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: Increasing the models utility with the SimpliRED D-dimer. Thromb. Haemost. 2000, 83, 416–420. [Google Scholar]
- Le Gal, G.; Righini, M.; Roy, P.M.; Sanchez, O.; Aujesky, D.; Bounameaux, H.; Perrier, A. Prediction of pulmonary embolism in the emergency department: The revised Geneva score. Ann. Intern. Med. 2006, 144, 165–171. [Google Scholar]
- Hu, Z.; He, R.; Zhao, Y.; Luo, M.; Fan, Y.; Li, J. Risk assessment models for PICC-related venous thrombosis in adult patients with cancer: A network meta-analysis. Thromb. Res. 2024, 239, 109030. [Google Scholar]
- Yue, J.; Zhang, Y.; Xu, F.; Mi, A.; Zhou, Q.; Chen, B.; Shin, L. A clinical study of peripherally inserted central catheter-related venous thromboembolism in patients with hematological malignancies. Sci. Rep. 2022, 12, 9871. [Google Scholar]
- Young, A.M.; Billingham, L.J.; Begum, G.; Kerr, D.J.; Hughes, A.I.; Rea, D.W.; Shepherd, S.; Stanley, A.; Sweeney, A.; Wilde, J.; et al. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): An open-label randomised trial. Lancet 2009, 373, 567–574. [Google Scholar] [PubMed]
- Fioretti, A.M.; Leopizzi, T.; La Forgia, D.; De Luca, R.; Oreste, D.; Inchingolo, R.; Scicchitano, P.; Oliva, S. Abelacimab in Cancer-Associated Thrombosis: The Right Drug at the Right Time for the Right Purpose. A Comprehensive Review. Rev. Cardiovasc. Med. 2023, 24, 295. [Google Scholar] [PubMed]
- Pfeffer, M.A.; Kohs, T.C.L.; Vu, H.H.; Jordan, K.R.; Wang, J.S.H.; Lorentz, C.U.; Tucker, E.I.; Puy, C.; Olson, S.R.; DeLoughery, T.G.; et al. Factor XI Inhibition for the Prevention of Catheter-Associated Thrombosis in Patients with Cancer Undergoing Central Line Placement: A Phase 2 Clinical Trial. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 290–299. [Google Scholar] [PubMed]
- Verso, M.; Agnelli, G.; Bertoglio, S.; Di Somma, F.C.; Paoletti, F.; Ageno, W.; Bazzan, M.; Parise, P.; Quintavalla, R.; Naglieri, E.; et al. Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: A double-blind, placebo-controlled, randomized study in cancer patients. J. Clin. Oncol. 2005, 23, 4057–4062. [Google Scholar]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2019, 380, 711–719. [Google Scholar]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef]
- Brandt, W.; Brown, C.; Wang, T.F.; Tagalakis, V.; Shivakumar, S.; Ciuffini, L.A.; Mallick, R.; Wells, P.S.; Carrier, M. Efficacy and safety of apixaban for primary prevention of thromboembolism in patients with cancer and a central venous catheter: A subgroup analysis of the AVERT Trial. Thromb. Res. 2022, 216, 8–10. [Google Scholar]
- Lv, S.; Liu, Y.; Wei, G.; Shi, X.; Chen, S.; Zhang, X. The anticoagulants rivaroxaban and low molecular weight heparin prevent PICC-related upper extremity venous thrombosis in cancer patients. Medicine 2019, 98, e17894. [Google Scholar]
- Ikesaka, R.; Siegal, D.; Mallick, R.; Wang, T.F.; Witham, D.; Webb, C.; Carrier, M. Canadian Venous Thromboembolism Research Network (CanVECTOR) Thromboprophylaxis with rivaroxaban in patients with malignancy and central venous lines (TRIM-Line): A two-center open-label pilot randomized controlled trial. Res. Pract. Thromb. Haemost. 2021, 5, e12517. [Google Scholar]
- D’Ambrosio, L.; Aglietta, M.; Grignani, G. Anticoagulation for central venous catheters in patients with cancer. N. Engl. J. Med. 2014, 371, 1362–1363. [Google Scholar] [PubMed]
- Kahale, L.A.; Tsolakian, I.G.; Hakoum, M.B.; Matar, C.F.; Barba, M.; Yosuico, V.E.; Terrenato, I.; Sperati, F.; Schünemann, H.; Akl, E.A. Anticoagulation for people with cancer and central venous catheters. Cochrane Database Syst. Rev. 2018, 6, CD006468. [Google Scholar] [PubMed]
- Pinelli, F.; Balsorano, P.; Mura, B.; Pittiruti, M. Reconsidering the GAVeCeLT Consensus on catheter-related thrombosis, 13 years later. J. Vasc. Access. 2021, 22, 501–508. [Google Scholar]
- Xiao, W. The curative effect analysis of peripherally inserted central venous catheter catheterization for tumor patients under the guidance of new medical guide wire. Eur. J. Med. Res. 2021, 26, 99. [Google Scholar]
- Seckold, T.; Walker, S.; Dwyer, T. A comparison of silicone and polyurethane PICC lines and postinsertion complication rates: A systematic review. J. Vasc. Access. 2015, 16, 167–177. [Google Scholar]
- Sheng, Y.; Yang, L.H.; Wu, Y.; Gao, W.; Dongye, S.Y. Implementation of Tunneled Peripherally Inserted Central Catheters Placement in Cancer Patients: A Randomized Multicenter Study. Clin. Nurs. Res. 2024, 33, 19–26. [Google Scholar] [CrossRef]
- Xiao, M.F.; Xiao, C.Q.; Li, J.; Dai, C.; Fan, Y.Y.; Cao, H.; Qin, H.-Y. Subcutaneous tunneling technique to improve outcomes for patients undergoing chemotherapy with peripherally inserted central catheters: A randomized controlled trial. J. Int. Med. Res. 2021, 49, 3000605211004517. [Google Scholar] [PubMed]
- Li, F.; Shen, H.; Wang, M.; Wang, Y. Peripheral insertion of reverse-tapered and non-tapered central catheters (PICC) in patients receiving tumor chemotherapy. J. Cancer. Res. Ther. 2021, 17, 1651–1655. [Google Scholar]
- Desjardins, B.; Hanley, M.; Steigner, M.L.; Aghayev, A.; Azene, E.M.; Bennett, S.J.; Chandra, A.; Hedgire, S.S.; Lo, B.M.; Mauro, D.M.; et al. ACR Appropriateness Criteria Suspected Upper Extremity Deep Vein Thrombosis. J. Am. Coll. Radiol. 2020, 17, S315–S322. [Google Scholar] [CrossRef]
- Brescia, F.; Annetta, M.G.; Pinelli, F.; Pittiruti, M. A GAVeCeLT bundle for PICC-port insertion: The SIP-Port protocol. J. Vasc. Access. 2024, 25, 1713–1720. [Google Scholar] [CrossRef]
- Annetta, M.G.; Bertoglio, S.; Biffi, R.; Brescia, F.; Giarretta, I.; Greca, A.; Panocchia, N.; Passaro, G.; Perna, F.; Pinelli, F.; et al. Management of antithrombotic treatment and bleeding disorders in patients requiring venous access devices: A systematic review and a GAVeCeLT consensus statement. J. Vasc. Access. 2022, 23, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, C.L.; Pan, C.Q.; Cui, X.W.; Dietrich, C.F. Risk of venous thromboembolism associated with totally implantable venous access ports in cancer patients: A systematic review and meta-analysis. J. Thromb. Haemost. 2020, 18, 2253–2273. [Google Scholar] [CrossRef] [PubMed]
- Burbridge, B.; Lim, H.; Dwernychuk, L.; Le, H.; Asif, T.; Sami, A.; Ahmed, S. Comparison of the Quality of Life of Patients with Breast or Colon Cancer with an Arm Vein Port (TIVAD) Versus a Peripherally Inserted Central Catheter (PICC). Curr. Oncol. 2021, 28, 1495–1506. [Google Scholar] [CrossRef]
- Clatot, F.; Fontanilles, M.; Lefebvre, L.; Lequesne, J.; Veyret, C.; Alexandru, C.; Leheurteur, M.; Guillemet, C.; Gouérant, S.; Petrau, C.; et al. Randomised phase II trial evaluating the safety of peripherally inserted catheters versus implanted port catheters during adjuvant chemotherapy in patients with early breast cancer. Eur. J. Cancer 2020, 126, 116–124. [Google Scholar] [CrossRef]
- Tippit, D.; Siegel, E.; Ochoa, D.; Pennisi, A.; Hill, E.; Merrill, A.; Rowe, M.; Henry-Tillman, R.; Ananthula, A.; Makhoul, I. Upper-Extremity Deep Vein Thrombosis in Patients with Breast Cancer With Chest Versus Arm Central Venous Port Catheters. Breast Cancer 2018, 12, 1178223418771909. [Google Scholar] [CrossRef]
- Shiono, M.; Takahashi, S.; Takahashi, M.; Yamaguchi, T.; Ishioka, C. Current situation regarding central venous port implantation procedures and complications: A questionnaire-based survey of 11,693 implantations in Japan. Int. J. Clin. Oncol. 2016, 21, 1172–1182. [Google Scholar] [CrossRef]
- Bertoglio, S.; Cafiero, F.; Meszaros, P.; Varaldo, E.; Blondeaux, E.; Molinelli, M.; Minuto, M. PICC-PORT totally implantable vascular access device in breast cancer patients undergoing chemotherapy. J. Vasc. Access. 2020, 21, 460–466. [Google Scholar] [CrossRef]
- Bertoglio, S.; Annetta, M.G.; Brescia, F.; Emoli, A.; Fabiani, F.; Fino, M.; Merlicco, D.; Musaro, A.; Orlandi, M.; Parisella, L.; et al. A multicenter retrospective study on 4480 implanted PICC-ports: A GAVeCeLT project. J. Vasc. Access. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Pinelli, F.; Barbani, F.; Defilippo, B.; Fundarò, A.; Nella, A.; Selmi, V.; Romagnoli, S.; Villa, G. Quality of lifein women with breast cancer undergoing neoadjuvant chemotherapy: Comparison between PICC and PICC-port. Breast Cancer 2024, 31, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Cominacini, M.; De Marchi, S.; Tosi, F.; Piccinno, E.; Dal Corso, A.; Dalla Grana, E.; Stefani, F.; Carbonare, L.D. Incidence and clinical progression of asymptomatic peripherally inserted central catheter-related thrombosis in solid neoplasm patients: Ultrasound insights from a prospective cohort study. Res. Pract. Thromb. Haemost. 2024, 8, 102391. [Google Scholar] [CrossRef]
- Zwicker, J.I.; Connolly, G.; Carrier, M.; Kamphuisen, P.W.; Lee, A.Y. Catheter-associated deep vein thrombosis of the upper extremity in cancer patients: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2014, 12, 796–800. [Google Scholar] [CrossRef]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [PubMed]
- Falanga, A.; Ay, C.; Di Nisio, M.; Gerotziafas, G.; Jara-Palomares, L.; Langer, F.; Lecumberri, R.; Mandala, M.; Maraveyas, A.; Pabinger, I.; et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 452–467. [Google Scholar]
- Alikhan, R.; Gomez, K.; Maraveyas, A.; Noble, S.; Young, A.; Thomas, M. Cancer-associated venous thrombosis in adults (second edition): A British Society for Haematology Guideline. Br. J. Haematol. 2024, 205, 71–87. [Google Scholar] [PubMed]
- Verso, M.; Agnelli, G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J. Clin. Oncol. 2003, 21, 3665–3675. [Google Scholar]
- Brescia, F.; Pittiruti, M.; Spencer, T.R.; Dawson, R.B. The SIP protocol update: Eight strategies, incorporating Rapid Peripheral Vein Assessment (RaPeVA), to minimize complications associated with peripherally inserted central catheter insertion. J. Vasc. Access. 2024, 25, 5–13. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, M.; Matovic, M.; Balestreri, L.; Steffan, A.; Pacenzia, R.; Malafronte, M.; Fantin, D.; Bertuzzi, C.A.; Fabiani, F.; Morassut, S.; et al. Early and short-term acenocumarine or dalteparin for the prevention of central vein catheter-related thrombosis in cancer patients: A randomized controlled study based on serial venographies. Ann. Oncol. 2009, 20, 1936–1942. [Google Scholar] [CrossRef]
- Debourdeau, P.; Lamblin, A.; Debourdeau, T.; Marcy, P.Y.; Vazquez, L. Venous thromboembolism associated with central venous catheters in patients with cancer: From pathophysiology to thromboprophylaxis, areas for future studies. J. Thromb. Haemost. 2021, 19, 2659–2673. [Google Scholar] [CrossRef]


| Pre-Insertion | At Insertion | Post Insertion |
|---|---|---|
| Ultrasound evaluation of the patency of the arm veins to rule out thrombosis | Silicone device material | Tip location at the superior vena cava/right atrium junction |
| Identification of the median nerve and the brachial artery | Small sample size needles | Assessment of the correct tip position by intracavitary electrocardiogram |
| Proper antiseptic techniques | Microintroducer kits | Proper securement |
| Vein (basilic, brachial) caliber selection with a catheter/vein ratio < 1/3 | Ultrasound-guided venipuncture and tip navigation | Appropriate protection of the exit site |
| Pocket creation in the green zone (Dawson’s ZIM) | Subcutaneous tunnelling and non-tapering | Ambulatory care and maintenance of PICC line by specialist nurse team |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioretti, A.M.; Scicchitano, P.; La Forgia, D.; De Luca, R.; Campello, E.; Tocchetti, C.G.; Di Nisio, M.; Oliva, S. Prevention of Peripherally Inserted Central Catheter (PICC)-Associated Vein Thrombosis in Cancer: A Narrative Review. Biomedicines 2025, 13, 786. https://doi.org/10.3390/biomedicines13040786
Fioretti AM, Scicchitano P, La Forgia D, De Luca R, Campello E, Tocchetti CG, Di Nisio M, Oliva S. Prevention of Peripherally Inserted Central Catheter (PICC)-Associated Vein Thrombosis in Cancer: A Narrative Review. Biomedicines. 2025; 13(4):786. https://doi.org/10.3390/biomedicines13040786
Chicago/Turabian StyleFioretti, Agnese Maria, Pietro Scicchitano, Daniele La Forgia, Raffaele De Luca, Elena Campello, Carlo Gabriele Tocchetti, Marcello Di Nisio, and Stefano Oliva. 2025. "Prevention of Peripherally Inserted Central Catheter (PICC)-Associated Vein Thrombosis in Cancer: A Narrative Review" Biomedicines 13, no. 4: 786. https://doi.org/10.3390/biomedicines13040786
APA StyleFioretti, A. M., Scicchitano, P., La Forgia, D., De Luca, R., Campello, E., Tocchetti, C. G., Di Nisio, M., & Oliva, S. (2025). Prevention of Peripherally Inserted Central Catheter (PICC)-Associated Vein Thrombosis in Cancer: A Narrative Review. Biomedicines, 13(4), 786. https://doi.org/10.3390/biomedicines13040786









