Oxidative Stress Biomarkers in Laryngeal Squamous Cell Carcinoma and Their Clinical Implications: Preliminary Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Evaluation
2.1.1. Patients’ Data Collection
2.1.2. Samples Harvesting
2.2. Biochemical Evaluation
2.2.1. Protein Extraction from Tissue Samples
2.2.2. Western Blot
2.3. Statistical Analysis
3. Results
3.1. Clinical Data
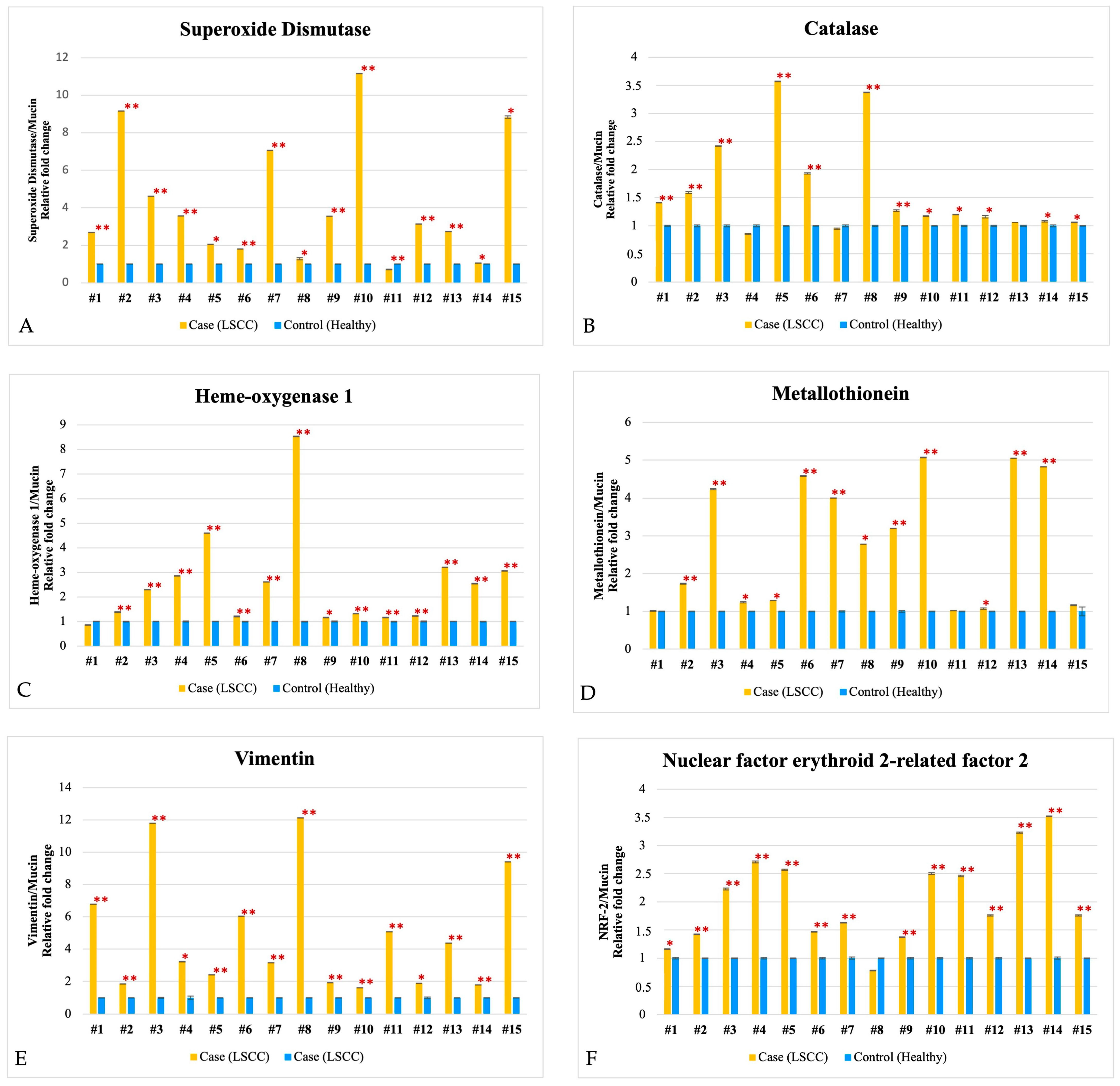
3.2. Biochemical Data
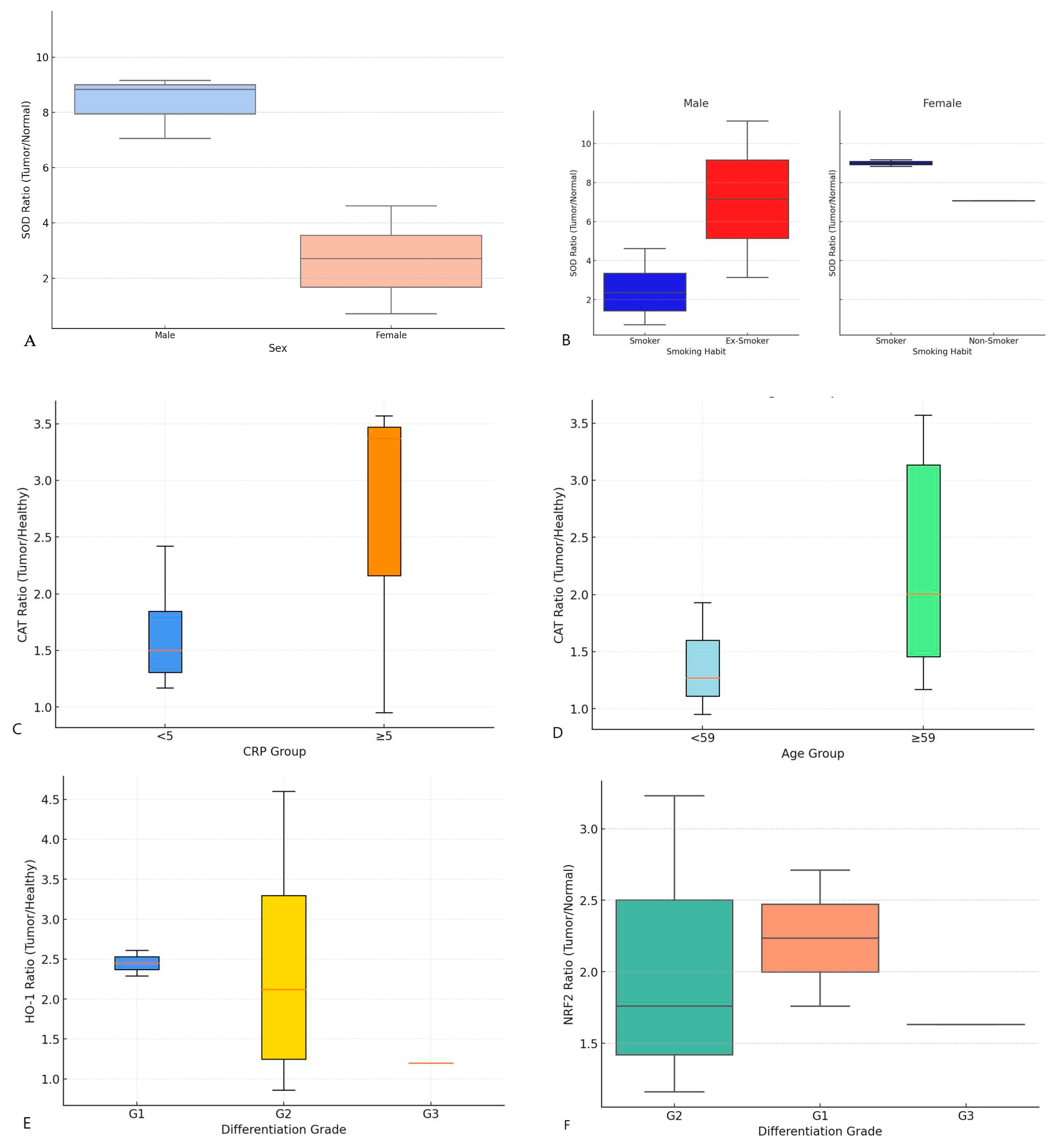
3.3. Clinical–Biochemical Correlation
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LSCC | Laryngeal Squamous Cell Carcinoma |
| HNC | Head And Neck Carcinomas |
| LPR | Laryngopharyngeal Reflux |
| ROS | Reactive Oxygen Species |
| AJCC | American Joint Committee on Cancer |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| TNM | Tumor-Nodes-Metastasis |
| HPV | Human Papilloma Virus |
| PD-L1 | Programmed Death-1 Ligand 1 |
| CRP | C-reactive protein |
| CPS | Combined Positive Score |
| RT | Radiotherapy |
| CT | Chemotherapy |
| PBS | Phosphate-Buffered Saline |
| RIPA | Radioimmunoprecipitation Assay |
| SDS | Sodium Dodecyl Sulfate |
| EDTA | Ethylenediaminetetraacetic Acid |
| PMSF | Phenylmethylsulfonyl Fluoride |
| HO-1 | Heme-Oxygenase 1 |
| MT | Metallothionein |
| VIM | Vimentin |
| NRF-2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| EMT | Epithelial-to-mesenchymal transition |
| RT | Room Temperature |
| SD | Standard Deviation |
| SEM | Standard Error of the Mean |
| OPHL | Open Partial Horizontal Laryngectomy |
| TLM | Transoral Laser Microsurgery |
| ESL | Endoscopic Supraglottic Laryngectomy |
| TL | Total Laryngectomy |
| CRT | Chemoradiotherapy |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.E.; Ilaghi, M.; Aslani, A.; Najafi, M.; Yekta, Z.; Nejadghaderi, S.A. Laryngeal cancer incidence trends in the United States over 2000-2020: A population-based analysis. Arch. Public Health 2024, 82, 106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.; Wu, H.; Wang, S.; Zhu, J. Expression and correlation of NRF2, KEAP1, NQO-1 and HO-1 in advanced squamous cell carcinoma of the larynx and their association with clinicopathologic features. Mol. Med. Rep. 2016, 14, 5171–5179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Divakar, P.; Davies, L. Trends in Incidence and Mortality of Larynx Cancer in the US. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 34–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Li, E.Y.; Kejner, A.E. Treatment modality and outcomes in larynx cancer patients: A sex-based evaluation. Head Neck 2019, 41, 3764–3774. [Google Scholar] [CrossRef] [PubMed]
- Tosakoon, S.; Lawrence, W.R.; Shiels, M.S.; Jackson, S.S. Sex Differences in Cancer Incidence Rates by Race and Ethnicity: Results from the Surveillance, Epidemiology, and End Results (SEER) Registry (2000–2019). Cancers 2024, 16, 989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramroth, H.; Dietz, A.; Becher, H. Intensity and inhalation of smoking in the aetiology of laryngeal cancer. Int. J. Environ. Res. Public Health 2011, 8, 976–984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verro, B.; Saraniti, G.; Fiumara, S.; Ottoveggio, G.; Saraniti, C. Smoking and alcohol habits in head and neck cancers: How many patients stop after diagnosis? J. Cancer Policy 2024, 41, 100498. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liberale, C.; Soloperto, D.; Marchioni, A.; Monzani, D.; Sacchetto, L. Updates on Larynx Cancer: Risk Factors and Oncogenesis. Int. J. Mol. Sci. 2023, 24, 12913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levesque, C.; Sanger, N.; Edalati, H.; Sohi, I.; Shield, K.D.; Sherk, A.; Stockwell, T.; Butt, P.R.; Paradis, C. A systematic review of relative risks for the relationship between chronic alcohol use and the occurrence of disease. Alcohol Clin. Exp. Res. 2023, 47, 1238–1255. [Google Scholar] [CrossRef] [PubMed]
- Ferster, A.P.O.; Schubart, J.; Kim, Y.; Goldenberg, D. Association Between Laryngeal Cancer and Asbestos Exposure: A Systematic Review. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Bolm-Audorff, U.; Hegewald, J.; Fishta, A.; Schlattmann, P.; Schmitt, J.; Seidler, A. Occupational polycyclic aromatic hydrocarbon exposure and risk of larynx cancer: A systematic review and meta-analysis. Occup. Environ. Med. 2015, 72, 226–233. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.A.; Samuels, T.L.; Battle, M.A.; Frolkis, T.; Blumin, J.H.; Bock, J.M.; Wells, C.; Yan, K.; Altman, K.W.; Johnston, N. H+/K+ATPase Expression in the Larynx of Laryngopharyngeal Reflux and Laryngeal Cancer Patients. Laryngoscope 2021, 131, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Garavello, W.; Lucenteforte, E.; Bosetti, C.; Talamini, R.; Levi, F.; Tavani, A.; Franceschi, S.; Negri, E.; La Vecchia, C. Diet diversity and the risk of laryngeal cancer: A case-control study from Italy and Switzerland. Oral Oncol. 2009, 45, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, J.Z. Oxidative stress induces mitochondrial DNA damage and cytotoxicity through independent mechanisms in human cancer cells. Biomed. Res. Int. 2013, 2013, 825065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuli, H.S.; Kaur, J.; Vashishth, K.; Sak, K.; Sharma, U.; Choudhary, R.; Behl, T.; Singh, T.; Sharma, S.; Saini, A.K.; et al. Molecular mechanisms behind ROS regulation in cancer: A balancing act between augmented tumorigenesis and cell apoptosis. Arch. Toxicol. 2023, 97, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Tang, P.; Li, H.; Ye, T.; Zhu, X.; He, W.; Cheng, L.; Cheng, H. Cucurbitacin E elicits apoptosis in laryngeal squamous cell carcinoma by enhancing reactive oxygen species-regulated mitochondrial dysfunction and endoplasmic reticulum stress. Am. J. Cancer Res. 2024, 14, 3905–3921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lubiński, J.; Jaworowska, E.; Derkacz, R.; Marciniak, W.; Białkowska, K.; Baszuk, P.; Scott, R.J.; Lubiński, J.A. Survival of Laryngeal Cancer Patients Depending on Zinc Serum Level and Oxidative Stress Genotypes. Biomolecules 2021, 11, 865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuzhalin, A.E.; Kutikhin, A.G. Inherited variations in the SOD and GPX gene families and cancer risk. Free Radic. Res. 2012, 46, 581–599. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, M.; Huen, K.; Manzanillo, P.; Chande, N.; Chen, C.; Balmes, J.R.; Tager, I.B.; Holland, N. Genotype-activity relationship for Mn-superoxide dismutase, glutathione peroxidase 1 and catalase in humans. Pharmacogenet. Genom. 2006, 16, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, S.; D’Anneo, A.; Calvaruso, G.; Cernigliaro, C.; Giuliano, M.; Lauricella, M. The Double-Edged Sword Profile of Redox Signaling: Oxidative Events As Molecular Switches in the Balance between Cell Physiology and Cancer. Chem. Res. Toxicol. 2018, 31, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Bozan, N.; Demir, H.; Gürsoy, T.; Özkan, H.; Düzenli, U.; Sarıkaya, E.; Turan, M.; Kiroglu, A.F.; Çankaya, H. Alterations in oxidative stress markers in laryngeal carcinoma patients. J. Chin. Med. Assoc. 2018, 81, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.D.; Echanique, K.A.; Yip, C.; Hsueh, W.D.; Baredes, S.; Park, R.C.W.; Eloy, J.A. Supraglottic Squamous Cell Carcinoma: A Population-Based Study of 22,675 Cases. Laryngoscope 2019, 129, 1822–1827. [Google Scholar] [CrossRef] [PubMed]
- Spector, J.G.; Sessions, D.G.; Haughey, B.H.; Chao, K.S.; Simpson, J.; El Mofty, S.; Perez, C.A. Delayed regional metastases, distant metastases, and second primary malignancies in squamous cell carcinomas of the larynx and hypopharynx. Laryngoscope 2001, 111, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirkman, H.N.; Gaetani, G.F. Mammalian catalase: A venerable enzyme with new mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes. Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Keefe, E.L.; DiNicolantonio, J.J.; O’Keefe, J.H.; Lavie, C.J. Alcohol and CV Health: Jekyll and Hyde J-Curves. Prog. Cardiovasc. Dis. 2018, 61, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- College of American Pathologists. Head and Neck Biomarker Reporting Template (Version 2.0.0.1). 2021. Available online: https://documents.cap.org/protocols/HN.Bmk_2.0.0.1.REL_CAPCP.pdf (accessed on 20 March 2024).
- Agilent Technologies. PD-L1 IHC 22C3 pharmDx Interpretation Manual: Head and Neck Squamous Cell Carcinoma (HNSCC). Available online: https://www.agilent.com/cs/library/usermanuals/public/29314_22c3_pharmDx_hnscc_interpretation_manual_us.pdf (accessed on 20 March 2024).
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferraguti, G.; Terracina, S.; Petrella, C.; Greco, A.; Minni, A.; Lucarelli, M.; Agostinelli, E.; Ralli, M.; de Vincentiis, M.; Raponi, G.; et al. Alcohol and Head and Neck Cancer: Updates on the Role of Oxidative Stress, Genetic, Epigenetics, Oral Microbiota, Antioxidants, and Alkylating Agents. Antioxidants 2022, 11, 145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Someah, M.S.; Golbabaei, F.; Arjomandi, R.; Semiromi, F.B.; Mohammadi, A. Oxidative Stress and DNA Damages Induced by Occupational Exposure to Asbestos: A Systematic Review. Iran. J. Public Health 2023, 52, 1613–1625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bulut, F.; Tetiker, A.T.; Çelikkol, A.; Yılmaz, A.; Ballica, B. Low Antioxidant Enzyme Levels and Oxidative Stress in Laryngopharyngeal Reflux (LPR) Patients. J. Voice 2023, 37, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- Verro, B.; Saraniti, C.; Carlisi, D.; Chiesa-Estomba, C.; Maniaci, A.; Lechien, J.R.; Mayo, M.; Fakhry, N.; Lauricella, M. Biomarkers in Laryngeal Squamous Cell Carcinoma: The Literature Review. Cancers 2023, 15, 5096. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Durak, I.; Işik, A.C.; Canbolat, O.; Akyol, O.; Kavutçu, M. Adenosine deaminase, 5’ nucleotidase, xanthine oxidase, superoxide dismutase, and catalase activities in cancerous and noncancerous human laryngeal tissues. Free Radic. Biol. Med. 1993, 15, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Inci, E.; Civelek, S.; Seven, A.; Inci, F.; Korkut, N.; Burçax, G. Laryngeal cancer: In relation to oxidative stress. Tohoku J. Exp. Med. 2003, 200, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, A.; Ozturk, A.; Ozturk, K.; Karagozoglu, E.; Dolanmaz, D. Superoxide dismutase and glutathione peroxidase enzyme activities in larynx carcinoma. Acta Otolaryngol. 2005, 125, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Biri, H.; Oztürk, H.S.; Kaçmaz, M.; Karaca, K.; Tokuçoğlu, H.; Durak, I. Activities of DNA turnover and free radical metabolizing enzymes in cancerous human prostate tissue. Cancer Investig. 1999, 17, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Sartori, S.; La Vecchia, C.; Serraino, D.; Maso, L.D.; Negri, E.; Smith, E.; Levi, F.; Boccia, S.; Cadoni, G.; et al. Hormone factors play a favorable role in female head and neck cancer risk. Cancer Med. 2017, 6, 1998–2007. [Google Scholar] [CrossRef]
- Roy, P.; Kandel, R.; Sawant, N.; Singh, K.P. Estrogen-induced reactive oxygen species, through epigenetic reprogramming, causes increased growth in breast cancer cells. Mol. Cell Endocrinol. 2024, 579, 112092. [Google Scholar] [CrossRef] [PubMed]
- Atasever Akkas, E.; Yucel, B. Prognostic value of systemic ımmune ınflammation ındex in patients with laryngeal cancer. Eur. Arch. Otorhinolaryngol. 2021, 278, 1945–1955. [Google Scholar] [CrossRef]
- Yücel, A.; Yücel, H.; Aydemir, F.; Mutaf, M.; Eryılmaz, M.A.; Arbağ, H. Development of Pharyngocutaneous Fistula after Total Laryngectomy: The Predictive Value of C-reactive Protein/Albumin Ratio. Acta Medica 2020, 63, 159–163. [Google Scholar] [CrossRef]
- Fu, J.; Yang, X. The Prognostic Value of the C-reactive Protein/Prognostic Nutritional Index Ratio in Stage III and IV Laryngeal Cancer Patients Treated with Radiotherapy. Cureus 2019, 11, e4648. [Google Scholar] [CrossRef]
- Zeng, Y.-C.; Xue, M.; Chi, F.; Xu, Z.-G.; Fan, G.-L.; Wu, R.; Fan, Y.-C.; Zhong, W.-Z.; Wang, S.-L.; Zhang, X.-Y.; et al. C-reactive protein level predicts prognosis in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. Tumour Biol. 2012, 33, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, X.; Song, D.M.; Niu, Y.H.; Wang, B.S. Inhibition of heme oxygenase-1 enhances the chemosensitivity of laryngeal squamous cell cancer Hep-2 cells to cisplatin. Apoptosis 2016, 21, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Tang, W.J.; Shi, X.L.; Li, W.P.; Zhou, H.; Lu, L.M.; Tao, L. Association of the microsatellite (GT)n repeat polymorphisms of the HO-1 gene promoter and corresponding serum levels with the risk of laryngeal squamous cell carcinoma. Acta Otolaryngol. 2016, 136, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xu, J.; Bao, X.; Shi, J.; Liu, B.; Chen, Y.; Li, J. Nuclear Nrf2 Activity in Laryngeal Carcinoma is Regulated by SENP3 After Cisplatin-Induced Reactive Oxygen Species Stress. J. Cancer 2019, 10, 3427–3434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nowinska, K.; Chmielewska, M.; Piotrowska, A.; Pula, B.; Pastuszewski, W.; Krecicki, T.; Podhorska-Okołow, M.; Zabel, M.; Dziegiel, P. Correlation between levels of expression of minichromosome maintenance proteins, Ki-67 proliferation antigen and metallothionein I/II in laryngeal squamous cell cancer. Int. J. Oncol. 2016, 48, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Pastuszewski, W.; Dziegiel, P.; Krecicki, T.; Podhorska-Okolow, M.; Ciesielska, U.; Gorzynska, E.; Zabel, M. Prognostic significance of metallothionein, p53 protein and Ki-67 antigen expression in laryngeal cancer. Anticancer Res. 2007, 27, 335–342. [Google Scholar] [PubMed]
- Ioachim, E.; Assimakopoulos, D.; Peschos, D.; Zissi, A.; Skevas, A.; Agnantis, N.J. Immunohistochemical expression of metallothionein in benign premalignant and malignant epithelium of the larynx: Correlation with p53 and proliferative cell nuclear antigen. Pathol. Res. Pract. 1999, 195, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, W.; Zhang, J.; You, Z.; Hu, T.; Shao, G.; Zhang, Z.; Xu, Z.; Yu, X. TWIST2 inhibits EMT and induces oxidative stress in lung cancer cells by regulating the FGF21-mediated AMPK/mTOR pathway. Exp. Cell Res. 2021, 405, 112661, Erratum in Exp. Cell Res. 2024, 436, 113782. https://doi.org/10.1016/j.yexcr.2023.113782. PMID: 34044016. [Google Scholar] [CrossRef] [PubMed]
- Håversen, L.; Sundelin, J.P.; Mardinoglu, A.; Rutberg, M.; Ståhlman, M.; Wilhelmsson, U.; Hultén, L.M.; Pekny, M.; Fogelstrand, P.; Bentzon, J.F.; et al. Vimentin deficiency in macrophages induces increased oxidative stress and vascular inflammation but attenuates atherosclerosis in mice. Sci. Rep. 2018, 8, 16973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pérez-Sala, D.; Oeste, C.L.; Martínez, A.E.; Carrasco, M.J.; Garzón, B.; Cañada, F.J. Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat. Commun. 2015, 6, 7287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karamagkiolas, S.; Giotakis, I.; Kyrodimos, E.; Giotakis, E.I.; Kataki, A.; Karagianni, F.; Lazaris, A.M. Expression of vimentin (VIM) and metastasis-associated 1 (MTA1) protein in laryngeal squamous cell carcinoma are associated with prognostic outcome of patients. Am. J. Otolaryngol. 2019, 40, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Mizdrak, I.; Mizdrak, M.; Racetin, A.; Bošković, B.; Benzon, B.; Durdov, M.G.; Vukojević, K.; Filipović, N. Expression of Connexins 37, 40 and 45, Pannexin 1 and Vimentin in Laryngeal Squamous Cell Carcinomas. Genes 2023, 14, 446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Characteristics | N (%) |
|---|---|
| Gender | |
| Female | 3 (20) |
| Male | 12 (80) |
| Age (years) | |
| Mean ± SD | 59.86 ± 10.16 |
| <59 years old | 6 (40) |
| ≥59 years old | 9 (60) |
| By gender | |
| Female (3) | 54 ± 12.8 |
| Male (12) | 61.33 ± 9.04 |
| Range | 43–79 |
| Risk factors | |
| Tobacco use | |
| Non-smoker | 1 (6.67) |
| Light smoker (<20 cigarettes/day) | 3 (20) |
| Heavy smoker (≥20 cigarettes/day) | 9 (60) |
| Former smoker | 2 (13.33) |
| Mean cigarettes/day ± SD | 23.33 ± 8.74 |
| Alcohol abuse | |
| Non-drinker | 9 (60) |
| Light drinker (<4 drinks/day) | 3 (20) |
| Heavy drinker (≥4 drinks/day) | 3 (20) |
| LPR | |
| Yes | 4 (26.67) |
| Not | 11 (73.33) |
| Family history of cancer (mother, father, sisters, brothers) | |
| Yes (lung, breast, prostate, lymphoma, melanoma, gallbladder) | 6 (40) |
| Not | 9 (60) |
| CRP (mg/L) | |
| Cut-off: <5 mg/L | |
| <5 | 10 (66.67) |
| ≥5 | 5 (33.33) |
| Total | 15 (100) |
| Parameters | N (%) |
|---|---|
| Laryngeal involvement | |
| Supraglottic SCC | 4 (26.67) |
| Glottis SCC | 4 (26.67) |
| Glottic-supraglottic SCC | 4 (26.67) |
| Trans-glottic SCC | 1 (6.66) |
| Pharyngo-laryngeal SCC | 2 (13.33) |
| pT | |
| Cis | 0 (0) |
| T1 | 2 (13.33) |
| T2 | 9 (60) |
| T3 | 0 (0) |
| T4a | 4 (26.67) |
| pN | |
| Nx | 4 (26.67) |
| N0 | 8 (53.33) |
| N1 | 1 (6.67) |
| N2c | 1 (6.67) |
| N3b | 1 (6.67) |
| PD-L1 | |
| CPS < 1 | 1 (6.67) |
| CPS ≥ 1 | 8 (53.33) |
| CPS ≥ 20 | 3 (20) |
| Miss data | 3 (20) |
| +p16 | |
| Negative | 10 (66.67) |
| Equivocal | 0 (0) |
| Positive | 1 (6.66) |
| Miss data | 4 (26.67) |
| Degree of differentiation | |
| G1 | 2 (13.33) |
| G2 | 9 (60) |
| G3 | 1 (6.67) |
| Miss data | 3 (20) |
| Therapy | |
| Surgery | |
| TLM (cordectomies, ESL) | 3 (20) |
| OPHL | 8 (53.33) |
| TL | 4 (26.67) |
| Non-surgical therapy | 0 (0) |
| Adjuvant therapy | |
| RT | 1 (6.67) |
| CT | 0 (0) |
| CRT | 3 (20) |
| Specimens | |
| Supraglottis | 9 (60) |
| Glottis | 5 (33.33) |
| Subglottis | 1 (6.67) |
| Total | 15 (100) |
| Parameters | SOD Ratio | CAT Ratio | HO-1 Ratio | MT Ratio | VIM Ratio | NRF-2 Ratio |
|---|---|---|---|---|---|---|
| Age | −0.46 (0.086) | 0.71 (0.03) | −0.16 (0.69) | 0.56 (0.15) | −0.26 (0.42) | −0.38 (0.35) |
| Sex | −0.64 (0.010) | −0.03 (0.94) | 0.28 (0.50) | −023 (0.59) | 0.25 (0.43) | −0.26 (0.53) |
| Smoking | −0.62 (0.013) | −0.43 (0.21) | 0.24 (0.57) | −0.70 (0.057) | −0.12 (0.69) | −0.02 (0.92) |
| Alcohol abuse | −0.30 (0.28) | 0.03 (0.93) | 0.40 (0.32) | 0.33 (0.43) | 0.15 (0.63) | 0.37 (0.35) |
| LPR | −0.33 (0.22) | −0.21 (0.58) | −0.25 (0.54) | −0.04 (0.92) | −0.10 (0.74) | −0.69 (0.053) |
| pTNM | 0.13 (0.63) | 0.15 (0.68) | 0.12 (0.77) | −0.63 (0.09) | −0.36 (0.25) | 0.04 (0.86) |
| Degree of differentiation | 0.38 (0.16) | 0.18 (0.61) | −0.73 (0.038) | −0.15 (0.73) | −0.28 (0.39) | −0.75 (0.03) |
| Laryngeal site | −0.26 (0.36) | −0.26 (0.36) | −0.50 (0.20) | −0.63 (0.09) | −0.49 (0.10) | 0.23 (0.57) |
| CRP | −0.27 (0.52) | 0.91 (0.001) | −0.17 (0.70) | −0.34 (0.58) | −0.45 (0.16) | 0.43 (0.27) |
| CPS | 0.16 (0.71) | 0.15 (0.78) | 0.32 (0.60) | 0.43 (0.47) | 0.14 (0.74) | 0.92 (0.07) |
| p16 | 0.13 (0.63) | N/A | N/A | N/A | −0.31 (0.40) | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verro, B.; Saraniti, C.; Di Liberto, D.; Pratelli, G.; Lauricella, M.; Carlisi, D. Oxidative Stress Biomarkers in Laryngeal Squamous Cell Carcinoma and Their Clinical Implications: Preliminary Results. Biomedicines 2025, 13, 667. https://doi.org/10.3390/biomedicines13030667
Verro B, Saraniti C, Di Liberto D, Pratelli G, Lauricella M, Carlisi D. Oxidative Stress Biomarkers in Laryngeal Squamous Cell Carcinoma and Their Clinical Implications: Preliminary Results. Biomedicines. 2025; 13(3):667. https://doi.org/10.3390/biomedicines13030667
Chicago/Turabian StyleVerro, Barbara, Carmelo Saraniti, Diana Di Liberto, Giovanni Pratelli, Marianna Lauricella, and Daniela Carlisi. 2025. "Oxidative Stress Biomarkers in Laryngeal Squamous Cell Carcinoma and Their Clinical Implications: Preliminary Results" Biomedicines 13, no. 3: 667. https://doi.org/10.3390/biomedicines13030667
APA StyleVerro, B., Saraniti, C., Di Liberto, D., Pratelli, G., Lauricella, M., & Carlisi, D. (2025). Oxidative Stress Biomarkers in Laryngeal Squamous Cell Carcinoma and Their Clinical Implications: Preliminary Results. Biomedicines, 13(3), 667. https://doi.org/10.3390/biomedicines13030667







