Changes in Serum Inflammatory Markers and in Clinical Periodontal Condition After Non-Surgical Periodontal Treatment in Hypertensive Patients
Abstract
1. Introduction
2. Materials and Methods
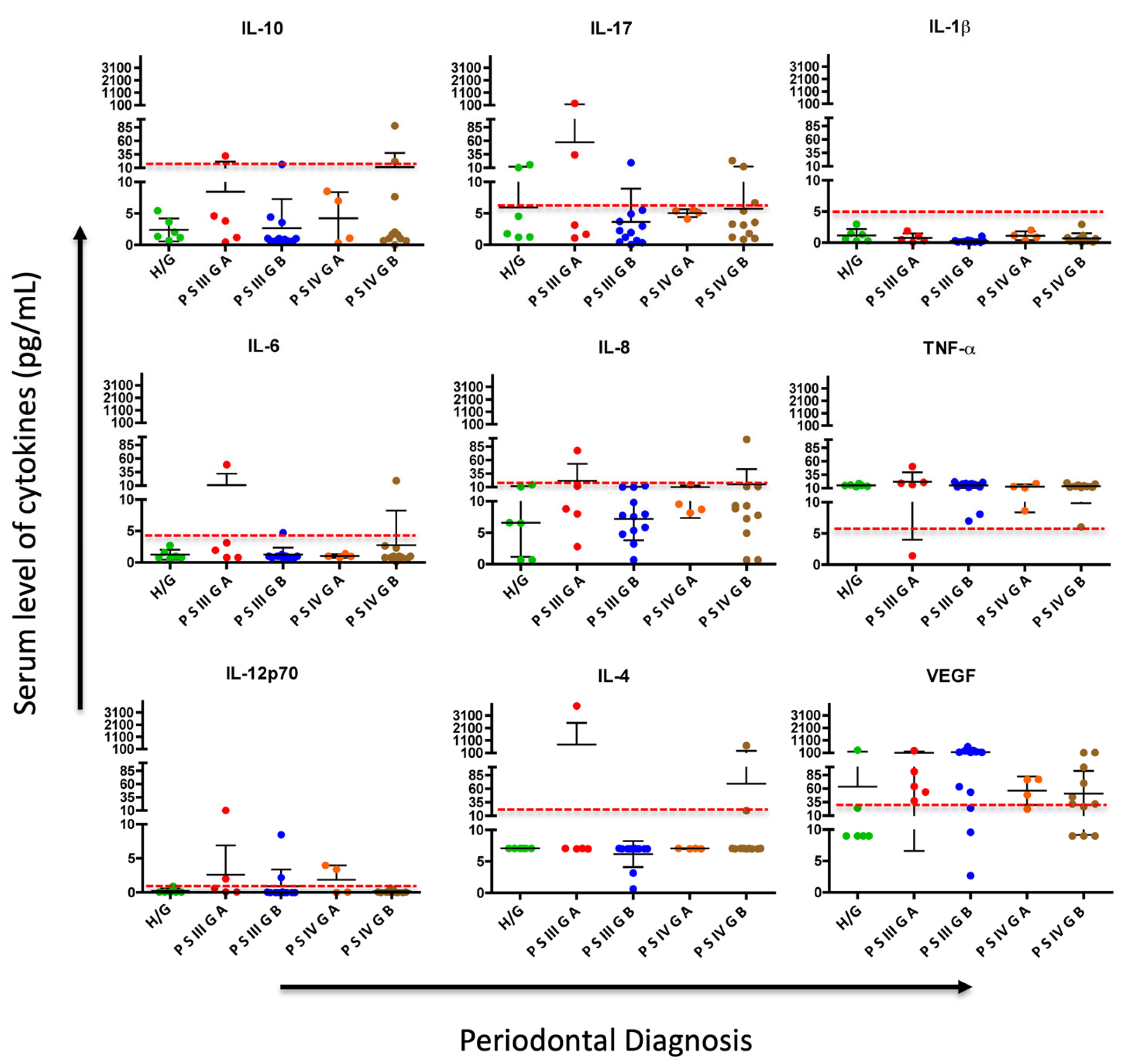
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Osmenda, G.; Siedlinski, M.; Nosalski, R.; Pelka, P.; Nowakowski, D.; Wilk, G.; Mikolajczyk, T.P.; Schramm-Luc, A.; Furtak, A.; et al. Causal association between periodontitis and hypertension: Evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur. Heart J. 2019, 40, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; D’Aiuto, F.; Deanfield, J. Cardiovascular prevention starts from your mouth. Eur. Heart J. 2019, 40, 1146–1148. [Google Scholar] [CrossRef]
- Rosa, R.A.C.; Rodrigues, J.V.S.; Claudio, M.M.; Franciscon, J.P.S.; Mulinari-Santos, G.; Cirelli, T.; de Molon, R.S.; Gouveia Garcia, V.; Theodoro, L.H. The Relationship between Hypertension and Periodontitis: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 5140. [Google Scholar] [CrossRef]
- Sanz, M.; Del Castillo, A.M.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases. Consensus Report. Glob. Heart 2020, 15, 1. [Google Scholar] [CrossRef]
- Munoz Aguilera, E.; Suvan, J.; Orlandi, M.; Miro Catalina, Q.; Nart, J.; D’Aiuto, F. Association Between Periodontitis and Blood Pressure Highlighted in Systemically Healthy Individuals: Results From a Nested Case-Control Study. Hypertension 2021, 77, 1765–1774. [Google Scholar] [CrossRef]
- Paddmanabhan, P.; Gita, B.; Chandrasekaran, S.C. Association between chronic periodontitis and hypertension in South Indian population: A cross-sectional study. J. Pharm. Bioallied Sci. 2015, 7, S543–S547. [Google Scholar] [CrossRef]
- Escobar Arregoces, F.M.; Del Hierro Rada, M.; Saenz Martinez, M.J.; Hernandez Meza, F.J.; Roa, N.S.; Velosa-Porras, J.; Latorre Uriza, C. Systemic inflammatory response to non-surgical treatment in hypertensive patients with periodontal infection. Medicine 2021, 100, e24951. [Google Scholar] [CrossRef]
- Goodyear, M.D.E.; Krleza-Jeric, K.; Lemmens, T. The Declaration of Helsinki. BMJ 2007, 335, 624–625. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Participants, E.F.P.W.; Methodological, C. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Latorre Uriza, C.; Roa, N.S.; Velosa-Porras, J.; Villamil Poveda, J.C.; Otero, L.; Ruiz, A.J.; Escobar Arregoces, F.M. Relationship between Carotid Intima-Media Thickness, Periodontal Disease, and Systemic Inflammation Biomarkers in an Adult Population. Biomedicines 2024, 12, 1425. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, U.; Jung, R.; Nierhaus, A.; Tsokos, M. Serial monitoring of interleukin-1beta, soluble interleukin-2 receptor and lipopolysaccharide binding protein levels after death A comparative evaluation of potential postmortem markers of sepsis. Int. J. Legal Med. 2005, 119, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Fayad, L.; Keating, M.J.; Reuben, J.M.; O’Brien, S.; Lee, B.N.; Lerner, S.; Kurzrock, R. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: Correlation with phenotypic characteristics and outcome. Blood 2001, 97, 256–263. [Google Scholar] [CrossRef]
- Polyak, S.J.; Khabar, K.S.; Rezeiq, M.; Gretch, D.R. Elevated levels of interleukin-8 in serum are associated with hepatitis C virus infection and resistance to interferon therapy. J. Virol. 2001, 75, 6209–6211. [Google Scholar] [CrossRef]
- Ethuin, F.; Delarche, C.; Gougerot-Pocidalo, M.A.; Eurin, B.; Jacob, L.; Chollet-Martin, S. Regulation of interleukin 12 p40 and p70 production by blood and alveolar phagocytes during severe sepsis. Lab. Invest. 2003, 83, 1353–1360. [Google Scholar] [CrossRef]
- Khorami SH, H.; Nejatollahi, F.; Davarpanah, M.A. Serum Levels of Interleukin-4, Interleukin-10 and Interferon-γ in Patients with Chronic Hepatitis B Infection. Hepat. Mon. 2018, 18, e60377. [Google Scholar] [CrossRef]
- Kut, C.; Mac Gabhann, F.; Popel, A.S. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br. J. Cancer 2007, 97, 978–985. [Google Scholar] [CrossRef]
- Ghahartars, M.; Sedaghat, F.; Khajavi, E.; Nejat, A.A.; Malekzadeh, M.; Ghaderi, A.; Fattahi, M.J. Investigation of IL-17A Serum Levels in Patients with Nonmelanoma Skin Cancer. Dermatol. Res. Pract. 2021, 2021, 5540163. [Google Scholar] [CrossRef]
- Jason, J.; Archibald, L.K.; Nwanyanwu, O.C.; Byrd, M.G.; Kazembe, P.N.; Dobbie, H.; Jarvis, W.R. Comparison of serum and cell-specific cytokines in humans. Clin. Diagn. Lab. Immunol. 2001, 8, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Albush, M.M.; Razan, K.K.; Raed, A.D. Effect of surgical and non-surgical periodontal debridement on vascular thrombotic markers in hypertensives. J. Indian. Soc. Periodontol. 2013, 17, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.V.S.; Claudio, M.M.; Franciscon, J.P.S.; Rosa, R.A.C.; Cirelli, T.; de Molon, R.S.; Figueredo, C.M.S.; Garcia, V.G.; Theodoro, L.H. The Effect of Non-Surgical Periodontal Treatment on Patients with Combined Refractory Arterial Hypertension and Stage III, Grade B Periodontitis: A Preliminary Prospective Clinical Study. J. Clin. Med. 2023, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Munoz Aguilera, E.; Suvan, J.; Buti, J.; Czesnikiewicz-Guzik, M.; Barbosa Ribeiro, A.; Orlandi, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc. Res. 2020, 116, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ye, H.; Liu, W.; Lv, Z.; Jia, Y.; Li, C.; Zhang, Y. Effect of periodontal treatments on blood pressure. Cochrane Database Syst. Rev. 2021, 12, CD009409. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ding, X.; Yue, Q.; Wang, X.; Chen, Z.; Cai, Z.; Li, W.; Cai, Z.; Chen, G.; Lan, Y.; et al. Triglyceride-glucose index trajectory and stroke incidence in patients with hypertension: A prospective cohort study. Cardiovasc. Diabetol. 2022, 21, 141. [Google Scholar] [CrossRef]
- Szczepaniak, P.; Mikolajczyk, T.P.; Czesnikiewicz-Guzik, M.; Guzik, T.J. Periodontitis as an inflammatory trigger in hypertension: From basic immunology to clinical implications. Kardiol. Pol. 2021, 79, 1206–1214. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Nosalski, R.; Mikolajczyk, T.P.; Vidler, F.; Dohnal, T.; Dembowska, E.; Graham, D.; Harrison, D.G.; Guzik, T.J. Th1-type immune responses to Porphyromonas gingivalis antigens exacerbate angiotensin II-dependent hypertension and vascular dysfunction. Br. J. Pharmacol. 2019, 176, 1922–1931. [Google Scholar] [CrossRef]
- Agita, A.; Alsagaff, M.T. Inflammation, Immunity, and Hypertension. Acta Med. Indones. 2017, 49, 158–165. [Google Scholar]
- D’Isidoro, O.; Perrotti, V.; Hui, W.L.; Piattelli, A.; Iaculli, F.; Quaranta, A. The impact of non-surgical therapy of periodontal disease on surrogate markers for cardiovascular disease: A literature review. Am. J. Dent. 2019, 32, 191–200. [Google Scholar]
- Touyz, R.M.; Lang, N.N.; Herrmann, J.; van den Meiracker, A.H.; Danser, A.H.J. Recent Advances in Hypertension and Cardiovascular Toxicities With Vascular Endothelial Growth Factor Inhibition. Hypertension 2017, 70, 220–226. [Google Scholar] [CrossRef]
- Giannakoulas, G.; Mouratoglou, S.A.; Gatzoulis, M.A.; Karvounis, H. Blood biomarkers and their potential role in pulmonary arterial hypertension associated with congenital heart disease. a systematic review. Int. J. Cardiol. 2014, 174, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.M.; Ribeiro, I.W.J.; Kampits, C.; Saffi, M.A.L.; Furtado, M.V.; Polanczyk, C.A.; Haas, A.N.; Rosing, C.K. Randomized controlled trial of the effect of periodontal treatment on cardiovascular risk biomarkers in patients with stable coronary artery disease: Preliminary findings of 3 months. J. Clin. Periodontol. 2019, 46, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Vicharenko, T.; Rozhko, M. Changes in the Levels of Pro-Inflammatory Cytokines in Patients with Generalized Periodontitis and Hypertension, Depending on the Method of Treatment. Galician Med. J. 2017, 24(4), 1–4. [Google Scholar] [CrossRef][Green Version]
- Caiazzo, E.; Sharma, M.; Rezig, A.O.M.; Morsy, M.I.; Czesnikiewicz-Guzik, M.; Ialenti, A.; Sulicka-Grodzicka, J.; Pellicori, P.; Crouch, S.H.; Schutte, A.E.; et al. Circulating cytokines and risk of developing hypertension: A systematic review and meta-analysis. Pharmacol. Res. 2024, 200, 107050. [Google Scholar] [CrossRef]
- Jockel-Schneider, Y.; Bechtold, M.; Haubitz, I.; Stork, S.; Fickl, S.; Harks, I.; Eigenthaler, M.; Vollrath, O.; Baulmann, J.; Schlagenhauf, U. Impact of anti-infective periodontal therapy on parameters of vascular health. J. Clin. Periodontol. 2018, 45, 354–363. [Google Scholar] [CrossRef]
- Paterno Holtzman, L.; Valente, N.A.; Vittorini Orgeas, G.; Copes, L.; Discepoli, N.; Clementini, M. Change in clinical parameters after subgingival instrumentation for the treatment of periodontitis and timing of periodontal re-evaluation: A systematic review and meta-analysis. J. Clin. Periodontol. 2025, 52, 137–158. [Google Scholar] [CrossRef]


| n | % | |
|---|---|---|
| Sex | ||
| Female | 25 | 60.9 |
| Male | 16 | 39.1 |
| Total | 41 | 100 |
| Media | CI 95% | |
| Age | 57.8 | 54.8–60.8 |
| Pre-Treatment | Post-Treatment | |||
|---|---|---|---|---|
| n | % | n | % | |
| Reduced healthy periodontium | 0 | 0 | 2 | 4.8 |
| Biofilm-induced gingivitis on reduced periodontium in a periodontally treated patient | 0 | 0 | 4 | 9.8 |
| Periodontitis stage III Grade A | 11 | 26.8 | 8 | 19.5 |
| Periodontitis stage III Grade B | 14 | 34.1 | 12 | 29.2 |
| Periodontitis stage IV Grade A | 5 | 12.2 | 4 | 9.8 |
| Periodontitis stage IV Grade B | 11 | 26.8 | 11 | 26.8 |
| TOTAL | 41 | 100 | 41 | 100 |
| Pre-Treatment | Post-Treatment | Media Difference | CI 95% | p Value | |||
|---|---|---|---|---|---|---|---|
| Media | S.D. | Media | S.D. | ||||
| Teeth number | 22.7 | 5.65 | 22.6 | 5.74 | 0.02 | −0.08–0.13 | 0.66 |
| Teeth with periodontitis | 14.5 | 6.05 | 8.80 | 6.14 | 5.73 | 3.99–7.46 | 0.00 |
| P.D. (mm) | 3.90 | 1.18 | 3.18 | 0.88 | 0.71 | 0.52–0.91 | 0.00 |
| CAL (mm) | 3.88 | 1.59 | 3.42 | 1.49 | 0.46 | 0.30–0.62 | 0.00 |
| PISA (mm2) | 956.1 | 586.5 | 439.3 | 412.2 | 516.7 | 403.3–630.2 | 0.00 |
| BOP (%) | 58.3 | 33.4 | 33.1 | 23.3 | 25.1 | 17.6–32.6 | 0.00 |
| Biofilm (%) | 59.0 | 25.3 | 34.9 | 21.3 | 24.0 | 17.5–30.5 | 0.00 |
| Pre-Treatment | Post-Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Media | S.D. | Media | S.D. | Media Difference | CI 95% | p Value | |
| Systolic | 131.1 | 14.7 | 126.5 | 14.4 | 4.62 | 0.57–8.66 | 0.02 |
| Dyastolic | 81.8 | 10.1 | 79.1 | 9.43 | 2.74 | 0.28–5.20 | 0.02 |
| Pre-Treatment | Post-Treatment | Media Difference | CI 95% | p Value | |||
|---|---|---|---|---|---|---|---|
| Media | S.D. | Media | S.D. | ||||
| Glucose | 98.0 | 10.3 | 96.6 | 9.92 | 1.46 | −1.20–4.13 | 0.27 |
| Total cholesterol | 193.9 | 39.1 | 186.1 | 34.5 | 7.75 | −1.19–16.7 | 0.08 |
| Triglycerides | 156.3 | 76.9 | 144.3 | 71.1 | 12.0 | −0.17–24.2 | 0.05 |
| HDL-C | 64.1 | 37.1 | 45.2 | 12.1 | 18.8 | 7.72–30.0 | 0.00 * |
| LDL-C | 119.6 | 33.4 | 113.2 | 23.3 | 6.32 | −2.00–14.6 | 0.13 |
| Pre-Treatment | Post-Treatment | Difference | p * Value | p ** Value | ||||
|---|---|---|---|---|---|---|---|---|
| Media | S.D. | Media | S.D. | Media | S.D. | |||
| IL-1β | 0.82 | 1.16 | 0.69 | 0.72 | 0.13 | 0.78 | 0.29 | 0.79 |
| IL-4 | 38.2 | 125.2 | 124.2 | 609.6 | −85.9 | 496.8 | 0.27 | 0.43 |
| IL-6 | 1.74 | 3.40 | 2.75 | 7.92 | −1.00 | 6.71 | 0.34 | 0.12 |
| IL-8 | 8.65 | 4.43 | 11.6 | 18.0 | −3.02 | 17.5 | 0.27 | 0.87 |
| IL-10 | 5.88 | 18.19 | 5.83 | 14.57 | 0.05 | 9.13 | 0.97 | 0.56 |
| IL-12p70 | 1.16 | 3.32 | 0.87 | 2.13 | 0.29 | 2.29 | 0.42 | 0.39 |
| IL-17A | 5.55 | 8.78 | 11.4 | 38.4 | −5.87 | 38.8 | 0.33 | 0.06 |
| TNF-α | 15.5 | 4.71 | 15.4 | 7.30 | 0.14 | 7.11 | 0.89 | 0.12 |
| VEGF | 109.9 | 134.5 | 88.5 | 117.2 | 21.3 | 141.2 | 0.33 | 0.00 |
| Cytokines | Periodontal Diagnosis | p Value * | |
|---|---|---|---|
| Healthy/gingivitis Ψ Mean (CI 95%) | Periodontitis ∝ Mean (CI 95%) | ||
| IL-1β | 1.16 (0.32–2.00) | 0.61(0.39–0.83) | 0.112 |
| IL-4 | 7.1 (0–¥) | 144.2 (−80.8–369.4) | 0.718 |
| IL-6 | 1.25 (0.60–1.90) | 3.01 (0.09–5.93) | 0.726 |
| IL-8 | 6.59 (2.11–11.0) | 12.5 (5.95–19.1) | 0.337 |
| IL-10 | 2.38 (0.88–3.88) | 6.42 (1.05–11.7) | 0.605 |
| IL-12p70 | 0.22 (−0.05–0.50) | 0.99 (0.20–1.77) | 0.061 |
| IL-17A | 5.93 (0.84–11.0) | 12.3 (−1.84–26.5) | 0.911 |
| TNF-α | 15.2 (13.4–16.9) | 15.4 (12.7–18.1) | 0.631 |
| VEGF | 63.7 (−40.8–168.2) | 92.8 (52.8–132.8) | 0.069 |
| PISA (A) | VEGF (A) | IL4 (A) | IL12p70 (A) | TNFA (A) | IL8 (A) | IL6 (A) | IL1B (A) | IL17A (A) | IL10 (A) | PISA (B) | VEGF (B) | IL4(B) | IL12p70 (B) | TNFA (B) | IL8 (B) | IL6 (B) | IL1B (B) | IL17A (B) | IL10 (B) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PISA (A) | 1.000 | |||||||||||||||||||
| VEGF (A) | −0.3808 | 1.000 | ||||||||||||||||||
| IL4 (A) | 0.0178 | −0.1620 | 1.000 | |||||||||||||||||
| IL12p70 (A) | −0.1383 | 0.1231 | 0.1300 | 1.000 | ||||||||||||||||
| TNFA (A) | −0.1044 | 0.2439 | −0.0758 | −0.0035 | 1.000 | |||||||||||||||
| IL8 (A) | 0.0420 | 0.2527 | 0.0079 | −0.0010 | 0.1312 | 1.000 | ||||||||||||||
| IL6 (A) | 0.5808 | −0.1737 | 0.2225 | −0.0243 | 0.1915 | 0.0698 | 1.000 | |||||||||||||
| IL1B (A) | 0.2335 | −0.1595 | −0.0107 | 0.2207 | 0.1033 | 0.0288 | 0.3753 | 1.000 | ||||||||||||
| IL17A (A) | −0.0440 | −0.0596 | 0.0123 | 0.2467 | 0.1943 | 0.2706 | 0.0632 | 0.1859 | 1.000 | |||||||||||
| IL10 (A) | 0.5740 | −0.1538 | −0.0324 | −0.0475 | 0.2332 | 0.1035 | 0.8938 | 0.4042 | 0.2949 | 1.000 | ||||||||||
| PISA (B) | 0.7955 | −0.3226 | 0.0382 | −0.0401 | −0.2603 | 0.1105 | 0.3891 | 0.0529 | −0.1686 | 0.3164 | 1.000 | |||||||||
| VEGF (B) | −0.2308 | 0.3773 | 0.3968 | 0.0854 | 0.1460 | −0.1776 | 0.0333 | −0.2020 | −0.1995 | −0.1493 | 0.2487 | 1.000 | ||||||||
| IL4 (B) | 0.0122 | −0.1455 | 0.9198 | 0.1465 | −0.1324 | 0.0762 | 0.2113 | −0.0093 | −0.0494 | −0.0147 | 0.0974 | 0.2340 | 1.000 | |||||||
| IL12p70 (B) | 0.0515 | −0.0383 | 0.6349 | 0.7279 | −0.0917 | −0.0165 | 0.0779 | 0.0913 | 0.0314 | −0.0726 | 0.1274 | 0.2032 | 0.6819 | 1.000 | ||||||
| TNFA (B) | 0.0601 | −0.1490 | 0.7960 | 0.0572 | 0.3635 | −0.0050 | 0.2338 | 0.0181 | −0.0078 | 0.0562 | 0.0316 | 0.3080 | 0.7459 | 0.5056 | 1.000 | |||||
| IL8 (B) | −0.0187 | −0.1861 | 0.5616 | 0.0293 | −0.1360 | 0.2304 | 0.0756 | 0.0316 | −0.0584 | −0.0197 | 0.1125 | −0.0119 | 0.5749 | 0.3588 | 0.3933 | 1.000 | ||||
| IL6 (B) | 0.2458 | −0.1755 | 0.8965 | 0.1209 | −0.0583 | 0.0518 | 0.5432 | 0.1488 | −0.0633 | 0.2991 | 0.2313 | 0.2444 | 0.9162 | 0.6249 | 0.7420 | 0.5324 | 1.000 | |||
| IL1B (B) | 0.5410 | −0.3336 | 0.2579 | −0.0144 | 0.0890 | 0.0718 | 0.5101 | 0.7528 | 0.1541 | 0.5086 | 0.2726 | −0.2239 | 0.2507 | 0.2216 | 0.3106 | 0.2421 | 0.4246 | 1.000 | ||
| IL17A (B) | 0.0190 | −0.1473 | 0.9502 | 0.1661 | −0.1078 | 0.0941 | 0.1955 | 0.0435 | 0.0770 | 0.0008 | 0.0647 | 0.2151 | 0.9693 | 0.6859 | 0.7666 | 0.5767 | 0.9114 | 0.3078 | 1.000 | |
| IL10 (B) | 0.5778 | −0.1995 | 0.2635 | −0.0241 | 0.1511 | 0.0913 | 0.9304 | 0.4346 | 0.0087 | 0.8674 | 0.3855 | −0.0783 | 0.2879 | 0.1547 | 0.2684 | 0.3375 | 0.5950 | 0.6227 | 0.2807 | 1.000 |
| Cutoff Points pg/mL | Pre-Treatment | Post-Treatment | p Value | |
|---|---|---|---|---|
| IL-10 | ≤13.68 >13.68 | 39 2 | 37 4 | 0.000 * |
| IL-1β | ≤5 >5 | 40 1 | 41 0 | Not calculable |
| IL-6 | ≤4.3 >4.3 | 38 3 | 38 3 | 0.000 * |
| IL-8 | ≤12.35 >12.35 | 35 6 | 35 6 | 0.161 |
| TNF-α | ≤6.11 >6.11 | 1 40 | 2 39 | 0.000 * |
| IL-4 | ≤17.76 >17.76 | 34 7 | 37 4 | 0.065 |
| IL-12p70 | <1.0 >1.0 | 33 8 | 35 6 | 0.000 * |
| IL-17A | ≤6.13 >6.13 | 32 9 | 32 9 | 0.000 * |
| VEGF | ≤30 >3.0 | 14 27 | 16 25 | 0.002 * |
| Cut-off Points pg/mL | PISA | |||
|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | p Value | ||
| IL-10 | ≤13.68 >13.68 | 892.7 (CI 95%: 737.4–1048) 2191.5 (CI 95%: −9977.8–14360) | 414.4 (CI 95%: 283.8–545) 669.5 (CI 95%: −266.3–1605.5) | 0.000 * 0.000 * |
| p value | 0.403 | 0.244 | ||
| IL-6 | ≤4.3 >4.3 | 888.7 (CI 95%: 729.3–1048) 1809 (CI 95%: −1082.2–4701.2) | 411.8 (CI 95%: 284.7–539) 786.8 (CI 95%: −850.6–2424.2) | 0.000 * 0.000 * |
| p value | 0.007 * | 0.131 | ||
| IL-8 | ≤12.35 >12.35 | 959.9 (CI 95% 747.4–1172.5) 933.6 (CI 95% 528.7–1338.4) | 383.6 (CI 95%: 259.1–508) 764.2 (CI 95%: 172.2–1356.1) | 0.000 0.1164 |
| p value | 0.920 | 0.034 * | ||
| TNF-α | ≤6.11 >6.11 | No calculable | 699 (CI 95%: −5226.4–6624.5) 426 (CI 95%: 294.8–557.1) | |
| p value | 0.367 | |||
| IL-4 | ≤17.76 >17.76 | 966.7 (CI 95%: 744.4–1188.9) 904.5 (CI 95%: 680.7–1128.4) | 406.4 (CI 95%: 274.1–538.7) 743.6 (CI 95%: −35.6–1522.9) | 0.000 * 0.0630 |
| p value | 0.667 | 0.121 | ||
| IL-12p70 | <1.0 >1.0 | 953.4 (CI 95%: 734.4–1172) 967 (CI 95%: 573.3–1360.8) | 405.8 (CI 95%: 259.9–551.6) 634.8 (CI 95%: 339.4–930.2) | 0.000 * 0.000 * |
| p value | 0.953 | 0.212 | ||
| IL-17A | ≤6.13 >6.13 | 1013.4 (CI 95%: 795.7–1231) 752.3 (CI 95%: 369.2–1135.5) | 450.2 (CI 95%: 299.6–600.9) 400.4 (CI 95%: 82.7–718) | 0.000 * 0.000 * |
| p value | 0.2430 | 0.753 | ||
| VEGF | ≤30 >30 | 1222.5 (CI 95%: 809.2–1635.7) 817.9 (CI 95%: 634.4–1001.4) | 538.5 (CI 95%: 270.7–806.3) 375.8 (CI 95%: 236.1–515.4) | 0.000 * 0.000 * |
| p value | 0.034 * | 0.221 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arregoces, F.M.E.; Roa, N.S.; Velosa-Porras, J.; Rodríguez, L.V.; Merchan, M.J.; Poveda, J.C.V.; Otero, L.; Ruiz, Á.J.; Uriza, C.L. Changes in Serum Inflammatory Markers and in Clinical Periodontal Condition After Non-Surgical Periodontal Treatment in Hypertensive Patients. Biomedicines 2025, 13, 374. https://doi.org/10.3390/biomedicines13020374
Arregoces FME, Roa NS, Velosa-Porras J, Rodríguez LV, Merchan MJ, Poveda JCV, Otero L, Ruiz ÁJ, Uriza CL. Changes in Serum Inflammatory Markers and in Clinical Periodontal Condition After Non-Surgical Periodontal Treatment in Hypertensive Patients. Biomedicines. 2025; 13(2):374. https://doi.org/10.3390/biomedicines13020374
Chicago/Turabian StyleArregoces, Francina María Escobar, Nelly S. Roa, Juliana Velosa-Porras, Lina Velásquez Rodríguez, María José Merchan, Jean Carlos Villamil Poveda, Liliana Otero, Álvaro J. Ruiz, and Catalina Latorre Uriza. 2025. "Changes in Serum Inflammatory Markers and in Clinical Periodontal Condition After Non-Surgical Periodontal Treatment in Hypertensive Patients" Biomedicines 13, no. 2: 374. https://doi.org/10.3390/biomedicines13020374
APA StyleArregoces, F. M. E., Roa, N. S., Velosa-Porras, J., Rodríguez, L. V., Merchan, M. J., Poveda, J. C. V., Otero, L., Ruiz, Á. J., & Uriza, C. L. (2025). Changes in Serum Inflammatory Markers and in Clinical Periodontal Condition After Non-Surgical Periodontal Treatment in Hypertensive Patients. Biomedicines, 13(2), 374. https://doi.org/10.3390/biomedicines13020374





