Humic Acid Derived from Vermicompost Inhibits Alveolar Bone Degradation and Protects Against Renal Injury in an Experimental Model of Periodontitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Composting, Vermicomposting, Extraction, and Characterization of Humic Acids (HA)
2.4. Histomorphometric Analyses: Assessment of Attachment Loss and Alveolar Bone Loss
2.5. Assessment of Osteocyte Density
2.6. Assessment of Alveolar Bone Loss and Morphological Analysis of Mandibular Bone Composition and Topography Using Scanning Electron Microscopy Coupled with Energy Dispersive X-Ray Spectroscopy (SEM/EDS)
2.7. Histopathological Evaluation of Liver and Kidney Tissues via Optical Microscopy
2.8. Statistical Analyses
3. Results
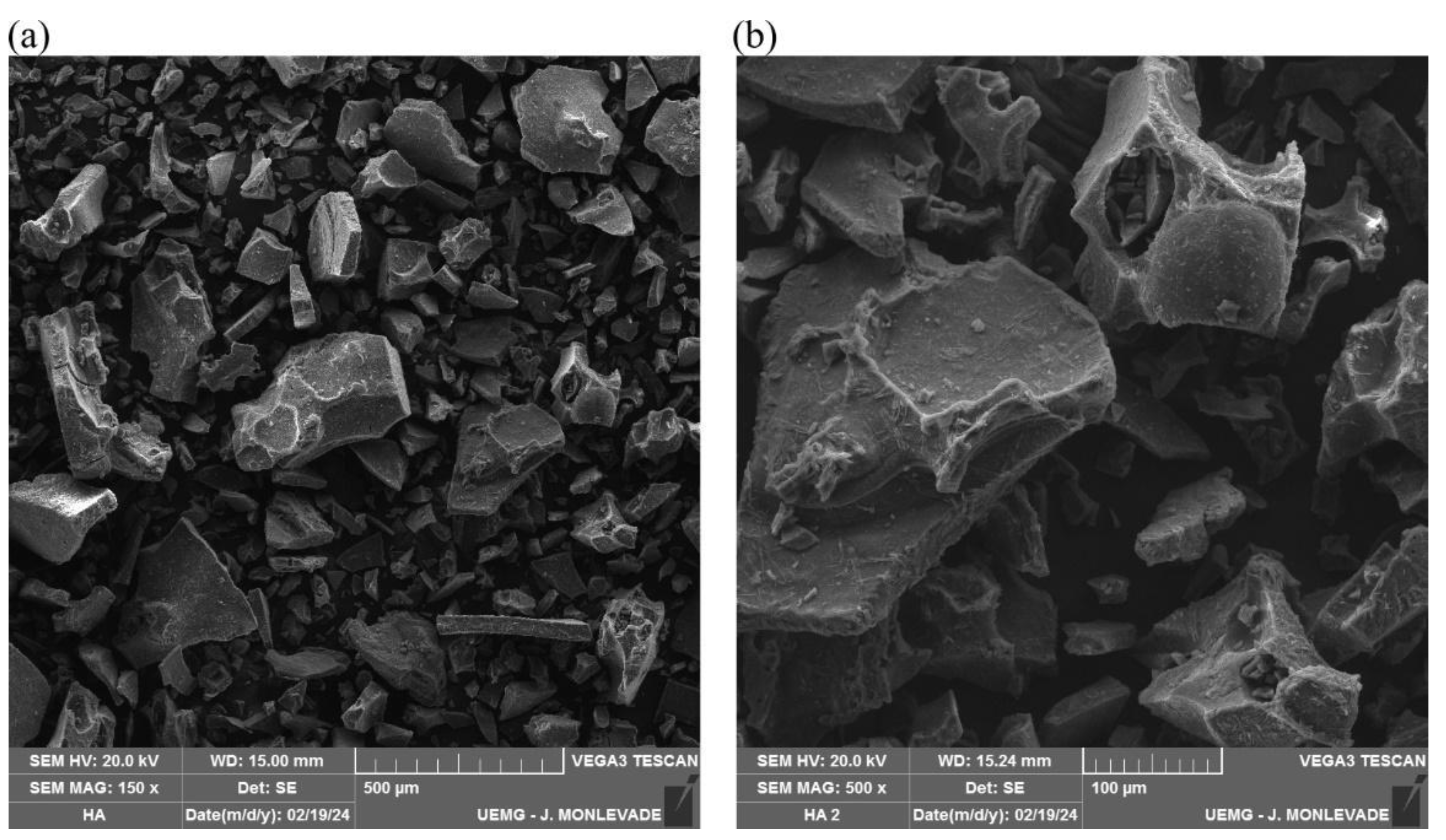
3.1. Scanning Electron Microscopy Analysis of Humic Acid (SEM/EDS)
3.2. Histomorphometric Analyses
3.3. Osteocyte Density
3.4. Alveolar Bone Loss (ABL) Assessment via SEM Images
3.5. Elemental Composition of the Alveolar Bone Surface
3.6. Topography of Alveolar Bone
3.7. Quantitative Analysis of Alveolar Bone Porosity Beneath the First Mandibular Molar
3.8. Histopathological Evaluation of Liver and Kidney Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef]
- Negrato, C.A.; Tarzia, O.; Jovanovic, L.; Chinellato, L.E.M. Periodontal disease and diabetes mellitus. J. Appl. Oral Sci. 2013, 21, 1–12. [Google Scholar] [CrossRef]
- Alhassani, A.A.; Hu, F.B.; Li, Y.; Rosner, B.A.; Willett, W.C.; Joshipura, K.J. The associations between major dietary patterns and risk of periodontitis. J. Clin. Periodontol. 2021, 48, 2–14. [Google Scholar] [CrossRef]
- Woelber, J.P.; Tennert, C. Chapter 13: Diet and Periodontal Diseases 2020. In The Impact of Nutrition and Diet on Oral Health; Karger Publishers: Basel, Switzerland, 2019; pp. 125–133. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, T.; Yang, L.; Loprinzi, P.D. Exercise as an Alternative Approach for Treating Smartphone Addiction: A Systematic Review and Meta-Analysis of Random Controlled Trials. Int. J. Environ. Res. Public Health 2019, 16, 3912. [Google Scholar] [CrossRef]
- Gamonal, J.; Bravo, J.; Malheiros, Z.; Stewart, B.; Morales, A.; Cavalla, F.; Gomez, M. Periodontal disease and its impact on general health in Latin America. Section I: Introduction part I. Braz. Oral Res. 2020, 34, 1. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontol. 2000 2017, 75, 152–188. [Google Scholar] [CrossRef]
- Feres, M.; Figueiredo, L.C.; Soares, G.M.S.; Faveri, M. Systemic antibiotics in the treatment of periodontitis. Periodontol. 2000 2015, 67, 131–186. [Google Scholar] [CrossRef]
- Balta, M.G.; Papathanasiou, E.; Blix, I.J.; Van Dyke, T.E. Host Modulation and Treatment of Periodontal Disease. J. Dent. Res. 2021, 100, 798–809. [Google Scholar] [CrossRef]
- Orlando, P.R.; Tavares, H.G.; Pereira, R.R.d.S.; Silva, G.; Carvalho, J.d.C.L.; Machado, A.R.T.; Dobbs, L.B.; Dias-Peixoto, M.F.; Pereira, L.J.; Andrade, E.F. Humic Acid Derived from Agricultural Biomass Mitigates Alveolar Bone Loss and Modulates Systemic Inflammatory Cytokines in Rats with Periodontitis. Curr. Top. Med. Chem. 2024, 24. in press. [Google Scholar] [CrossRef]
- Vašková, J.; Stupák, M.; Vidová Ugurbaş, M.; Žatko, D.; Vaško, L. Therapeutic Efficiency of Humic Acids in Intoxications. Life 2023, 13, 971. [Google Scholar] [CrossRef]
- Murbach, T.S.; Glávits, R.; Endres, J.R.; Clewell, A.E.; Hirka, G.; Vértesi, A.; Béres, E.; Pasics Szakonyiné, I. A toxicological evaluation of a fulvic and humic acids preparation. Toxicol. Rep. 2020, 7, 1242–1254. [Google Scholar] [CrossRef]
- Thiel, K.D.; Helbig, B.; Klöcking, R.; Wutzler, P.; Sprössig, M.; Schweizer, H. Comparison of the in vitro activities of ammonium humate and of enzymically oxidized chlorogenic and caffeic acids against type 1 and type 2 human herpes virus (author’s transl). Die Pharm. 1981, 36, 50–53. [Google Scholar]
- Çalışır, M.; Akpınar, A.; Poyraz, Ö.; Göze, F.; Çınar, Z. Humic Acid, a Polyphenolic Substance, Decreases Alveolar Bone Loss in Experimental Periodontitis in Rats. J. Vet. Dent. 2019, 36, 257–265. [Google Scholar] [CrossRef]
- Dai, J.; Ma, Y.; Shi, M.; Cao, Z.; Zhang, Y.; Miron, R.J. Initial changes in alveolar bone volume for sham-operated and ovariectomized rats in ligature-induced experimental periodontitis. Clin. Oral Investig. 2016, 20, 581–588. [Google Scholar] [CrossRef]
- Messora, M.R.; Oliveira, L.F.F.; Foureaux, R.C.; Taba, M.; Zangerônimo, M.G.; Furlaneto, F.A.C.; Pereira, L.J. Probiotic Therapy Reduces Periodontal Tissue Destruction and Improves the Intestinal Morphology in Rats With Ligature-Induced Periodontitis. J. Periodontol. 2013, 84, 1818–1826. [Google Scholar] [CrossRef]
- Peinado, B.R.R.; Frazão, D.R.; Chemelo, V.S.; Matos-Souza, J.M.; Ferreira, R.d.O.; Bittencourt, L.O.; Balbinot, G.d.S.; Collares, F.M.; Fernandes, L.M.P.; Maia, C.S.F.; et al. Physical training mitigates alveolar bone and blood enzymatic antioxidants defense impairment induced by binge ethanol consumption in rats. Biomed. Pharmacother. 2024, 174, 116554. [Google Scholar] [CrossRef]
- Martins, C.S.; Leitão, R.F.C.; Costa, D.V.S.; Melo, I.M.; Santos, G.S.; Lima, V.; Baldim, V.; Wong, D.V.T.; Bonfim, L.E.; Melo, C.B.; et al. Topical HPMC/S-Nitrosoglutathione Solution Decreases Inflammation and Bone Resorption in Experimental Periodontal Disease in Rats. PLoS ONE 2016, 11, e0153716. [Google Scholar] [CrossRef]
- Pereira, R.R.d.S.; Castro, G.B.d.; Magalhães, C.O.D.; Costa, K.B.; Garcia, B.C.C.; Silva, G.; Carvalho, J.d.C.L.; Machado, A.R.T.; Vieira, E.R.; Cassilhas, R.C.; et al. High-intensity interval training mitigates the progression of periodontitis and improves behavioural aspects in rats. J. Clin. Periodontol. 2024, 51, 1222–1235. [Google Scholar] [CrossRef]
- Çalışır, M.; Akpınar, A.; Poyraz, Ö.M.E.R.; Göze, F.; Çınar, Z. The histopathological and morphometric investigation of the effects of systemically administered humic acid on alveolar bone loss in ligature-induced periodontitis in rats. J. Periodontal Res. 2016, 51, 499–507. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Okorokova-Façanha, A.L.; Façanha, A.R. Humic Acids Isolated from Earthworm Compost Enhance Root Elongation, Lateral Root Emergence, and Plasma Membrane H+-ATPase Activity in Maize Roots. Plant Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef]
- Kamar Zaman, A.M.; Yaacob, J.S. Exploring the potential of vermicompost as a sustainable strategy in circular economy: Improving plants’ bioactive properties and boosting agricultural yield and quality. Environ. Sci. Pollut. Res. 2022, 29, 12948–12964. [Google Scholar] [CrossRef]
- Ghabbour, E.A.; Davies, G.; Ghali, N.K.; Mulligan, M.D. The effect of temperature on tight metal binding by peat and soil derived solid humic acids. Can. J. Soil Sci. 2001, 8, 331–336. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical Structure and Biological Activity of Humic Substances Define Their Role as Plant Growth Promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Schiefke, I. Humic Acids in Patients with Diarrhoea-Predominant Irritable Bowel Syndrome: Results from A Randomised Controlled Trial. Biomed. J. Sci. Tech. Res. 2021, 33, 25584–25591. [Google Scholar] [CrossRef]
- Mullender, M.G.; Tan, S.D.; Vico, L.; Alexandre, C.; Klein-Nulend, J. Differences in Osteocyte Density and Bone Histomorphometry Between Men and Women and Between Healthy and Osteoporotic Subjects. Calcif. Tissue Int. 2005, 77, 291–296. [Google Scholar] [CrossRef]
- Huang, X.; Xie, M.; Xie, Y.; Mei, F.; Lu, X.; Li, X.; Chen, L. The roles of osteocytes in alveolar bone destruction in periodontitis. J. Transl. Med. 2020, 18, 479. [Google Scholar] [CrossRef]
- Komori, T. Functions of the osteocyte network in the regulation of bone mass. Cell Tissue Res. 2013, 352, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Rybalka, M.A.; Stepchenko, L.M.; Shuleshko, O.O.; Zhorina, L.V. The impact of humic acid additives on mineral metabolism of rabbits in the postnatal period of ontogenesis. Regul. Mech. Biosyst. 2020, 11, 289–293. [Google Scholar] [CrossRef]
- Spencer, H.; Fuller, H.; Norris, C.; Williams, D. Effect of magnesium on the intestinal absorption of calcium in man. J. Am. Coll. Nutr. 1994, 13, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Garcia-Mina, J.M.; Proctor, M.; Yvin, J.C. Humic Acid and Glucan: Protection Against Liver Injury Induced by Carbon Tetrachloride. J. Med. Food 2015, 18, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.L.; Liu, X.-Y.; Meng, X.; Zhao, R.Q.; Ou, L.L.; Li, B.Z.; Xing, T. Periodontitis Exacerbates and Promotes the Progression of Chronic Kidney Disease Through Oral Flora, Cytokines, and Oxidative Stress. Front. Microbiol. 2021, 12, 656372. [Google Scholar] [CrossRef] [PubMed]
- Akbas, A.; Silan, C.; Gulpinar, M.T.; Sancak, E.B.; Ozkanli, S.S.; Cakir, D.U. Renoprotective Effect of Humic Acid on Renal Ischemia-Reperfusion Injury: An Experimental Study in Rats. Inflammation 2015, 38, 2042–2048. [Google Scholar] [CrossRef]








| Elements | Normalized Mass of Elements, % | Statistical Error (σ), % |
|---|---|---|
| Carbon | 40.5 | 0.2 |
| Oxygen | 40.5 | 0.2 |
| Sodium | 10.9 | 0.1 |
| Bromine | 3.5 | 0.0 |
| Silicon | 2.0 | 0.0 |
| Potassium | 1.2 | 0.0 |
| Calcium | 0.4 | 0.0 |
| Iron | 0.8 | 0.0 |
| Titanium | 0.2 | 0.0 |
| Groups | Kidney | Liver | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Animal | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| G1 | + | + | + | + | + | + | + | + | + | + | + | + |
| G2 | +++ | +++ | +++ | +++ | +++ | + | + | + | + | + | + | + |
| G3 | +++ | +++ | +++ | ++ | + | + | + | + | + | + | + | + |
| G4 | + | ++ | ++ | ++ | + | + | + | + | + | + | + | + |
| G5 | +++ | ++++ | ++ | + | + | ++ | + | + | + | + | + | + |
| G6 | ++++ | ++ | +++ | + | + | + | + | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, K.R.; Tavares, H.G.; Pereira, R.R.d.S.; Carvalho, J.d.C.L.; Botelho, R.d.O.; Reis Spuri, A.C.; Dobbss, L.B.; Machado, A.R.T.; Orlando, D.R.; Remédio, R.N.; et al. Humic Acid Derived from Vermicompost Inhibits Alveolar Bone Degradation and Protects Against Renal Injury in an Experimental Model of Periodontitis. Biomedicines 2024, 12, 2710. https://doi.org/10.3390/biomedicines12122710
Lima KR, Tavares HG, Pereira RRdS, Carvalho JdCL, Botelho RdO, Reis Spuri AC, Dobbss LB, Machado ART, Orlando DR, Remédio RN, et al. Humic Acid Derived from Vermicompost Inhibits Alveolar Bone Degradation and Protects Against Renal Injury in an Experimental Model of Periodontitis. Biomedicines. 2024; 12(12):2710. https://doi.org/10.3390/biomedicines12122710
Chicago/Turabian StyleLima, Karen Rodrigues, Hugo Giordano Tavares, Ramona Ramalho de Souza Pereira, Jaqueline do Carmo Lima Carvalho, Roberta de Oliveira Botelho, Aline Chaves Reis Spuri, Leonardo Barros Dobbss, Alan Rodrigues Teixeira Machado, Débora Ribeiro Orlando, Rafael Neodini Remédio, and et al. 2024. "Humic Acid Derived from Vermicompost Inhibits Alveolar Bone Degradation and Protects Against Renal Injury in an Experimental Model of Periodontitis" Biomedicines 12, no. 12: 2710. https://doi.org/10.3390/biomedicines12122710
APA StyleLima, K. R., Tavares, H. G., Pereira, R. R. d. S., Carvalho, J. d. C. L., Botelho, R. d. O., Reis Spuri, A. C., Dobbss, L. B., Machado, A. R. T., Orlando, D. R., Remédio, R. N., Paiva, S. M. d., Moura, R. F. d., Dias-Peixoto, M. F., Pereira, L. J., & Andrade, E. F. (2024). Humic Acid Derived from Vermicompost Inhibits Alveolar Bone Degradation and Protects Against Renal Injury in an Experimental Model of Periodontitis. Biomedicines, 12(12), 2710. https://doi.org/10.3390/biomedicines12122710






