Differentially Expressed miRNA of Prostate Cancer Compared with Benign Prostatic Hyperplasia Tissues: VAMP Associated Protein B Could Be Used for New Targets and Biomarkers of Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
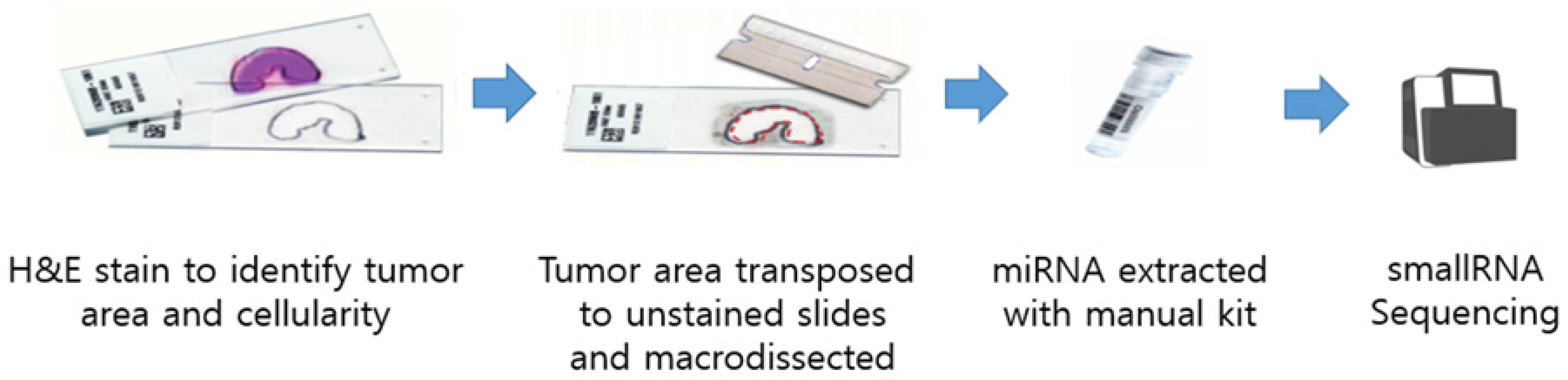
2.1. Patient Recruitment and Sample Collection
2.2. Library Preparation and miRNA Sequencing
2.3. Differential miRNA Expression Analysis
2.4. Target Prediction and Pathway Enrichment
2.5. Statistical Analysis
TCGA-PRAD Public Dataset Analysis
3. Results
3.1. Patient Characteristics
3.2. Differential miRNA Expression
3.3. Pathway and Disease Ontology Analysis
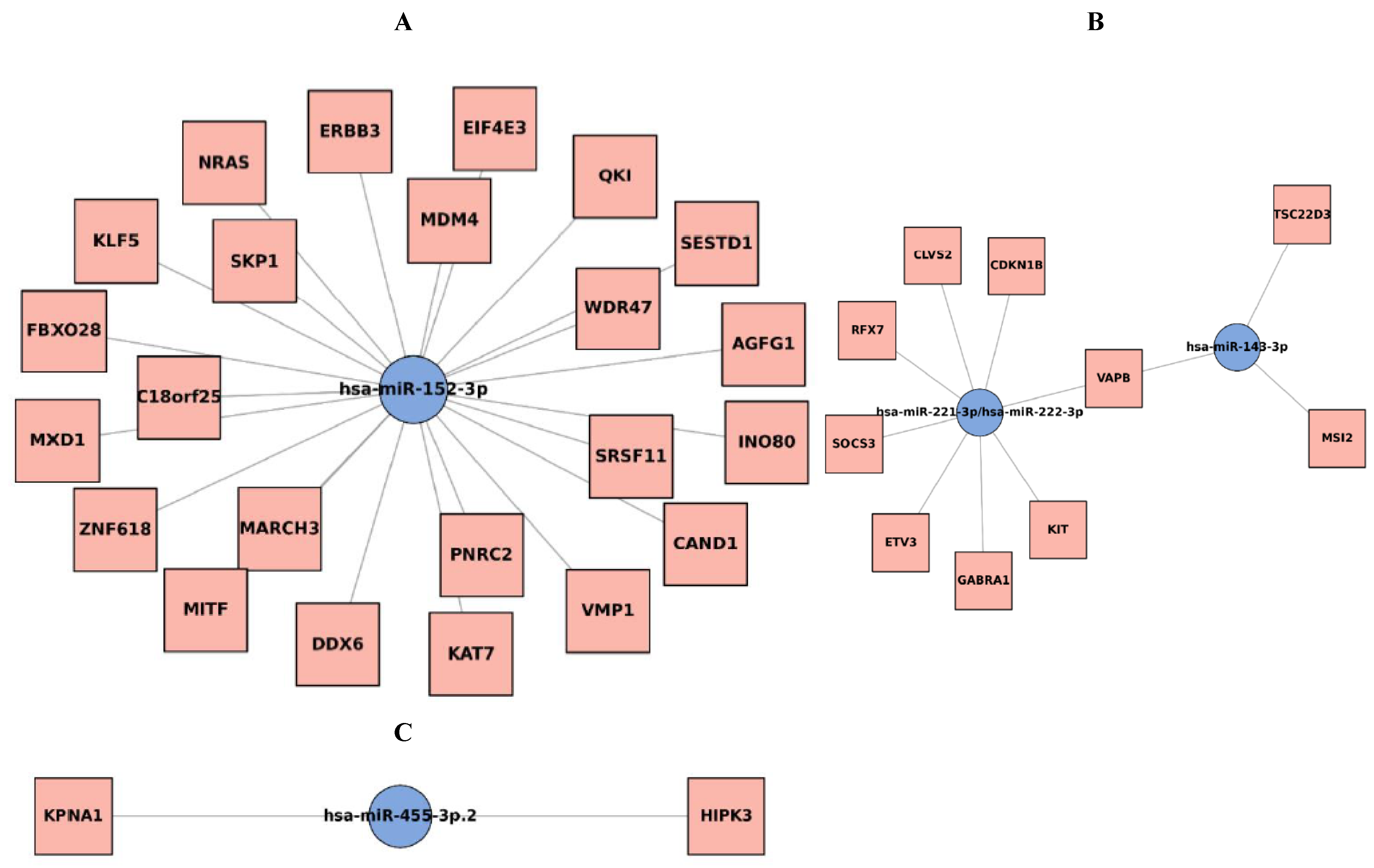
3.4. miRNA–mRNA Interaction Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPH | Benign prostatic hyperplasia |
| VAPB | The protein vesicle-associated membrane protein-associated protein B (VAPB) |
| PSA | Prostate-specific antigen (PSA) |
| miRNAs | MicroRNAs (miRNAs) |
| NGS | Next-generation sequencing (NGS) |
| FFPE | Formalin-fixed paraffin-embedded (FFPE) |
| H&E | Hematoxylin–eosin (H&E) |
| PCa | Prostate cancer (PCa) |
References
- Malvezzi, M.; Carioli, G.; Bertuccio, P.; Rosso, T.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann. Oncol. 2016, 27, 725–731. [Google Scholar] [CrossRef]
- Klotz, L. Active surveillance for prostate cancer: For whom? J. Clin. Oncol. 2005, 23, 8165–8169. [Google Scholar] [CrossRef]
- Walz, J.; Gallina, A.; Saad, F.; Montorsi, F.; Perrotte, P.; Shariat, S.F.; Jeldres, C.; Graefen, M.; Bénard, F.; McCormack, M.; et al. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J. Clin. Oncol. 2007, 25, 3576–3581. [Google Scholar] [CrossRef]
- Sabbatini, P.; Larson, S.M.; Kremer, A.; Zhang, Z.F.; Sun, M.; Yeung, H.; Imbriaco, M.; Horak, I.; Conolly, M.; Ding, C.; et al. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J. Clin. Oncol. 1999, 17, 948–957. [Google Scholar] [CrossRef]
- Loberg, R.; Gayed, B.; Olson, K.; Pienta, K. A paradigm for the treatment of prostate cancer bone metastases based on an understanding of tumor cell–microenvironment interactions. J. Cell. Biochem. 2005, 96, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Zamore, P.D.; Haley, B. Ribo-gnome: The big world of small RNAs. Science 2005, 309, 1519–1524. [Google Scholar] [CrossRef]
- Mattick, J.S.; Makunin, I.V. Non-coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Li, L.; Lodish, H.F.; Bartel, D.P. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.; Berlin, A.; Bristow, R.G.; van der Kwast, T. Genomic, pathological, and clinical heterogeneity as drivers of personalized medicine in prostate cancer. Urol. Oncol. 2015, 33, 85–94. [Google Scholar] [CrossRef]
- Lan, H.; Lu, H.; Wang, X.; Jin, H. MicroRNAs as potential biomarkers in cancer: Opportunities and challenges. BioMed Res. Int. 2015, 2015, 125094. [Google Scholar] [CrossRef]
- Gordanpour, A.; Nam, R.K.; Sugar, L.; Seth, A. MicroRNAs in prostate cancer: From biomarkers to molecularly-based therapeutics. Prostate Cancer Prostatic Dis. 2012, 15, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Yadav, T.; Rani, V. Exploring miRNA based approaches in cancer diagnostics and therapeutics. Crit. Rev. Oncol. Hematol. 2016, 98, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, P.V.; Reinsbach, S.E.; Müller, A.; Nicot, N.; Philippidou, D.; Vallar, L.; Kreis, S. Interplay of microRNAs, transcription factors and target genes: Linking dynamic expression changes to function. Nucleic Acids Res. 2013, 41, 2817–2831. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Q.; Yan, J.; Wang, Y.; Zhu, C.; Chen, C.; Zhao, X.; Xu, M.; Sun, Q.; Deng, R.; et al. miRNA-296-3p–ICAM-1 axis promotes metastasis of prostate cancer by possibly enhancing survival of natural killer cell-resistant circulating tumour cells. Cell Death Dis. 2013, 4, e928. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, T.; Liu, C.; Badeaux, M.A.; Liu, B.; Liu, R.; Jeter, C.; Chen, X.; Vlassov, A.V.; Tang, D.G. miRNA-128 suppresses prostate cancer by inhibiting BMI-1 to reduce tumor-initiating cell potential. Cancer Res. 2014, 74, 4183–4195. [Google Scholar] [CrossRef]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.A.; Broyles, D.; Head, T.; Deo, S.K. MicroRNA detection: Current technology and research strategies. Annu. Rev. Anal. Chem. 2015, 8, 217–237. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Garcia, D.M.; Baek, D.; Shin, C.; Bell, G.W.; Grimson, A.; Bartel, D.P. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat. Struct. Mol. Biol. 2011, 18, 1139–1146. [Google Scholar] [CrossRef]
- Colaprico, A.; Silva, T.C.; Olsen, C.; Garofano, L.; Cava, C.; Garolini, D.; Sabedot, T.S.; Malta, T.M.; Pagnotta, S.M.; Castiglioni, I.; et al. TCGAbiolinks: An R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016, 44, e71. [Google Scholar] [CrossRef]
- Song, Q.; An, Q.; Niu, B.; Lu, X.; Zhang, N.; Cao, X. Role of miR-221/222 in tumor development and the underlying mechanism. J. Oncol. 2019, 2019, 7252013. [Google Scholar] [CrossRef]
- Goto, Y.; Kojima, S.; Nishikawa, R.; Kurozumi, A.; Kato, M.; Enokida, H.; Matsushita, R.; Yamazaki, K.; Ishida, Y.; Nakagawa, M.; et al. MicroRNA expression signature of castration-resistant prostate cancer: The microRNA-221/222 cluster functions as a tumor suppressor and disease progression marker. Br. J. Cancer 2015, 113, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Fuse, M.; Kojima, S.; Enokida, H.; Chiyomaru, T.; Yoshino, H.; Nohata, N.; Kinoshita, T.; Sakamoto, S.; Naya, Y.; Nakagawa, M.; et al. Tumor-suppressive microRNAs (miR-222 and miR-31) regulate molecular pathways based on microRNA expression signature in prostate cancer. J. Hum. Genet. 2012, 57, 691–699. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Gan, R.; Zhao, L.; Li, W.; Zhou, H.; Wang, X.; Wang, X.; Lu, J.; Meng, Q.H. Down-regulation of miR-221 and miR-222 restrains prostate cancer cell proliferation and migration that is partly mediated by activation of SIRT1. PLoS ONE 2014, 9, e98833. [Google Scholar] [CrossRef]
- Xu, B.; Niu, X.; Zhang, X.; Tao, J.; Wu, D.; Wang, Z.; Li, P.; Zhang, W.; Wu, H.; Feng, N.; et al. miR-143 decreases prostate cancer cell proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol. Cell Biochem. 2011, 350, 207–213. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, M.; Yun, Y.; Zhang, J.; Zhang, R.; Li, Y.; Wu, X.; Liu, Q.; Miao, W.; Jiang, H. MicroRNA-455-3p functions as a tumor suppressor by targeting eIF4E in prostate cancer. Oncol. Rep. 2017, 37, 2449–2458. [Google Scholar] [CrossRef]
- Maehama, T.; Taylor, G.S.; Dixon, J.E. PTEN and myotubularin: Novel phosphoinositide phosphatases. Annu. Rev. Biochem. 2001, 70, 247–279. [Google Scholar] [CrossRef]
- Gürbüz, V.; Sozen, S.; Bilen, C.Y.; Konac, E. miR-148a, miR-152 and miR-200b promote prostate cancer metastasis by targeting DNMT1 and PTEN expression. Oncol. Lett. 2021, 22, 805. [Google Scholar] [CrossRef]
- Catanzaro, G.; Besharat, Z.M.; Carai, A.; Jager, N.; Splendiani, E.; Colin, C.; Po, A.; Chiacchiarini, M.; Citarella, A.; Gianno, F.; et al. MiR-1248: A new prognostic biomarker able to identify supratentorial hemispheric pediatric low-grade gliomas patients associated with progression. Biomark. Res. 2022, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Deng, Z.; Xu, C.; Yu, Y.; Li, Y.; Yang, C.; Chen, J.; Liu, Z.; Huang, G.; Li, L.-C.; et al. miR-663 induces castration-resistant prostate cancer transformation and predicts clinical recurrence. J. Cell Physiol. 2014, 229, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-F.; Chen, Z.-Y.; Wang, L.; Wang, M.; Liu, X.-H. miR-142-3p functions as an oncogene in prostate cancer by targeting FOXO1. J. Cancer 2020, 11, 1614–1624. [Google Scholar] [CrossRef]
- Yan, Z.; Hong, S.; Song, Y.; Bi, M. miR-4449 promotes colorectal cancer cell proliferation via regulation of SOCS3 and activation of STAT3 signaling. Cancer Manag. Res. 2021, 13, 3029–3039. [Google Scholar] [CrossRef] [PubMed]
- Teuling, E.; Ahmed, S.; Haasdijk, E.; Demmers, J.; Steinmetz, M.O.; Akhmanova, A.; Jaarsma, D.; Hoogenraad, C.C. Motor neuron disease-associated mutant vesicle-associated membrane protein-associated protein (VAP) B recruits wild-type VAPs into endoplasmic reticulum-derived tubular aggregates. J. Neurosci. 2007, 27, 9801–9815. [Google Scholar] [CrossRef] [PubMed]
- Lev, S.; Halevy, D.B.; Peretti, D.; Dahan, N. The VAP protein family: From cellular functions to motor neuron disease. Trends Cell Biol. 2008, 18, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Leal, S.S.; Ben Halevy, D.; Gomes, C.M.; Lev, S. Structural requirements for VAP-B oligomerization and their implication in amyotrophic lateral sclerosis-associated VAP-B (P56S) neurotoxicity. J. Biol. Chem. 2010, 285, 13839–13849. [Google Scholar] [CrossRef]
- Gkogkas, C.; Middleton, S.; Kremer, A.M.; Wardrope, C.; Hannah, M.; Gillingwater, T.H.; Skehel, P. VAPB interacts with and modulates the activity of ATF6. Hum. Mol. Genet. 2008, 17, 1517–1526. [Google Scholar] [CrossRef]
- Mórotz, G.M.; De Vos, K.J.; Vagnoni, A.; Ackerley, S.; Shaw, C.E.; Miller, C.C. Amyotrophic lateral sclerosis-associated mutant VAPB^P56S perturbs calcium homeostasis to disrupt axonal transport of mitochondria. Hum. Mol. Genet. 2012, 21, 1979–1988. [Google Scholar] [CrossRef]
- Assoni, A.F.; Mitsugi, T.G.; Wardenaar, R.; Ferreira, R.O.; Jandrey, E.H.; Novaes, G.M.; Granha, I.F.; Bakker, P.; Kaid, C.; Zatz, M.; et al. Neurodegeneration-associated protein VAPB regulates proliferation in medulloblastoma. Sci. Rep. 2023, 13, 19481. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef]
- GEO Accession Viewer. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE46602 (accessed on 24 November 2025).
- Tabach, Y.; Kogan-Sakin, I.; Buganim, Y.; Solomon, H.; Goldfinger, N.; Hovland, R.; Ke, X.; Oyan, A.M.; Kalland, K.; Rotter, V.; et al. Amplification of the 20q Chromosomal Arm Occurs Early in Tumorigenic Transformation and May Initiate Cancer. PLoS ONE 2011, 6, e14632. [Google Scholar] [CrossRef]
- Xiao, L.; Wei, Y.; Qin, Y.; Guo, B. The role of mitochondria-endoplasmic reticulum crosstalk in colorectal cancer. Genes Dis. 2025, 13, 101766. [Google Scholar] [CrossRef]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef]
- Gordanpour, A.; Stanimirovic, A.; Nam, R.K.; Moreno, C.S.; Sherman, C.; Sugar, L.; Seth, A. miR-221 is down-regulated in TMPRSS2:ERG fusion-positive prostate cancer. Anticancer Res. 2011, 31, 403–410. [Google Scholar] [PubMed]
- Sun, T.; Yang, M.; Chen, S.; Balk, S.; Pomerantz, M.; Hsieh, C.-L.; Brown, M.; Lee, G.-S.M.; Kantoff, P.W. The altered expression of miR-221/222 and miR-23b/27b is associated with the development of human castration-resistant prostate cancer. Prostate 2012, 72, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Galardi, S.; Mercatelli, N.; Farace, M.G.; Ciafrè, S.A. The microRNA-221/222 cluster functions as a tumor suppressor and disease progression marker in trichotomy of prostate cancer. Br. J. Cancer 2015, 113, 1055–1065. [Google Scholar]
- Zhou, P.; Chen, W.; Li, X. MicroRNA-143 aqcts as a tumor suppressor by targeting hexokinase 2 in human prostate cancer. Am. J. Cancer Res. 2015, 5, 2056–2063. [Google Scholar] [PubMed]
- Feng, Y.; Cao, H.; Zhao, W.; Chen, L.; Wang, D.; Gao, R. miR-143 mediates abiraterone acetate resistance by regulating the JNK/Bcl-2 signaling pathway in prostate cancer. J. Cancer 2022, 13, 3652–3659. [Google Scholar] [CrossRef]
- Schaefer, A.; Jung, M.; Mollenkopf, H.J.; Wagner, I.; Stephan, C.; Jentzmik, F.; Miller, K.; Lein, M.; Kristiansen, G.; Jung, K. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int. J. Cancer 2010, 126, 1167–1176. [Google Scholar] [CrossRef]
- Kojima, S.; Enokida, H.; Yoshino, H.; Itesako, T.; Chiyomaru, T.; Kinoshita, T.; Fuse, M.; Nishikawa, R.; Goto, Y.; Naya, Y.; et al. The tumor-suppressive microRNA-143/145 cluster inhibits cell migration and invasion by targeting GOLM1 in prostate cancer. J. Hum. Genet. 2014, 59, 78–87. [Google Scholar] [CrossRef]
- Ramalho-Carvalho, J.; Gonçalves, C.S.; Graça, I.; Bidarra, D.; Pereira-Silva, E.; Salta, S.; Godinho, M.I.; Gomez, A.; Esteller, M.; Costa, B.M.; et al. A multi-platform approach identifies miR-152-3p as a epigenetically silenced tumor suppressor in prostate cancer, targeting T MEM97. Clin. Epigenet. 2018, 10, 40. [Google Scholar] [CrossRef]
- Li, X.; Qian, B.; Chen, X.; Shen, M.; Zhao, S.; Zhang, X.; He, J. The role of miR-152 in urological tumors: Potential biomarkers and therapeutic targets. Front. Immunol. 2024, 15, 1464327. [Google Scholar] [CrossRef] [PubMed]
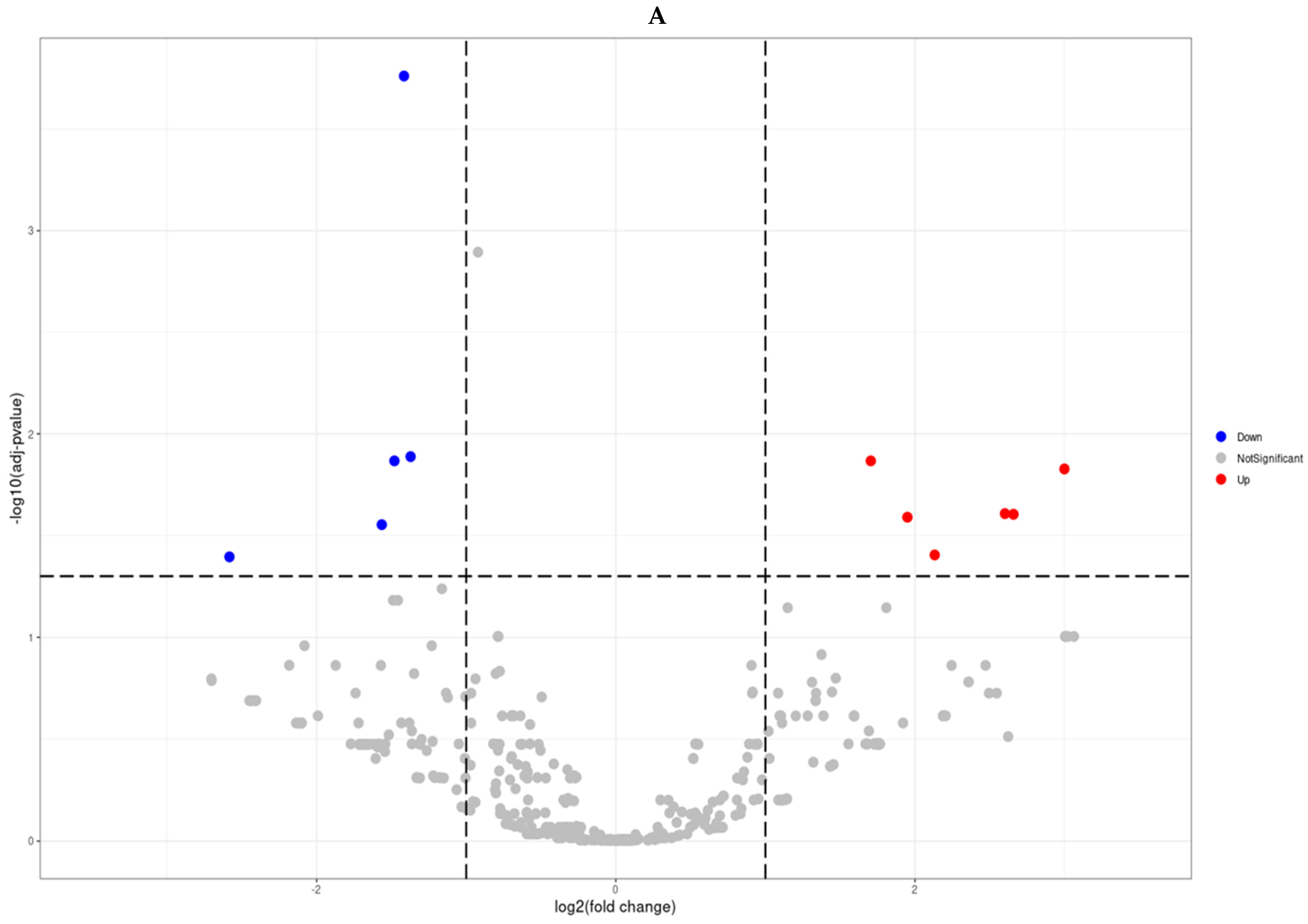


| miRNA(mature_precursor) | LogFC | p Value | FDR (Q Value) |
|---|---|---|---|
| has-miR-10524-5p_hsa-mir-10524 | 2.99852617 | 0.00018072 | 0.01487925 |
| has-miR-1248_hsa-mir-1248 | 2.65823893 | 0.00040184 | 0.02487541 |
| has-miR-9901_hsa-mir-9901 | 2.6009562 | 0.0003498 | 0.02468574 |
| has-miR-663a_hsa-mir-663a | 2.13141469 | 0.00087813 | 0.03943605 |
| has-miR-142-3p_hsa-mir-142 | 1.94898106 | 0.00046858 | 0.02571969 |
| has-miR-4449_hsa-mir-4449 | 1.70424794 | 0.00013744 | 0.01357934 |
| has-miR-221-3p_hsa-mir-221 | −1.37198402 | 7.86 × 10−5 | 0.01293899 |
| has-miR-143-3p_hsa-mir-143 | −1.4160143 | 3.51 × 10−7 | 0.00017321 |
| has-miR-222-3p_hsa-mir-222 | −1.48020016 | 0.00012267 | 0.01357934 |
| has-miR-455-3p_hsa-mir-455 | −1.56457919 | 0.00056648 | 0.02798432 |
| has-miR-152-3p_hsa-mir-152 | −2.58295908 | 0.00097907 | 0.04030488 |
| Benign Prostatic Hyperplasia | Prostate Cancer | p Value | |
|---|---|---|---|
| Age | 70.6 ± 1.2 (n = 5) | 68.6 ± 2.5 (n = 9) | >0.05 |
| PSA | 1.7 ± 0.1 (n = 5) | 22.8 ± 8.8 (n = 9) | <0.05 |
| Gleason grade | NA | 2.4 ± 0.6 (n = 9) | |
| Risk stratification | NA | 1.6 ± 0.2 (n = 9) | |
| Pathologic T stage | NA | 1.6 ± 0.2 (n = 9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Moon, A.; Song, M.; Lee, K.W.; Seo, S.M.; Kim, H.J.; Pefianco, L.A.; Andrean, K.; Ryu, S.; Song, Y.-S. Differentially Expressed miRNA of Prostate Cancer Compared with Benign Prostatic Hyperplasia Tissues: VAMP Associated Protein B Could Be Used for New Targets and Biomarkers of Prostate Cancer. Biomedicines 2025, 13, 2922. https://doi.org/10.3390/biomedicines13122922
Kim JH, Moon A, Song M, Lee KW, Seo SM, Kim HJ, Pefianco LA, Andrean K, Ryu S, Song Y-S. Differentially Expressed miRNA of Prostate Cancer Compared with Benign Prostatic Hyperplasia Tissues: VAMP Associated Protein B Could Be Used for New Targets and Biomarkers of Prostate Cancer. Biomedicines. 2025; 13(12):2922. https://doi.org/10.3390/biomedicines13122922
Chicago/Turabian StyleKim, Jae Heon, Ahrim Moon, Miho Song, Kwang Woo Lee, Su Min Seo, Hui Ji Kim, Luis Alfonso Pefianco, Kevin Andrean, Seongho Ryu, and Yun-Seob Song. 2025. "Differentially Expressed miRNA of Prostate Cancer Compared with Benign Prostatic Hyperplasia Tissues: VAMP Associated Protein B Could Be Used for New Targets and Biomarkers of Prostate Cancer" Biomedicines 13, no. 12: 2922. https://doi.org/10.3390/biomedicines13122922
APA StyleKim, J. H., Moon, A., Song, M., Lee, K. W., Seo, S. M., Kim, H. J., Pefianco, L. A., Andrean, K., Ryu, S., & Song, Y.-S. (2025). Differentially Expressed miRNA of Prostate Cancer Compared with Benign Prostatic Hyperplasia Tissues: VAMP Associated Protein B Could Be Used for New Targets and Biomarkers of Prostate Cancer. Biomedicines, 13(12), 2922. https://doi.org/10.3390/biomedicines13122922







