1. Introduction
Current prostate cancer diagnostic pathways still hinge on serum PSA prompting targeted or systematic biopsy, although PSA’s limited specificity and frequent benign or inflammatory elevations drive avoidable procedures and overdiagnosis [
1]. This persistent performance ceiling has accelerated interest in non-invasive matrices capable of refining pre-biopsy risk stratification. Urine, readily obtainable, repeatable, and directly influenced by prostatic secretions, retains molecular and extracellular vesicle (EV) cargo often diluted or confounded in blood [
1,
2]. EV fractions protect proteins, lipids, and nucleic acids that mirror tumor phenotypes and preserve their integrity during handling, creating a resilient substrate for multiplex biosensing [
3,
4]. Methodological advances (size exclusion, microfluidic immunocapture, nano-flow cytometry, label-free electrical transduction) reinforce analytical yield and scalability [
5]. Early demonstrations include urinary detection of Engrailed-2 using hybrid aptamer-antibody HCR-ELONA and graphene transistor platforms, and PCA3 via aptamer-based electrochemical or impedimetric sensors, confirming feasibility in native, minimally processed urine [
2,
6,
7]. SELEX-derived aptamers combine high affinity and specificity with batch-consistent chemical synthesis and modular functionalization suited to miniaturized multiplex devices, even within complex urinary matrices [
3,
8]. Collectively, the convergence of PSA’s diagnostic ceiling, the biochemical richness of urine (including its EV compartment), and the engineering flexibility of aptamer systems delineates a translational opportunity for multiplex urinary signatures to refine biopsy triage and reduce unnecessary invasive sampling [
1,
2,
3,
4,
5,
6,
7,
8].
Beyond biomarker-oriented evidence, several foundational studies on SELEX optimization, aptamer engineering, and biosensing technologies have shaped the methodological landscape exploited in this review [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31].
To date, no systematic review has quantitatively synthesized the impact of AI integration in SELEX workflows for urinary aptamer-based prostate cancer diagnostics.
Despite recognition of PSA’s constraints, clinical practice remains slow to operationalize robust adjunctive tools, and current pre-biopsy decision algorithms still underperform in discriminating indolent from clinically significant disease [
9,
10,
11,
12,
13,
14]. A key unmet need is an integrated panel that couples analytically stable urinary EV targets with rapid, low-cost signal transduction to deliver actionable post-PSA risk refinement. Existing single-analyte or dual-marker assays seldom exploit the full composite information space (protein conformation states, small RNA cargo, and low-abundance transcription factors) [
7,
8,
15,
16]. Aptamer platforms can bridge this gap: iterative SELEX optimization (including negative selection against benign prostatic hyperplasia matrices), tunable dissociation constants into the low-nanomolar or sub-nanomolar range, and orthogonal labeling strategies (electroactive tags, enzymatic amplification, nanomaterial conjugation) enable layered specificity while maintaining manufacturability [
1,
2,
3,
5,
6]. Strategic development priorities now include (i) standardization of pre-analytical urine handling for EV integrity, (ii) head-to-head benchmarking of aptamer-EV signatures versus established kallikrein or exosomal RNA panels, (iii) analytical-clinical bridging studies reporting harmonized metrics (AUC with confidence intervals, paired sensitivity/specificity, decision-curve analysis, and calibration), and (iv) prospective validation within MRI-integrated pathways [
10,
11,
12,
13,
14]. Framing these steps within transparent reporting (STARD, QUADAS-2/PROBAST) will accelerate regulatory credibility and facilitate clinical adoption.
Primary objective (Population/Index/Comparator/Outcomes/Study designs):
Population: individuals undergoing evaluation for suspected prostate cancer prior to biopsy.
Index tests: urinary aptamer-based assays (single or multiplex), including those incorporating extracellular vesicle (EV) cargo.
Comparator: histopathological biopsy (reference standard) and, where available, established adjunct tests (e.g., kallikrein panels, exosomal RNA assays).
Outcomes: diagnostic accuracy metrics (sensitivity, specificity, AUC with 95% confidence intervals, likelihood ratios, diagnostic odds ratio, HSROC parameters).
Study designs: diagnostic accuracy investigations (prospective, retrospective, proof-of-concept with extractable 2 × 2 data).
Secondary objectives: (i) quantify SELEX performance metrics (dissociation constant Kd, number of enrichment cycles, time-to-hit, stability/chemistry) and relate them to assay accuracy; (ii) assess the influence of pre-analytical factors (urine handling, EV isolation) and analytical choices (aptamer chemistry/modifications, transduction modality, AI-driven optimization) on diagnostic yield; (iii) benchmark multiplex aptamer-EV signatures versus established serum and urine panels using harmonized endpoints (AUC with 95% CIs, paired sensitivity/specificity, LR+, LR−, DOR, calibration, decision-curve analysis); (iv) appraise risk of bias and applicability (diagnostic studies: QUADAS-2; prediction models, if present: PROBAST) and report completeness using a focused SELEX-AI checklist.
Tertiary objective: characterize bibliometric trends (publication trajectory, collaboration networks, thematic clustering spanning EV analytics, electrical transduction, and AI-guided SELEX).
Methodological contribution: A PRISMA-compliant systematic review integrated with a quantitative bibliometric landscape; searches (PRISMA-S) will span multiple databases and gray literature sources; records will be de-duplicated, screened in duplicate with agreement statistics (Cohen’s κ), and extracted via a pre-specified template covering diagnostic, SELEX, and implementation endpoints (turnaround time, cost per test, internal vs. external validation); meta-analysis will apply Reitsma’s bivariate model with HSROC plotting, plus sensitivity and heterogeneity analyses and publication bias assessment (Deeks’ test where applicable); bibliometrics (VOSviewer/Bibliometrix) will map keyword co-occurrence, temporal overlays, venues, and collaborations to identify thematic clusters (EV analytics, electrical transduction, AI-guided SELEX) and field dynamics.
This review will be conducted and reported in accordance with PRISMA 2020. PRISMA-S statement: All search strategies and information sources will be fully documented and reported in accordance with the PRISMA-S extension.
2. Methodology
2.1. Registration, Sources, and Search Strategy (PRISMA/PRISMA-S)
The protocol was prospectively registered (OSF: osf.io/b2y7u, accessed on 3 November 2025), before any study selection; no deviations were introduced. The
Supplementary Materials Checklist is provided to ensure full transparency of search, screening, and extraction procedures. The covered period spans 1 January 2010 to 24 August 2025 (inclusive). The data lock was set at 2025-08-24T23:47Z (UTC) and this timestamp is reported consistently (Methods, PRISMA flow legend, Appendix). The following three bibliographic databases were queried: PubMed/MEDLINE, Scopus, and Web of Science Core Collection. Trial registries, preprints (medRxiv, bioRxiv), and patent databases were excluded by strategy (structural heterogeneity, peer-review robustness objective).
The multiblock search combined controlled vocabulary (MeSH, Emtree, WoS categories) and free-text terms covering aptamers/SELEX, urinary biomarkers (including extracellular vesicles/exosomes), prostate cancer, and diagnostic terminology (sensitivity, specificity, ROC, AUC, Kd, LoD). Truncations, orthographic variants, phrase searching, and proximity operators (NEAR/3) were used to maximize recall while limiting thematic drift. Minor harmonizations (exosom*, specific*, aptamer*) preceded execution. The strategy underwent internal peer review by an independent information specialist; no substantive modification was required (syntax-only adjustments). Execution timeline (UTC): PubMed 2025-08-24T21:05Z; Scopus 2025-08-24T21:32Z; Web of Science 2025-08-24T22:10Z.
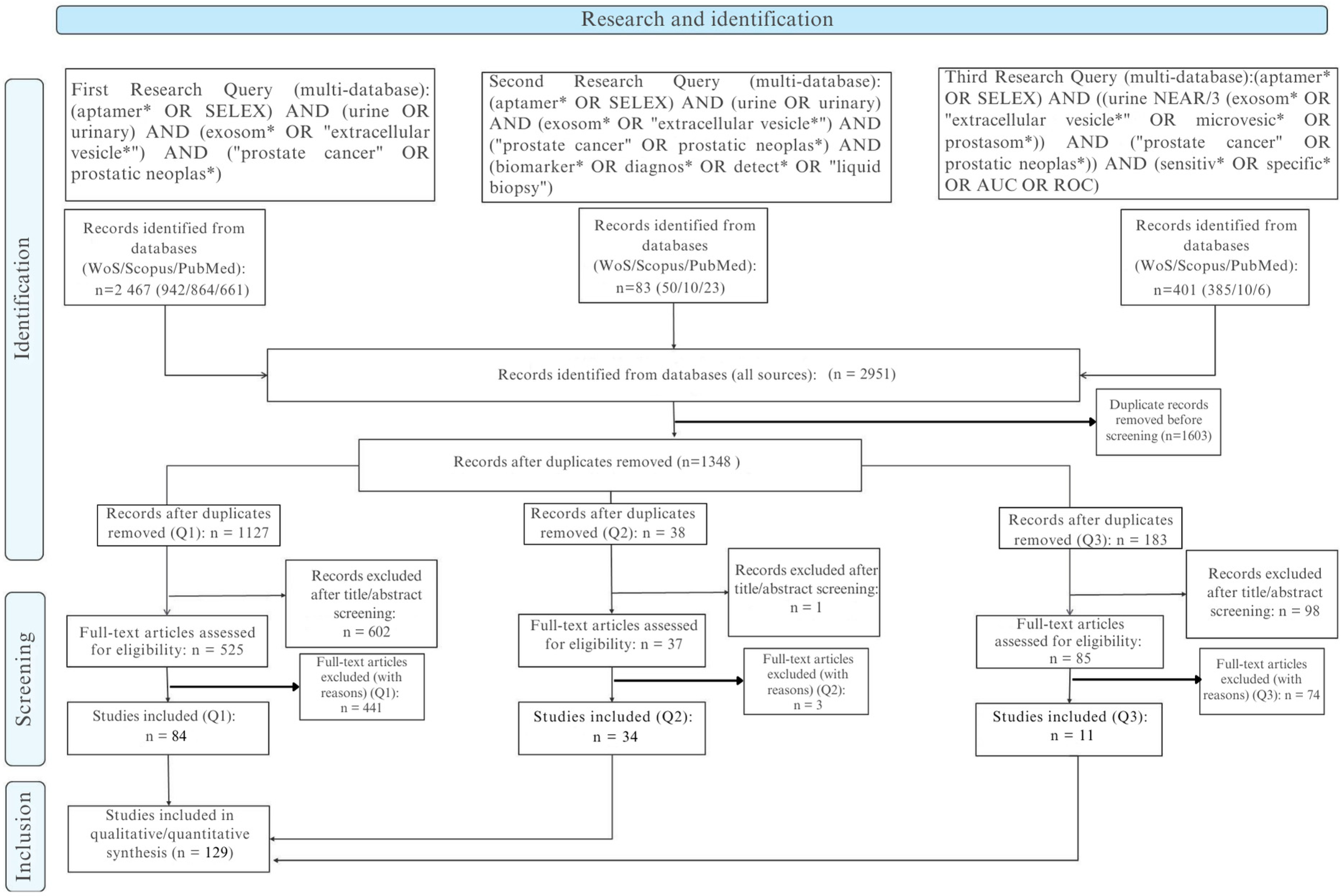
Across the three predefined query blocks (Q1–Q3), we retrieved a total of 2951 records: Q1 (core intersection) 2467, Q2 (biomarker/diagnostic extension) +83, and Q3 (performance-focused: sensitivity, specificity, AUC, ROC) 401. Following hierarchical deduplication (normalized DOI, then composite key: title/author/year, then fuzzy title similarity with a threshold ≥0.90 and manual adjudication; retention priority: PubMed > Scopus > Web of Science), 1348 unique records advanced to title and abstract screening. All exportable metadata (title, abstract, DOI, authors, affiliations, author keywords, controlled terms, year, source, document type) were consolidated into a unified workspace, with each procedural element (query log, field mapping, merge rules) fully version-tracked to ensure reproducibility.
The report follows PRISMA 2020 (doi:10.1136/bmj.n71) and PRISMA-S (doi:10.5195/jmla.2021.962).
Figure 1 (PRISMA flow) includes the note: “Six included full texts lacked extractable quantitative metrics and were retained qualitatively; they were excluded from pooled analyses.”
2.2. Eligibility Criteria and Selection
The bibliometric metadata are presented in
Table 1, after initial screening (1348 records), 701 title/abstract exclusions left 647 full texts. Of these 647, 518 were excluded for categorized reasons (
Table 2), yielding 135 full-text studies included. Of the 135, 129 had extractable quantitative data (diagnostic, analytical, and/or affinity) and constitute the “evaluable” subset; six (4 from Q1, 2 from Q2) were retained qualitatively without metric integration. Post-deduplication distribution by block: Q1 1127; Q2 38; Q3 183. Full texts included Q1 88 (84 evaluable), Q2 36 (34 evaluable), Q3 11 (all evaluable).
Inclusion criteria: original studies using urinary samples (cell-free whole urine, urinary extracellular vesicles/exosomes, exfoliated cells) and/or aptamers derived from a SELEX process applied to diagnosis or risk stratification of prostate cancer, reporting at least one quantitative measure (diagnostic performance, analytical metric, affinity constant). Exclusions: secondary literature, purely therapeutic applications without diagnostic metric, platform descriptions lacking a relevant cohort/specimen, non-urinary or non-prostate matrices, absence of an aptamer (non-conforming index test), non-extractable data (absence of Se, Sp, AUC, LoD, Kd, or equivalents), redundant cohorts without added value, retraction/inaccessibility.
Screening was performed in duplicate (piloted form); disagreements resolved by consensus. Inter-reviewer reliability: observed agreement 93.2%; kappa (κ) = 0.689 (95% CI 0.628–0.746); prevalence index 0.75; bias index 0.05. No retrospective author contact (pre-specified).
2.3. PICOS Framework
Population: Men evaluated for prostate cancer (initial biopsy, active surveillance, confirmed cases), native or derivative urinary samples (EV, exfoliated cells); for selection/affinity studies: cell lines or cellular structures contextually relevant to the prostate urinary axis (where diagnostic intent is explicit).
Intervention/Index test: Aptamer-based tests, sensors, or signatures (electrochemistry EIS/DPV/FET, fluorescence/amplification, SPR, microfluidic platforms, nanopore, nanomaterial hybrids) and SELEX processes (cell-SELEX, protein-SELEX, hybrids, in silico refinements).
Comparators: Histopathology (biopsy) and/or standard-of-care biomarkers (PSA, %free PSA, PHI, PCA3, TMPRSS2:ERG, mpMRI) or clinically established thresholds; for purely analytical performance: internal standards or spiked samples (not treated as diagnostic comparators).
Outcomes: Diagnostic performance (Se, Sp, AUC, LR+, LR−, DOR, PPV, NPV when 2 × 2 data available), analytical metrics (LoD, LoQ, dynamic range, assay time, stability, reproducibility), affinity (Kd, log10(Kd), ΔG), SELEX parameters (cycles, counter-selection).
Designs: Experimental studies, analytical validations, diagnostic accuracy studies (prospective, retrospective, cross-sectional, case–control), engineering, or optimization of aptamers with urinary prostate anchoring.
Exclusions: Reviews/secondary reports, purely therapeutic approaches, in silico without empirical validation, non-urinary matrices, absence of quantifiable metric.
2.4. Data Extraction and Target Variables
Two reviewers independently extracted (structured template) the following: publication metadata; study type; matrix (native urine, artificial urine, buffer, urinary EV, exfoliated cells); cohort description (total size, cases, controls/benign); pre-analytical context if reported (collection, digital rectal exam status, storage); index platform (electrochemical subtype, optical, SPR, amplification, microfluidic, hybrid).
Diagnostic: Se, Sp, AUC, PPV, NPV (only if 2 × 2 data), LR+, LR−, DOR (2 × 2 required), 95% CI, cut-off definition (pre-specified vs. derived), validation level (internal simple, cross-validation, external cohort), reproducibility (intra/inter-assay), stability (storage, matrix perturbations).
Analytical: LoD, LoQ (if explicit), dynamic range (min–max, converted to molar where possible), calibration coefficient (R2), span (RangeMax/RangeMin), ratio (RangeMin/LoD) to estimate effective sensitivity exploitation, assay time (sample-to-result), and total workflow duration.
Affinity/SELEX: Target (class: protein, cell surface, nucleic, metabolite, EV), aptamer identifier, length (nt), chemical modifications (2′F, LNA, PEG, thiol, biotin, locked bases), selection mode (cell, protein, hybrid, in silico), cycles, counter-selection strategy, Kd (units verified), log10(Kd [M]), with default T = 298 K; recalculated at experimental T (277 or 310 K) when explicitly reported. Approximate values (digitized) marked “*” and excluded from high-confidence sensitivity analyses. Qualitative non-quantifiable affinities categorized (E1–E5) to map gaps.
No imputation; unavailable field is “NR”. AUCs arising solely from internal cross-validation are annotated to distinguish from external validations.
2.5. Risk of Bias Assessment
Diagnostic accuracy studies were assessed with QUADAS-2 (patient selection, index test, reference standard, flow/timing) plus applicability concerns. Multivariate signatures or predictive modeling approaches (if present) were examined with PROBAST (participants, predictors, outcome, analysis). Non-randomized methodological comparisons with potential confounding were contextually described using ROBINS-I (qualitative only, not replacing QUADAS-2 judgments). An internal SELEX transparency framework (cycles, enrichment kinetics, counter-selection, library complexity management, algorithmic/AI augmentation, validation modality) generated reporting flags (adequate vs. partial/insufficient).
Dual assessment with calibration phase (n = 10). No composite score; “uncertain” only when textual insufficiency is explicit. High-risk domains or applicability concerns feed sensitivity exclusions. GRADE-DTA not applied (high extrinsic heterogeneity).
2.6. Evidence Synthesis Strategy and Bibliometric Analyses
Bibliometrics: A distinct corpus (n = 756) was constituted (after pre-analytical exclusion of 136 out-of-scope or incomplete records) for mapping: annual output (2010–2025*), source dispersion, co-author networks (fractional counting), international collaborations, keyword co-occurrence. Tools: VOSviewer (v1.x) and Bibliometrix (R 2024). Citation counts normalized to “citations per year” to mitigate recency bias. The compound annual growth rate (CAGR) is calculated as
Diagnostic synthesis: Metrics (Se, Sp, AUC, LR+, LR−, DOR) are reported descriptively. Quantitative pooling is considered only if ≥5 studies evaluate the same biomarker-platform construct in a clinically comparable context (population spectrum, matrix, threshold). Modeling: bivariate (Reitsma) with hierarchical summary ROC (HSROC), 95% CI, and prediction regions. Continuity correction (0.5) for zero cells. No formal publication bias assessment if <10 studies per cell. Formulas:
DOR excludes studies lacking 2 × 2 data. AUCs derived exclusively from internal self-validation are not aggregated (optimism bias mitigation).
Affinity: Distribution of Kd (high-confidence subset) by target class: median, IQR, range; ΔG reported at 298 K and at assay temperature when provided (expected effect: more negative ΔG at higher T when . No meta-aggregation (non-harmonizable variability: ionic strength, buffer, format).
Analytical: LoD harmonized to molar when conversions are unambiguous; dynamic range presented (lower bound, upper bound), span, and ratio (RangeMin/LoD) to estimate effective sensitivity leverage. Narrative synthesis is due to format and unit diversity.
Missing data handling: “NR” without imputation. Pre-specified sensitivity analyses: exclusion (i) multi-domain high risk of bias studies; (ii) assays on artificial matrices without validation on native urine.
Limitations: Exclusion of gray literature (risk of publication bias), under-reporting of pre-analytics (collection, storage conditions), matrix and platform heterogeneity limiting quantitative aggregations, absence of kinetic parameters (k_on, k_off) impairing fine mechanistic interpretation of ΔG.
Eligibility criteria encompassed original investigations reporting urinary biomarkers (cell-free urine, urinary extracellular vesicles, or exfoliated urinary cells) and/or aptamer- or SELEX-based approaches applied to prostate cancer diagnosis or risk stratification. Studies were required to provide experimental data or quantitative diagnostic performance measures (e.g., sensitivity, specificity, AUC, or affinity constants such as Kd). Exclusion criteria comprised secondary or overview literature (reviews, editorials, commentaries), therapeutic aptamer applications without a diagnostic component, methodological or platform papers lacking a clinical or biological cohort, and studies with non-extractable or incomplete outcome data.
Two reviewers independently screened records in a dual-review workflow at the title/abstract and full-text stages using a piloted, standardized form. Discrepancies were resolved through consensus. Agreement between reviewers was quantified using Cohen’s κ. At the title/abstract stage (n = 1348 unique records after de-duplication), the overall inclusion rates were 9.6% for Reviewer 1 and 15.0% for Reviewer 2. Observed agreement was 93.2%, with Cohen’s κ = 0.689 (95% CI 0.628–0.746), indicating substantial inter-reviewer reliability. The prevalence index (0.75) reflected the expected predominance of exclusions, while the bias index (0.05) indicated minimal systematic imbalance between reviewers.
From 2951 initial records (Scopus, Web of Science, and PubMed combined), 1603 duplicates were removed, leaving 1348 records for screening. Of these, 701 were excluded at the title/abstract stage and 647 underwent full-text assessment. A total of 518 full texts were excluded (secondary literature, non-prostate focus, methodological platforms without diagnostic data, or insufficient extractable outcomes), yielding 129 studies included in the final synthesis. Detailed distributions of exclusion reasons are provided in
Supplementary Table S1.
2.7. Software
All bibliometric analyses were performed using VOSviewer v1.6.20 (Leiden University, The Netherlands), Bibliometrix/Biblioshiny v4.2.2 (University of Naples, Italy), RStudio v2023.09 (Boston, MA, USA), and Microsoft Excel 365 (Microsoft Corporation, USA).
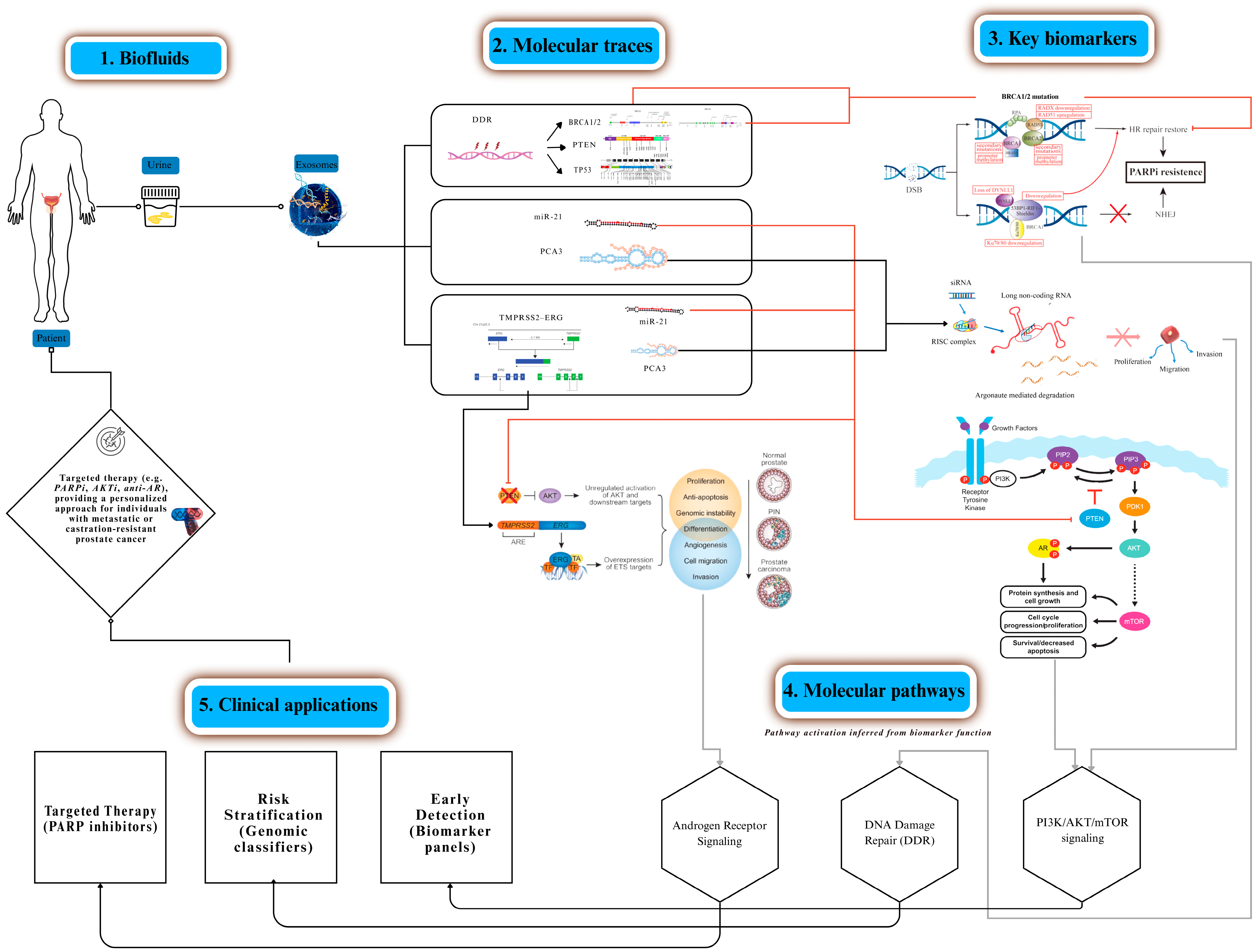
Figure 2 was created using Canva Pro (Canva Pty Ltd., Sydney, Australia), and
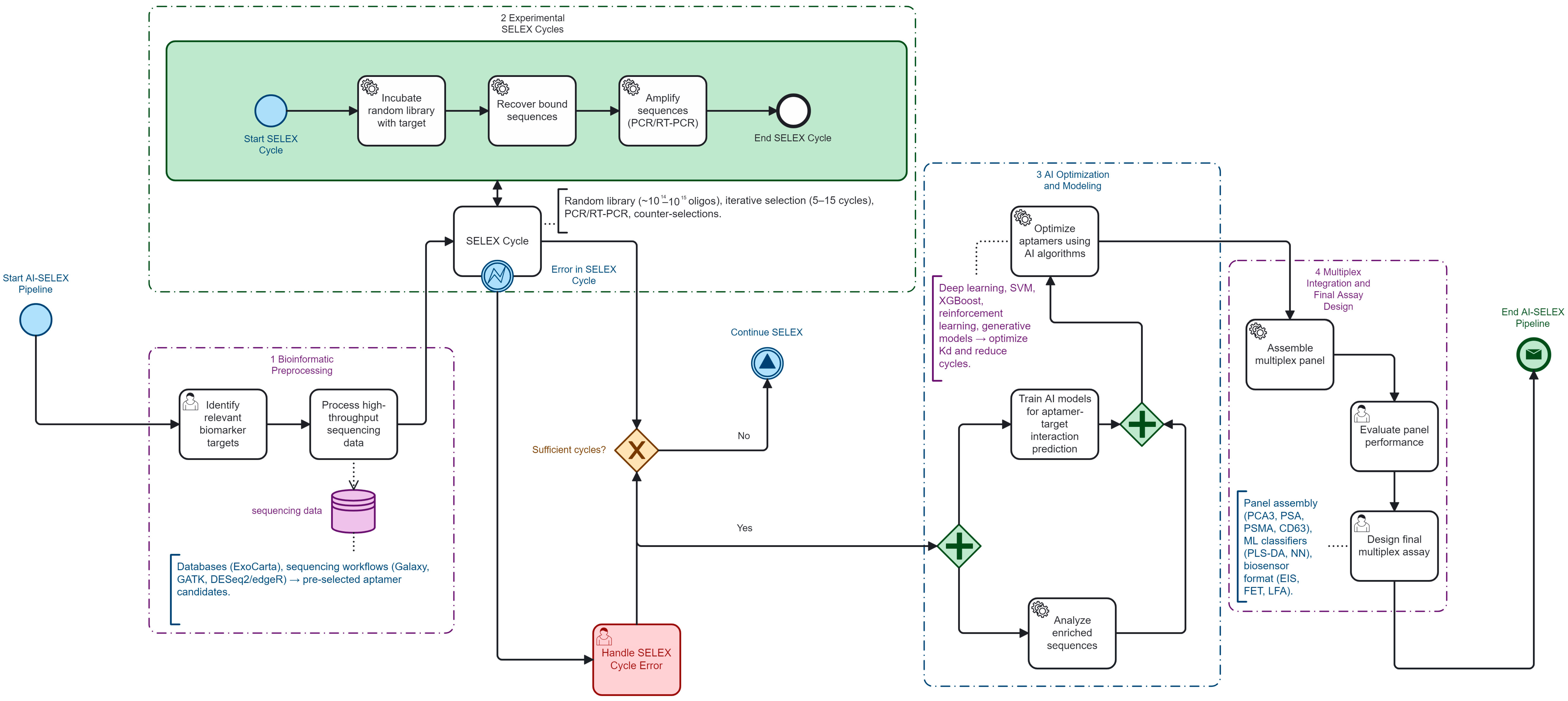
Figure 3 was created using Camunda Modeler v5.18 (Camunda Services GmbH, Berlin, Germany).
9. Conclusions
This systematic and bibliometric review demonstrates that urinary aptamer as-says—particularly when integrated with extracellular vesicle (EV) fractions—offer a credible path to refine pre-biopsy stratification in prostate cancer. Across 129 included studies, single-marker assays such as PCA3 and EN2 achieved proof-of-concept diag-nostic performance, with limits of detection in the femtomolar to attomolar range [
1,
2,
3,
75,
124,
125]. Multiplex prototypes combining PCA3, EN2, PSA derivatives, sarcosine, and exosomal miRNAs provided complementary information streams but rarely pro-gressed beyond internal validation [
7,
9,
36,
65].
SELEX performance analysis highlighted AI-assisted workflows as a turning point. Compared with conventional 12–15 cycle protocols, machine-learning-driven priori-tization compressed enrichment to 5–7 cycles (≈40–55% relative reduction), delivered affinity gains up to 100-fold, and shortened calendar time through NGS-integrated stopping rules [
23,
25,
66,
96,
126,
127]. Yet, the translational bridge remains incomplete: many computationally optimized aptamers were not validated in clinical urine co-horts, and diagnostic endpoints such as AUC or calibration curves were underreported [
19,
21,
27].
Taken together, evidence synthesis underscores a dual signal: (i) analytical feasi-bility—aptamers can reach clinically relevant detection thresholds in urine—and (ii) pipeline fragility—heterogeneity, missing 2 × 2 data, and lack of external validation currently limit regulatory translation.
Several priorities emerge for the next phase of urinary aptamer research. When it comes to urinary multi-cancer panels, the molecular diversity of urine supports ex-pansion beyond prostate cancer. Early studies have demonstrated aptamer binding to colorectal, ovarian, and bladder cancer vesicles [
28,
72,
128], suggesting a multi-cancer urine panel is technically feasible. Comparative head-to-head benchmarks across tu-mor types will be critical to demonstrate added value over PSA-only pathways. Speaking of integration with MRI and AI workflows, radiogenomic pipelines already couple mpMRI with urinary signatures to stratify indolent versus aggressive disease. Embedding AI-SELEX multiplex panels into these decision algorithms could improve pre-biopsy specificity and reduce unnecessary sampling [
10,
11,
12,
13,
14]. AI integration will also require infrastructure for reproducibility, version control, and regulatory audit. When it comes to open data and reproducibility, transparent deposition of SELEX se-quences, affinity constants, and diagnostic datasets into repositories such as OSF or Zenodo should become standard. Open-source benchmarks will allow reproducibility, reduce duplicative effort, and accelerate cross-cohort validation. Harmonized report-ing (PRISMA, QUADAS-2, PROBAST, SELEX-AI checklist) will support evidence syn-thesis and downstream guideline adoption, in line with recent community-level rec-ommendations on aptamer development [
129].
AI-assisted SELEX is a promising strategy for accelerating high-affinity aptamer discovery and assembling multiplex urinary panels for prostate cancer, with consistent cycle reduction from 12 to 15 to 5 to 7 (≈40–55% relative reduction). Current evidence remains early phase, methodologically heterogeneous, and largely single center. Priori-ties include standardized uEV processing, complete 2 × 2 diagnostic reporting with confidence intervals, multicenter external validation, calibration and decision impact analyses, and harmonized LoD and Kd reporting frameworks. Without these, the promise of non-invasive, low-cost urinary aptamer diagnostics will remain underexploited; with them, the field is poised to deliver clinically actionable, globally scalable tools for precision oncology.