Stromal COL11A1: Mechanisms of Stroma-Driven Multidrug Resistance in Breast Cancer and Biomarker Potential
Abstract
1. Introduction
2. Materials and Methods
3. Breast Cancer Subtypes and Stromal Drivers of Therapeutic Resistance
4. COL11A1 and Classical Immunohistochemical Markers
5. COL11A1 an Important Stromal Factor in Breast Cancer
5.1. COL11A1-Tumor Microenvironment
5.2. COL11A1-Signaling Pathways in Breast Cancer
5.3. The Effect of COL11A1 on the Immune System
5.4. Resistance to Hormone Therapy
5.5. Chemotherapy Resistance
6. Discussion
6.1. Biomarker Development
6.2. Challenges of Clinical Translation
6.3. Limitations
6.4. Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IHC | Immunohistochemistry |
| COL11A1 | Collagen type XI α1 chain |
| BRCA | Breast Cancer Gene |
| DCIS | Ductal Carcinoma In Situ |
| NST | No Specific type |
| BMI | Body Mass Index |
| ADH | Atypical Ductal Hyperplasia |
| ER | Estrogen Receptor |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| ECM | Extracellular Matrix |
| CAFs | Cancer-associated fibroblasts |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| CP | Chronic Pancreatitis |
| IAPs | Apoptosis Inhibitory Proteins |
| OSM | Oncostatin M |
| TGF-β | Transforming Growth Factor Beta |
| SERM | Selective Estrogen Receptor Modulator |
| HR | Hormone Receptors |
| DFS | Disease-Free Survival |
| OS | Overall Survival |
| TβRI | Transforming Growth Factor Beta Receptor 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| TNBC | Triple-Negative Breast Cancer |
| IM | Immunomodulatory |
| HRT | Hormone Replacement Therapy |
| BL | Basal-like |
| MSL | Mesenchymal Stem-like |
| LAR | Luminal Androgen Receptor |
References
- 20-Breast-Fact-Sheet.pdf [Internet]. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/20-breast-fact-sheet.pdf (accessed on 15 July 2025).
- Luo, Q.; Li, J.; Su, X.; Tan, Q.; Zhou, F.; Xie, S. COL11A1 serves as a biomarker for poor prognosis and correlates with immune infiltration in breast cancer. Front. Genet. 2022, 13, 935860. [Google Scholar] [CrossRef]
- Malla, R.R.; Vasudevaraju, P.; Vempati, R.K.; Rakshmitha, M.; Merchant, N.; Nagaraju, G.P. Regulatory T cells: Their role in triple-negative breast cancer progression and metastasis. Cancer 2022, 128, 1171–1183. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.J. Collagen Type XI Alpha 1 (COL11A1): A Novel Biomarker and a Key Player in Cancer. Cancers 2021, 13, 935. [Google Scholar] [CrossRef]
- Shi, W.; Chen, Z.; Liu, H.; Miao, C.; Feng, R.; Wang, G.; Chen, G.; Chen, Z.; Fan, P.; Pang, W.; et al. COL11A1 as an novel biomarker for breast cancer with machine learning and immunohistochemistry validation. Front. Immunol. 2022, 13, 937125. [Google Scholar] [CrossRef]
- Freire, J.; García-Berbel, P.; Caramelo, B.; García-Berbel, L.; Ovejero, V.J.; Cadenas, N.; Azueta, A.; Gómez-Román, J. Usefulness of COL11A1 as a Prognostic Marker of Tumor Infiltration. Biomedicines 2023, 11, 2496. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J. The Dynamic Changes of COL11A1 Expression During the Carcinogenesis and Development of Breast Cancer and as a Candidate Diagnostic and Prognostic Marker. Breast J. 2025, 2025, 7861864. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Chou, C.Y. Collagen XI Alpha 1 Chain, a Novel Therapeutic Target for Cancer Treatment. Front. Oncol. 2022, 12, 925165. Available online: https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2022.925165/full (accessed on 14 October 2025). [CrossRef] [PubMed]
- Fu, C.; Duan, S.; Zhou, X.; Meng, Y.; Chen, X. Overexpression of COL11A1 confers tamoxifen resistance in breast cancer. npj Breast Cancer 2024, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Liu, Z.; Deng, N.; Tan, T.Z.; Huang, R.Y.J.; Taylor-Harding, B.; Cheon, D.-J.; Lawrenson, K.; Wiedemeyer, W.R.; Walts, A.E.; et al. A COL11A1-correlated pan-cancer gene signature of activated fibroblasts for the prioritization of therapeutic targets. Cancer Lett. 2016, 382, 203–214. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Erber, R.; Hartmann, A. Histology of Luminal Breast Cancer. Breast Care 2020, 15, 327–336. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Dogra, A.K.; Prakash, A.; Gupta, S.; Gupta, M. Prognostic Significance and Molecular Classification of Triple Negative Breast Cancer: A Systematic Review. Eur. J. Breast Health 2025, 21, 101–114. Available online: https://eurjbreasthealth.com/articles/prognostic-significance-and-molecular-classification-of-triple-negative-breast-cancer-a-systematic-review/ejbh.galenos.2025.2024-10-2 (accessed on 13 October 2025). [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Das, D.; Pandey, M. Understanding genetic variations associated with familial breast cancer. World J. Surg. Oncol. 2024, 22, 271. [Google Scholar] [CrossRef]
- Mubarak, F.; Kowkabany, G.; Popp, R.; Bansal, S.; Ahmed, S.H.; Sharan, S.; Sukniam, K.B.; Raikot, S.R.; Jimenez, P.B.; Popp, K.; et al. Early Stage Breast Cancer: Does Histologic Subtype (Ductal vs. Lobular) Impact 5 Year Overall Survival? Cancers 2024, 16, 1509. [Google Scholar] [CrossRef] [PubMed]
- Sağdıç, M.F.; Özaslan, C. Rare Histological Types of Breast Cancer: A Single-Center Experience. Breast J. 2025, 2025, 1179914. [Google Scholar] [CrossRef]
- Conti, M.; Morciano, F.; Amodeo, S.; Gori, E.; Romanucci, G.; Belli, P.; Tommasini, O.; Fornasa, F.; Rella, R. Special Types of Breast Cancer: Clinical Behavior and Radiological Appearance. J. Imaging 2024, 10, 182. [Google Scholar] [CrossRef]
- Berg, T.; Jensen, M.B.; Celik, A.; Talman, M.L.; Misiakou, M.A.; Knoop, A.S.; Nielsen, F.C.; Ejlertsen, B.; Rossing, M. Molecular subtyping improves breast cancer diagnosis in the Copenhagen Breast Cancer Genomics Study. JCI Insight 2024, 9, e178114. Available online: https://insight.jci.org/articles/view/178114?utm_source=chatgpt.com (accessed on 13 October 2025). [CrossRef]
- Ades, F.; Zardavas, D.; Bozovic-Spasojevic, I.; Pugliano, L.; Fumagalli, D.; de Azambuja, E.; Viale, G.; Sotiriou, C.; Piccart, M. Luminal B breast cancer: Molecular characterization, clinical management, and future perspectives. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2794–2803. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Cheang, M.C.U.; Martín, M.; Parker, J.S.; Carrasco, E.; Caballero, R.; Tyldesley, S.; Gelmon, K.; Bernard, P.S.; Nielsen, T.O.; et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 203–209. [Google Scholar] [CrossRef]
- Lammers, S.W.M.; Geurts, S.M.E.; Hermans, K.E.P.E.; Kooreman, L.F.S.; Swinkels, A.C.P.; Smorenburg, C.H.; van der Sangen, M.; Kroep, J.; Honkoop, A.; Berkmortel, F.v.D.; et al. The prognostic and predictive value of the luminal-like subtype in hormone receptor-positive breast cancer: An analysis of the DATA trial. ESMO Open 2025, 10, 104154. [Google Scholar] [CrossRef]
- Nak, D.; Kivrak, M. Mastectomy, HER2 Receptor Positivity, NPI, Late Stage and Luminal B-Type Tumor as Poor Prognostic Factors in Geriatric Patients with Breast Cancer. Diagnostics 2025, 15, 13. [Google Scholar] [CrossRef]
- Rediti, M.; Venet, D.; Joaquin Garcia, A.; Maetens, M.; Vincent, D.; Majjaj, S.; El-Abed, S.; Di Cosimo, S.; Ueno, T.; Izquierdo, M.; et al. Identification of HER2-positive breast cancer molecular subtypes with potential clinical implications in the ALTTO clinical trial. Nat. Commun. 2024, 15, 10402. [Google Scholar] [CrossRef]
- Intrieri, T.; Manneschi, G.; Caldarella, A. 10-year survival in female breast cancer patients according to ER, PR and HER2 expression: A cancer registry population-based analysis. J. Cancer Res. Clin. Oncol. 2023, 149, 4489–4496. [Google Scholar] [CrossRef] [PubMed]
- Susini, T.; Renda, I.; Giani, M.; Vezzosi, V.; Baroni, G.; Bianchi, S. Immunohistochemical Evaluation of COL11A1 and FGD3 Expression in Invasive Breast Cancer. Med. Res. Arch. 2023, 11. Available online: https://esmed.org/MRA/mra/article/view/3593 (accessed on 10 July 2025). [CrossRef]
- Jin, M.; Fang, J.; Peng, J.; Wang, X.; Xing, P.; Jia, K.; Hu, J.; Wang, D.; Ding, Y.; Wang, X.; et al. PD-1/PD-L1 immune checkpoint blockade in breast cancer: Research insights and sensitization strategies. Mol. Cancer 2024, 23, 266. [Google Scholar] [CrossRef]
- Toss, M.S.; Miligy, I.M.; Gorringe, K.L.; Aleskandarany, M.A.; Alkawaz, A.; Mittal, K.; Azueta, A.; Gómez-Román, J. Collagen (XI) alpha-1 chain is an independent prognostic factor in breast ductal carcinoma in situ. Mod. Pathol. 2019, 32, 1460–1472. [Google Scholar] [CrossRef] [PubMed]
- García-Pravia, C.; Galván, J.A.; Gutiérrez-Corral, N.; Solar-García, L.; García-Pérez, E.; García-Ocaña, M.; Del Amo-Iribarren, J.; Menéndez-Rodríguez, P.; García-García, J.; de los Toyos, J.R.; et al. Overexpression of COL11A1 by Cancer-Associated Fibroblasts: Clinical Relevance of a Stromal Marker in Pancreatic Cancer. PLoS ONE 2013, 8, e78327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, S.; Lu, T.; Han, D.; Zhang, K.; Gan, L.; Wu, X.; Li, Y.; Zhao, X.; Li, Z.; et al. Single-cell analysis reveals the COL11A1+ fibroblasts are cancer-specific fibroblasts that promote tumor progression. Front. Pharmacol. 2023, 14, 1121586. [Google Scholar] [CrossRef]
- Ryan, R.E.; Martin, B.; Mellor, L.; Jacob, R.B.; Tawara, K.; McDougal, O.M.; Oxford, J.T.; Jorcyk, C.L. Oncostatin M binds to extracellular matrix in a bioactive conformation: Implications for inflammation and metastasis. Cytokine 2015, 72, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Yong-hao, Y.; Xian-guo, W.; Ming, X.; Jin-ping, Z. Expression and clinical significance of miR-139-5p in non-small cell lung cancer. J. Int. Med. Res. 2019, 47, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, Y.; Qian, C.; Liu, J.; Li, G.; Li, Z. Over-expression of CDX2 alleviates breast cancer by up-regulating microRNA let-7b and inhibiting COL11A1 expression. Cancer Cell Int. 2020, 20, 13. [Google Scholar] [CrossRef]
- Gu, S.Q.; Luo, J.H.; Yao, W.X. The regulation of miR-139-5p on the biological characteristics of breast cancer cells by targeting COL11A1. Math. Biosci. Eng. MBE 2019, 17, 1428–1441. [Google Scholar] [CrossRef]
- Bou-Dargham, M.J.; Sha, L.; Sarker, D.B.; Krakora-Compagno, M.Z.; Chen, Z.; Zhang, J.; Sang, Q.-X.A. TCGA RNA-Seq and Tumor-Infiltrating Lymphocyte Imaging Data Reveal Cold Tumor Signatures of Invasive Ductal Carcinomas and Estrogen Receptor-Positive Human Breast Tumors. Int. J. Mol. Sci. 2023, 24, 9355. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Omoto, Y.; Iwase, H.; Yamashita, H.; Gustafsson, J.-Å. Differential expression of estrogen receptor α, β1, and β2 in lobular and ductal breast cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 1933–1938. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, L.; Li, Z.; Zhang, H.; Wang, Q.; Huang, J.; Wang, B.; Mohan, C.D.; Sethi, G.; Wang, G. The role of the tumor microenvironment in endocrine therapy resistance in hormone receptor-positive breast cancer. Front. Endocrinol. 2023, 14, 1261283. Available online: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2023.1261283/full (accessed on 14 October 2025). [CrossRef]
- Jeselsohn, R.; Buchwalter, G.; De Angelis, C.; Brown, M.; Schiff, R. ESR1 mutations—A mechanism for acquired endocrine resistance in breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Thurn, K.T.; Biçaku, E.; Marchion, D.C.; Münster, P.N. Addition of a histone deacetylase inhibitor redirects tamoxifen-treated breast cancer cells into apoptosis, which is opposed by the induction of autophagy. Breast Cancer Res. Treat. 2011, 130, 437–447. [Google Scholar] [CrossRef]
- Garcia-Martinez, L.; Zhang, Y.; Nakata, Y.; Chan, H.L.; Morey, L. Epigenetic mechanisms in breast cancer therapy and resistance. Nat. Commun. 2021, 12, 1786. [Google Scholar] [CrossRef]
- Raheem, F.; Karikalan, S.A.; Batalini, F.; El Masry, A.; Mina, L. Metastatic ER+ Breast Cancer: Mechanisms of Resistance and Future Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 16198. [Google Scholar] [CrossRef]
- Lloyd, M.R.; Spring, L.M.; Bardia, A.; Wander, S.A. Mechanisms of Resistance to CDK4/6 Blockade in Advanced Hormone Receptor-positive, HER2-negative Breast Cancer and Emerging Therapeutic Opportunities. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 821–830. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.Q.; Chen, Q.; Yu, T.Y.; Zhang, S.L.; Guo, W.H.; Luo, L.H.; Zhao, G.; Yin, D.C.; Zhang, C.Y. MiR-4458-loaded gelatin nanospheres target COL11A1 for DDR2/SRC signaling pathway inactivation to suppress the progression of estrogen receptor-positive breast cancer. Biomater. Sci. 2022, 10, 4596–4611. [Google Scholar] [CrossRef] [PubMed]
- Prihantono Faruk, M. Breast cancer resistance to chemotherapy: When should we suspect it and how can we prevent it? Ann. Med. Surg. 2021, 70, 102793. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Chang, T.H.; Huang, Y.F.; Chen, C.C.; Chou, C.Y. COL11A1 confers chemoresistance on ovarian cancer cells through the activation of Akt/c/EBPβ pathway and PDK1 stabilization. Oncotarget 2015, 6, 23748–23763. [Google Scholar] [CrossRef]
- Rada, M.; Nallanthighal, S.; Cha, J.; Ryan, K.; Sage, J.; Eldred, C.; Ullo, M.; Orsulic, S.; Cheon, D.-J. Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene 2018, 37, 4809–4820. [Google Scholar] [CrossRef]
- Wu, Y.H.; Huang, Y.F.; Chang, T.H.; Chou, C.Y. Activation of TWIST1 by COL11A1 promotes chemoresistance and inhibits apoptosis in ovarian cancer cells by modulating NF-κB-mediated IKKβ expression. Int. J. Cancer 2017, 141, 2305–2317. [Google Scholar] [CrossRef]
- Tia, S.T.; Luo, M.; Fan, W. Mapping the Role of P-gp in Multidrug Resistance: Insights from Recent Structural Studies. Int. J. Mol. Sci. 2025, 26, 4179. [Google Scholar] [CrossRef]
- Tadayyon, H.; Sannachi, L.; Gangeh, M.J.; Kim, C.; Ghandi, S.; Trudeau, M.; Pritchard, K.; Tran, W.T.; Slodkowska, E.; Sadeghi-Naini, A.; et al. A priori Prediction of Neoadjuvant Chemotherapy Response and Survival in Breast Cancer Patients using Quantitative Ultrasound. Sci. Rep. 2017, 7, 45733. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and hot tumors: From molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Franzoi, M.A.; Agostinetto, E.; Perachino, M.; Del Mastro, L.; de Azambuja, E.; Vaz-Luis, I.; Partridge, A.H.; Lambertini, M. Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol. 2021, 22, e303–e313. [Google Scholar] [CrossRef] [PubMed]
- Freire, J.; García-Berbel, L.; García-Berbel, P.; Pereda, S.; Azueta, A.; García-Arranz, P.; De Juan, A.; Vega, A.; Hens, Á.; Enguita, A.; et al. Collagen Type XI Alpha 1 Expression in Intraductal Papillomas Predicts Malignant Recurrence. BioMed Res. Int. 2015, 2015, 812027. [Google Scholar] [CrossRef]
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Galván, J.A.; García-Martínez, J.; Vázquez-Villa, F.; García-Ocaña, M.; García-Pravia, C.; Menéndez-Rodríguez, P.; González-del Rey, C.; Barneo-Serra, L.; De Los Toyos, J.R. Validation of COL11A1/procollagen 11A1 expression in TGF-β1-activated immortalised human mesenchymal cells and in stromal cells of human colon adenocarcinoma. BMC Cancer 2014, 14, 867. [Google Scholar] [CrossRef]
- Lo Buglio, G.; Lo Cicero, A.; Campora, S.; Ghersi, G. The Multifaced Role of Collagen in Cancer Development and Progression. Int. J. Mol. Sci. 2024, 25, 13523. [Google Scholar] [CrossRef]
- Wu, Y.H.; Huang, Y.F.; Chang, T.H.; Wu, P.Y.; Hsieh, T.Y.; Hsiao, S.Y.; Huang, S.C.; Chou, C.Y. miR-335 Restrains the Aggressive Phenotypes of Ovarian Cancer Cells by Inhibiting COL11A1. Cancers 2021, 13, 6257. [Google Scholar] [CrossRef]
- Giussani, M.; Landoni, E.; Merlino, G.; Turdo, F.; Veneroni, S.; Paolini, B.; Cappelletti, V.; Miceli, R.; Orlandi, R.; Triulzi, T.; et al. Extracellular matrix proteins as diagnostic markers of breast carcinoma. J. Cell Physiol. 2018, 233, 6280–6290. [Google Scholar] [CrossRef]
- Raglow, Z.; Thomas, S.M. Tumor matrix protein collagen XIα1 in cancer. Cancer Lett. 2015, 357, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Huseni, M.A.; Wang, L.; Klementowicz, J.E.; Yuen, K.; Breart, B.; Orr, C.; Liu, L.-F.; Li, Y.; Gupta, V.; Li, C.; et al. CD8+ T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Rep. Med. 2023, 4, 100878. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.W.; Kozielski, A.J.; Qian, W.; Zhou, J.; Anselme, A.C.; Chan, A.A.; Pan, P.-Y.; Lee, D.J.; Chang, J.C. Tocilizumab overcomes chemotherapy resistance in mesenchymal stem-like breast cancer by negating autocrine IL-1A induction of IL-6. npj Breast Cancer 2022, 8, 30. [Google Scholar] [CrossRef]
- Siersbæk, R.; Scabia, V.; Nagarajan, S.; Chernukhin, I.; Papachristou, E.K.; Broome, R.; Johnston, S.J.; Joosten, S.E.; Green, A.R.; Kumar, S.; et al. IL6/STAT3 Signaling Hijacks Estrogen Receptor α Enhancers to Drive Breast Cancer Metastasis. Cancer Cell 2020, 38, 412–423.e9. [Google Scholar] [CrossRef]
- Piotrzkowska-Wróblewska, H.; Dobruch-Sobczak, K.; Klimonda, Z.; Karwat, P.; Roszkowska-Purska, K.; Gumowska, M.; Litniewski, J. Monitoring breast cancer response to neoadjuvant chemotherapy with ultrasound signal statistics and integrated backscatter. PLoS ONE 2019, 14, e0213749. [Google Scholar] [CrossRef]
- Galbo, P.M.; Zang, X.; Zheng, D. Molecular Features of Cancer-associated Fibroblast Subtypes and their Implication on Cancer Pathogenesis, Prognosis, and Immunotherapy Resistance. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 2636–2647. [Google Scholar] [CrossRef]
- Anurag, M.; Zhu, M.; Huang, C.; Vasaikar, S.; Wang, J.; Hoog, J.; Burugu, S.; Gao, D.; Suman, V.; Zhang, X.H.; et al. Immune Checkpoint Profiles in Luminal B Breast Cancer (Alliance). J. Natl. Cancer Inst. 2020, 112, 737–746. [Google Scholar] [CrossRef]
- Wang, Z.; You, P.; Yang, Z.; Xiao, H.; Tang, X.; Pan, Y.; Li, X.; Gao, F. PD-1/PD-L1 immune checkpoint inhibitors in the treatment of unresectable locally advanced or metastatic triple negative breast cancer: A meta-analysis on their efficacy and safety. BMC Cancer 2024, 24, 1339. [Google Scholar] [CrossRef]
- Vargas, A.C.; McCart Reed, A.E.; Waddell, N.; Lane, A.; Reid, L.E.; Smart, C.E.; Cocciardi, S.; da Silva, L.; Song, S.; Chenevix-Trench, G.; et al. Gene expression profiling of tumour epithelial and stromal compartments during breast cancer progression. Breast Cancer Res. Treat. 2012, 135, 153–165. [Google Scholar] [CrossRef]
- Cords, L.; Tietscher, S.; Anzeneder, T.; Langwieder, C.; Rees, M.; de Souza, N.; Bodenmiller, B. Cancer-associated fibroblast classification in single-cell and spatial proteomics data. Nat. Commun. 2023, 14, 4294. [Google Scholar] [CrossRef] [PubMed]
- Karaglani, M.; Toumpoulis, I.; Goutas, N.; Poumpouridou, N.; Vlachodimitropoulos, D.; Vasilaros, S.; Rizos, I.; Kroupis, C. Development of novel real-time PCR methodology for quantification of COL11A1 mRNA variants and evaluation in breast cancer tissue specimens. BMC Cancer 2015, 15, 694. [Google Scholar] [CrossRef] [PubMed]
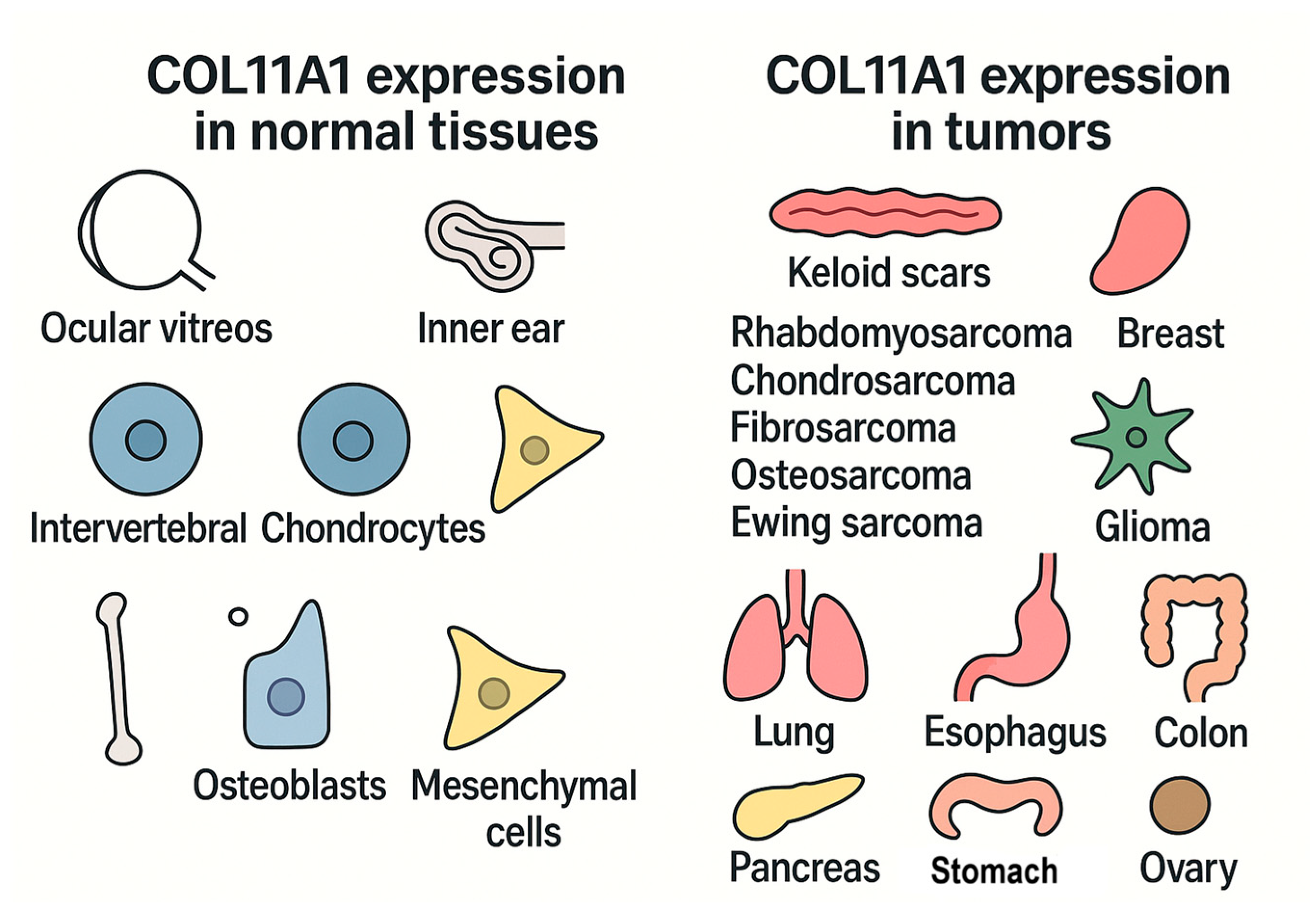
| No. | Reference | Cohort | Method | Key Outcome |
|---|---|---|---|---|
| 1 | Luo et al., 2022 [2] | Breast samples | Bioinformatics + IHC | High COL11A1 associated with shorter OS and aggressive phenotypes |
| 2 | Shi et al., 2022 [5] | Six breast cancer datasets | Machine learning on transcriptomic data + IHC | Correlates with poor prognosis and altered immune infiltrate |
| 3 | Freire et al., 2023 [6] | Small clinical biopsies (including breast) | IHC vs. histology | COL11A1 may predict tumor infiltration; potential clinical utility |
| 4 | Wang et al., 2025 [7] | Samples breast cancer tissue, benign breast tumors, normal breast tissue and lymph nodes with metastases | IHC and real-time reverse transcription PCR (RT-PCR) | COL11A1 mRNA is significantly higher in primary breast cancer tissues than in adjacent normal tissue |
| 5 | Wu et al., 2022 [8] | Previous studies | Analyze and synthesize the results | Increased expression is frequently associated with aggressive tumors |
| 6 | Fu et al., 2024 [9] | MCF-7/T47D cells and MCF-7/COL11A1 and T47D/COL11A1 cells | IHC + in vivo functional assays | High COL11A1 linked to poor tamoxifen response; in vivo resistance confirmed |
| 7 | Jia et al., 2016 [10] | Data from The Cancer Genome Atlas (TCGA) for 13 types of primary carcinoma | Transcriptomic, functional analysis and IHC validation. | COL11A1 has been identified as a specific biomarker for activated CAFs in multiple cancer types. |
| Implications | ER/PR/HER2/Ki-67 | COL11A1 |
| Application | Standard | Experimental |
| Marker type | Tumor cell | Peritumoral stroma |
| Diagnostic | Molecular subtype, classification | Confirmation of invasion |
| Prognostic value | Yes | Yes |
| Targeted therapy | Hormonal therapy, anti-HER2 therapy | Future treatment option |
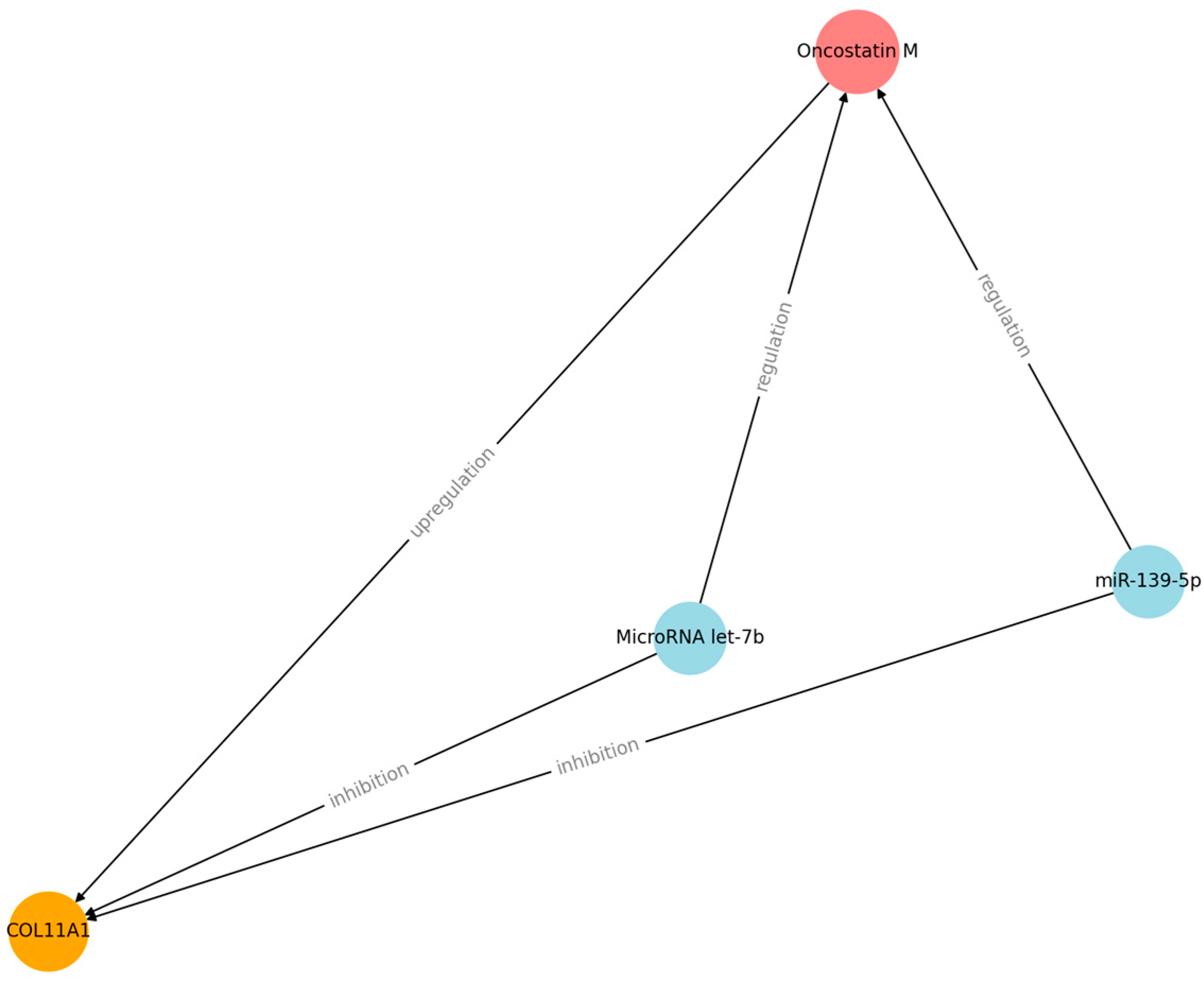
| Regulator | Effect |
|---|---|
| miR-139-5p | Increases cell proliferation and inhibits apoptosis |
| MicroRNA let-7b | Increases cell proliferation, migration, invasion, and metastasis |
| Oncostatin M | Increases inflammation and metastasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onofrei, A.; Mihailuta, F.; Mihalache, D.; Vodă, C.C.; Jurja, S.; Deacu, S.; Mehedinți, M.C. Stromal COL11A1: Mechanisms of Stroma-Driven Multidrug Resistance in Breast Cancer and Biomarker Potential. Biomedicines 2025, 13, 2905. https://doi.org/10.3390/biomedicines13122905
Onofrei A, Mihailuta F, Mihalache D, Vodă CC, Jurja S, Deacu S, Mehedinți MC. Stromal COL11A1: Mechanisms of Stroma-Driven Multidrug Resistance in Breast Cancer and Biomarker Potential. Biomedicines. 2025; 13(12):2905. https://doi.org/10.3390/biomedicines13122905
Chicago/Turabian StyleOnofrei (Popa), Andreea, Felicia Mihailuta, Daniela Mihalache, Cristina Chelmu Vodă, Sanda Jurja, Sorin Deacu, and Mihaela Cezarina Mehedinți. 2025. "Stromal COL11A1: Mechanisms of Stroma-Driven Multidrug Resistance in Breast Cancer and Biomarker Potential" Biomedicines 13, no. 12: 2905. https://doi.org/10.3390/biomedicines13122905
APA StyleOnofrei, A., Mihailuta, F., Mihalache, D., Vodă, C. C., Jurja, S., Deacu, S., & Mehedinți, M. C. (2025). Stromal COL11A1: Mechanisms of Stroma-Driven Multidrug Resistance in Breast Cancer and Biomarker Potential. Biomedicines, 13(12), 2905. https://doi.org/10.3390/biomedicines13122905






