From Semantic Modeling to Precision Radiotherapy: An AI Framework Linking Radiobiology, Oncology, and Public Health Integration
Abstract
1. Introduction
2. Methods
3. Results
3.1. Corpus Construction and Harmonization
3.2. Topic Modeling and Co-Occurrence Analysis
- Topic_0t: cancer, radiotherapy, patient, head, neck, breast, prostate—concentration on specific tumor types treated with radiotherapy, including advanced cases and toxicity.
- Topic_1t: radiobiology, clinical, oncology—integration of biological foundations with clinical practice.
- Topic_2t: cancer, breast, carcinoma, prostate, esophageal—comparative studies among tumor types.
- Topic_3t: radiation, oncology, biology, molecular—mechanisms of radiation action.
- Topic_4t: stereotactic, body, radiosurgery, lung—SBRT literature in pulmonary neoplasms.
- Topic_5t: radiation, beam, ion, proton—dose delivery physics and tissue protection.
- Topic_6t: tumor, brain, model, imaging—modeling and preclinical studies of brain tumors.
- Topic_7t: tumor, cell, DNA, repair, pathway—molecular biology of DNA damage.
- Topic_8t: cell, human, expression, gene—in vitro experimentation.
- Topic_9t: dose, brachytherapy, model, radiobiological—dose modeling and brachytherapy.
- Topic_0a: model, dose, imaging, flash—imaging-based modeling and planning for FLASH-RT.
- Topic_1a: cell, tumor, DNA, repair, damage—biological mechanisms of radiation.
- Topic_2a: proton, ion, RBE, particle—particle therapy literature.
- Topic_3a: patient, survival, RT, surgery—prognostic clinical studies.
- Topic_4a: toxicity, breast, risk, Gy, SBRT—toxicity in breast cancer treated with precision radiotherapy.
- Topic_5a: trial, immunotherapy, preclinical, targeted—immunoradiotherapy and combination therapies.
- Topic_6a: expression, gene, protein, blood—molecular biomarker research.
- Topic_7a: dose, Gy, plan, mouse—preclinical trials and validation in animal models.
- Topic_8a: clinical, therapy, oncology, development—institutional or editorial content.
- Topic_9a: dose, fraction, tissue, effect—dose fractionation studies in normal tissues.
3.3. Thematic Axes and Translational Integration
- Cancer and radiotherapy function as structuring axes of recent scientific production.
- The growth of terms such as cell, dose, effect, and treatment reflects the emphasis on therapeutic personalization, mechanisms of action, and clinical efficacy.
- The co-occurrence of tumor, DNA, repair, and survival indicates intensified research in precision medicine and response biomarkers.
- The dense network connectivity confirms the transversal nature of the field, integrating molecular biology, medical physics, and clinical practice into a unified scientific ecology.
- Class 1—DNA Repair and Molecular Response:
- Class 2—Precision Oncology and Genomic Modeling:
- Class 3—Individual Radiosensitivity and Clinical Risk:
- Class 4—Radioresistance and Associated Mechanisms:
- Class 5—Advanced Technologies and Innovative Radiotherapy:
- Class 1 (n = 6): 0.4693 ± 0.1542 (0.3151–0.6235)
- Class 2 (n = 6): 0.5387 ± 0.0439 (0.4948–0.5826)
- Class 3 (n = 18): 0.5520 ± 0.0289 (0.5231–0.5810)
- Class 4 (n = 4): 0.4592 ± 0.0869 (0.3723–0.5460)
- Class 5 (n = 3): 0.5167 ± 0.2541 (0.2626–0.7708)
3.4. Operational Pathways for Dose Personalization and AI-Driven Adaptation
3.4.1. Genomic Dose Personalization (RSI/GARD)
3.4.2. Voxel-Level Dose Painting
3.4.3. AI-Driven Adaptive Radiotherapy
3.5. Case Studies
- Class 1—DNA Repair and Molecular Response.
- Class 2—Precision Oncology and Genomic Modeling.
- Class 3—Individual Radiosensitivity and Clinical Risk
- Class 4—Radioresistance and Associated Mechanisms
- Class 5—Advanced Technologies and Innovative Radiotherapy
3.6. Implementation Plan in Precision Radiotherapy
- Class 3—Individual Radiosensitivity and Clinical Risk
- Class 4—Radioresistance and Associated Mechanisms
- Class 5—Advanced Technologies and Innovative Radiotherapy
Synthesis and Implementation Framework
- (i)
- Scientific and clinical validation focuses on biomarker qualification and the harmonization of data standards across institutions.
- (ii)
- The translational phase emphasizes interoperability, real-world pilot programs within national health systems, and the establishment of governance standards for AI-assisted radiotherapy.
- (iii)
- The consolidation phase centers on the integration of adaptive, biomarker-driven protocols and AI ethics frameworks into public policy and clinical guidelines.
4. Discussion
4.1. Overview and Conceptual Integration
4.2. Translational Framework: The Precision Radiotherapy Implementation Plan (PRIP)
4.2.1. Structure and Thematic Domains
4.2.2. Translational Role and Health-System Relevance
4.3. Clinical and Operational Implications
4.3.1. Predictive and Adaptive Applications
4.3.2. Methodological Constraints and Data Scope
4.3.3. Data Integrity and Model Sensitivity
4.4. Validation, Barriers, and Regulatory Frameworks
4.4.1. Translational Gaps and Real-World Constraints
4.4.2. Ethical and Practical Barriers
4.4.3. Governance and Explainable AI
4.4.4. Harmonization and Collaborative Oversight
4.5. Future Perspectives and Roadmap
4.5.1. Methodological and Computational Advances
4.5.2. Clinical Translation and Adaptive Integration
4.5.3. Toward a Learning Health Ecosystem
4.6. Historical and Analytical Context
4.6.1. Thematic Domains and Case Analyses
- (1)
- DNA repair and molecular responses;
- (2)
- Precision oncology and genomic modeling;
- (3)
- Individual radiosensitivity and clinical risk;
- (4)
- Mechanisms of radioresistance;
- (5)
- Advanced technologies and innovation in radiotherapy.
4.6.2. Conceptual and Methodological Implications
4.7. Limitations and Methodological Boundaries
5. Conclusions
- DNA repair and molecular response.
- Precision oncology and genomic modeling.
- Individual radiosensitivity and clinical risk.
- Mechanisms of radioresistance.
- Innovative radiotherapy technologies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
Abbreviations
| Abbreviation/Term | Meaning | Context/Explanation |
| ≥3 Toxicity | Grade 3 or Higher Adverse Events | Represents severe clinical side effects per standardized toxicity grading. |
| ≥40 Gy/s | Dose-Rate Threshold for FLASH-RT | Represents ultrafast radiation rate preserving normal tissue integrity. |
| 0.291–0.669 (~0.378 span) | Range of Observed Cosine Scores | Captures the minimum-to-maximum similarity observed between LLM outputs. |
| 0.5235 ± 0.0271 (95% CI 0.4964–0.5506) | Aggregate Similarity Statistics | Demonstrates moderate overall alignment and high reproducibility of language/semantic structure between models. |
| 15 Standardized Terms | Controlled Vocabulary Set | Used for harmonizing semantic meaning across Scopus, PubMed, and Web of Science records. |
| 2014/2015–2024 windows | Temporal limits of corpus | Ensure focus on contemporary, translational radiotherapy. |
| 2014–2025 Analytic Window | Defined Temporal Scope of Corpus | Restriction ensuring semantic stability and consistent term usage across databases. |
| 2D/3D | Two-dimensional/Three-dimensional | Cell-culture models compared for radiation response. |
| 3D Spheroids | Three-Dimensional Cell Cultures | In vitro tumor analogs reproducing microenvironmental gradients and resistance. |
| 60Co/192Ir | Cobalt-60/Iridium-192 Isotopes | Common brachytherapy sources; compared for efficacy, RBE, and safety. |
| a | Abstracts Corpus | The suffix “a” in Topic_0a–Topic_9a refers to topic models generated from abstracts rather than titles. |
| Adaptive Clinical Protocols | Dynamically Updated Treatment Guidelines | Clinical pathways that adjust based on biomarker or imaging feedback. |
| Adaptive Radiotherapy Planning | Treatment Optimization Based on Patient Response | Future goal using AI-derived biomarkers for closed-loop, real-time dose adjustment. |
| Adjacency definition | Mathematical rule for linking nodes | Directed adjacency alters numeric scales of network metrics. |
| Advanced Radiotherapy Technologies | Emerging Modalities (FLASH-RT, Brachytherapy, Particle Therapy) | High-precision and/or ultrafast radiation technologies providing enhanced therapeutic index. |
| Advanced Technologies Cluster | Thematic Category in LDA Model | Identified area with limited samples causing wider uncertainty in validation. |
| AGENT | Therapeutic Agent | Denotes a drug, molecule, or compound used in combination with radiation therapy. |
| AI | Artificial Intelligence | Central methodological pillar of the study; refers to machine-learning and computational modeling approaches. |
| AI Models (Validated) | Certified Predictive Algorithms | Used to plan or adjust treatments, ensuring regulatory and scientific reliability. |
| AI synthesis | Artificial Intelligence synthesis | Refers to AI-based interpretation and summarization phase of the pipeline. |
| AI-assisted | Artificial Intelligence–Assisted | Denotes the use of AI in processing, interpreting, and modeling scientific literature. |
| AI-based | Artificial Intelligence-based | Refers to the study’s computational modeling methods. |
| AI-based methods | Artificial-intelligence analytical techniques | Include topic modeling, text mining, and co-occurrence network analysis. |
| AI-based Modeling | Artificial Intelligence–Based Modeling | Framework for harmonizing heterogeneous datasets into unified evidence models. |
| AI-derived Biomarkers | Machine-Learned Predictive Molecular Indicators; Artificial Intelligence–Generated Predictive Features | Proposed for real-time, adaptive radiotherapy planning linked to patient-specific biological response; used for adaptive treatment and real-time learning protocols. |
| Alpha/Beta (α/β) | LQ model tissue parameters | Reported in pediatric medulloblastoma for tailoring dose/fractionation. |
| ATM | Ataxia-Telangiectasia Mutated | DNA-damage response kinase; nucleoshuttling linked to tissue radiosensitivity. |
| ATR | Ataxia Telangiectasia and Rad3-Related Protein | Parallel kinase to ATM; monitors replication stress and coordinates DNA repair. |
| Average Degree = 9.5 | Mean number of links per node | Quantifies average lexical connectivity. |
| Average Weighted Degree = 1962.2 | Mean sum of edge weights | Captures total co-occurrence frequency per term. |
| BER | Base Excision Repair | DNA repair pathway correcting small, non-helix-distorting base lesions caused by ionizing radiation. |
| BIOLOGICAL | Biological Effect or Endpoint | Used in Topic_2a for biophysical modeling linking energy deposition to cellular effect. |
| Biomarker signature | Composite molecular indicator | Selection criterion for case-study inclusion and stratification. |
| Biomedical Stopwords | Domain-specific uninformative terms | Filtered to improve semantic precision. |
| Biomedical subject areas | Domain filter in Scopus | Restricts search to clinically relevant literature. |
| Blue–Red Color Bar | Visual encoding of co-occurrence strength | Blue = weak association; red = strong association. |
| BMAL1, CLOCK, PER, CRY | Core Circadian Genes | Regulate cellular timing of DNA repair; targeted in chronoradiotherapy to reduce toxicity. |
| Brachytherapy | Internal radiotherapy using implanted sources | Another advanced modality considered in the framework. |
| Brachytherapy dose escalation | Targeted increase in internal RT dose | Inclusion term; linked to voxel-level and TCP modeling. |
| Carcinoma | Cancer Type Originating from Epithelial Cells | Frequently used term in radiation oncology literature (breast, esophageal, prostate). |
| CD8+ Lymphocytes | Cytotoxic T Cells | Immune effectors preserved after FLASH-RT, supporting anti-tumor immunity. |
| Cell-Fit-HD | High-Density Cell Microbeam Technology | Experimental system quantifying DNA-damage kinetics and repair precision. |
| Central node | Highest-connectivity vertex | “Cancer” identified as the core of the radiotherapy supergraph. |
| cGAS–STING | Cyclic GMP–AMP Synthase/Stimulator of Interferon Genes | Cytosolic DNA-sensing pathway linking radiation damage to immune activation. |
| ChatGPT | Generative Pretrained Transformer (GPT-5 model, OpenAI) | AI-based language model used under human supervision to improve clarity, grammar, and precision during manuscript preparation. |
| Chronoradiotherapy | Time-Synchronized Radiation Delivery | Aligns irradiation with circadian repair peaks to enhance efficacy and minimize side effects. |
| CI | Confidence Interval | Statistical interval representing uncertainty around the mean cosine-similarity estimates (95% CI: 0.4964–0.5506). |
| CiteSpace | Citation Space Mapping Tool | Bibliometric software for co-authorship and trend analysis. |
| Class 1—DNA Repair and Molecular Response | Class 1 Category | Focused on DNA damage signaling, repair pathways (HR, NHEJ, NER, BER), and checkpoint activation; aligns molecular biology with therapeutic personalization. |
| Class 2—Precision Oncology and Genomic Modeling | Class 2 Category | Integrates genomic profiling, machine-learning models, and patient stratification to personalize radiation therapy. |
| Class 3—Individual Radiosensitivity and Clinical Risk | Class 3 Category | Addresses variability in patient radiation response, genetic predisposition, and predictive biomarkers. |
| Class 4—Radioresistance and Associated Mechanisms | Class 4 Category | Explores resistance mechanisms (hypoxia, metabolism, stemness, viral integration) and corresponding therapeutic interventions. |
| Class 5—Advanced Technologies and Innovative Radiotherapy | Class 5 Category | Encompasses next-generation technologies including FLASH-RT, hadron therapy, voxel-based analysis, and high-Z nanoparticle radiosensitization. |
| CLINICAL/ONCOLOGY/RADIOBIOLOGY/MEDICAL/DEVELOPMENT/ELSEVIER/RIGHT/RESERVED | Publishing or Institutional Terms | Topic_8a includes frequent non-scientific tokens derived from editorial metadata, likely due to text-parsing of abstracts from publishers (Elsevier, etc.). |
| Clinical trial phase | Trial stage indicator | Used to filter translational maturity of studies. |
| Clinical–Anatomical Axis | Axis describing patient- and site-specific features; dimension grouping disease sites, therapies, and outcomes | Encompasses disease sites, therapy types, outcomes; derived from topic modeling to describe clinical themes. |
| Clustering coefficient | Likelihood that connected nodes form closed triangles | High value shows local semantic cohesion. |
| Co-Occurrence Supergraph | Graph of term co-appearances; combined network of all significant co-occurring terms | Reveals structural relationships among concepts in the literature; integrates molecular, clinical, and technological vocabularies into one semantic structure. |
| Combined modality | Multimodal treatment (e.g., RT + IO) | Inclusion term bridging lab and clinic. |
| COMPLETE (trial) | HolistiC early respOnse assessMent for oroPharyngeaL cancEr paTiEnts | Protocol integrating imaging and early-response assessment in oropharyngeal cancer. |
| COMPLETE Protocol | Combined Multiparametric Imaging, Radiomics and Machine-Learning Framework | Integrates imaging and omics data for personalized radiotherapy planning. |
| Computational Oncology | Field Integrating AI and Cancer Research | The disciplinary frame under which the study’s integrative modeling approach is positioned. |
| COPPE | Instituto Alberto Luiz Coimbra de Pós-Graduação e Pesquisa de Engenharia | UFRJ’s graduate engineering institute; associated with the Nanotechnology Engineering Program. |
| Cosine Similarity | Vector-Space Similarity Measure | Quantifies the semantic and lexical overlap between two text representations (range 0–1). Used here to assess alignment between outputs of different LLMs. |
| Cost per Controlled Case | Economic Indicator | Total treatment cost divided by number of patients with complete or partial sustained response; measures cost-effectiveness. |
| COVID-19 | Coronavirus Disease 2019 | Systemic factor influencing radiosensitivity and patient management. |
| Cross-Model Consistency | Comparative Validation Concept | Indicates methodological reliability by reproducing semantically equivalent results across distinct LLM architectures. |
| CSV | Comma-Separated Values | Log format for timestamped model inferences and auditing. |
| CTCAE | Common Terminology Criteria for Adverse Events | Global clinical-trial standard for grading radiotherapy-related adverse events. |
| Data Repositories | Centralized Databases for Clinical and Molecular Information | National archives enabling interoperability and machine-learning-based outcome prediction. |
| DAY | Treatment Day/Fraction Day | Represents time variable in radiotherapy fractionation (Topic_7a). |
| Decision-Ready Framework | Actionable Computational Infrastructure | Describes the output: a structured knowledge base usable for policy, funding, and clinical translation. |
| Deduplication | Removal of duplicate entries | 594 records removed by ID and 25 by title → 3343 unique publications. |
| Density | Ratio of existing to possible edges; ratio of actual to possible edges | 0.5 in the directed setup, signifying a tightly connected lexical field; Indicates a dense and cohesive co-occurrence network. |
| DER/SER/RBE | Dose Enhancement Ratio/Sensitization Enhancement Ratio/Relative Biological Effectiveness | Quantitative radiobiological indices comparing biological responses between radiation modalities. |
| Diameter | Longest shortest path in the network; Longest shortest-path length | Here equals 1 due to directed adjacency; reflects condensed connectivity; reflects immediate connectivity among principal terms in a directed adjacency setup. |
| Directed/Weighted Graph | Graph with edge orientation and numerical weights | Models term co-occurrence frequency and directionality in the semantic network. |
| DNA | Deoxyribonucleic Acid | Central mechanistic concept related to damage, repair, and radiosensitivity. |
| DNA Repair | Deoxyribonucleic Acid Repair | Core biological process for maintaining genomic integrity after radiation exposure; major research theme representing molecular response to radiation. |
| DOI | Digital Object Identifier | Persistent identifier ensuring record uniqueness and traceability. |
| Dose–Response Model | Quantitative relation between dose and effect; mathematical link between dose and biological effect | Appears among inclusion keywords; supports mechanistic theme; underpins LDA topics and clinical correlation analysis. |
| E2 | HPV Regulatory Protein | Loss disrupts viral genome control; restoration of E2-associated checkpoints observed with carbon-ion irradiation. |
| Edge Thickness | Graph-visualization parameter | Increases proportionally with co-occurrence weight. |
| Editorial Workflow (“Llama → GPT-4o”) | Sequential Model Use | Denotes the pipeline where Llama3 generated drafts and GPT-4o refined phrasing, without altering data or analytic interpretations. |
| Effect | Biological or Clinical Outcome | Used to represent both therapeutic effects and adverse effects of radiation exposure. |
| ENERGY | Beam Energy | Physical parameter determining penetration depth and RBE of particle beams (Topic_2a). |
| EORTC | European Organisation for Research and Treatment of Cancer | European body with harmonized toxicity and clinical-trial reporting frameworks. |
| Eq. | Equation | Used to identify model equations from Equations (A11)–(A20). |
| Equations (A1)–(A20) (Appendix A and Appendix B) | Numerical LDA Output Tables | Provide word probabilities and model parameters for transparency. |
| Equity/Access Metrics | Health Policy Indicators | Includes survival, toxicity, cost per controlled case, and hospitalizations for public health alignment. |
| EXPRESSION/GENE/PROTEIN/BLOOD | Biomarker-related Terms | In Topic_6a, these describe molecular and clinical biomarkers used for radiosensitivity prediction. |
| Factor | Biological or Statistical Factor | Represents either biological modifiers (e.g., hypoxia) or regression factors in predictive models. |
| FANCA | Fanconi Anemia Complementation Group A | Germline carrier state linked to hypersensitivity to chemoradiation. |
| Feasibility/Translational Potential | Applied Assessment Metrics | Used to evaluate how physical models and clinical data can be realistically implemented within current technological and clinical constraints. |
| Feature Space | Multidimensional Representation of Texts | Conceptual domain in which cosine similarity evaluates orientation and semantic overlap between document vectors. |
| FIU | Florida International University | U.S. collaborator institution for computational and AI analysis. |
| FLASH | Ultrafast Radiotherapy (FLASH-RT); Ultrafast radiotherapy at ultra-high dose rates; ultrafast (>40 Gy s−1) radiation-delivery technique; Ultrafast (≥40 Gy s−1) radiotherapy | High-dose-rate radiation mode producing reduced normal-tissue toxicity; investigated for normal-tissue sparing and tumor control; one of the advanced technologies highlighted in Class 5; modern modality delivering high-dose-rate pulses with reduced normal-tissue damage. |
| FLASH Radiotherapy and Brachytherapy | Advanced Techniques | Explored for synergistic effects in tissue protection and immune modulation. |
| FRACTION | Dose Fraction | In Topic_9a, refers to division of total dose into multiple treatment sessions—key variable in radiobiological modeling. |
| Fraction/Fractionation | Division of Total Radiation Dose into Multiple Sessions | Appears among frequent title/abstract terms; optimization variable flagged by abstracts analysis. |
| Frequency Trimming | Vocabulary Cutoff Procedure | Eliminates extremely rare or overly common tokens. |
| G ≥ 3 | Grade 3 or Higher Toxicity | Severe adverse-event category per CTCAE or RTOG/EORTC scales; measures safety profile. |
| GARD | Genomic Adjusted Radiation Dose | Integrates RSI and gene-expression data to personalize dose prescription. |
| Genomic/Proteomic Modeling | Molecular Data Integration Approaches | Computational modeling of genomic (gene-level) and proteomic (protein-level) datasets to predict radiosensitivity and outcomes. |
| Genomic Classifier | Model Assigning Molecular Subtypes | Used for precision oncology decisions. |
| Genomic Modeling | Predictive Modeling Based on Genetic Data; Mechanistic to Machine-Learning Frameworks | Used for individualized therapy planning in precision oncology; used to personalize radiotherapy (predict response/toxicity). |
| Gephi | Open-source Network-Analysis Software | Used for calculating density, diameter, clustering, and modularity of the co-occurrence network. |
| GPT-4o | OpenAI’s Multimodal Large Language Model (Omni Version) | Used to refine language, maintain consistency, and ensure terminological precision; served only in editorial and validation steps. |
| GPT-5 | Generative Pretrained Transformer, Version 5 | Model used within ChatGPT for generative text refinement and consistency checking. |
| Grammarly (v.2025) | AI-Powered English Language Writing Assistant | Utilized for grammatical and stylistic correction to ensure readability for international readers. |
| Gy | Gray | SI unit of absorbed radiation dose (1 Gy = 1 J/kg); frequent in topics 4a, 7a, 9a related to dosimetry and toxicity. |
| Gy/Gy s−1 | Gray/Gray per second | SI units of absorbed dose and dose rate. |
| H2O2 | Hydrogen Peroxide | Central to radiosensitization concepts exploiting oxidative effects (low-LET contexts). |
| Hadron Therapy | Particle-Beam Radiotherapy (Protons, Carbon Ions); Particle-Based Radiotherapy (Using Protons or Heavy Ions) | Emerging high-precision radiation technique; advanced technology referenced in the framework as part of “Innovative Radiotherapy Technologies.” |
| Head and Neck/Breast/Prostate Cancer | Disease-Site Descriptors | Define clinical–anatomical axes in Topic_0t and related clusters. |
| Heavy Charged Particles | Proton/Helium/Carbon/Oxygen Ion Beams | “Next-generation multi-scale” characterization and high-LET effects. |
| HIF | Hypoxia-Inducible Factor | Transcription factor activated by VHL loss; mediates hypoxia-related radioresistance. |
| High-End Modalities | Advanced Treatment Technologies | Includes FLASH-RT, hadron therapy, and other precision radiotherapy innovations. |
| High-LET | High Linear Energy Transfer | Radiation that deposits dense energy tracks (e.g., heavy ions); generates complex DNA lesions. |
| High-LET Modalities | Heavy-Ion or Particle Therapies | Used in Class 4 to reverse resistant phenotypes via dense-ionization damage. |
| High-LET Radiation | High Linear Energy Transfer Radiation | Mentioned indirectly in prior results, relevant here as part of the “system-level dynamic modeling” refinement. |
| High-Z | High Atomic-Number Elements | Nanoparticles used to enhance dose deposition and radiosensitization. |
| High-Z Nanoparticles | High Atomic-Number Nanomaterials | Act as radiosensitizers by amplifying local dose and reactive-species generation. |
| High-Z Nanotechnologies | High Atomic Number Nanomaterials | Proposed future pathway to couple with ultrafast radiotherapy for dose amplification and radiosensitization. |
| High-Z Platforms | High Atomic Number Platforms | Refers to nanomaterials designed to enhance radiotherapy through dose amplification. |
| Hospitalization Rate | Frequency of Radiotherapy-Related Admissions | Indicator of adverse-event management effectiveness. |
| HPV | Human Papillomavirus | Oncogenic virus associated with cervical-cancer radioresistance; carbon-ion therapy shown to overcome it. |
| HPV/E2 | Human Papillomavirus/Regulatory Gene | Integration/E2 disruption associated with cervical radioresistance; carbon ions reported to overcome it. |
| HR | Homologous Recombination | Accurate double-strand-break repair pathway; its inhibition increases radiosensitization. |
| Hybrid Modeling/Model Fusion | Combined Output Strategy | Not used in this study; models were compared for agreement, not merged to produce shared outputs. |
| Hypotheses 1–4 | Structural Inferences Derived from Topology | Define conceptual axes: (1) cancer + radiotherapy centrality; (2) personalization trends; (3) biomarker emphasis; (4) transversal field integration. |
| ID Numbers | Unique Database Identifiers (e.g., PMID, DOI, EID) | Used for automated deduplication across sources. |
| IIPPR | Integrated Implementation Plan in Precision Radiotherapy | The overarching operational and translational framework structuring radiotherapy research into five classes with progressive integration into health policy. |
| IMA | Instituto de Macromoléculas Professora Eloisa Mano | UFRJ institute specializing in polymers and macromolecular science. |
| Imaging | Medical Imaging | Used for diagnosis, planning, and verification in radiotherapy (e.g., MRI, CT, PET). |
| Immunoradiotherapy | Radiotherapy Combined with Immune Modulation | Emergent interdisciplinary theme. |
| Immunotherapy | Immune-Based Cancer Therapy | Appears in Topic_5a; integration of radiation with immunotherapy strategies. |
| In vitro | Laboratory-Based Experiments | Denotes cellular studies that feed mechanistic insight. |
| Individualized Radiosensitivity Assessment | Patient-Specific Radiation Response Evaluation | Quantifies how individual genetic and biological profiles modify treatment tolerance and efficacy. |
| Integrated Implementation Plan in Precision Radiotherapy (IIPPR) | Translational Framework | Unifies molecular, clinical, and technological insights into five interdependent operational blocks for precision oncology and public-health integration. |
| Interdisciplinary Maturity | Cross-Disciplinary Integration | Refers to the field’s evolution into a coherent system combining biology, physics, and clinical practice. |
| Ion | Ion Beam | Refers to charged particle beams (proton, helium, carbon, oxygen) used in particle radiotherapy. |
| ION/PARTICLE/PROTON/CARBON/PHOTON | Ion-Beam and Particle Radiation Terms | Terms appearing together in Topic_2a; describe physical particles used in hadron therapy. |
| Irradiation | Exposure to Ionizing Radiation | Common in Topics 6a and 7a; refers to experimental or clinical exposure processes. |
| k = 10 Topics | Number of LDA Topics | Defines model granularity; ten topics selected for interpretability and stability. |
| Kidney Cancer VHL/HIF Axis | von Hippel–Lindau/Hypoxia-Inducible Factor | Mechanistic route to pseudohypoxia and resistance. |
| KORTUC II | Kochi Oxidative Radiotherapy for Unresectable Carcinomas Type II | Technique using hydrogen peroxide (H2O2) to increase tumor oxygenation and radiosensitivity. |
| LaBioS | Laboratório de Biopolímeros e Sustentabilidade | Laboratory supporting the experimental and organizational phases of the study. |
| LabOPTIMA | Laboratory for Optimization, Data Analytics, and Artificial Intelligence in Materials Science | UFRJ-based lab responsible for data-centric analysis and computational modeling; mentioned as a contributor to data processing and validation. |
| LDA | Latent Dirichlet Allocation | Unsupervised topic-modeling algorithm used for semantic extraction of dominant research axes. |
| LDR | Low-Dose-Rate | Brachytherapy context (e.g., prostate cancer TCP modeling). |
| LDR Brachytherapy Sources 60Co/192Ir | Cobalt-60/Iridium-192 | Compared radiobiologically (e.g., cervical cancer HDR; RBE differences). |
| Lemmatization | Word-Form Normalization | Reduces inflectional variants to base forms for stable modeling. |
| LET | Linear Energy Transfer | Energy deposition per track length; key in radiobiology and particle therapy. |
| LEVEL | Expression Level/Dosimetric Level | Indicates quantitative measurement of gene, protein, or dose levels (Topic_6a). |
| LINE | Cell Line | Experimental model system used for in vitro radiation studies (Topic_1a). |
| Linear | Linear Model (Linear-Quadratic Model) | Refers to the linear component of the LQ model used to describe cell survival curves in radiotherapy modeling. |
| Llama-3 (8B) | Large Language Model (8 Billion Parameters); Open-Source Large Language Model (8 Billion Parameters) | Referenced as a semantic-processing model used during analysis, highlighting technical limitations and multilingual challenges; used for initial draft generation and offline semantic analysis; suitable for secure, local computation. |
| LLM | Large Language Model | AI model trained on massive text corpora; in this study, Llama-3 (8B) and GPT-4o were compared. |
| LLMs | Large Language Models | Used for semantic synthesis under fixed prompts; outputs logged for auditability; models (like GPT-type) used in PaperProcessor for semantic synthesis and summarization. |
| Local Control/Systemic Control | On-Site vs. Distant Tumor Suppression | Metrics evaluating therapeutic success at tumor site and metastasis prevention. |
| Machine Learning | AI Technique for Pattern Recognition and Prediction | Supports risk stratification, biomarker identification, and outcome modeling. |
| Mean (μ) | Arithmetic Average | Represents central tendency of similarity values (~0.52 overall). |
| Mean Clustering Coefficient = 0.5 | Local Cohesion Metric | Reveals that many terms form tightly knit clusters (≈1140 triangles). |
| Mechanism/Molecular | Mechanistic and Molecular Basis | Indicates interest in the cellular and sub-cellular mechanisms of radiation injury and repair. |
| Mechanistic–Molecular Axis | Axis Describing Biological Mechanisms; Dimension Encompassing DNA Repair and Biomarkers | Encompasses cellular responses, DNA repair, and biomarkers; describes mechanistic research focus across decades. |
| Median Cohort | Summary Statistic in Cohort Analyses | Appears in abstract-level signals of methodological layer. |
| MeSH | Medical Subject Headings | Controlled vocabulary in PubMed employed for precise field-restricted searching. |
| Metadata | Supplementary Bibliographic Descriptors | Retained for reproducibility and cross-referencing (authors, year, source title, etc.). |
| MicroRNAs/Cytokines | Small Regulatory RNAs/Signaling Proteins | Linked to radiation toxicity and inflammatory responses in prostate cancer. |
| MMRd | Mismatch Repair Deficiency | Genetic defect leading to hypermutation; associated with increased immunogenicity and favorable prognosis. |
| Model | Computational or Predictive Model | Refers to mathematical frameworks (e.g., dose-response, LQ, biological modeling). |
| Modularity | Measure of Community Segregation; Community-Segregation Index | Low modularity indicates overlapping thematic communities; very low value (≈0.02) shows themes overlap strongly with one weakly connected component. |
| MOUSE | Animal Model | Indicates in vivo preclinical testing in mice (Topic_7a). |
| Multi-Omic Biomarkers | Combined Genomic, Transcriptomic, and Proteomic Indicators | Used to stratify patients and personalize treatment strategies. |
| Multilingual Biomedical Corpora | Multi-Language Scientific Datasets | Describes the heterogeneity of textual sources posing semantic-alignment challenges. |
| n | Sample Size | Number of text pairs analyzed per class (Class 1: n = 6; Class 2: n = 6; Class 3: n = 18; Class 4: n = 4; Class 5: n = 3). |
| NER | Nucleotide Excision Repair | DNA repair process that removes bulky helix-distorting lesions; important for radiation and oxidative stress responses. |
| NER/SER/DER Metrics | Nucleotide Excision Repair/Sensitization Enhancement Ratio/Dose Enhancement Ratio | Quantitative parameters for evaluating biological amplification of radiation effects. |
| NHEJ | Non-Homologous End Joining | Fast, error-prone DNA repair mechanism; hyperactivation linked to radioresistance. |
| no_below = 2–5 /no_above = 0.5 | Frequency-Trimming Parameters | Filter words appearing in fewer than 2–5 articles or in >50 % of texts, ensuring vocabulary stability. |
| Node/Edge | Vertex/Connection | Represent keywords and their co-occurrence relations. |
| Normal | Normal Tissue | Opposed to tumor tissue; represents non-targeted biological matter whose protection is critical in treatment planning. |
| Normalization/Standardization | Data-Cleaning Processes | Harmonized author, title, and metadata formats across databases before merging. |
| NSCLC | Non-Small Cell Lung Cancer | Disease context in studies on radiotherapy benefits. |
| NSMP | No Specific Molecular Profile | Endometrial cancer subtype lacking defined genomic markers; used for risk-based treatment calibration. |
| O | Overall (e.g., Overall Survival—OS) | Shortened reference to clinical endpoints (Topic_3a); likely from expressions such as “overall survival.” |
| Offline Analyses | Local Computation Without Internet Connectivity | Performed using Llama-3 for data security, sovereignty, and reproducibility. |
| Omics Data | Integrated Genomic, Transcriptomic, Proteomic Datasets | Used across classes for multi-scale modeling of tumor behavior. |
| Oncology | Medical Field of Cancer Diagnosis and Treatment | Provides the clinical component in the interdisciplinary framework. |
| OpenAI | Artificial Intelligence Research Organization | Developer of ChatGPT; referenced for model attribution. |
| OS | Overall Survival | Proportion of patients alive at specified follow-up times (1/3/5 years) after radiotherapy. |
| P | p-value (Statistical Significance) | Appears in Topic_3a and 4a; denotes statistical significance in clinical or survival analyses. |
| PaperProcessor | LLM-Guided Semantic Extraction Script | Pipeline component for document-level summarization and tagging. |
| Parameter/Radiobiological | Model Coefficients/Domain Qualifier | Signal the quantitative calibration layer in abstracts. |
| Particle Therapy | Proton or Heavy-Ion Radiation Treatment | High-precision, high-LET modality for deep or radioresistant tumors. |
| passes = 10 | Number of LDA Training Iterations | Controls convergence during topic-model optimization. |
| Pathway | Biological Pathway | Indicates molecular signaling cascades affected by radiation (e.g., DNA damage-repair pathways). |
| Pearson Correlation Coefficient | Statistical Measure (r); Statistical Correlation Index | Indicates robustness of network stability across runs (r > 0.9); confirms stability of node/community ordering between directed and undirected graphs. |
| PENt | Programa de Engenharia da Nanotecnologia (Nanotechnology Engineering Program, COPPE/UFRJ) | Academic home of the AI and semantic-modeling pipeline described. |
| Peripheral Nodes | Lower-Degree Vertices at Network Edges | Terms like proton, stereotactic, imaging anchor technology subfields. |
| Personalized/Protocol | Individualized Plans/Standardized Procedures | Bridge terms in co-occurrence graphs linking themes. |
| PFS | Progression-Free Survival | Interval during which a patient remains free from tumor progression or recurrence. |
| PLAN/DOS | Treatment Planning and Dosimetry | In Topic_7a, “plan” and “dos” (truncated for dose or dosimetry) refer to planning and dose-distribution modeling. |
| PNG | Portable Network Graphics | Raster format for graphical visualization outputs. |
| POLE | DNA Polymerase Epsilon (Mutated Subtype) | Mutation defining a favorable genomic subtype in endometrial cancer; used in precision oncology stratification. |
| PORTEC-3/PORTEC-4 | Post-operative Radiation Therapy for Endometrial Carcinoma Trials | Landmark clinical studies incorporating molecular subtyping into radiotherapy decision frameworks. |
| Precision Oncology | Data-Driven Personalized Cancer Care; Data-Driven Personalized Cancer Treatment | Cross-cutting theme linking biomarkers to clinical protocols; integrates molecular profiling and computational modeling to guide clinical decisions. |
| Precision Radiotherapy | Data-Guided, Patient-Specific Treatment Framework | Conceptual endpoint of the integrated ecosystem. |
| Precision Radiotherapy Corpus | Consolidated Dataset | Final ≈ 45 deduplicated records informing thematic and network analyses. |
| Precision Radiotherapy Implementation Plan (PRIP) | Operational Framework; AI-Derived Operational Framework | Practical output of the study—semantic and molecular insights into five actionable clinical classes; translates computational and molecular insights into clinical strategies. |
| Preclinical | Preclinical Study; Experimental (Non-Clinical) Phase | Experimental phase prior to human clinical trials; linked to animal or in vitro testing (Topic_5a); denotes laboratory or animal studies preceding human trials. |
| Prediction/Response/Normal | High-Centrality Nodes | “Semantic hinges” connecting mechanistic and clinical themes in the supergraph. |
| Prediction Models | Computational or Statistical Outcome-Forecasting Tools | Represent the data-driven dimension emerging after 2010. |
| Predictive Systems | AI-Based Forecasting Models | Refer to evolving models that anticipate treatment response and guide dynamic planning. |
| Progression-Free Interval | Time to Recurrence | Quantitative measure for disease stability post-therapy. |
| Proteomics | Large-Scale Protein Analysis | Identifies functional markers of radioresistance and therapeutic targets. |
| Proton/Ion Beams | Particle-Beam Radiotherapy Modalities | Core of hadron-therapy and dose-delivery-physics studies. |
| Proton Therapy | Particle-Based Radiotherapy; Proton Therapy; Charged-Particle Radiation Modality | Advanced technique using proton beams for accurate dose delivery with minimal collateral damage; part of the technological wave correlated with publication surge. |
| Public Health Integration | Application of Precision Frameworks to Health-System Policies | Links AI-guided radiotherapy models to equity and access. |
| PubMed | U.S. National Library of Medicine Database (MEDLINE) | Primary biomedical source queried with MeSH and free-text terms; provided ≈ 66% of final records. |
| PubMed, Scopus, Web of Science (WoS) | Scientific Databases | Used for bibliometric data collection and harmonization. |
| Quantitative Metrics | Numerical Parameters or Indicators | Include dose, LET, RBE, similarity scores, or statistical validation values supporting model precision. |
| RAD51/BRCA1 | DNA Repair Genes | Over-expression correlates with radioresistance; potential biomarkers for therapeutic targeting. |
| RAD51, PARP1, CHK1, MAPK15 | Key DNA Repair/Stress Response Proteins | Biomarkers and therapeutic targets integrated into proteomic and genomic modeling layers. |
| Radiat/Cell/Dose/Tumor/Patient/Cancer/Radiobiolog | High-Frequency Lexical Stems | Most recurrent words in the processed corpus; define thematic backbone. |
| Radiobiological | Related to Radiation Biology | Describes models or parameters linking radiation dose to biological effects. |
| Radiobiological Integration | Coupling Biological Models and Clinical Protocols | Incorporates biological parameters (e.g., radiosensitivity indices) into planning. |
| Radiobiological Modeling | Quantitative Biological Modeling of Dose–Response | Used to predict tissue effects and optimize treatment. |
| Radiobiology | Study of Biological Effects of Ionizing Radiation | Core discipline analyzed alongside radiotherapy and oncology. |
| Radiomics | Quantitative Image-Feature Extraction | Supports predictive modeling and risk assessment integrated into adaptive radiotherapy. |
| Radioresistance Mechanisms | Molecular and Cellular Resistance Pathways | Refers to signaling and repair mechanisms that make tumor cells less responsive to radiation. |
| Radiotherapy (RT) | Use of Ionizing Radiation to Treat Cancer | Central disciplinary axis in all queries. |
| RadRes | Radiation Research (Journal) | Appears in a title summarizing 75 years of the field. |
| random_state = 42 | Random-Seed Setting | Ensures reproducible topic-model results. |
| Rate (Dose-Rate) | Dose Per Unit Time | Critical radiobiological variable, particularly relevant for FLASH-RT and LDR contexts. |
| RBE | Relative Biological Effectiveness | Quantifies biological potency of one radiation type relative to another. |
| Repair | DNA or Cellular Repair | Biological process restoring damaged DNA or cellular integrity after irradiation. |
| Reproducibility | Methodological Consistency; Methodological Standards | Refers to achieving consistent quantitative and terminological results across independent AI systems; ensures that pre-processing and modeling steps can be independently verified. |
| RESPONSE | Biological or Clinical Response | Describes radiation-induced effects, gene-expression responses, or treatment outcomes. |
| Risk/Volume | Dosimetric and Anatomical Covariates | Terms indicating planning and outcome modeling foci. |
| RSI | Radiosensitivity Index | Genomic predictor of individual radiosensitivity used for dose calibration. |
| RT | Radiotherapy | Appears in several topics and class definitions. |
| RTOG | Radiation Therapy Oncology Group | U.S. co-operative group providing standardized toxicity and outcome reporting criteria. |
| SARS-CoV-2/COVID-19 | Virus/Disease | Examined for potential changes in individual radiation sensitivity. |
| SBRT | Stereotactic Body Radiotherapy | Highly conformal, high-precision modality for small or lung tumors; advanced form of external beam radiotherapy delivering high-precision doses to small tumor volumes (directly referenced in Topic_4t). |
| Scopus | Elsevier’s Multidisciplinary Abstract and Citation Database | Used for automated retrieval of bibliographic records on radiotherapy, radiobiology, and oncology. |
| Scopus/PubMed/Web of Science (WoS) | Major Bibliographic Databases | Primary data sources for corpus construction (1964–2025); used for refining, validating, and harmonizing corpus selection. |
| SD/± | Standard Deviation | Expresses the dispersion of cosine-similarity values within each class. |
| Semantic Supergraph Analysis | Advanced Network Modeling Approach | Suggested future technique for identifying underexplored or emerging clusters within the scientific corpus. |
| Stereotactic Body Radiotherapy (SBRT) | High-Precision, Image-Guided Radiation Technique | Marks transition to modern radiotherapy (post-2010 growth phase). |
| Stopword Filtering | Removal of High-Frequency Function Words | Biomedical-specific list applied before modeling. |
| Supergraph | Global Integrated Co-Occurrence Network | Encompasses molecular, clinical, and technological domains. |
| Supergraph Inset | Central Visual in Figure 1 | Depicts term relationships with colored weighted edges (blue = weak, red = strong). |
| SURVIVAL | Cellular or Patient Survival | Appears in Topics 1a and 3a, referring to both cell-survival assays and patient outcome metrics. |
| SUS/Unified Health System (SUS) | Sistema Único de Saúde (Brazil’s Unified Health System) | Framework for pilot implementation, ensuring scalability, equity, and cost-effectiveness; Brazil’s public-health system alignment target. |
| SVG | Scalable Vector Graphics | Visualization format for network maps. |
| t | Titles Corpus | The suffix “t” in Topic_0t–Topic_9t designates topics derived from article titles rather than abstracts. |
| Table 3/Figure 4 | Plan Visualization Elements | Table summarizes indicators; figure visualizes network linking plan classes, health metrics, and strategic goals. |
| TARGET | Radiation Target Volume | Anatomical or biological region receiving prescribed radiation dose (Topic_7a). |
| TARGETED (THERAPY) | Molecularly Targeted Therapy | Used in Topic_5a; describes agents designed to act on specific molecular pathways. |
| TCGA | The Cancer Genome Atlas | Genomic classification system defining molecular subtypes across cancers; applied for adaptive radiotherapy. |
| TCP/NTCP | Tumor Control Probability/Normal Tissue Complication Probability | Radiobiological models estimating treatment success vs. toxicity. |
| Temporal Evolution Curve | Publication-Frequency Over Time | Depicts output growth and term-usage expansion (sharp post-2010 increase). |
| Term-Frequency Curve | Temporal Plot of Keyword Occurrence | Used to link bibliometric evolution to network topology. |
| TF-IDF | Term Frequency–Inverse Document Frequency | Weighting scheme representing articles numerically for cosine-similarity computation; emphasizes discriminative words. |
| Therap/Radiotherapi (Radiotherapy Lemmatized Form) | Lemmatized Word Stems | Generated during text preprocessing before word-cloud and LDA analysis. |
| Therapeutic Window | Efficacy–Toxicity Balance | Keyword guiding selection of translationally relevant articles. |
| Tissue/Normal | Biological Material (Tumor vs. Normal) | Distinction between targeted tumor tissue and healthy tissue, relevant to toxicity models (Topic_9a). |
| TITLE-ABS-KEY | Title–Abstract–Keyword Query Field in Scopus | Syntax specifying that Boolean terms be searched in all three metadata fields. |
| Topic_0a–Topic_9a | Abstract-Based LDA Topics | Capture methodological and biological dimensions (e.g., FLASH-RT, RBE, immunotherapy). |
| Topic_0t–Topic_9t | Title-Based LDA Topics | Identify clusters such as tumor sites, radiation types, or molecular mechanisms; each topic represents a statistically derived keyword cluster from titles. |
| Toxicity | Measure of Treatment-Induced Adverse Effects | One of the outcome indicators for therapy optimization. |
| TP53/p53abn | Tumor Protein 53/Abnormal TP53 Subtype | Gene regulating cell cycle and apoptosis; mutation indicates poor prognosis in endometrial and cervical cancers. |
| Translational Continuity | Bridging Experimental and Clinical Domains | Describes how findings progress from bench to bedside within a single analytical cycle. |
| Translational Publication Types/Translational Trials | Research Bridging Lab and Clinic/Studies Bridging Lab Findings and Clinical Implementation | Filters applied in PubMed to maximize relevance; reflected by terms “clinic,” “trial,” “meta,” “evalu.” |
| Transparency | Disclosure of AI Tool Use | Explicitly described to comply with academic-integrity and reproducibility standards. |
| Two-Dimensional Thematic Landscape | Dual-Axis Conceptual Map | Represents integration of clinical and molecular radiotherapy research. |
| UFRJ | Universidade Federal do Rio de Janeiro (Federal University of Rio de Janeiro) | Institutional affiliation of multiple authors. |
| Undirected, Thresholded Version | Simplified Graph with Bidirectional Edges and Minimum-Weight Cutoff | Used to test robustness of results. |
| VHL | von Hippel–Lindau Gene | Mutation induces pseudohypoxia and pro-survival signaling in renal carcinoma. |
| Vitro (from in vitro) | In Vitro Studies | Laboratory studies conducted outside living organisms, often in cell cultures, used to study radiation-response mechanisms. |
| VOSviewer | Visualization of Similarities Viewer | Software for bibliometric and co-occurrence network visualization. |
| Voxel/Voxel-Based Analysis/Voxel-Based Mapping/Voxel-Level Analytics | Volumetric Pixel and Spatial Dose–Response Modeling Methods | Voxel = smallest unit in 3D medical imaging and dosimetry; used for dose/response mapping and adaptive planning; links radiobiological effects to 3-D anatomical regions for spatially resolved optimization. |
| Weakly Connected Component | Subgraph with at Least One Directional Path Between All Nodes | Indicates overall semantic unity of the dataset. |
| Web of Science (WoS) | Clarivate’s Multidisciplinary Citation Index | Used with direct keyword search to complement PubMed and Scopus coverage. |
| Weighted Degree | Sum of Edge Weights for a Node | Counts total co-occurrence frequency rather than binary presence. |
| Word Cloud | Frequency-Scaled Visual Representation of Keywords | Summarizes lexical prominence from titles and abstracts. |
| α-Parameter | Linear Component of the LQ Model | Represents cell-killing probability per unit dose in radiobiological modeling. |
| α/β Values | Linear–Quadratic Model Parameters | Describe tissue-specific radiation response; used in pediatric and comparative modeling. |
| γH2AX | Phosphorylated Histone H2AX | Biomarker of DNA double-strand breaks; persistence indicates inefficient repair. |
Appendix A. Topics from Titles (t)
Appendix B. Topics from Abstracts (a)
References
- Song, Z.; Hwang, G.-Y.; Zhang, X.; Huang, S.; Park, B.-K. A Scientific-Article Key-Insight Extraction System Based on Multi-Actor of Fine-Tuned Open-Source Large Language Models. Sci. Rep. 2025, 15, 1608. [Google Scholar] [CrossRef] [PubMed]
- Dritsas, E.; Trigka, M. Exploring the Intersection of Machine Learning and Big Data: A Survey. Mach. Learn. Knowl. Extr. 2025, 7, 13. [Google Scholar] [CrossRef]
- Elahi, M.M.A.; Hossain, M.; Karim, M.R.; Zain, M.F.M.; Shearer, C. A Review on Alkali-Activated Binders: Materials Composition and Fresh Properties of Concrete. Constr. Build. Mater. 2020, 260, 119788. [Google Scholar] [CrossRef]
- Shi, F.; Evans, J. Surprising Combinations of Research Contents and Contexts Are Related to Impact and Emerge with Scientific Outsiders from Distant Disciplines. Nat. Commun. 2023, 14, 1641. [Google Scholar] [CrossRef]
- Masseroli, M.; Mons, B.; Bongcam-Rudloff, E.; Ceri, S.; Kel, A.; Rechenmann, F.; Lisacek, F.; Romano, P. Integrated Bio-Search: Challenges and Trends for the Integration, Search and Comprehensive Processing of Biological Information. BMC Bioinform. 2014, 15, S2. [Google Scholar] [CrossRef]
- Hong, Z.; Ward, L.; Chard, K.; Blaiszik, B.; Foster, I. Challenges and Advances in Information Extraction from Scientific Literature: A Review. JOM 2021, 73, 3383–3400. [Google Scholar] [CrossRef]
- Vuković, D.B.; Dekpo-Adza, S.; Matović, S. AI Integration in Financial Services: A Systematic Review of Trends and Regulatory Challenges. Humanit. Soc. Sci. Commun. 2025, 12, 562. [Google Scholar] [CrossRef]
- Giamellaro, M.; Buxton, C.; Taylor, J.; Ayotte-Beaudet, J.-P.; L’Heureux, K.; Beaudry, M.-C. The Landscape of Research on Contextualized Science Learning: A Bibliometric Network Review. Sci. Educ. 2025, 109, 851–875. [Google Scholar] [CrossRef]
- Neumann, E.; Thomas, J. Knowledge Assembly for the Life Sciences. Drug Discov. Today 2002, 7, s160–s162. [Google Scholar] [CrossRef]
- Zheng, X.; Peng, P.; Wang, Y.; Bian, L.; Zhao, K.; Shi, A.; Jiang, Z.; Zhao, L.; Jiang, J.; Zhang, S. The Impact of Exercise during Radiotherapy on Treatment-Related Side Effects in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2025, 163, 104990. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Louie, A.V.; Kotecha, R.; Saxena, A.; Zhang, Y.; Guckenberger, M.; Kim, M.-S.; Scorsetti, M.; Slotman, B.J.; Lo, S.S.; et al. Stereotactic Body Radiotherapy for Non-Spine Bone Metastases: A Meta-Analysis and International Stereotactic Radiosurgery Society (ISRS) Clinical Practice Guidelines. Radiother. Oncol. 2025, 205, 110717. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.U.; Sun, Y.; Tree, A.C.; Hall, E.; Dearnaley, D.; Catton, C.N.; Lukka, H.R.; Pond, G.; Lee, W.R.; Sandler, H.M.; et al. Hypofractionated Radiotherapy for Prostate Cancer (HYDRA): An Individual Patient Data Meta-Analysis of Randomised Trials in the MARCAP Consortium. Lancet Oncol. 2025, 26, 459–469. [Google Scholar] [CrossRef]
- Javadnia, P.; Bahadori, A.R.; Ghanaatpisheh, A.; Dahaghin, S.; Rajabi, M.; Davari, A.; Sheikhvatan, M.; Ranji, S.; Shafiee, S.; Tafakhori, A. The Safety and Efficacy of Robotic Radiosurgery and Radiotherapy in the Management of Skull Base Tumors: A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2025, 48, 39. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.S.; Chow, R.; Benour, A.; Chen, D.; Boldt, G.; Wallis, C.J.D.; Swaminath, A.; Simone, C.B.; Lock, M.; Raman, S. Comparative Efficacy and Safety of Ablative Therapies in the Management of Primary Localised Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Lancet Oncol. 2025, 26, 387–398. [Google Scholar] [CrossRef]
- Guo, Y.; Hao, S.; Huang, Q.; Di, C.; Gan, L.; Xie, Y.; Li, Q.; Si, J. Unraveling the Dual Nature of FLASH Radiotherapy: From Normal Tissue Sparing to Tumor Control. Cancer Lett. 2025, 630, 217895. [Google Scholar] [CrossRef]
- Tan, V.S.; Padayachee, J.; Rodrigues, G.B.; Navarro, I.; Shah, P.S.; Palma, D.A.; Barry, A.; Fazelzad, R.; Raphael, J.; Helou, J. Stereotactic Ablative Radiotherapy for Oligoprogressive Solid Tumours: A Systematic Review and Meta-Analysis. Radiother. Oncol. 2024, 200, 110505. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.L.F.; Tos, S.M.; Shaaban, A.; Mantziaris, G.; Trifiletti, D.M.; Sheehan, J. Proton Beam and Carbon Ion Radiotherapy in Skull Base Chordoma: A Systematic Review, Meta-Analysis and Meta-Regression with Trial Sequential Analysis. Neurosurg. Rev. 2024, 47, 893. [Google Scholar] [CrossRef]
- Huo, X.; Zhao, X.; Liu, X.; Zhang, Y.; Tian, J.; Li, M. Treatment Options for Unilateral Vestibular Schwannoma: A Network Meta-Analysis. BMC Cancer 2024, 24, 1490. [Google Scholar] [CrossRef]
- Duckett, K.A.; Kassir, M.F.; Nguyen, S.A.; Brennan, E.A.; Chera, B.S.; Sterba, K.R.; Halbert, C.H.; Hill, E.G.; McCay, J.; Puram, S.V.; et al. Factors Associated with Head and Neck Cancer Postoperative Radiotherapy Delays: A Systematic Review and Meta-analysis. Otolaryngol.–Head Neck Surg. 2024, 171, 1265–1282. [Google Scholar] [CrossRef]
- Daloiso, A.; Cazzador, D.; Concheri, S.; Tealdo, G.; Zanoletti, E. Long-Term Hearing Outcome For Vestibular Schwannomas After Microsurgery And Radiotherapy: A Systematic Review and Meta-Analysis. Otolaryngol.–Head Neck Surg. 2024, 171, 1670–1681. [Google Scholar] [CrossRef]
- Boterberg, T.; Dunlea, C.; Harrabi, S.; Janssens, G.; Laprie, A.; Whitfield, G.; Gaze, M. Contemporary Paediatric Radiation Oncology. Arch. Dis. Child. 2023, 108, 332–337. [Google Scholar] [CrossRef]
- Waqar, M.; Trifiletti, D.M.; McBain, C.; O’Connor, J.; Coope, D.J.; Akkari, L.; Quinones-Hinojosa, A.; Borst, G.R. Early Therapeutic Interventions for Newly Diagnosed Glioblastoma: Rationale and Review of the Literature. Curr. Oncol. Rep. 2022, 24, 311–324. [Google Scholar] [CrossRef]
- Kempson, I. Nanoparticle-Based Radiosensitization. Int. J. Mol. Sci. 2020, 21, 2879. [Google Scholar] [CrossRef]
- Sidibe, I.; Biau, J.; Graff, P. Carcinomes épidermoïdes de la cavité buccale et du pharyngo-larynx. Quels volumes ganglionnaires traiter en radiothérapie externe? Cancer/Radiothérapie 2019, 23, 696–700. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, T.; Robinson, L.; Newton-Hughes, A.; Strudwick, R. A Review of Visual Ethnography: Radiography Viewed through a Different Lens. Radiography 2019, 25, S9–S13. [Google Scholar] [CrossRef]
- Bleiker, J.; Morgan-Trimmer, S.; Knapp, K.; Hopkins, S. Navigating the Maze: Qualitative Research Methodologies and Their Philosophical Foundations. Radiography 2019, 25, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Clementel, E.; Eaton, D.J.; Greer, P.B.; Haworth, A.; Ishikura, S.; Kry, S.F.; Lehmann, J.; Lye, J.; Monti, A.F.; et al. A Virtual Dosimetry Audit—Towards Transferability of Gamma Index Analysis between Clinical Trial QA Groups. Radiother. Oncol. 2017, 125, 398–404. [Google Scholar] [CrossRef]
- Cosset, J.-M. L’aube de la radiothérapie, entre coups de génie, drames et controverses. Cancer/Radiothérapie 2016, 20, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Cai, D.; Zhong, Q.; Dou, W.; Yuan, B. Impact of Breast Size on Dosimetry and Radiobiology of VMAT Left-sided Breast-conserving Conventional Fractionation Radiotherapy under Setup Errors. J. Appl. Clin. Med. Phys. 2025, 26, e70151. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, R.; Shen, G.; Tuo, C.; Dong, Y.; Wang, W.; Zhang, M.; Tong, G.; Zhang, H.; Yuan, B.; et al. The Space Radiobiological Exposure Facility on the China Space Station. Astrobiology 2025, 25, 32–41. [Google Scholar] [CrossRef]
- Xu, T.; Liu, F.; He, J.; Xu, P.; Qu, J.; Wang, H.; Yue, J.; Yang, Q.; Wu, W.; Zeng, G.; et al. Leveraging Zebrafish Models for Advancing Radiobiology: Mechanisms, Applications, and Future Prospects in Radiation Exposure Research. Environ. Res. 2025, 266, 120504. [Google Scholar] [CrossRef]
- Taliaferro, L.P.; Brenner, D.J.; Amundson, S.A.; Garty, G.; Zhang, Y.; Davies, E.W.; Carrier, F.; Ross, J.R.; Cline, J.M.; Chao, N.J. Centers for Medical Countermeasures against Radiation Consortium: Past, Present, and Beyond. Radiat. Res. 2025, 204, 238–252. [Google Scholar] [CrossRef]
- Rovituso, M.; Groenendijk, C.F.; Van Der Wal, E.; Van Burik, W.; Ibrahimi, A.; Rituerto Prieto, H.; Brown, J.M.C.; Weber, U.; Simeonov, Y.; Fontana, M.; et al. Characterisation of the HollandPTC R&D Proton Beamline for Physics and Radiobiology Studies. Phys. Medica 2025, 130, 104883. [Google Scholar] [CrossRef]
- Pouget, J.-P.; Gabina, P.M.; Herrmann, K.; Deandreis, D.; Konijnenberg, M.; Taieb, D.; Van Leeuwen, F.W.B.; Kurth, J.; Eberlein, U.; Lassmann, M.; et al. EANM Expert Opinion: How Can Lessons from Radiobiology Be Applied to the Design of Clinical Trials? Part I: Back to the Basics of Absorbed Dose–Response and Threshold Absorbed Doses. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 1210–1222. [Google Scholar] [CrossRef]
- Oliveira Dias, J.; Sampaio Fagundes, I.; Bisio, M.D.C.; Da Silva Barboza, V.; Jacinto, A.A.; Altei, W.F. Extracellular Vesicles as the Common Denominator among the 7 Rs of Radiobiology: From the Cellular Level to Clinical Practice. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2025, 1880, 189315. [Google Scholar] [CrossRef]
- Mendes, F.; Terry, S.Y.A.; Spiegelberg, D.; Cornelissen, B.; Bolcaen, J.; Nonnekens, J.; On behalf of the European Working Group of Radiobiology of Molecular Radionuclide Therapy. Recommendations for Reporting Preclinical Radiobiological Studies in Targeted Radionuclide Therapy. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 3066–3070. [Google Scholar] [CrossRef] [PubMed]
- Kazmierska-Grebowska, P.; Jankowski, M.M.; Obrador, E.; Kolodziejczyk-Czepas, J.; Litwinienko, G.; Grebowski, J. Nanotechnology Meets Radiobiology: Fullerenols and Metallofullerenols as Nano-Shields in Radiotherapy. Biomed. Pharmacother. 2025, 184, 117915. [Google Scholar] [CrossRef]
- Hill, M.A.; Silvestre Patallo, I.; Aitkenhead, A.H.; Bazalova-Carter, M.; Carter, R.; Nill, S.; Nisbet, A.; Ghita-Pettigrew, M.; Poirier, Y.; Prise, K.M.; et al. The Importance of Standardization and Challenges of Dosimetry in Conventional Preclinical Radiation Biology Research. Br. J. Radiol. 2025, 98, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Shang, Y.; Zhang, S.; Fan, S. The Interplay between RNA m6A Modification and Radiation Biology of Cancerous and Non-Cancerous Tissues: A Narrative Review. Cancer Biol. Med. 2025, 21, 1120–1140. [Google Scholar] [CrossRef]
- Charalampopoulou, A.; Barcellini, A.; Magro, G.; Bellini, A.; Borgna, S.S.; Fulgini, G.; Ivaldi, G.B.; Mereghetti, A.; Orlandi, E.; Pullia, M.G.; et al. Advancing Radiobiology: Investigating the Effects of Photon, Proton, and Carbon-Ion Irradiation on PANC-1 Cells in 2D and 3D Tumor Models. Curr. Oncol. 2025, 32, 49. [Google Scholar] [CrossRef]
- Azevedo, T.A.; Abrantes, A.M.; Carvalho, J. Radiobiological Modeling with Monte Carlo Tools—Simulating Cellular Responses to Ionizing Radiation. Technol. Cancer Res. Treat. 2025, 24, 15330338251350909. [Google Scholar] [CrossRef]
- Akolawala, Q.; Accardo, A. Engineered Cell Microenvironments: A Benchmark Tool for Radiobiology. ACS Appl. Mater. Interfaces 2025, 17, 5563–5577. [Google Scholar] [CrossRef]
- Abbasi, F.; Ghorbani, M.; Seif, F.; Deevband, M.R.; Pursamimi, M.; Babai, S.; Tavakoli, M. Comparison Study between Different Treatment Planning Techniques for Medulloblastoma in Terms of Dosimetry, Radiobiology, and Secondary Cancer Risk. J. Cancer Res. Ther. 2025, 21, 165–179. [Google Scholar] [CrossRef]
- Punshon, L.D.; Fabbrizi, M.R.; Phoenix, B.; Green, S.; Parsons, J.L. Current Insights into the Radiobiology of Boron Neutron Capture Therapy and the Potential for Further Improving Biological Effectiveness. Cells 2024, 13, 2065. [Google Scholar] [CrossRef]
- Gardner, L.L.; Thompson, S.J.; O’Connor, J.D.; McMahon, S.J. Modelling Radiobiology. Phys. Med. Biol. 2024, 69, 18TR01. [Google Scholar] [CrossRef]
- Zhou, Z.; Guan, B.; Xia, H.; Zheng, R.; Xu, B. Particle Radiotherapy in the Era of Radioimmunotherapy. Cancer Lett. 2023, 567, 216268. [Google Scholar] [CrossRef] [PubMed]
- Tuleasca, C.; Tripathi, M.; Starnoni, D.; Daniel, R.T.; Reyns, N.; Levivier, M. Radiobiology of Radiosurgery for Neurosurgeons. Neurol. India 2023, 71, S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.C. Pulsed Low Dose-Rate Radiotherapy: Radiobiology and Dosimetry. Phys. Med. Biol. 2022, 67, 03TR01. [Google Scholar] [CrossRef] [PubMed]
- Barcellos-Hoff, M.H. The Radiobiology of TGFβ. Semin. Cancer Biol. 2022, 86, 857–867. [Google Scholar] [CrossRef]
- Morris, Z.S.; Wang, A.Z.; Knox, S.J. The Radiobiology of Radiopharmaceuticals. Semin. Radiat. Oncol. 2021, 31, 20–27. [Google Scholar] [CrossRef]
- Sgouros, G.; Hobbs, R.; Josefsson, A. Dosimetry and Radiobiology of Alpha-Particle Emitting Radionuclides. Curr. Radiopharm. 2018, 11, 209–214. [Google Scholar] [CrossRef]
- Thiagarajan, A.; Yamada, Y. Radiobiology and Radiotherapy of Brain Metastases. Clin. Exp. Metastasis 2017, 34, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Valenza, C.; Saldanha, E.F.; Gong, Y.; De Placido, P.; Gritsch, D.; Ortiz, H.; Trapani, D.; Conforti, F.; Cremolini, C.; Peters, S.; et al. Circulating Tumor DNA Clearance as a Predictive Biomarker of Pathologic Complete Response in Patients with Solid Tumors Treated with Neoadjuvant Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Ann. Oncol. 2025, 36, 726–736. [Google Scholar] [CrossRef]
- Soong, R.Y.; Low, C.E.; Ong, V.; Sim, I.; Lee, C.; Lee, F.; Chew, L.; Yau, C.E.; Lee, A.R.Y.B.; Chen, M.Z. Exercise Interventions for Depression, Anxiety, and Quality of Life in Older Adults With Cancer: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2025, 8, e2457859. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Han, H.; Li, H.; Wei, L.; Wu, H.; Li, C.; Xie, X.; Zhang, B.; Li, Z.; Chen, X.; et al. Cancer Risk Subsequent to Cardiovascular Disease: A Prospective Population-Based Study and Meta-Analysis. BMC Med. 2025, 23, 192. [Google Scholar] [CrossRef]
- Rischin, D.; Porceddu, S.; Day, F.; Brungs, D.P.; Christie, H.; Jackson, J.E.; Stein, B.N.; Su, Y.B.; Ladwa, R.; Adams, G.; et al. Adjuvant Cemiplimab or Placebo in High-Risk Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2025, 393, 774–785. [Google Scholar] [CrossRef]
- Mamounas, E.P.; Bandos, H.; White, J.R.; Julian, T.B.; Khan, A.J.; Shaitelman, S.F.; Torres, M.A.; Vicini, F.A.; Ganz, P.A.; McCloskey, S.A.; et al. Omitting Regional Nodal Irradiation after Response to Neoadjuvant Chemotherapy. N. Engl. J. Med. 2025, 392, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Kuerer, H.M.; Valero, V.; Smith, B.D.; Krishnamurthy, S.; Diego, E.J.; Johnson, H.M.; Lin, H.; Shen, Y.; Lucci, A.; Shaitelman, S.F.; et al. Selective Elimination of Breast Surgery for Invasive Breast Cancer: A Nonrandomized Clinical Trial. JAMA Oncol. 2025, 11, 529. [Google Scholar] [CrossRef]
- Hwang, E.S.; Hyslop, T.; Lynch, T.; Ryser, M.D.; Weiss, A.; Wolf, A.; Norris, K.; Witten, M.; Grimm, L.; Schnitt, S.; et al. Active Monitoring With or Without Endocrine Therapy for Low-Risk Ductal Carcinoma In Situ: The COMET Randomized Clinical Trial. JAMA 2025, 333, 972. [Google Scholar] [CrossRef]
- Haddad, R.; Fayette, J.; Teixeira, M.; Prabhash, K.; Mesia, R.; Kawecki, A.; Dechaphunkul, A.; Dinis, J.; Guo, Y.; Masuda, M.; et al. Atezolizumab in High-Risk Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Randomized Clinical Trial. JAMA 2025, 333, 1599. [Google Scholar] [CrossRef]
- Galsky, M.D.; Witjes, J.A.; Gschwend, J.E.; Milowsky, M.I.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; et al. Adjuvant Nivolumab in High-Risk Muscle-Invasive Urothelial Carcinoma: Expanded Efficacy From CheckMate 274. J. Clin. Oncol. 2025, 43, 15–21. [Google Scholar] [CrossRef]
- Fekrvand, S.; Abolhassani, H.; Esfahani, Z.H.; Fard, N.N.G.; Amiri, M.; Salehi, H.; Almasi-Hashiani, A.; Saeedi-Boroujeni, A.; Fathi, N.; Mohtashami, M.; et al. Cancer Trends in Inborn Errors of Immunity: A Systematic Review and Meta-Analysis. J. Clin. Immunol. 2025, 45, 34. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Vardy, J.L.; O’Callaghan, C.J.; Gill, S.; Friedenreich, C.M.; Wong, R.K.S.; Dhillon, H.M.; Coyle, V.; Chua, N.S.; Jonker, D.J.; et al. Structured Exercise after Adjuvant Chemotherapy for Colon Cancer. N. Engl. J. Med. 2025, 393, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, L.; Pang, Y.; Shi, J.; Gao, H.; Sun, Y.; Chen, J.; Fu, H.; Cai, J.; Yu, L.; et al. 68Ga-MY6349 PET/CT Imaging to Assess Trop2 Expression in Multiple Types of Cancer. J. Clin. Investig. 2025, 135, e185408. [Google Scholar] [CrossRef] [PubMed]
- Bettariga, F.; Galvao, D.A.; Taaffe, D.R.; Bishop, C.; Lopez, P.; Maestroni, L.; Quinto, G.; Crainich, U.; Verdini, E.; Bandini, E.; et al. Association of Muscle Strength and Cardiorespiratory Fitness with All-Cause and Cancer-Specific Mortality in Patients Diagnosed with Cancer: A Systematic Review with Meta-Analysis. Br. J. Sports Med. 2025, 59, 722–732. [Google Scholar] [CrossRef]
- Ye, F.; Huang, Y.; Zeng, L.; Li, N.; Hao, L.; Yue, J.; Li, S.; Deng, J.; Yu, F.; Hu, X. The Genetically Predicted Causal Associations between Circulating 3-Hydroxybutyrate Levels and Malignant Neoplasms: A Pan-Cancer Mendelian Randomization Study. Clin. Nutr. 2024, 43, 137–152. [Google Scholar] [CrossRef]
- Yan, Q.; Zhu, C.; Li, L.; Li, Y.; Chen, Y.; Hu, X. The Effect of Targeted Palliative Care Interventions on Depression, Quality of Life and Caregiver Burden in Informal Caregivers of Advanced Cancer Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Nurs. Stud. 2024, 160, 104895. [Google Scholar] [CrossRef]
- Quan, L.; Liu, J.; Wang, Y.; Yang, F.; Yang, Z.; Ju, J.; Shuai, Y.; Wei, T.; Yue, J.; Wang, X.; et al. Exploring Risk Factors for Endocrine-Related Immune-Related Adverse Events: Insights from Meta-Analysis and Mendelian Randomization. Hum. Vaccines Immunother. 2024, 20, 2410557. [Google Scholar] [CrossRef]
- Nichols, A.C.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; De Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Radiotherapy Versus Transoral Robotic Surgery for Oropharyngeal Squamous Cell Carcinoma: Final Results of the ORATOR Randomized Trial. J. Clin. Oncol. 2024, 42, 4023–4028. [Google Scholar] [CrossRef]
- Ludmir, E.B.; Sherry, A.D.; Fellman, B.M.; Liu, S.; Bathala, T.; Haymaker, C.; Medina-Rosales, M.N.; Reuben, A.; Holliday, E.B.; Smith, G.L.; et al. Addition of Metastasis-Directed Therapy to Systemic Therapy for Oligometastatic Pancreatic Ductal Adenocarcinoma (EXTEND): A Multicenter, Randomized Phase II Trial. J. Clin. Oncol. 2024, 42, 3795–3805. [Google Scholar] [CrossRef]
- Long, Y.; Zhou, Z.; Zhou, S.; Zhang, G. The Effectiveness of Different Non-Pharmacological Therapies on Cancer-Related Fatigue in Cancer Patients:A Network Meta-Analysis. Int. J. Nurs. Stud. 2024, 160, 104904. [Google Scholar] [CrossRef]
- Baker, J.; Noguchi, N.; Marinovich, M.L.; Sprague, B.L.; Salisbury, E.; Houssami, N. Atypical Ductal or Lobular Hyperplasia, Lobular Carcinoma in-Situ, Flat Epithelial Atypia, and Future Risk of Developing Breast Cancer: Systematic Review and Meta-Analysis. Breast 2024, 78, 103807. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, D.; Cacciagrano, D.; Piangerelli, M. Explainability and Interpretability in Concept and Data Drift: A Systematic Literature Review. Algorithms 2025, 18, 443. [Google Scholar] [CrossRef]
- Bibri, S.E.; Huang, J.; Jagatheesaperumal, S.K.; Krogstie, J. The Synergistic Interplay of Artificial Intelligence and Digital Twin in Environmentally Planning Sustainable Smart Cities: A Comprehensive Systematic Review. Environ. Sci. Ecotechnol. 2024, 20, 100433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wang, S.; Ouyang, C.; Chen, M.; Liu, C.; Zhang, J.; Yu, L.; Wang, F.; Xie, Y.; Li, J.; et al. Artificial Intelligence for Geoscience: Progress, Challenges, and Perspectives. Innovation 2024, 5, 100691. [Google Scholar] [CrossRef]
- Gomes Souza, F.; Bhansali, S.; Pal, K.; Silveira Maranhão, F.D.; Santos Oliveira, M.; Valladão, V.S.; Brandão e Silva, D.S.; Silva, G.B. A 30-Year Review on Nanocomposites: Comprehensive Bibliometric Insights into Microstructural, Electrical, and Mechanical Properties Assisted by Artificial Intelligence. Materials 2024, 17, 1088. [Google Scholar] [CrossRef]
- Valladão, V.S.; da Silva, E.O.; Souza, F.G., Jr. A Data Mining Study of Zinc Oxide Nanostructures Utilization in Drug Delivery Nanosystems. Macromol. Symp. 2024, 413, 2300141. [Google Scholar] [CrossRef]
- Gomes, F.; Bhansali, S.; Valladão, V.; Brandão, D.; Silva, G.; Maranhão, F.; Pal, K.; Thiré, R.; Araújo, J.; Batista, A.; et al. Advancing Dye-Sensitized Solar Cells: Synergistic Effects of Polyaniline, Graphene Oxide, and Carbon Nanotubes for Enhanced Efficiency and Sustainability Developments. Recent Pat. Nanotechnol. 2025. [Google Scholar] [CrossRef]
- Gomes Souza, F.; Pal, K.; Maranhão, F.; Zanoni, C.; Brandão, D.; Colão, M.; Silva, G.; Ampah, J.; Velasco, K. Advancing Hybrid Nanocatalyst Research: A Python-based Visualization of Similarity Analysis for Interdisciplinary and Sustainable Development. Curr. Nanosci. 2024, 20, 830–856. [Google Scholar] [CrossRef]
- Almeida, T.M.D.; Souza, F.G.D.; Pal, K. Bibliometric Analysis of the Hot Theme “Phytosynthesized Nanoparticles. ” Arch. Biomed. Eng. Biotechnol. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Gomes Souza, F.; Pal, K.; Ampah, J.D.; Dantas, M.C.; Araújo, A.; Maranhão, F.; Domingues, P. Biofuels and Nanocatalysts: Python Boosting Visualization of Similarities. Materials 2023, 16, 1175. [Google Scholar] [CrossRef]
- Santos, J.F.; Del Rocío Silva-Calpa, L.; De Souza, F.G.; Pal, K. Central Countries’ and Brazil’s Contributions to Nanotechnology. Curr. Nanomater. 2024, 9, 109–147. [Google Scholar] [CrossRef]
- Daher, E.; Souza, F.G., Jr.; Carelo, J.; Brandão, V. Drug Delivery Polymers: An Analysis Based on Literature Text Mining. Braz. J. Exp. Des. Data Anal. Inferent. Stat. 2021, 1, 40–55. [Google Scholar] [CrossRef]
- Souza, F.G., Jr.; Velasco, K.; Cunha, S.; Santos, J.; Aboelkheir, M.G.; Sumini, M.; Thiré, R.; Duarte, P.C.; Andrade, A.J.P.; Diaz-Martin, R.D.; et al. Leveraging Large Language Models for Accelerated Learning and Innovation in Biogenic Tissue-Engineered Vascular Grafts. J. Drug Deliv. Sci. Technol. 2025, 108, 106935. [Google Scholar] [CrossRef]
- Souza, F.G., Jr.; Barradas, T.N.; Caetano, V.F.; Becerra, A. Nanoparticles Improving Polyaniline Electrical Conductivity: A Meta-Analysis Study. Braz. J. Exp. Des. Data Anal. Inferent. Stat. 2022, 2, 25–58. [Google Scholar] [CrossRef]
- Costa, V.C.; Souza, F.G., Jr.; Thomas, S.; Filho, R.D.T.; Sousa, L.D.C.; Filho, S.T.; Carvalho, F.V.D.; Maranhão, F.D.S.; Aboelkheir, M.G.; de Lima, N.R.B.; et al. Nanotechnology in Concrete: A Bibliometric Review. Braz. J. Exp. Des. Data Anal. Inferent. Stat. 2021, 1, 100–113. [Google Scholar] [CrossRef]
- Daher, E.; Souza, F.G., Jr.; Brandão, V.; Carelo, J. Poly(Lactic Acid)-PLA: An Analysis Based on Literature Text Mining. Braz. J. Exp. Des. Data Anal. Inferent. Stat. 2021, 1, 56–68. [Google Scholar] [CrossRef]
- Valladão, V.S.; Souza, F.G.d.; Thode Filho, S.; Maranhão, F.d.S.; Ribeiro, L.d.S.; Carneiro, M.E.d.S.; Santos, R.K.d.S. dos Sentiment Mapping of Microplastic Awareness in Educational Environments. Polímeros 2025, 35, e20250017. [Google Scholar] [CrossRef]
- Gomes, F.; Bhansali, S.; Valladão, V.; Maranhão, F.; Brandão, D.; Delfino, C.; Asthana, N. Sustainable Catalysts: Advances in Geopolymer-Catalyzed Reactions and Their Applications. J. Mol. Struct. 2025, 1336, 142017. [Google Scholar] [CrossRef]
- Delfino, C.S.C.; Perez Cordovés, A.I.; Souza, F.G., Jr. The Use of Biosensor as a New Trend in Cancer: Bibliometric Analysis from 2007 to 2017. Res. Dev. Mat. Sci. 2018, 7, 1–15. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Unlocking the Future: Carbon Nanotubes as Pioneers in Sensing Technologies. Chemosensors 2025, 13, 225. [Google Scholar] [CrossRef]
- de Souza, F.G., Jr. Up-and-Coming Oil-Sorbing Green Fibers: A Text Mining Study. Braz. J. Exp. Des. Data Anal. Inferent. Stat. 2021, 1, 114–129. [Google Scholar] [CrossRef]
- Gomes, F.; Bhansali, S.; Maranhão, F.; Valladão, V.; Velasco, K. What Are the Future Directions for Microplastics Characterization? A Regex-Llama Data Mining Approach for Identifying Emerging Trends. An. Acad. Bras. Ciênc. 2025, 97, e20241345. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, Y.; Wang, F. Radiomics in Head and Neck Cancer: A Web of Science-Based Bibliometric and Visualized Analysis. MEDS Clin. Med. 2025, 6, 060413. [Google Scholar] [CrossRef]
- Andrei, V.; Arandjelović, O. Complex Temporal Topic Evolution Modelling Using the Kullback-Leibler Divergence and the Bhattacharyya Distance. J. Bioinform. Sys. Biol. 2016, 2016, 16. [Google Scholar] [CrossRef]
- Tabibi, S.; Ali, L.; Jyothi, H.; Asasfeh, E.; Abdelgani, W. Integration of Artificial Intelligence in Predicting Radiotherapy Outcomes for Glioblastoma Multiforme (GBM). Int. J. Multidiscip. Res. 2025, 7, 51688. [Google Scholar] [CrossRef]
- Lastrucci, A.; Wandael, Y.; Ricci, R.; Maccioni, G.; Giansanti, D. The Integration of Deep Learning in Radiotherapy: Exploring Challenges, Opportunities, and Future Directions through an Umbrella Review. Diagnostics 2024, 14, 939. [Google Scholar] [CrossRef]
- Altamimi, A. Evaluating Topic Modeling for Saudi Newspapers Texts Using LDA: A Computational Linguistics Study. J. Umm Al-Qura Univ. Lang. Sci. Lit. 2022, 29, 24–33. [Google Scholar] [CrossRef]
- Quang Trung, H.; Bui, X. A Stochastic Algorithm for Solving the Posterior Inference Problem in Topic Models. Telecommun. Comput. Electron. Control 2022, 20, 971. [Google Scholar] [CrossRef]
- Narozhnyi, V.; Kharchenko, V. Semantic Clustering Method Using Integration of Advanced LDA Algorithm and BERT Algorithm. Innov. Technol. Sci. Solut. Ind. 2024, 140–153. [Google Scholar] [CrossRef]
- Journal, I. Topic Modelling of Web Pages with Latent Dirichlet Allocation Methods. Int. J. Sci. Res. Eng. Manag. 2023, 7, 1–11. [Google Scholar] [CrossRef]
- Maanicshah, K.; Amayri, M.; Bouguila, N. Novel Mixture Allocation Models for Topic Learning. Comput. Intell. 2024, 40, e12641. [Google Scholar] [CrossRef]
- Ajinaja, M.O.; Adetunmbi, A.O.; Ugwu, C.C.; Popoola, O.S. Semantic Similarity Measure for Topic Modeling Using Latent Dirichlet Allocation and Collapsed Gibbs Sampling. Iran J. Comput. Sci. 2023, 6, 81–94. [Google Scholar] [CrossRef]
- Momin, S.; Fu, Y.; Lei, Y.; Roper, J.; Bradley, J.D.; Curran, W.J.; Liu, T.; Yang, X. Knowledge-Based Radiation Treatment Planning: A Data-Driven Method Survey. J. Appl. Clin. Med. Phys. 2021, 22, 16–44. [Google Scholar] [CrossRef]
- Trifiletti, D.M.; Showalter, T.N. Big Data and Comparative Effectiveness Research in Radiation Oncology: Synergy and Accelerated Discovery. Front. Oncol. 2015, 5, 274. [Google Scholar] [CrossRef]
- Souza, F.G., Jr. BiDAVis: Bibliometric Data Visualization Software. Patent Application No.: BR512025000496-6, 15 February 2024. [Google Scholar]
- Souza, F.G., Jr. PaperProcessor: Text Extraction, NLP, and Topic Modeling for Academic Reports. Patent Application No.: BR512025000497-4, 28 May 2024. [Google Scholar]
- Souza, F.G., Jr. SLAT: Scientific Literature Analysis Tool. Patent Application No.: BR512025000495-8, 5 July 2023. [Google Scholar]
- Souza, F.G., Jr. VOSDataAnalyzer: Network Analysis and Term Co-Occurrence Visualization. Patent Application No.: BR512025000498-2, 10 April 2023. [Google Scholar]
- Souza, F.G., Jr. WordSpectrum: Keyword Frequency and Cosine Similarity Analysis. Patent Application No.: BR512025000494-0, 20 August 2023. [Google Scholar]
- Li, J.; He, H.-G.; Guan, C.; Ding, Y.; Hu, X. Dynamic Joint Prediction Model of Severe Radiation-Induced Oral Mucositis among Nasopharyngeal Carcinoma: A Prospective Longitudinal Study. Radiother. Oncol. 2025, 209, 110993. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Chen, Y.; Qiu, B.; Ji, Z.; Jiang, P.; Wang, J. Interventional Radiotherapy: CT-Sim Guided Modern Minimally Invasive Technique. Adv. Radiother. Nucl. Med. 2024, 2, 3781. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Li, Y.; Qin, Y.; Zhou, Z.; Yang, H.; Sun, Y. Oxygen-Independent Radiodynamic Therapy: Radiation-Boosted Chemodynamics for Reprogramming the Tumor Immune Environment and Enhancing Antitumor Immune Response. ACS Appl. Mater. Interfaces 2024, 16, 21546–21556. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Song, J.; Liu, X.; Liu, S.; Yang, N.; Wang, L.; Liu, Y.; Zhao, Y.; Zhou, W.; et al. Tumor Cell-Targeting and Tumor Microenvironment–Responsive Nanoplatforms for the Multimodal Imaging-Guided Photodynamic/Photothermal/Chemodynamic Treatment of Cervical Cancer. Int. J. Nanomed. 2024, 19, 5837–5858. [Google Scholar] [CrossRef]
- Story, M.D.; Davis, A.J.; Sishc, B.J. Current Trends and Future Perspectives in Hadron Therapy: Radiobiology. Health Technol. 2024, 14, 867–872. [Google Scholar] [CrossRef]
- Bermúdez-Guzmán, L.; Blanco-Saborío, A.; Ramírez-Zamora, J.; Lovo, E. The Time for Chronotherapy in Radiation Oncology. Front. Oncol. 2021, 11, 687672. [Google Scholar] [CrossRef]
- Martin, O.A.; Sykes, P.J.; Lavin, M.; Engels, E.; Martin, R.F. What’s Changed in 75 Years of RadRes?–An Australian Perspective on Selected Topics. Radiat. Res. 2024, 202, 309–327. [Google Scholar] [CrossRef]
- Luo, Y. Radiobiological Perspective on Metrics for Quantifying Dose Enhancement Effects of High-Z Nanoparticles. Front. Nanotechnol. 2025, 7, 1603334. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Z.; Perentesis, J.P.; Khuntia, D.; Pfister, S.X.; Sharma, R.A. Can Rational Combination of Ultra-High Dose Rate FLASH Radiotherapy with Immunotherapy Provide a Novel Approach to Cancer Treatment? Clin. Oncol. 2021, 33, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Dokic, I.; Mairani, A.; Niklas, M.; Zimmermann, F.; Chaudhri, N.; Krunic, D.; Tessonnier, T.; Ferrari, A.; Parodi, K.; Jäkel, O.; et al. Next Generation Multi-Scale Biophysical Characterization of High Precision Cancer Particle Radiotherapy Using Clinical Proton, Helium-, Carbon- and Oxygen Ion Beams. Oncotarget 2016, 7, 56676–56689. [Google Scholar] [CrossRef]
- Calvaruso, M.; Pucci, G.; Alberghina, C.; Minafra, L. Radiation Therapy Personalization in Cancer Treatment: Strategies and Perspectives. Int. J. Mol. Sci. 2025, 26, 6375. [Google Scholar] [CrossRef]
- Tini, P.; Nardone, V.; Pastina, P.; Pirtoli, L.; Correale, P.; Giordano, A. The Effects of Radiotherapy on the Survival of Patients with Unresectable Non-Small Cell Lung Cancer. Expert Rev. Anticancer. Ther. 2018, 18, 593–602. [Google Scholar] [CrossRef]
- Willers, H.; Keane, F.K.; Kamran, S.C. Toward a New Framework for Clinical Radiation Biology. Hematol./Oncol. Clin. North Am. 2019, 33, 929–945. [Google Scholar] [CrossRef] [PubMed]
- Mery, B.; Rancoule, C.; Rowinski, E.; Bosacki, C.; Vallard, A.; Guy, J.-B.; Magné, N. Cancer du rein et radiothérapie: Radiorésistance et au-delà. Bull. Cancer 2018, 105, S280–S285. [Google Scholar] [CrossRef] [PubMed]
- Arians, N.; Nicolay, N.H.; Brons, S.; Koerber, S.A.; Jaschke, C.; Vercruysse, M.; Daffinger, S.; Rühle, A.; Debus, J.; Lindel, K. Carbon-Ion Irradiation Overcomes HPV-Integration/E2 Gene-Disruption Induced Radioresistance of Cervical Keratinocytes. J. Radiat. Res. 2019, 60, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Cozzarini, C.; Olivieri, M.; Magli, A.; Cante, D.; Noris Chiorda, B.; Munoz, F.; Faiella, A.; Olivetta, E.; Deantoni, C.; Fodor, A.; et al. Accurate Prediction of Long-Term Risk of Biochemical Failure after Salvage Radiotherapy Including the Impact of Pelvic Node Irradiation. Radiother. Oncol. 2022, 175, 26–32. [Google Scholar] [CrossRef]
- Jazmati, D.; Sohn, D.; Hörner-Rieber, J.; Qin, N.; Bölke, E.; Haussmann, J.; Schwarz, R.; Niggemeier, N.D.; Borkhardt, A.; Babor, F.; et al. Alpha/Beta Values in Pediatric Medulloblastoma: Implications for Tailored Approaches in Radiation Oncology. Radiat. Oncol. 2025, 20, 17. [Google Scholar] [CrossRef]
- Chekhun, V.F.; Domina, E.A. Can SARS-CoV-2 Change Individual Radiation Sensitivity of the Patients Recovered from COVID-19? (Experimental and Theoretical Background). Exp. Oncol. 2021, 43, 277–280. [Google Scholar] [CrossRef]
- Raitanen, J.; Barta, B.; Hacker, M.; Georg, D.; Balber, T.; Mitterhauser, M. Comparison of Radiation Response between 2D and 3D Cell Culture Models of Different Human Cancer Cell Lines. Cells 2023, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, M.; Stanojkovic, T. Current Aspects of Radiobiology in Modern Radiotherapy—Our Clinical Experience. Srp. Arh. Celok. Lek. 2022, 150, 732–736. [Google Scholar] [CrossRef]
- Kang, J.; Coates, J.T.; Strawderman, R.L.; Rosenstein, B.S.; Kerns, S.L. Genomics Models in Radiotherapy: From Mechanistic to Machine Learning. Med. Phys. 2020, 47, e203–e217. [Google Scholar] [CrossRef]
- Sirák, I.; Šinkorová, Z.; Šenkeříková, M.; Špaček, J.; Laco, J.; Vošmiková, H.; John, S.; Petera, J. Hypersensitivity to Chemoradiation in FANCA Carrier with Cervical Carcinoma—A Case Report and Review of the Literature. Rep. Pract. Oncol. Radiother. 2015, 20, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y. Paradigm Shift in Radiation Biology/Radiation Oncology—Exploitation of the “H2O2 Effect” for Radiotherapy Using Low-LET (Linear Energy Transfer) Radiation Such as X-Rays and High-Energy Electrons. Cancers 2016, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Kerns, S.L.; Chuang, K.-H.; Hall, W.; Werner, Z.; Chen, Y.; Ostrer, H.; West, C.; Rosenstein, B. Radiation Biology and Oncology in the Genomic Era. Br. J. Radiol. 2018, 91, 20170949. [Google Scholar] [CrossRef]
- Her, E.J.; Reynolds, H.M.; Mears, C.; Williams, S.; Moorehouse, C.; Millar, J.L.; Ebert, M.A.; Haworth, A. Radiobiological Parameters in a Tumour Control Probability Model for Prostate Cancer LDR Brachytherapy. Phys. Med. Biol. 2018, 63, 135011. [Google Scholar] [CrossRef]
- Verduijn, G.M.; Capala, M.E.; Sijtsema, N.D.; Lauwers, I.; Hernandez Tamames, J.A.; Heemsbergen, W.D.; Sewnaik, A.; Hardillo, J.A.; Mast, H.; Van Norden, Y.; et al. The COMPLETE Trial: HolistiC Early respOnse assessMent for oroPharyngeaL cancEr paTiEnts; Protocol for an Observational Study. BMJ Open 2022, 12, e059345. [Google Scholar] [CrossRef]
- McWilliam, A.; Palma, G.; Abravan, A.; Acosta, O.; Appelt, A.; Aznar, M.; Monti, S.; Onjukka, E.; Panettieri, V.; Placidi, L.; et al. Voxel-Based Analysis: Roadmap for Clinical Translation. Radiother. Oncol. 2023, 188, 109868. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, N.; Farkas, G.; Székely, G.; Kocsis, Z.S.; Kelemen, P.B.; Fodor, J.; Polgár, C.; Jurányi, Z. Progressive Breast Fibrosis Caused by Extreme Radiosensitivity: Oncocytogenetic Diagnosis and Treatment by Reconstructive Flap Surgery. Cancer Rep. 2019, 2, e1126. [Google Scholar] [CrossRef]
- Dayyani, M.; Hoseinian-Azghadi, E.; Miri-Hakimabad, H.; Rafat-Motavalli, L.; Abdollahi, S.; Mohammadi, N. Radiobiological Comparison between Cobalt-60 and Iridium-192 High-dose-rate Brachytherapy Sources: Part I—Cervical Cancer. Med. Phys. 2021, 48, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Granzotto, A.; Benadjaoud, M.A.; Vogin, G.; Devic, C.; Ferlazzo, M.L.; Bodgi, L.; Pereira, S.; Sonzogni, L.; Forcheron, F.; Viau, M.; et al. Influence of Nucleoshuttling of the ATM Protein in the Healthy Tissues Response to Radiation Therapy: Toward a Molecular Classification of Human Radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 450–460. [Google Scholar] [CrossRef]
- Perico, D.; Mauri, P. Deciphering Radiotherapy Resistance: A Proteomic Perspective. Proteomes 2025, 13, 25. [Google Scholar] [CrossRef]
- Hong, M.; Zhang, C.J.; Jiang, D. Semantic-Augmented Latent Topic Modeling with LLM-in-the-Loop 2025. arXiv 2025, arXiv:2507.08498. [Google Scholar]
- Essue, B.M.; Gheorghe, A.; Rodin, G.; Sullivan, R. Re-Envisioning the Value Proposition for Investment in Cancer Care. JNCI J. Natl. Cancer Inst. 2025, djaf199. [Google Scholar] [CrossRef]
- Fadiel, A.; Fadel, S.; Butt, W.; Eichenbaum, K.D.; Abbasi, M.; Elbialy, A.; Odunsi, K. Abstract 1049: Advancing Health Equity in Oncology through AI, GIS, and Data-Driven Tools. Cancer Res. 2025, 85, 1049. [Google Scholar] [CrossRef]
- Horgan, D.; Tanner, M.; Aggarwal, C.; Thomas, D.; Grover, S.; Basel-Salmon, L.; Dienstmann, R.; Tan, T.J.Y.; Park, W.-Y.; Abu Rasheed, H.M.; et al. Precision Oncology: A Global Perspective on Implementation and Policy Development. JCO Glob. Oncol. 2025, 11, e2400416. [Google Scholar] [CrossRef]
- Kouhen, F.; Naciri, M.; El Gouache, H.; Errafiy, N.; Maghous, A. The Promise of Artificial Intelligence-Assisted Radiotherapy for Prostate Cancer in Morocco: A Transformational Opportunity. Front. Med. 2025, 12, 1577034. [Google Scholar] [CrossRef]
- Montoya-Quintero, K.F.; Galván-Barrios, J.; Martinez-Guevara, D.; Dueñas, D.; Montenegro, J.; Liscano, Y. Bridging the Gap: Cancer Scientific Equity, Global Child Health, and Distribution of CAR T-Cell Therapy Clinical Trials in Childhood Cancer. Front. Pediatr. 2025, 13, 1611187. [Google Scholar] [CrossRef]
- Puthenpura, V.; Hunter, M.; Marks, A.M. “Techquity” in Pediatric, Adolescent, and Young Adult Oncology: Addressing Inequities Through Artificial Intelligence and Immersive Technologies. Pediatr. Blood Cancer 2025, 72, e31909. [Google Scholar] [CrossRef]
- Ranger, S.; Birhiray, M.N.; Birhiray, R.E. Advancing Racial Representation in Clinical Trials: An Analysis of 53 ASCO Plenary and Late-Breaking Abstracts Using the DRIVE Score. J. Clin. Oncol. 2025, 43, e23007. [Google Scholar] [CrossRef]
- Riaz, I.B.; Khan, M.A.; Osterman, T.J. Artificial Intelligence across the Cancer Care Continuum. Cancer 2025, 131, e70050. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Singh, A.; Singh, S.; Rivers, B.; Lillard, J.W.; Singh, R. Revolutionizing Oncology Through AI: Addressing Cancer Disparities by Improving Screening, Treatment, and Survival Outcomes via Integration of Social Determinants of Health. Cancers 2025, 17, 2866. [Google Scholar] [CrossRef]
- Vasquez, V.M.; McCabe, M.; McKee, J.C.; Siby, S.; Hussain, U.; Faizuddin, F.; Sheikh, A.; Nguyen, T.; Mayer, G.; Grier, J.; et al. Transforming Cancer Care: A Narrative Review on Leveraging Artificial Intelligence to Advance Immunotherapy in Underserved Communities. J. Clin. Med. 2025, 14, 5346. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Sedor, G.; Ellsworth, P.; Scarborough, J.A.; Ahmed, K.A.; Oliver, D.E.; Eschrich, S.A.; Kattan, M.W.; Torres-Roca, J.F. Pan-Cancer Prediction of Radiotherapy Benefit Using Genomic-Adjusted Radiation Dose (GARD): A Cohort-Based Pooled Analysis. Lancet Oncol. 2021, 22, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Stecklein, S.; White, J.; Yoder, R.; Staley, J.; Schmitt, Z.; O’Dea, A.; Nye, L.; Satelli, D.; Crane, G.; Madan, R.; et al. Abstract PS16-09: Analysis of the Radiosensitivity Index/Genomic-Adjusted Radiation Dose (RSI-GARD) in Paired Pre- and Post-Treatment TNBC Samples: Implications for Biomarker-Guided Radiotherapy. Cancer Res. 2024, 84, PS16-09. [Google Scholar] [CrossRef]
- Ahmed, K.; Washington, I.; Mills, M.; DeJesus, M.; Kim, Y.; Nanda, R.; Torres-Roca, J.; Eschrich, S.; Iglesia, J.D.L.; Puskas, J.; et al. Abstract PO3-20-05: Phase II Study of Genomically Guided Radiation Dose Personalization in the Management of Triple Negative Breast Cancer. Cancer Res. 2024, 84, PO3-20-05. [Google Scholar] [CrossRef]
- Xia, H.; Li, Z.; Lin, Y.; Lin, Y.; Zeng, L.; Xu, B.; Yao, Q.; Zheng, R. Validation of a Genome-Based Model for Adjusting Radiotherapy Dose (GARD) in Patients with Locally Advanced Rectal Cancer. Sci. Rep. 2024, 14, 21572. [Google Scholar] [CrossRef]
- Yuan, Z.; Frazer, M.; Ahmed, K.A.; Naqvi, S.M.H.; Schell, M.J.; Felder, S.; Sanchez, J.; Dessureault, S.; Imanirad, I.; Kim, R.D.; et al. Modeling Precision Genomic-Based Radiation Dose Response in Rectal Cancer. Future Oncol. 2020, 16, 2411–2420. [Google Scholar] [CrossRef]
- Ahmed, K.; Venkat, P.; Scott, J.; Diaz, R.; Fulp, W.; Torres-Roca, J. Abstract P3-12-03: Utilizing the Genomically Adjusted Dose (GAD) to Personalize Radiotherapy in Adjuvant Breast Cancer Management. Cancer Res. 2016, 76, P3-12-03. [Google Scholar] [CrossRef]
- Ho, E.; De Cecco, L.; Eschrich, S.A.; Cavalieri, S.; Sedor, G.; Hoebers, F.; Brakenhoff, R.H.; Scheckenbach, K.; Poli, T.; Yang, K.; et al. Personalized Treatment in HPV+ Oropharynx Cancer Using Genomic Adjusted Radiation Dose. J. Clin. Investig. 2025, 135, e194073. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.; Chan, K.S.K.; Li, H.; Ng, W.T.; Chow, J.C.H.; Choi, H.C.W.; Lam, K.O.; Lee, V.H.F.; Ngan, R.K.C.; Lee, A.W.M.; et al. Using the Genomic Adjusted Radiation Dose (GARD) to Personalize the Radiation Dose in Nasopharyngeal Cancer. Radiother. Oncol. 2024, 196, 110287. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Scott, J.; Johnstone, P.; Fulp, W.; Torres-Roca, J.; Caudell, J. Rtrb-01 utilizing the genomically adjusted dose (gad) to identify patients for adjuvant radiation dose escalation in glioblastoma. Neuro. Oncol. 2015, 17, v195. [Google Scholar] [CrossRef]
- Scott, J.G.; Berglund, A.; Schell, M.J.; Mihaylov, I.; Fulp, W.J.; Yue, B.; Welsh, E.; Caudell, J.J.; Ahmed, K.; Strom, T.S.; et al. A Genome-Based Model for Adjusting Radiotherapy Dose (GARD): A Retrospective, Cohort-Based Study. Lancet Oncol. 2017, 18, 202–211. [Google Scholar] [CrossRef]
- Cai, B.; Banks, T.I.; Shen, C.; Prasad, R.; Bal, G.; Lin, M.-H.; Godley, A.; Pompos, A.; Garant, A.; Westover, K.; et al. Strategies for Offline Adaptive Biology-Guided Radiotherapy (BgRT) on a PET-Linac Platform. Cancers 2025, 17, 2470. [Google Scholar] [CrossRef]
- Calvaruso, M.; Panizza, D.; Colciago, R.R.; Faccenda, V.; Pucci, G.; Ponti, E.D.; Forte, G.I.; Russo, G.; Minafra, L.; Arcangeli, S. A Biological-Driven Approach to Explore Dose-Escalated Ultra-Hypofractionation in Breast Cancer Radiotherapy. Biomedicines 2025, 13, 2154. [Google Scholar] [CrossRef]
- Hanna, G.J.; Gupta, T.R.; Trippa, L.; Kim, E.; Droznin, A.D.; Minken, J.; Gunasti, L.; Rettig, E.M.; Margalit, D.N.; Tishler, R.B.; et al. Risk-Adapted Therapy Guided by Human Papillomavirus (HPV) Circulating Tumor DNA in Patients with HPV-Positive Oropharyngeal Cancer (ReACT 1.0). J. Clin. Oncol. 2025, 43, 6009. [Google Scholar] [CrossRef]
- Kaida, A.; Nojima, H.; Miura, M. Effects of P53 Mutation on Tumor Radiosensitivity Estimated by Predictive Models. Radiat. Res. 2025, 204, 259–265. [Google Scholar] [CrossRef]
- Khatri, N.; Chalar, R.; Samad, S.; Stessin, A.; Damaghi, M. Abstract 4694: (R)Evolutionary Approach to Predicting Individual Response to Radiation Therapy in Breast Cancer. Cancer Res. 2025, 85, 4694. [Google Scholar] [CrossRef]
- Meattini, I.; Coles, C.E.; Tramm, T.; Borghesi, S.; Krug, D.; Montero, A.; Nardone, V.; Salvestrini, V.; Valzano, M.; Valentini, V.; et al. Biomarker-Directed Radiotherapy in Breast Cancer: A Narrative Review. JAMA Oncol. 2025, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Sambi, M.; Shekari, F.; Satari, K.; Ghafoury, R.; Ashayeri, N.; Eversole, P.; Baluch, N.; Harless, W.W.; Muscarella, L.A.; et al. A Comprehensive Oncological Biomarker Framework Guiding Precision Medicine. Biomolecules 2025, 15, 1304. [Google Scholar] [CrossRef]
- Morelli, I.; Banini, M.; Greto, D.; Visani, L.; Garlatti, P.; Loi, M.; Aquilano, M.; Valzano, M.; Salvestrini, V.; Bertini, N.; et al. Integrating Radiomics and Artificial Intelligence (AI) in Stereotactic Body Radiotherapy (SBRT)/Stereotactic Radiosurgery (SRS): Predictive Tools for Tailored Cancer Care. Cancers 2025, 17, 2906. [Google Scholar] [CrossRef]
- Weiner, A.B.; Proudfoot, J.A.; Aker, M.; Cardenas, M.; Gonzalez, S.; Kelly, E.; Davicioni, E.; Sisk, A.E.; Brisbane, W.G.; Marks, L.S. Genomic Biomarker for Prostate Cancer Focal Therapy: Post Hoc Assessment of a Phase II Clinical Trial. JCO Precis. Oncol. 2025, 9, e2500535. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Wu, J.; Zhang, H.; Cai, H.; Liu, X.; Li, Q. Hypoxia-guided Treatment Planning for Lung Cancer with Dose Painting by Numbers. J. Appl. Clin. Med. Phys. 2025, 26, e14609. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Chen, S.; Krauss, D.J.; Chen, P.Y.; Chinnaiyan, P.; Wilson, G.D. Tumor Voxel Dose-Response Matrix and Dose Prescription Function Derived Using 18F-FDG PET/CT Images for Adaptive Dose Painting by Number. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 207–218. [Google Scholar] [CrossRef]
- Shiradkar, R.; Podder, T.K.; Algohary, A.; Viswanath, S.; Ellis, R.J.; Madabhushi, A. Radiomics Based Targeted Radiotherapy Planning (Rad-TRaP): A Computational Framework for Prostate Cancer Treatment Planning with MRI. Radiat. Oncol. 2016, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Thorwarth, D. Biologically adapted radiation therapy. Z. Für Med. Phys. 2018, 28, 177–183. [Google Scholar] [CrossRef]
- Almeldin, D.S.; Sr, M.G.; Manolopoulos, S.; Karis, S.; Mancini, L.; Bisdas, S.; Kosmin, M. The Feasibility of Dose-Escalated Radiation Therapy for Glioblastoma Using Biological Image Guided Adaptive Radiotherapy (BIGART). Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, e83. [Google Scholar] [CrossRef]
- Naghavi, A.O.; Bryant, J.M.; Kim, Y.; Weygand, J.; Redler, G.; Sim, A.J.; Miller, J.; Coucoules, K.; Michael, L.T.; Gloria, W.E.; et al. Habitat Escalated Adaptive Therapy (HEAT): A Phase 2 Trial Utilizing Radiomic Habitat-Directed and Genomic-Adjusted Radiation Dose (GARD) Optimization for High-Grade Soft Tissue Sarcoma. BMC Cancer 2024, 24, 437. [Google Scholar] [CrossRef]
- Balvasi, E.; Mahmoudi, F.; Geraily, G.; Farnia, P.; Jafari, F. Modeling the Impact of Intercellular Signaling on Dose Metrics and Therapeutic Outcomes in Spatially Fractionated Radiation Therapy (SFRT) for Lung Cancer. Sci. Rep. 2025, 15, 28592. [Google Scholar] [CrossRef]
- Dongrong, Y.; Xinyi, L.; Sua, Y.; Rachel, B.; Susan, M.; Sarah, S.; Paul, S.; Wu, Q.J.; Yang, S. Breast Radiation Therapy Fluence Painting with Multi-agent Deep Reinforcement Learning. Med. Phys. 2025, 52, 2015–2024. [Google Scholar] [CrossRef]
- Dulley, L.; Ameen, A.; Almond, E.; Farmakidis, D.; Greenwood, J.; Ulewich, M. Are there benefits in treating glioblastomas with hyperarc versus rapidarc? Neuro-Oncol. 2025, 27, ii23–ii24. [Google Scholar] [CrossRef]
- Komisopoulos, G.; Mavroidis, P.; Simopoulou, F.; Kyrgias, G.; Pant, K.; Roussakis, Y.; Theodorou, K. Use of NTCP Metrics to Identify Unbalanced Levels of Dose Trade-off between Left Lung and Heart in Lung Cancer Radiotherapy. J. Appl. Clin. Med. Phys. 2025, 26, e70207. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, X.; Ding, J.; Shen, L.; Li, M.; Yi, J.; Dai, J. A Decision-Making Method for Photon/Proton Selection for Nasopharyngeal Cancer Based on Dose Prediction and NTCP. Cancers 2025, 17, 2620. [Google Scholar] [CrossRef]
- Menne Guricová, K.; Draulans, C.; Pos, F.J.; Kerkmeijer, L.G.W.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; De Boer, H.C.J.; Van Der Voort Van Der Zyp, J.R.N.; Haustermans, K.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: 10-Year Outcomes of the FLAME Trial. J. Clin. Oncol. 2025, 43, 3065–3069. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Li, J.; Li, W.; Lin, Y.; Qin, B.; Gao, H. NTCP-constrained Combined Proton-photon Treatment Planning to Maximize Utilization of Limited Proton Slots. Med. Phys. 2025, 52, e17969. [Google Scholar] [CrossRef]
- Yousefi, A.; Ketabi, S.; Moreno, A.C.; Abedi, I.; Kim, Y.; Gholami, S. An Innovative Process for Efficient Automated Optimizing IMRT Knowledge-based Planning (KBP). Med. Phys. 2025, 52, e18055. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Luo, J.; Xu, H.; Jiao, S.; Chen, C. The Improved Features to Dose Correlation for Dose Volumes Prediction in Multidisease Radiation Therapy Plans. Sci. Rep. 2025, 15, 32799. [Google Scholar] [CrossRef]
- Bentzen, S.M. Theragnostic Imaging for Radiation Oncology: Dose-Painting by Numbers. Lancet Oncol. 2005, 6, 112–117. [Google Scholar] [CrossRef]
- Bibault, J.-E.; Fumagalli, I.; Ferté, C.; Chargari, C.; Soria, J.-C.; Deutsch, E. Personalized Radiation Therapy and Biomarker-Driven Treatment Strategies: A Systematic Review. Cancer Metastasis Rev. 2013, 32, 479–492. [Google Scholar] [CrossRef]
- Kim, Y.; Tomé, W.A. Dose-Painting IMRT Optimization Using Biological Parameters. Acta Oncol. 2010, 49, 1374–1384. [Google Scholar] [CrossRef]
- Søvik, Å.; Malinen, E.; Olsen, D.R. Strategies for Biologic Image-Guided Dose Escalation: A Review. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Janke, F.; Stritzke, F.; Dvornikovich, K.; Franke, H.; Angeles, A.K.; Riediger, A.L.; Ogrodnik, S.; Gerhardt, S.; Regnery, S.; Schröter, P.; et al. Early Circulating Tumor DNA Changes Predict Outcomes in Head and Neck Cancer Patients under Re-radiotherapy. Int. J. Cancer 2025, 156, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Forouzannezhad, P.; Maes, D.; Hippe, D.S.; Thammasorn, P.; Iranzad, R.; Han, J.; Duan, C.; Liu, X.; Wang, S.; Chaovalitwongse, W.A.; et al. Multitask Learning Radiomics on Longitudinal Imaging to Predict Survival Outcomes Following Risk-Adaptive Chemoradiation for Non-Small Cell Lung Cancer. Cancers 2022, 14, 1228. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, V.; Aggarwal, C. Toward Response-Adaptive Therapy in Locally Advanced NSCLC: Integrating ctDNA and Radiomics for Risk Stratification. Cancer Discov. 2025, 15, 1534–1536. [Google Scholar] [CrossRef]
- Salhab, A.; Blumenfeld, P.A.; Arbit, E.; Feldman, J.; Berger, A.; Weizman, N.; Hillman, Y.; Popovtzer, A. Initial Clinical Experience Using Daily Artificial Intelligence-Assisted Adaptive Radiotherapy on Cone-Beam CT for Cancer of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, e622. [Google Scholar] [CrossRef]
- Sher, D.J.; Avkshtol, V.; Lin, M.H.; Hughes, R.; Wang, J.; Dohopolski, M.; Van Pelt, J.; Liao, C.Y.; Moon, D.H. Acute Toxicity and Efficiency Outcomes in the DARTBOARD Randomized Trial of Daily Adaptive Radiotherapy for Head and Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, e6. [Google Scholar] [CrossRef]
- Blumenfeld, P.A.; Feldman, J.; Arbit, E.; Weizman, N.; Berger, A.; Hillman, Y.; Wygoda, M.R.; Popovtzer, A. Daily Artificial Intelligence-Assisted Adaptive Radiotherapy on Cone-Beam CT for Cancer of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, e590–e591. [Google Scholar] [CrossRef]
- Dona Lemus, O.M.; Cao, M.; Cai, B.; Cummings, M.; Zheng, D. Adaptive Radiotherapy: Next-Generation Radiotherapy. Cancers 2024, 16, 1206. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Nishimura, Y.; Ishikawa, K.; Inada, M.; Matsumoto, K.; Doi, H.; Monzen, H. Online Adaptive Radiotherapy for Pharyngeal Cancer: Dose-Volume Histogram Analysis between Adapted and Scheduled Plan. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, e729. [Google Scholar] [CrossRef]
- Guo, C.; Huang, P.; Yang, B.; Jing, H.; Xia, W.; Yang, Z.; Men, K.; Dai, J. Longitudinal Evaluation of Workflow Optimization in Radiotherapy: A 4-year Retrospective Study. J. Appl. Clin. Med. Phys. 2025, 26, e70252. [Google Scholar] [CrossRef]
- Horowitz, D.P.; Wang, Y.-F.; Lee, A.; Kachnic, L.A. Optimizing Treatment Precision: Role of Adaptive Radiotherapy in Modern Anal Cancer Management. Cancers 2025, 17, 2478. [Google Scholar] [CrossRef]
- Li, R.; Lin, M.-H.; Nguyen, N.C.; Su, F.-C.; Parsons, D.; Salcedo, E.; Phillips, E.; Domal, S.; Garant, A.; Hannan, R.; et al. Clinical Implementation of PSMA-PET Guided Tumor Response-Based Boost Adaptation in Online Adaptive Radiotherapy for High-Risk Prostate Cancer. Cancers 2025, 17, 2893. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Wang, G.; Yang, Y.; Su, Y.; Zhang, H.; Liu, J.; Cui, P.; Fan, X.; Yang, J.; et al. Optimizing esophageal cancer treatment: Personalized CTDNA improves residual disease detection after neoadjuvant chemoradiotherapy and guides adaptive adjuvant immunotherapy. Dis. Esophagus 2025, 38, doaf061.046. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, Q.; Yuan, J.; Dai, L.; Luo, R.; Huang, T.; Wu, Y. Evaluation of ctDNA-Guided Adjuvant Therapy de-Escalation in Head and Neck Squamous Cell Carcinoma: A Comparative Cohort Study. Front. Immunol. 2025, 16, 1576042. [Google Scholar] [CrossRef] [PubMed]
- Balázs, Z.; Balermpas, P.; Ivanković, I.; Willmann, J.; Gitchev, T.; Bryant, A.; Guckenberger, M.; Krauthammer, M.; Andratschke, N. Longitudinal Cell-Free DNA Characterization by Low-Coverage Whole-Genome Sequencing in Patients Undergoing High-Dose Radiotherapy. Radiother. Oncol. 2024, 197, 110364. [Google Scholar] [CrossRef]
- Blomain, E.S.; Moding, E.J. Liquid Biopsies for Molecular Biology-Based Radiotherapy. Int. J. Mol. Sci. 2021, 22, 11267. [Google Scholar] [CrossRef]
- Suppan, C.; Brcic, I.; Tiran, V.; Mueller, H.D.; Posch, F.; Auer, M.; Ercan, E.; Ulz, P.; Cote, R.J.; Datar, R.H.; et al. Untargeted Assessment of Tumor Fractions in Plasma for Monitoring and Prognostication from Metastatic Breast Cancer Patients Undergoing Systemic Treatment. Cancers 2019, 11, 1171. [Google Scholar] [CrossRef]
- Kato, S.; Li, B.; Adashek, J.J.; Cha, S.W.; Bianchi-Frias, D.; Qian, D.; Kim, L.; So, T.W.; Mitchell, M.; Kamei, N.; et al. Serial Changes in Liquid Biopsy-Derived Variant Allele Frequency Predict Immune Checkpoint Inhibitor Responsiveness in the Pan-Cancer Setting. OncoImmunology 2022, 11, 2052410. [Google Scholar] [CrossRef]
- Thress, K.S.; Paweletz, C.P.; Felip, E.; Cho, B.C.; Stetson, D.; Dougherty, B.; Lai, Z.; Markovets, A.; Vivancos, A.; Kuang, Y.; et al. Acquired EGFR C797S Mutation Mediates Resistance to AZD9291 in Non–Small Cell Lung Cancer Harboring EGFR T790M. Nat. Med. 2015, 21, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yuan, M.; Yang, Y.; Xu, X.S. Improving Prediction of Survival and Progression in Metastatic Non-Small Cell Lung Cancer Following Immunotherapy through Machine Learning of Circulating Tumor DNA. JCO Precis. Oncol. 2024, 8, e2300718. [Google Scholar] [CrossRef] [PubMed]
- Onieva, J.L.; Pérez-Ruiz, E.; Figueroa-Ortiz, L.C.; Jurado, J.M.; Martínez-Gálvez, B.; Benitez, J.C.; Rueda-Domínguez, A.; Barragán, I. Integrative Multi-Omic Profiling of cfDNA Methylation and EV-miRNAs Identifies Immunotherapy-Outcome Molecular Subtypes in NSCLC. medRxiv preprint 2025. [Google Scholar] [CrossRef]
- Holanek, M.; Selingerova, I.; Fabian, P.; Coufal, O.; Zapletal, O.; Petrakova, K.; Kazda, T.; Hrstka, R.; Poprach, A.; Zvarikova, M.; et al. Biomarker Dynamics and Long-Term Treatment Outcomes in Breast Cancer Patients with Residual Cancer Burden after Neoadjuvant Therapy. Diagnostics 2022, 12, 1740. [Google Scholar] [CrossRef] [PubMed]
- Garma, L.D.; Pernas, S.; Baz, D.V.; Campelo, R.G.; Terrasa, J.; Nasarre, B.; García, R.V.; Bermejo, B.; Santiago, S.G.; Fullana, B.; et al. Longitudinal Clinical, Physiological and Molecular Profiling of Female Metastatic Cancer Patients: Protocol and Feasibility of a Multicenter High-Definition Oncology Study. medRxiv 2025. [Google Scholar] [CrossRef]
- Karlíková, M.; Čurillová, M.; Pecen, L.; Karnos, V.; Karnos, V.; Topolčan, O. Evaluation of Serum Cytokeratines, Thymidine Kinase, and Growth Factors as Cancer Biomarkers in Colorectal Cancer. Anticancer Res. 2024, 44, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Altreuter, J.; Bodapati, S.; Cristea, S.; Wong, C.J.; Wu, C.J.; Michor, F. Predicting Patient Outcomes after Treatment with Immune Checkpoint Blockade: A Review of Biomarkers Derived from Diverse Data Modalities. Cell Genom. 2024, 4, 100444. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.C.; Sivapalan, L.; Hummelink, K.; Balan, A.; White, J.R.; Niknafs, N.; Rhymee, L.; Pereira, G.; Rao, N.; Weksler, B.; et al. Elucidating the Heterogeneity of Immunotherapy Response and Immune-Related Toxicities by Longitudinal ctDNA and Immune Cell Compartment Tracking in Lung Cancer. Clin. Cancer Res. 2024, 30, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Cai, Y.; Yuan, H.; Chen, Q.; Zhao, S.W.; Yang, J.; Liu, M.; Arreola, A.X.; Ren, Y.; Xu, C.; et al. Evaluation of Ferroptosis as a Biomarker to Predict Treatment Outcomes of Cancer Immunotherapy. Cancer Res. Commun. 2025, 5, 1288–1297. [Google Scholar] [CrossRef]
- Juric, D.; Castel, P.; Griffith, M.; Griffith, O.L.; Won, H.H.; Ellis, H.; Ebbesen, S.H.; Ainscough, B.J.; Ramu, A.; Iyer, G.; et al. Convergent Loss of PTEN Leads to Clinical Resistance to a PI(3)Kα Inhibitor. Nature 2015, 518, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Yang, X. Early Tumor Shrinkage and Depth of Response as Predictive Markers of Treatment Response and Prognosis in Solid Tumors. Cancer Med. 2025, 14, e71251. [Google Scholar] [CrossRef]
- Carter, S.L.; Eklund, A.C.; Kohane, I.S.; Harris, L.N.; Szallasi, Z. A Signature of Chromosomal Instability Inferred from Gene Expression Profiles Predicts Clinical Outcome in Multiple Human Cancers. Nat. Genet. 2006, 38, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, Y.X.; Lian, J.J.; Ng, H.-Y.; Wang, S.S.Y. Personalized Treatment Using Predictive Biomarkers in Solid Organ Malignancies: A Review. Tumori J. 2024, 110, 386–404. [Google Scholar] [CrossRef]
- Rashid, N.U.; Luckett, D.J.; Chen, J.; Lawson, M.T.; Wang, L.; Zhang, Y.; Laber, E.B.; Liu, Y.; Yeh, J.J.; Zeng, D.; et al. High-Dimensional Precision Medicine From Patient-Derived Xenografts. J. Am. Stat. Assoc. 2021, 116, 1140–1154. [Google Scholar] [CrossRef] [PubMed]
- Magbanua, M.J.M.; Li, W.; Van ’T Veer, L.J. Integrating Imaging and Circulating Tumor DNA Features for Predicting Patient Outcomes. Cancers 2024, 16, 1879. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A Clinically Applicable Molecular-Based Classification for Endometrial Cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef]
- Van Den Heerik, A.S.V.M.; Horeweg, N.; De Boer, S.M.; Bosse, T.; Creutzberg, C.L. Adjuvant Therapy for Endometrial Cancer in the Era of Molecular Classification: Radiotherapy, Chemoradiation and Novel Targets for Therapy. Int. J. Gynecol. Cancer 2021, 31, 594–604. [Google Scholar] [CrossRef]
- León-Castillo, A.; Gilvazquez, E.; Nout, R.; Smit, V.T.; McAlpine, J.N.; McConechy, M.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; et al. Clinicopathological and Molecular Characterisation of ‘Multiple-classifier’ Endometrial Carcinomas. J. Pathol. 2020, 250, 312–322. [Google Scholar] [CrossRef]
- Vermij, L.; Smit, V.; Nout, R.; Bosse, T. Incorporation of Molecular Characteristics into Endometrial Cancer Management. Histopathology 2020, 76, 52–63. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Levine, D.A. Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Yen, T.-T.; Wang, T.-L.; Fader, A.N.; Shih, I.-M.; Gaillard, S. Molecular Classification and Emerging Targeted Therapy in Endometrial Cancer. Int. J. Gynecol. Pathol. 2020, 39, 26–35. [Google Scholar] [CrossRef]
- Goulder, A.; Gaillard, S.L. Molecular Classification of Endometrial Cancer: Entering an Era of Precision Medicine. J. Gynecol. Oncol. 2022, 33, e47. [Google Scholar] [CrossRef]
- Bosse, T.; Nout, R.A.; McAlpine, J.N.; McConechy, M.K.; Britton, H.; Hussein, Y.R.; Gonzalez, C.; Ganesan, R.; Steele, J.C.; Harrison, B.T.; et al. Molecular Classification of Grade 3 Endometrioid Endometrial Cancers Identifies Distinct Prognostic Subgroups. Am. J. Surg. Pathol. 2018, 42, 561–568. [Google Scholar] [CrossRef]
- Puechl, A.M.; Spinosa, D.; Berchuck, A.; Secord, A.A.; Drury, K.E.; Broadwater, G.; Wong, J.; Whitaker, R.; Devos, N.; Corcoran, D.L.; et al. Molecular Classification to Prognosticate Response in Medically Managed Endometrial Cancers and Endometrial Intraepithelial Neoplasia. Cancers 2021, 13, 2847. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Bosse, T.; McAlpine, J.N. The Emerging Role of Molecular Pathology in Directing the Systemic Treatment of Endometrial Cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211035959. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Mortagy, H.; Dwarshuis, N.; Wang, J.; Yang, P.; Holder, A.L.; Gupta, S.; Kamaleswaran, R. Improving Clinical Decision Support through Interpretable Machine Learning and Error Handling in Electronic Health Records. J. Am. Med. Inform. Assoc. 2025, ocaf058. [Google Scholar] [CrossRef]
- Bauer, J.M.; Michalowski, M. Human-Centered Explainability Evaluation in Clinical Decision-Making: A Critical Review of the Literature. J. Am. Med. Inform. Assoc. 2025, 32, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-da-Silva, R.; Cruz-Correia, R.; Ribeiro, I. Beyond Black Boxes: Using Explainable Causal Artificial Intelligence to Separate Signal from Noise in Pharmacovigilance. Int. J. Clin. Pharm. 2025. [Google Scholar] [CrossRef]
- Kumar, R.; Sporn, K.; Waisberg, E.; Ong, J.; Paladugu, P.; Vadhera, A.S.; Amiri, D.; Ngo, A.; Jagadeesan, R.; Tavakkoli, A.; et al. Navigating Healthcare AI Governance: The Comprehensive Algorithmic Oversight and Stewardship Framework for Risk and Equity. Health Care Anal. 2025, 1–25. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Zheng, J.; Xu, C.; Wang, D. Improving Explainability and Integrability of Medical AI to Promote Health Care Professional Acceptance and Use: Mixed Systematic Review. J. Med. Internet Res. 2025, 27, e73374. [Google Scholar] [CrossRef]
- Mascarenhas, M.; Mendes, F.; Martins, M.; Ribeiro, T.; Afonso, J.; Cardoso, P.; Ferreira, J.; Fonseca, J.; Macedo, G. Explainable AI in Digestive Healthcare and Gastrointestinal Endoscopy. J. Clin. Med. 2025, 14, 549. [Google Scholar] [CrossRef] [PubMed]
- Mitra, J.; Ghose, S.; Thawani, R. Clinically Explainable Prediction of Immunotherapy Response Integrating Radiomics and Clinico-Pathological Information in Non-Small Cell Lung Cancer. Cancers 2025, 17, 2679. [Google Scholar] [CrossRef]
- Muñoz-Ordóñez, J.; Cobos, C.; Vidal-Rojas, J.C.; Herrera, F. A Maturity Model for Practical Explainability in Artificial Intelligence-Based Applications: Integrating Analysis and Evaluation (MM4XAI-AE) Models. Int. J. Intell. Syst. 2025, 2025, 4934696. [Google Scholar] [CrossRef]
- Pahde, F.; Wiegand, T.; Lapuschkin, S.; Samek, W. Ensuring Medical AI Safety: Interpretability-Driven Detection and Mitigation of Spurious Model Behavior and Associated Data. Mach. Learn. 2025, 114, 206. [Google Scholar] [CrossRef]
- Vani, M.S.; Sudhakar, R.V.; Mahendar, A.; Ledalla, S.; Radha, M.; Sunitha, M. Personalized Health Monitoring Using Explainable AI: Bridging Trust in Predictive Healthcare. Sci. Rep. 2025, 15, 31892. [Google Scholar] [CrossRef]
- Ahmad, R. Developing Trustworthy and Ethically-Based Healthcare Systems. Appl. Comput. Inform. 2025, 1–13. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y. MLXOps4Medic: A Service Framework for Machine Learning and Explainability Operations in Medical Imaging AI Development. IEEE Access 2025, 13, 158149–158169. [Google Scholar] [CrossRef]
- Lockhart-Jones, H.; Biddle, J.; Lee, A.; Vau, J.; Bale, M.; Thompson, S. Enabling Federated Access to Administrative Data and Beyond. Int. J. Popul. Data Sci. 2025, 10. [Google Scholar] [CrossRef]
- Chornenky, D.; Haddad, T.C.; Stetson, P.D.; Goodman, C.S. Artificial Intelligence in Cancer Care: Opportunities, Challenges, and Governance. J. Natl. Compr. Cancer Netw. 2025, 23, e255006. [Google Scholar] [CrossRef]
- Diab, A.R.; Lotter, W. Distinguishing between Rigor and Transparency in FDA Marketing Authorization of AI-Enabled Medical Devices. Radiol. Artif. Intell. 2025, e250369. [Google Scholar] [CrossRef]
- El Arab, R.A.; Al Moosa, O.A.; Sagbakken, M. Economic, Ethical, and Regulatory Dimensions of Artificial Intelligence in Healthcare: An Integrative Review. Front. Public Health 2025, 13, 1617138. [Google Scholar] [CrossRef]
- Evangelou, K.; Kotsantis, I.; Kalyvas, A.; Kyriazoglou, A.; Economopoulou, P.; Velonakis, G.; Gavra, M.; Psyrri, A.; Boviatsis, E.J.; Stavrinou, L.C. Artificial Intelligence in the Diagnosis and Treatment of Brain Gliomas. Biomedicines 2025, 13, 2285. [Google Scholar] [CrossRef] [PubMed]
- Pesapane, F.; Nicosia, L.; D’Amelio, L.; Quercioli, G.; Pannarale, M.R.; Priolo, F.; Marinucci, I.; Farina, M.G.; Penco, S.; Dominelli, V.; et al. Artificial Intelligence-Driven Personalization in Breast Cancer Screening: From Population Models to Individualized Protocols. Cancers 2025, 17, 2901. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Mitchell, S.; Coad, G.; Michels, D. Safe and Responsible Use of Artificial Intelligence in Health Care: Current Regulatory Landscape and Considerations for Regulatory Policy. JCO Clin. Cancer Inform. 2025, 9, e2500123. [Google Scholar] [CrossRef]
- Windecker, D.; Baj, G.; Shiri, I.; Kazaj, P.M.; Kaesmacher, J.; Gräni, C.; Siontis, G.C.M. Generalizability of FDA-Approved AI-Enabled Medical Devices for Clinical Use. JAMA Netw. Open 2025, 8, e258052. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Wang, F.; Zhang, Y.; Huang, D. Addressing the Current Challenges in the Clinical Application of AI-Based Radiomics for Cancer Imaging. Front. Med. 2025, 12, 1674397. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, A.; Berretta, M.; Santorsola, M.; Ferrara, G.; Picone, C.; Savarese, G.; Ottaiano, A. Towards Post-Genomic Oncology: Embracing Cancer Complexity via Artificial Intelligence, Multi-Targeted Therapeutics, Drug Repurposing, and Innovative Study Designs. Int. J. Mol. Sci. 2025, 26, 7723. [Google Scholar] [CrossRef]
- Kisia, N. Abstract A007: Artificial Intelligence in Radiation Therapy: Bringing in a New Era of Cancer Therapy, Accurate and Fast. Clin. Cancer Res. 2025, 31, A007. [Google Scholar] [CrossRef]
- Li, R. Abstract IA05: Toward Multi-Modal Foundation AI for Precision Oncology. Clin. Cancer Res. 2025, 31, IA05. [Google Scholar] [CrossRef]
- Rai, P.; Mahajan, A. Radiomics in Head and Neck Squamous Cell Carcinoma—A Leap towards Precision Oncology. J. Immunother. Cancer 2025, 13, e011692. [Google Scholar] [CrossRef] [PubMed]
- Traverso, A.; Tonon, G.; Mahon, P.; Ciliberto, G.; Dekker, A.; Fernández, X.; Albini, A. Abstract 3372: Building Precision Oncology via Real-World Data Standardization, Privacy Preserving Infrastructures, and Collaboration among IT and Clinical Experts: The DIGICORE Network Experience. Cancer Res. 2025, 85, 3372. [Google Scholar] [CrossRef]
- Tripathi, A.G.; Waqas, A.; Yilmaz, Y.; Schabath, M.B.; Rasool, G. Abstract 3641: Predicting Treatment Outcomes Using Cross-Modality Correlations in Multimodal Oncology Data. Cancer Res. 2025, 85, 3641. [Google Scholar] [CrossRef]
- Yazdani, E.; Neizehbaz, A.; Karamzade-Ziarati, N.; Emami, F.; Vosoughi, H.; Asadi, M.; Mahmoudi, A.; Sadeghi, M.; Kheradpisheh, S.R.; Geramifar, P. Transforming [177Lu]Lu-PSMA-617 Treatment Planning: Machine Learning-based Radiodosiomics and Swin UNETR Using Pretherapy PSMA Positron Emission Tomography/Computed Tomography (PET/CT). Med. Phys. 2025, 52, e70030. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, E.; Shi, J.; Wu, X.; Cao, S.; Huang, S.; Ai, Z.; Su, J. Individualized Treatment Recommendations for Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma Utilizing Deep Learning. Front. Med. 2025, 11, 1478842. [Google Scholar] [CrossRef] [PubMed]
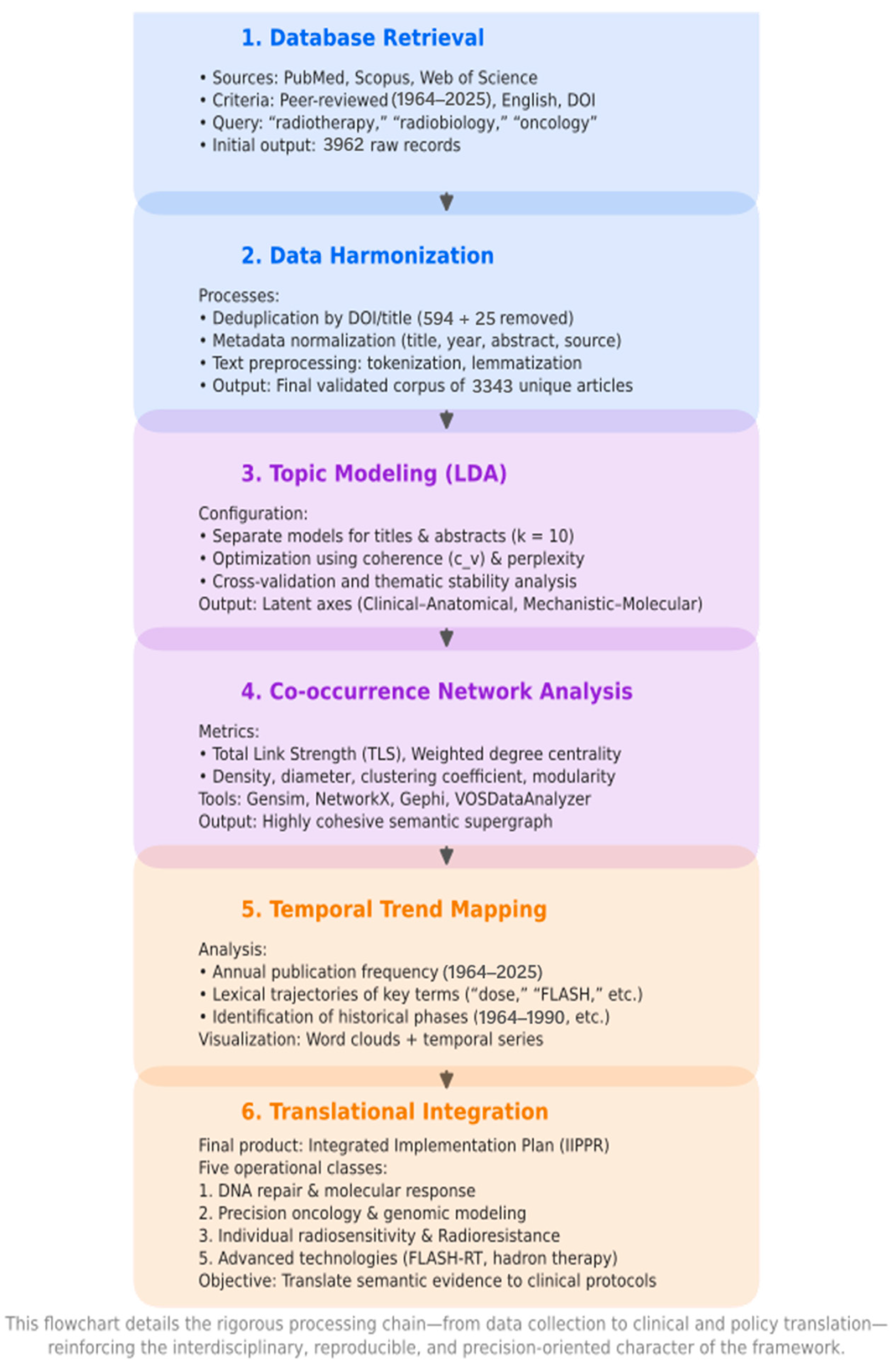
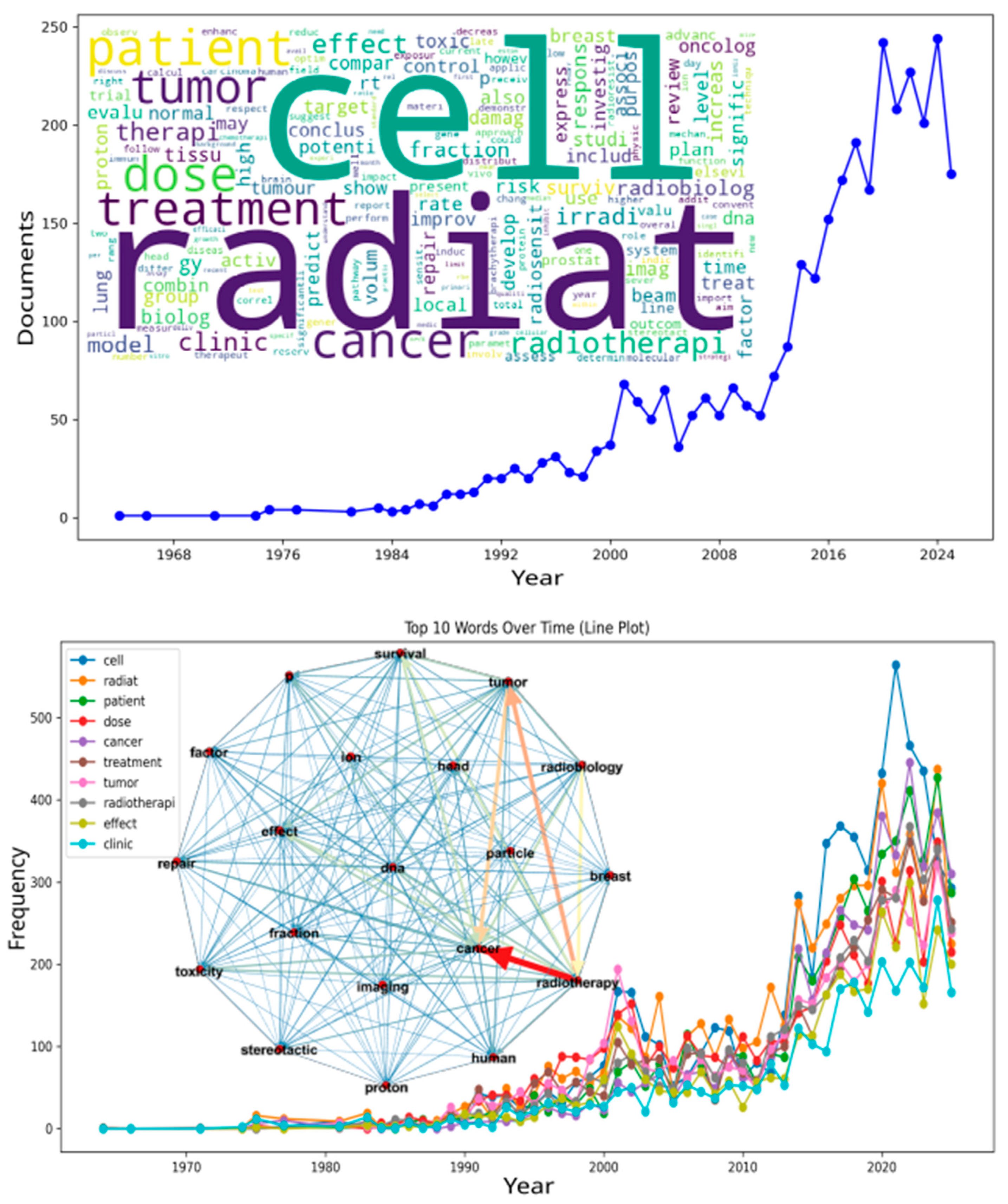

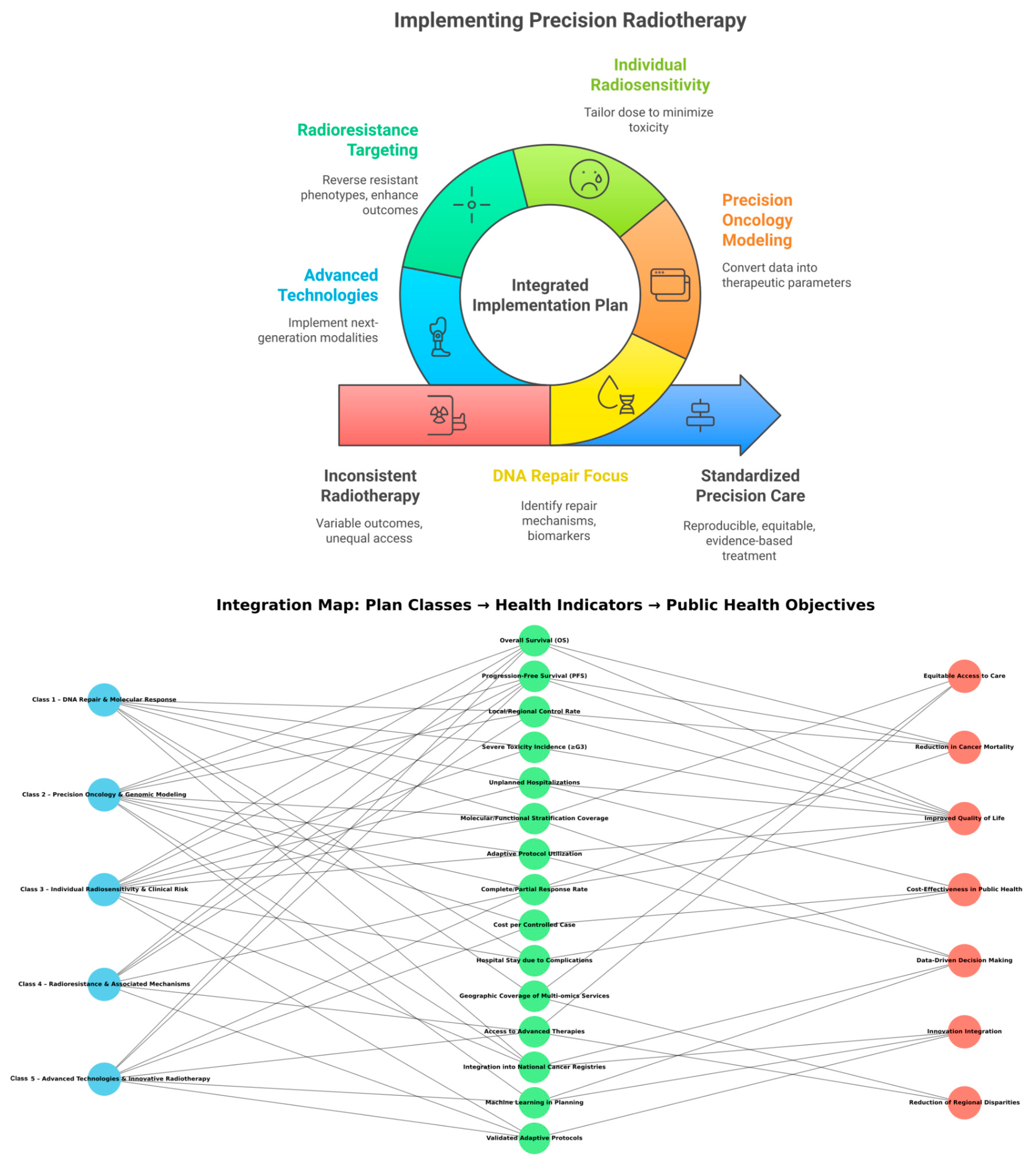
| # | DOI | Title | Reference | Year | Terms Found | Classes * |
|---|---|---|---|---|---|---|
| 1 | 10.1007/s12553-024-00895-y | Current trends and future perspectives in hadron therapy: radiobiology | [115] | 2024 | DNA repair | (1) |
| 2 | 10.3389/fonc.2021.687672 | The Time for Chronotherapy in Radiation Oncology | [116] | 2021 | DNA repair | (1) |
| 3 | 10.1667/RADE-24-00037.1 | What’s changed in 75 years of RadRes?—An Australian perspective on selected topics | [117] | 2024 | DNA repair | (1) |
| 4 | 10.3389/fnano.2025.1603334 | Radiobiological perspective on metrics for quantifying dose enhancement effects of High-Z nanoparticles | [118] | 2025 | DNA repair, precision oncology | (1, 2) |
| 5 | 10.1016/j.clon.2021.09.003 | Can Rational Combination of Ultra-high Dose Rate FLASH Radiotherapy with Immunotherapy Provide a Novel Approach to Cancer Treatment? | [119] | 2021 | flash radiotherapy | (5) |
| 6 | 10.1016/j.canlet.2025.217895 | Unraveling the dual nature of FLASH radiotherapy: From normal tissue sparing to tumor control | [15] | 2025 | flash radiotherapy, radiosensitivity | (5, 3) |
| 7 | 10.18632/oncotarget.10996 | Next generation multi-scale biophysical characterization of high precision cancer particle radiotherapy using clinical proton, helium-, carbon- and oxygen ion beams | [120] | 2016 | precision oncology | (2) |
| 8 | 10.3390/ijms26136375 | Radiation Therapy Personalization in Cancer Treatment: Strategies and Perspectives | [121] | 2025 | precision oncology | (2) |
| 9 | 10.1080/14737140.2018.1458615 | The effects of radiotherapy on the survival of patients with unresectable non-small cell lung cancer | [122] | 2018 | precision oncology | (2) |
| 10 | 10.1016/j.hoc.2019.07.001 | Toward a New Framework for Clinical Radiation Biology | [123] | 2019 | precision oncology | (2) |
| 11 | 10.1016/S0007-4551(18)30383-7 | Kidney cancer and radiotherapy: Radioresistance and beyond | [124] | 2018 | radioresistance | (4) |
| 12 | 10.1093/jrr/rrz048 | Carbon-ion irradiation overcomes HPV-integration/E2 gene-disruption induced radioresistance of cervical keratinocytes | [125] | 2019 | radioresistance, radiosensitivity | (4, 3) |
| 13 | 10.1016/j.radonc.2022.08.001 | Accurate prediction of long-term risk of biochemical failure after salvage radiotherapy including the impact of pelvic node irradiation | [126] | 2022 | radiosensitivity | (3) |
| 14 | 10.1186/s13014-024-02566-8 | Alpha/beta values in pediatric medulloblastoma: implications for tailored approaches in radiation oncology | [127] | 2025 | radiosensitivity | (3) |
| 15 | 10.32471/exp-oncology.2312-8852.vol-43-no-3.16554 | Can SARS-CoV-2 change individual radiation sensitivity of the patients recovered from COVID-19? (experimental and theoretical background). | [128] | 2021 | radiosensitivity | (3) |
| 16 | 10.3390/cells12030360 | Comparison of Radiation Response between 2D and 3D Cell Culture Models of Different Human Cancer Cell Lines | [129] | 2023 | radiosensitivity | (3) |
| 17 | 10.2298/SARH220131085N | Current aspects of radiobiology in modern radiotherapy—our clinical experience | [130] | 2022 | radiosensitivity | (3) |
| 18 | 10.1002/mp.13751 | Genomics models in radiotherapy: From mechanistic to machine learning | [131] | 2020 | radiosensitivity | (3) |
| 19 | 10.1016/j.rpor.2014.11.006 | Hypersensitivity to chemoradiation in FANCA carrier with cervical carcinoma-A case report and review of the literature. | [132] | 2015 | radiosensitivity | (3) |
| 20 | 10.3390/cancers8030028 | Paradigm Shift in Radiation Biology/Radiation Oncology Exploitation of the H2O2 Effect for Radiotherapy Using Low-LET (Linear Energy Transfer) Radiation such as X-rays and High-Energy Electrons | [133] | 2016 | radiosensitivity | (3) |
| 21 | 10.1259/bjr.20170949 | Radiation biology and oncology in the genomic era | [134] | 2018 | radiosensitivity | (3) |
| 22 | 10.1088/1361-6560/aac814 | Radiobiological parameters in a tumor control probability model for prostate cancer LDR brachytherapy | [135] | 2018 | radiosensitivity | (3) |
| 23 | 10.1136/bmjopen-2021-059345 | The COMPLETE trial: HolistiC early respOnse assessMent for oroPharyngeaL cancEr paTiEnts; Protocol for an observational study | [136] | 2022 | radiosensitivity | (3) |
| 24 | 10.1016/j.radonc.2023.109868 | Voxel-based analysis: Roadmap for clinical translation | [137] | 2023 | radiosensitivity | (3) |
| 25 | 10.1002/cnr2.1126 | Progressive breast fibrosis caused by extreme radiosensitivity: Oncocytogenetic diagnosis and treatment by reconstructive flap surgery. | [138] | 2019 | radiosensitivity, DNA repair | (3, 1) |
| 26 | 10.1002/mp.15177 | Radiobiological comparison between Cobalt-60 and Iridium-192 high-dose-rate brachytherapy sources: Part I—cervical cancer | [139] | 2021 | radiosensitivity, hypoxia-induced | (3, 5) |
| 27 | 10.1016/j.ijrobp.2015.11.013 | Influence of Nucleoshuttling of the ATM Protein in the Healthy Tissues Response to Radiation Therapy: Toward a Molecular Classification of Human Radiosensitivity | [140] | 2016 | radiosensitivity, radioresistance | (3, 4) |
| 28 | 10.3390/proteomes13020025 | Deciphering Radiotherapy Resistance: A Proteomic Perspective | [141] | 2025 | radiosensitivity, radioresistance, DNA repair, precision oncology | (3, 4, 1, 2) |
| Category | Specific Biomarker | Mechanism/Clinical Interpretation | Detection Modality | Associated Cancers | Predictive Performance | Limitations | References |
|---|---|---|---|---|---|---|---|
| Circulating Tumor DNA (ctDNA) | ctDNA (absolute levels or tumor fraction) | Early reduction → regression; increase/persistence → progression or resistance | Targeted sequencing/plasma NGS | NSCLC, colorectal, breast, gastroesophageal | High sensitivity for early detection of response or resistance (days to weeks before imaging) | Low sensitivity in “low-shedding” tumors; dependence on personalized panels | [206] |
| ctDNA—Variant Allele Frequency (VAF) | Rapid VAF decline after therapy onset → favorable response | ddPCR or NGS | NSCLC, melanoma, colorectal | Strong correlation with PFS and OS | Affected by tumor heterogeneity and clonal evolution | [207] | |
| ctDNA—emerging resistance mutations (e.g., EGFR C797S) | Detection of resistance mutations → impending therapeutic failure | Longitudinal sequencing | NSCLC | High specificity for acquired resistance | Requires prior knowledge of mutational profile | [208] | |
| ctDNA—personalized “fingerprint” panels | Multi-mutation tracking per patient → enhanced coverage of tumor heterogeneity | WES + customized panels | Multiple solid tumors | Better sensitivity vs. fixed panels | Higher cost and logistical complexity | [209] | |
| Epigenomic/Extracellular Vesicle Markers | cfDNA methylation profiles | Distinct methylation patterns linked to molecular subtypes and immune response | Bisulfite sequencing or methylation arrays | NSCLC, colorectal | High prognostic accuracy; predicts immunotherapy response | Requires advanced bioinformatics | [210] |
| EV-miRNAs (extracellular vesicle miRNAs) | Post-transcriptional regulation of immune and oncogenic pathways | EV RNA-seq or qPCR | NSCLC, breast | Complements cfDNA; enhances stratification | Low stability; isolation variability | [210] | |
| Proteomic/Serum Markers | sHER2, CEA, CA15.3 | Serum levels correlate with tumor burden and treatment response | ELISA/immunoassay | Breast, colorectal | Useful for continuous monitoring; low cost | Limited specificity when used alone | [211] |
| Plasma proteomic signatures (aptamer-based) | Dynamic profiles reflect tumor activity and immune response | Aptamer proteomics (e.g., SomaScan) | NSCLC | High temporal resolution; predicts immune-related adverse events (irAEs) | Requires broad clinical validation | [212] | |
| Cytokeratins, TK1, growth factors | Indicators of proliferation and aggressiveness | Immunoassay | Colorectal | Associated with stage and prognosis | Less sensitive than ctDNA | [213] | |
| Immunologic Markers | Tumor-infiltrating lymphocytes (TILs) | High density → regression; exhaustion → progression | Tissue IHC or RNA-seq | Pan-cancer | Robust prognostic value | Invasive; not suitable for serial monitoring | [214] |
| T-cell receptor (TCR) repertoire | Early clonal expansion → immunotherapy response | Blood TCR sequencing | Melanoma, HNSCC | Predictive for immune checkpoint blockade | Analytical complexity | [215] | |
| Ferroptosis-related gene activity | Ferroptotic death → enhanced immunogenicity | Transcriptomic scoring (ferroptosis score) | NSCLC, melanoma | Adds value beyond TMB/PD-L1 | Mechanism under functional validation | [216] | |
| PTPRD/PTPRT mutations | Loss of function → inflammatory tumor microenvironment → better ICB response | Genomic sequencing | Pan-cancer | Genomic biomarker for pan-cancer response | Variable frequency among tumors | [217] | |
| Imaging Biomarkers | Early Tumor Shrinkage (ETS) | ≥20% reduction within 6–8 weeks → regression | CT/MRI (RECIST) | Colorectal, NSCLC | Correlates with PFS/OS | Limited by pseudoprogression or stable disease | [218] |
| Depth of Response (DpR) | Maximum tumor shrinkage → improved prognosis | Continuous imaging analysis | Various solid tumors | More informative than binary response | Depends on standardized imaging protocols | [218] | |
| Genomic/Transcriptomic Signatures | Chromosomal instability (CIN) | High CIN → poor prognosis | Gene expression profiling (e.g., Carter et al.) | Breast, ovarian, colorectal | Independent prognostic value | Static; based on single biopsy | [219] |
| Progression Gene Signatures (PGS) | Activation of proliferation/metastasis pathways → progression | RNA-seq + ML modeling | Multiple cancers | High predictive power in cohorts | Requires prospective validation | [220] | |
| Kinome profiling | Activation of tumor-promoting kinase pathways | Functional proteomics + RNA | Colon | Identifies therapeutic targets | Technically complex; limited clinical use | [221] | |
| Integrated Models | ctDNA + imaging | Combines molecular dynamics with anatomical response | ML-based multimodal integration | Breast, NSCLC | Superior predictive accuracy vs. single biomarkers | Requires computational infrastructure | [222] |
| ctDNA + proteomics + immunophenotyping | Comprehensive profile of tumor–host evolution | Longitudinal multi-omics | NSCLC | High predictive and stratification accuracy | High cost; lack of standardization | [210] |
| Indicator | Related Plan Class(es) | Description and Measurement Method | Expected Public Health Impact |
|---|---|---|---|
| Overall survival (OS) at 1, 3, and 5 years | 2, 3, 4, 5 | Percentage of patients alive 1, 3, and 5 years after radiotherapy, stratified by tumor type and treatment protocol. | Improved longevity and reduction in cancer-specific mortality, particularly in high-risk tumors. |
| Progression-free survival (PFS) | 2, 3, 4, 5 | Mean time to recurrence or disease progression after personalized radiotherapy. | Greater disease control, reduced retreatment, and lower hospital costs. |
| Local and regional control ratel | 1, 2, 3, 4 | Proportion of patients without recurrence in the tumor bed or regional lymph nodes after 12 and 24 months. | Increased therapeutic efficacy and improved population prognosis. |
| Incidence of acute and late toxicities (grade ≥ 3) | 1, 2, 3 | Standardized reporting (RTOG/EORTC or CTCAE) of severe adverse events related to radiotherapy. | Reduced disabling side effects and improved post-treatment quality of life. |
| Rate of unplanned hospitalizations related to treatment | 1, 3 | Percentage of patients requiring emergency hospitalization due to radiotherapy complications. | Savings in hospital resources and reduced burden on inpatient units. |
| Percentage of patients undergoing molecular and functional stratification prior to treatment | 1, 2, 3 | Proportion of patients who underwent genomic, proteomic, or functional testing before radiotherapy initiation. | Greater precision in therapeutic planning and optimization of resource allocation. |
| Proportion of treatments with adaptive protocols | 2, 3, 5 | Percentage of patients whose radiotherapy plan was adjusted during treatment based on clinical response and/or biomarkers. | Reduced toxicity, improved efficacy, and more rational use of technology. |
| Complete and partial response rate | 2, 4, 5 | Percentage of patients with complete disappearance or significant reduction in tumor after treatment. | Direct indicator of personalized protocol effectiveness. |
| Average cost per patient with tumor control achieved | 2, 5 | Total treatment cost divided by the number of patients with sustained complete or partial response. | Increased economic efficiency of the public health system. |
| Mean hospital stay due to treatment complications | 1, 3 | Average number of days of hospitalization caused by radiotherapy-related adverse events. | Reduced hospital bed usage and expanded treatment capacity. |
| Geographic coverage of services with multi-omic and radiobiological capacity | 1, 2, 3 | Percentage of health regions with infrastructure for multi-omic testing and radiobiological integration. | Reduction in regional inequalities in access to advanced treatments. |
| Percentage of patients with access to advanced therapies in the public health system (SUS) | 4, 5 | Proportion of patients receiving modalities such as FLASH-RT, optimized brachytherapy, or particle therapy. | Equity in access to innovative technologies across the national territory. |
| Proportion of cases integrated into national registries with molecular and radiomic data | 1, 2, 3, 4, 5 | Percentage of patients with clinical, molecular, and imaging data stored in national repositories. | Strengthened epidemiological surveillance and translational research. |
| Utilization rate of machine learning in clinical planning | 2, 3, 5 | Percentage of cases planned or adjusted using validated AI models. | Standardization of evidence-based practices and increased outcome predictability. |
| Number of adaptive protocols validated and incorporated into national guidelines | 3, 4, 5 | Number of adaptive clinical protocols formally approved and implemented in the public health system. | Continuous improvement of care quality and systematic technological updating. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Jr., F.G.; Aliaga Jr., J.M.; Duarte Jr., P.C.; Crispilho, S.; Delfino, C.; Brandão, D.; Zamprogno e Silva, F. From Semantic Modeling to Precision Radiotherapy: An AI Framework Linking Radiobiology, Oncology, and Public Health Integration. Biomedicines 2025, 13, 2862. https://doi.org/10.3390/biomedicines13122862
de Souza Jr. FG, Aliaga Jr. JM, Duarte Jr. PC, Crispilho S, Delfino C, Brandão D, Zamprogno e Silva F. From Semantic Modeling to Precision Radiotherapy: An AI Framework Linking Radiobiology, Oncology, and Public Health Integration. Biomedicines. 2025; 13(12):2862. https://doi.org/10.3390/biomedicines13122862
Chicago/Turabian Stylede Souza Jr., Fernando Gomes, José Maria Aliaga Jr., Paulo C. Duarte Jr., Shirley Crispilho, Carolina Delfino, Daniele Brandão, and Fernando Zamprogno e Silva. 2025. "From Semantic Modeling to Precision Radiotherapy: An AI Framework Linking Radiobiology, Oncology, and Public Health Integration" Biomedicines 13, no. 12: 2862. https://doi.org/10.3390/biomedicines13122862
APA Stylede Souza Jr., F. G., Aliaga Jr., J. M., Duarte Jr., P. C., Crispilho, S., Delfino, C., Brandão, D., & Zamprogno e Silva, F. (2025). From Semantic Modeling to Precision Radiotherapy: An AI Framework Linking Radiobiology, Oncology, and Public Health Integration. Biomedicines, 13(12), 2862. https://doi.org/10.3390/biomedicines13122862