Herbal Neuroprotection Meets Stress-Induced Neuropathology: Bojungikgi-Tang Modulates the Hypothalamic–Pituitary–Adrenal Axis and GABAergic Pathways in Post-Traumatic Stress Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
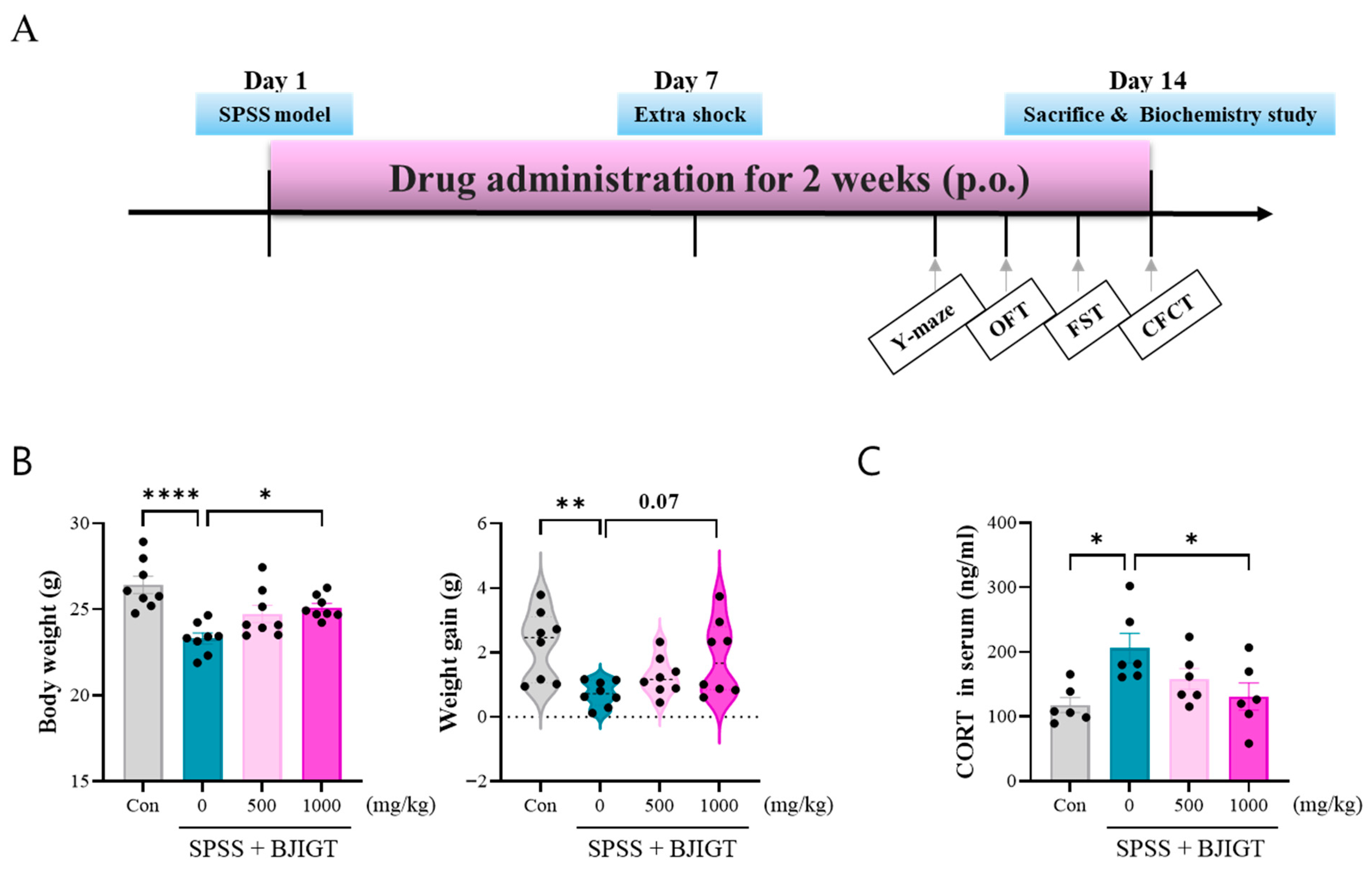
2.2. Drug Administration
2.3. SPSS-Induced PTSD Animal Model
2.4. Behavioral Tests
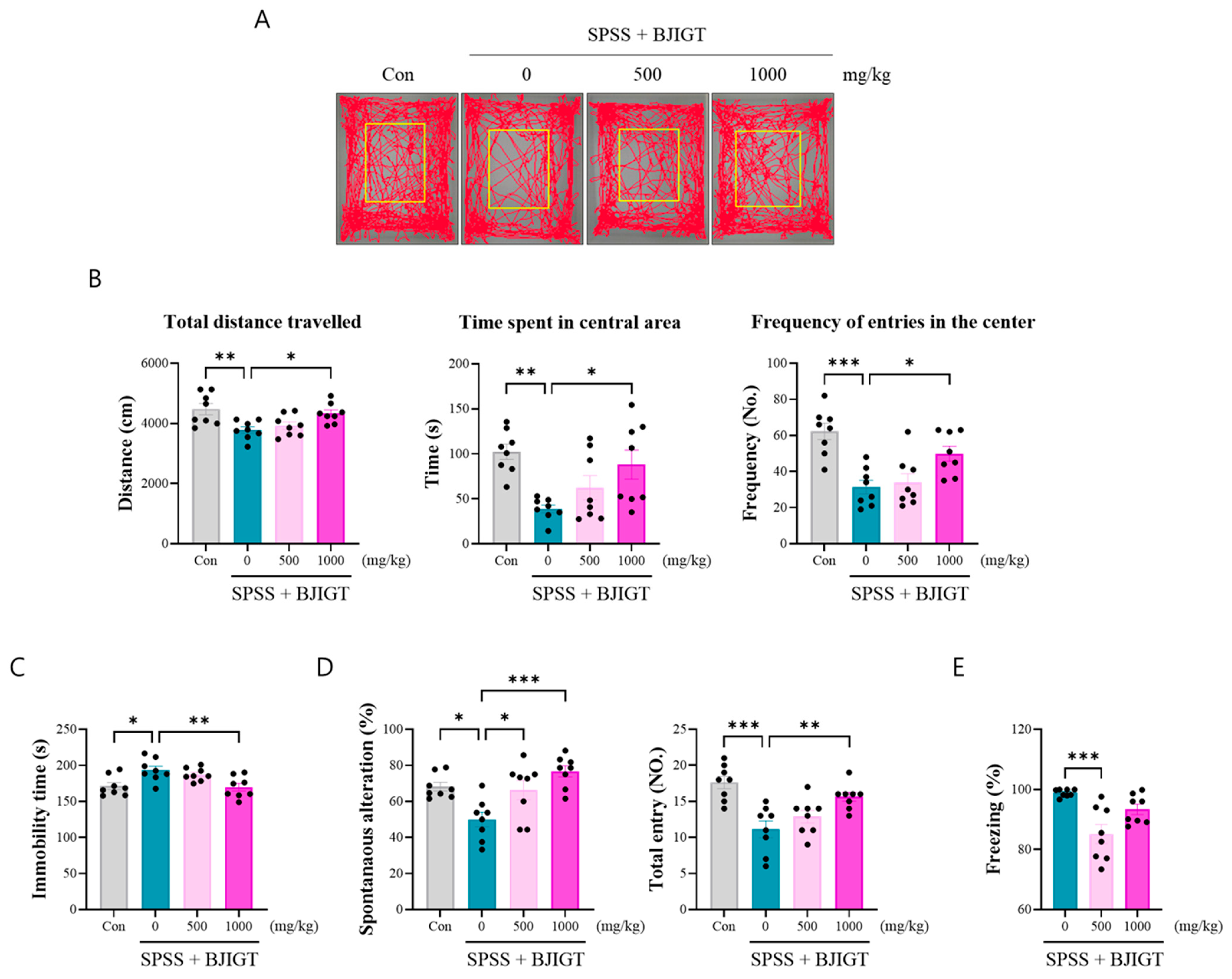
2.4.1. Open Field Test (OFT)
2.4.2. Forced Swimming Test (FST)
2.4.3. Y-Maze Test
2.4.4. Contextual Fear Conditioning Test (CFCT)
2.5. Tissue Preparation
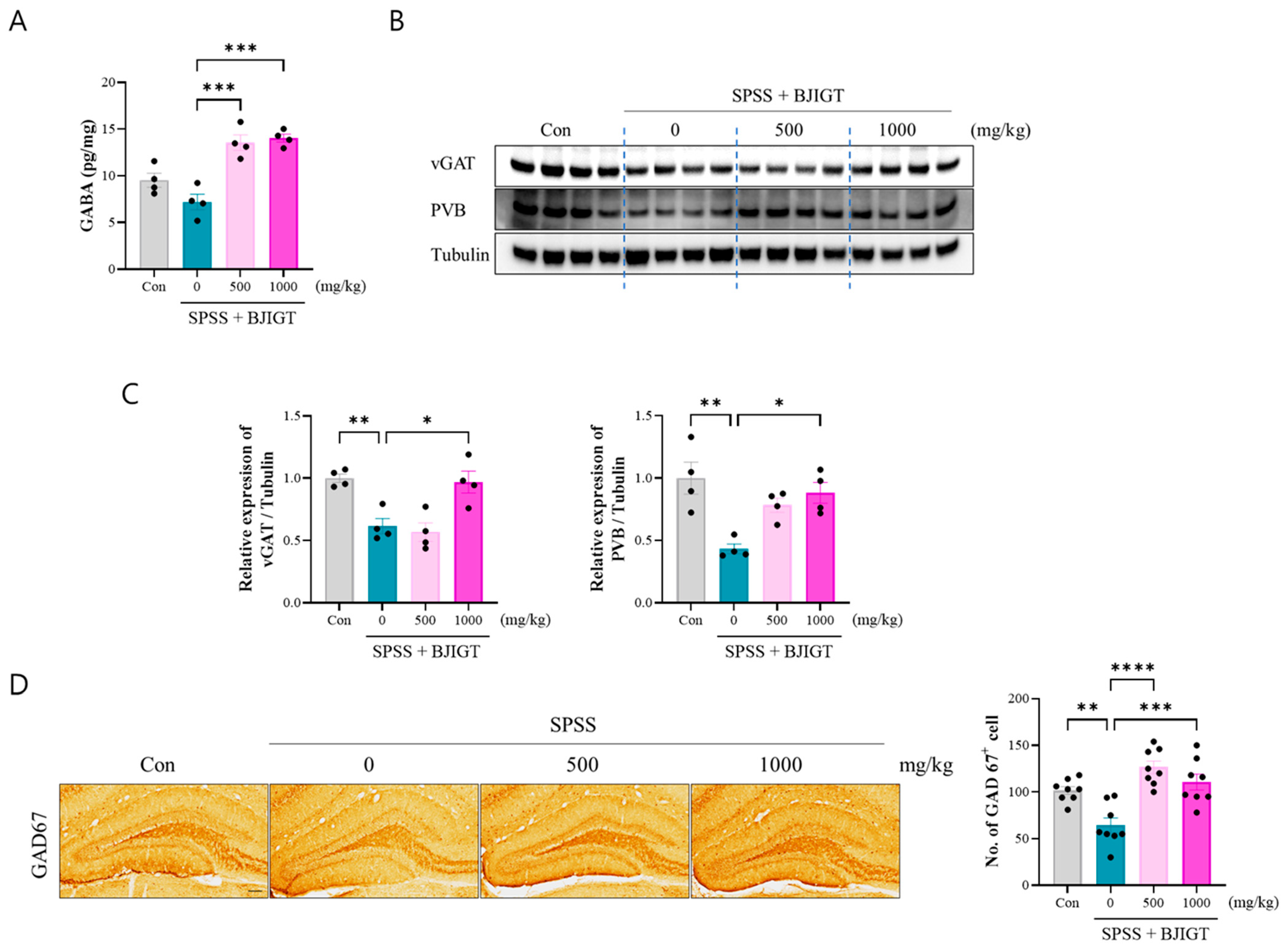
2.6. Corticosterone and GABA ELISA
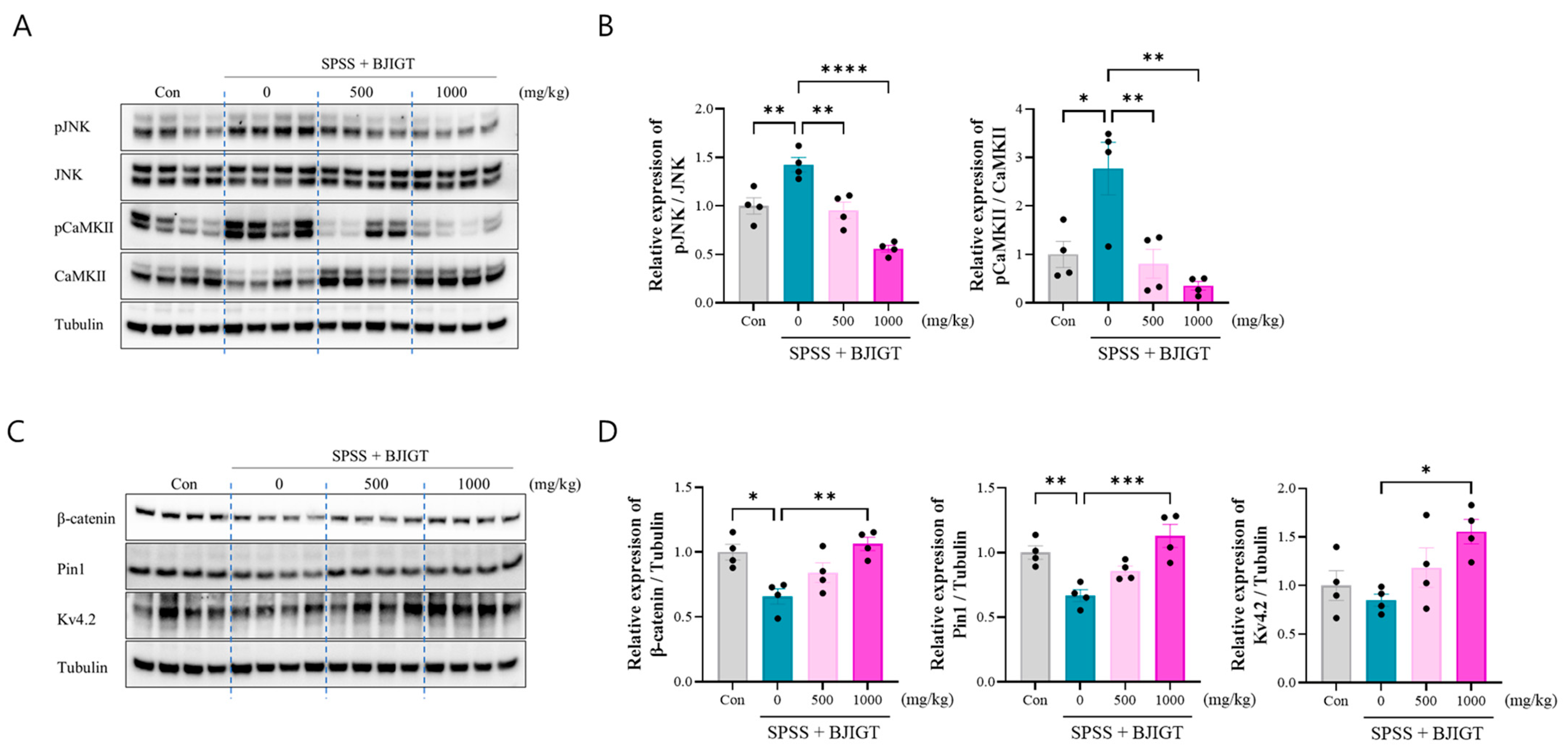
2.7. Western Blotting
2.8. Immunohistochemistry (IHC)
2.9. Statistical Analysis
3. Results
3.1. BJIGT Administration Regulates Stress Response
3.2. BJIGT Administration Alleviates Emotional and Cognitive Decline
3.3. BJIGT Administration Modulates Neuronal Function
3.4. BJIGT Administration Regulates GABAergic Neurotransmission
3.5. BJIGT Administration Restores Intracellular Signaling Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSRIs | Selective serotonin reuptake inhibitors |
| BJIGT | Bojungikgi-tang |
| BDNF | Brain-derived neurotrophic factor |
| CAM | Complementary and alternative medicine |
| CFCT | Contextual fear conditioning test |
| CORT | Corticosterone |
| FST | Forced swimming test |
| GAD67 | Glutamate decarboxylase 67 |
| HPA | Hypothalamic–pituitary–adrenal |
| OFT | Open field test |
| PTSD | Post-traumatic stress disorder |
| SPSS | Single-prolonged stress with shock |
| vGAT | Vesicular GABA transporter |
References
- Battle, D.E. Diagnostic and Statistical Manual of Mental Disorders (DSM). Codas 2013, 25, 191–192. [Google Scholar]
- Zhang, H.; Hu, Y.; Yu, Y.; Zhou, Z.; Sun, Y.; Qi, C.; Yang, L.; Xie, H.; Zhang, J.; Zhu, H. The value of multimodal neuroimaging in the diagnosis and treatment of post-traumatic stress disorder: A narrative review. Transl. Psychiatry 2025, 15, 208. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Phillips, N.J.; Stein, D.J.; Ipser, J.C. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst. Rev. 2022, 3, CD002795. [Google Scholar] [CrossRef] [PubMed]
- Nohr, A.K.; Eriksson, H.; Hobart, M.; Moltke, I.; Buller, R.; Albrechtsen, A.; Lindgreen, S. Predictors and trajectories of treatment response to SSRIs in patients suffering from PTSD. Psychiatry Res. 2021, 301, 113964. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Park, H.R.; Yang, E.J. Nutraceutical Interventions for Post-Traumatic Stress Disorder in Animal Models: A Focus on the Hypothalamic-Pituitary-Adrenal Axis. Pharmaceuticals 2022, 15, 898. [Google Scholar] [CrossRef]
- Traina, G.; Tuszynski, J.A. The Neurotransmission Basis of Post-Traumatic Stress Disorders by the Fear Conditioning Paradigm. Int. J. Mol. Sci. 2023, 24, 16327. [Google Scholar] [CrossRef]
- Huang, J.; Xu, F.; Yang, L.; Tuolihong, L.; Wang, X.; Du, Z.; Zhang, Y.; Yin, X.; Li, Y.; Lu, K.; et al. Involvement of the GABAergic system in PTSD and its therapeutic significance. Front. Mol. Neurosci. 2023, 16, 1052288. [Google Scholar] [CrossRef]
- Park, H.R.; Cai, M.; Yang, E.J. Herbal Formula Extract Ameliorates Anxiety and Cognitive Impairment via Regulation of the Reelin/Dab-1 Pathway in a Murine Model of Post-Traumatic Stress Disorder. Pharmaceutics 2024, 16, 1150. [Google Scholar] [CrossRef]
- Hourigan, B.; Balay, S.D.; Yee, G.; Sharma, S.; Tan, Q. Capicua regulates the development of adult-born neurons in the hippocampus. Sci. Rep. 2021, 11, 11725. [Google Scholar] [CrossRef]
- Besnard, A.; Sahay, A. Adult Hippocampal Neurogenesis, Fear Generalization, and Stress. Neuropsychopharmacology 2016, 41, 24–44. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, Y.J.; Sohn, E.; Yoon, J.; Kim, B.Y.; Jeong, S.J. Bojungikgi-Tang, a Traditional Herbal Formula, Exerts Neuroprotective Effects and Ameliorates Memory Impairments in Alzheimer’s Disease-Like Experimental Models. Nutrients 2018, 10, 1952. [Google Scholar] [CrossRef]
- Cai, M.; Lee, S.H.; Yang, E.J. Bojungikgi-tang Improves Muscle and Spinal Cord Function in an Amyotrophic Lateral Sclerosis Model. Mol. Neurobiol. 2019, 56, 2394–2407. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J. Combined Treatment with Bojungikgi-tang (Buzhong Yiqi Decoction) and Riluzole Attenuates Cell Death in TDP-43-Expressing Cells. Chin. J. Integr. Med. 2024, 30, 616–622. [Google Scholar] [CrossRef]
- Lee, B.; Ko, M.M.; Ahn, Y.M.; Park, H.J.; Jung, S.Y.; Jung, H.A.; Lee, H.; Kim, P.W.; Choi, Y.; Han, K.; et al. Effect of herbal medicine Bojungikgi-tang on gut microbiome and symptoms in anorexic patients with atopic dermatitis: A randomized controlled trial. Front. Pharmacol. 2025, 16, 1593477. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Park, H.R.; Yang, E.J. Hominis Placenta modulates PTSD-like behaviors in SPSS-induced PTSD mice: Regulating energy metabolism and neuronal activity. Biomed. Pharmacother. 2024, 178, 117243. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Zheng, H.; Wang, H.N.; Jin, X.; Chen, Y.C.; Zheng, L.N.; Luo, X.X.; Tan, Q.R. A modified single-prolonged stress model for post-traumatic stress disorder. Neurosci. Lett. 2008, 441, 237–241. [Google Scholar] [CrossRef]
- Lisieski, M.J.; Eagle, A.L.; Conti, A.C.; Liberzon, I.; Perrine, S.A. Single-Prolonged Stress: A Review of Two Decades of Progress in a Rodent Model of Post-traumatic Stress Disorder. Front. Psychiatry 2018, 9, 196. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Cryan, J.F.; Markou, A.; Lucki, I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol. Sci. 2002, 23, 238–245. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, M.S.; Kong, C.H.; Min, H.S.; Kang, W.C.; Park, K.; Jung, S.Y.; Bae, H.J.; Park, S.J.; Lee, J.Y.; et al. 4-Methoxycinnamic acid ameliorates post-traumatic stress disorder-like behavior in mice by antagonizing the CRF type 1 receptor. Life Sci. 2025, 361, 123271. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, 52587. [Google Scholar]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar]
- More, J.; Casas, M.M.; Sanchez, G.; Hidalgo, C.; Haeger, P. Contextual Fear Memory Formation and Destabilization Induce Hippocampal RyR2 Calcium Channel Upregulation. Neural Plast. 2018, 2018, 5056181. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Cai, M.; Yang, E.J. Novel Psychopharmacological Herbs Relieve Behavioral Abnormalities and Hippocampal Dysfunctions in an Animal Model of Post-Traumatic Stress Disorder. Nutrients 2023, 15, 3815. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Park, H.R.; Yang, E.J. Electroacupuncture modulates glutamate neurotransmission to alleviate PTSD-like behaviors in a PTSD animal model. Transl. Psychiatry 2023, 13, 357. [Google Scholar] [CrossRef]
- Hu, J.H.; Malloy, C.; Tabor, G.T.; Gutzmann, J.J.; Liu, Y.; Abebe, D.; Karlsson, R.M.; Durell, S.; Cameron, H.A.; Hoffman, D.A. Activity-dependent isomerization of Kv4.2 by Pin1 regulates cognitive flexibility. Nat. Commun. 2020, 11, 1567. [Google Scholar] [CrossRef]
- Verbitsky, A.; Dopfel, D.; Zhang, N. Rodent models of post-traumatic stress disorder: Behavioral assessment. Transl. Psychiatry 2020, 10, 132. [Google Scholar] [CrossRef]
- Shin, H.Y.; Shin, C.H.; Shin, T.Y.; Lee, E.J.; Kim, H.M. Effect of bojungikki-tang on lipopolysaccharide-induced cytokine production from peripheral blood mononuclear cells of chronic fatigue syndrome patients. Immunopharmacol. Immunotoxicol. 2003, 25, 491–501. [Google Scholar] [CrossRef]
- Lee, J.A.; Jang, S.; Jun, J.H.; Lee, M.S.; Lee, E.; Kim, N.; Lee, D.H. Herbal medicine (Bojungikki-tang) for allergic rhinitis: A protocol for a systematic review of controlled trials. Medicine 2018, 97, e9551. [Google Scholar] [CrossRef]
- Hu, L.; Chen, G.; Chen, J.; Zou, Z.; Qiu, Y.; Du, J.; Tong, X.; Chen, J.; Yao, X.; Lin, P.; et al. Quantitative ternary network-oriented discovery of Q-markers from traditional Chinese medicine prescriptions: Bu-Zhong-Yi-Qi-Tang as a case study. Phytomedicine 2024, 133, 155918. [Google Scholar] [CrossRef]
- Kandhwal, M.; Grewal, A.K.; Singh, V.; Sharma, O.; Khan, H.; Singh, M.; Kumar, A.; Singh, T.G.; Singh, T.; Ahmad, S.F.; et al. Hesperidin Attenuates Chronic Stress-Induced Depression via 5-HT2A-Linked Modulation of Neurochemical, Oxidative, and Inflammatory Pathways: Experimental and In Silico Evidence. Neurochem. Res. 2025, 50, 310. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Vadia, N.; Ballal, S.; Joshi, K.K.; Maharana, L.; Chauhan, A.S.; Abomughaid, M.M.; Lakhanpal, S.; Avinash, D.; Thakur, K.; et al. Decoding the neuroprotective potential of hesperidin: Insights into Alzheimer’s disease. Neuroscience 2025, 589, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yuan, J.; Zhou, H.; Li, Z.; Yao, X.; Wu, H.; Shi, H.; Huang, F.; Wu, X. Antidepressant potential of total flavonoids from Astragalus in a chronic stress mouse model: Implications for myelination and Wnt/beta-catenin/Olig2/Sox10 signaling axis modulation. J. Ethnopharmacol. 2024, 325, 117846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Wang, X.B.; Xue, R.R.; Gao, X.X.; Li, W. Ginsenoside Rg1 attenuates chronic unpredictable mild stress-induced depressive-like effect via regulating NF-kappaB/NLRP3 pathway in rats. NeuroReport 2019, 30, 893–900. [Google Scholar] [CrossRef]
- Lawrence, S.; Scofield, R.H. Post traumatic stress disorder associated hypothalamic-pituitary-adrenal axis dysregulation and physical illness. Brain Behav. Immun. Health 2024, 41, 100849. [Google Scholar] [CrossRef]
- Perrine, S.A.; Eagle, A.L.; George, S.A.; Mulo, K.; Kohler, R.J.; Gerard, J.; Harutyunyan, A.; Hool, S.M.; Susick, L.L.; Schneider, B.L.; et al. Severe, multimodal stress exposure induces PTSD-like characteristics in a mouse model of single prolonged stress. Behav. Brain Res. 2016, 303, 228–237. [Google Scholar] [CrossRef]
- Lee, B.; Choi, G.M.; Sur, B. Silibinin prevents depression-like behaviors in a single prolonged stress rat model: The possible role of serotonin. BMC Complement. Med. Ther. 2020, 20, 70. [Google Scholar] [CrossRef]
- Gong, Q.; Yan, X.J.; Lei, F.; Wang, M.L.; He, L.L.; Luo, Y.Y.; Gao, H.W.; Feng, Y.L.; Yang, S.L.; Li, J.; et al. Proteomic profiling of the neurons in mice with depressive-like behavior induced by corticosterone and the regulation of berberine: Pivotal sites of oxidative phosphorylation. Mol. Brain 2019, 12, 118. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Wong, A. The hypothalamic-pituitary-adrenal axis in PTSD: Pathophysiology and treatment interventions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 361–379. [Google Scholar] [CrossRef]
- Shi, H.J.; Wang, S.; Wang, X.P.; Zhang, R.X.; Zhu, L.J. Hippocampus: Molecular, Cellular, and Circuit Features in Anxiety. Neurosci. Bull. 2023, 39, 1009–1026. [Google Scholar] [CrossRef]
- Domschke, K.; Zwanzger, P. GABAergic and endocannabinoid dysfunction in anxiety—Future therapeutic targets? Curr. Pharm. Des. 2008, 14, 3508–3517. [Google Scholar] [CrossRef]
- Mikkelsen, J.D.; Bundzikova, J.; Larsen, M.H.; Hansen, H.H.; Kiss, A. GABA regulates the rat hypothalamic-pituitary-adrenocortical axis via different GABA-A receptor alpha-subtypes. Ann. N. Y. Acad. Sci. 2008, 1148, 384–392. [Google Scholar] [CrossRef]
- Trousselard, M.; Lefebvre, B.; Caillet, L.; Andruetan, Y.; de Montleau, F.; Denis, J.; Canini, F. Is plasma GABA level a biomarker of Post-Traumatic Stress Disorder (PTSD) severity? A preliminary study. Psychiatry Res. 2016, 241, 273–279. [Google Scholar] [CrossRef]
- Perova, Z.; Delevich, K.; Li, B. Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. J. Neurosci. 2015, 35, 3201–3206. [Google Scholar] [CrossRef]
- Rossetti, A.C.; Paladini, M.S.; Colombo, M.; Gruca, P.; Lason-Tyburkiewicz, M.; Tota-Glowczyk, K.; Papp, M.; Riva, M.A.; Molteni, R. Chronic Stress Exposure Reduces Parvalbumin Expression in the Rat Hippocampus through an Imbalance of Redox Mechanisms: Restorative Effect of the Antipsychotic Lurasidone. Int. J. Neuropsychopharmacol. 2018, 21, 883–893. [Google Scholar] [CrossRef]
- Fogaca, M.V.; Duman, R.S. Cortical GABAergic Dysfunction in Stress and Depression: New Insights for Therapeutic Interventions. Front. Cell Neurosci. 2019, 13, 87. [Google Scholar] [CrossRef]
- Albrecht, A.; Redavide, E.; Regev-Tsur, S.; Stork, O.; Richter-Levin, G. Hippocampal GABAergic interneurons and their co-localized neuropeptides in stress vulnerability and resilience. Neurosci. Biobehav. Rev. 2021, 122, 229–244. [Google Scholar] [CrossRef]
- Houston, C.M.; He, Q.; Smart, T.G. CaMKII phosphorylation of the GABA(A) receptor: Receptor subtype- and synapse-specific modulation. J. Physiol. 2009, 587, 2115–2125. [Google Scholar] [CrossRef]
- An, S.; Wang, J.; Zhang, X.; Duan, Y.; Xu, Y.; Lv, J.; Wang, D.; Zhang, H.; Richter-Levin, G.; Klavir, O.; et al. alphaCaMKII in the lateral amygdala mediates PTSD-Like behaviors and NMDAR-Dependent LTD. Neurobiol. Stress 2021, 15, 100359. [Google Scholar] [CrossRef]
- Gong, X.; Fan, Z.; Xu, H.; Qu, Y.; Li, B.; Li, L.; Yan, Y.; Wu, L.; Yan, C. GABAergic interneurons in the hippocampal CA1 mediate contextual fear generalization in PTSD rats. J. Neurochem. 2024, 168, 2587–2600. [Google Scholar] [CrossRef]
- Wang, Y.; Gai, S.; Zhang, W.; Huang, X.; Ma, S.; Huo, Y.; Wu, Y.; Tu, H.; Pin, J.P.; Rondard, P.; et al. The GABA(B) receptor mediates neuroprotection by coupling to G(13). Sci. Signal. 2021, 14, eaaz4112. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, W.; Zhi, Y.; Yu, J.; Lu, D.; Luo, Z.; Yuan, L.; Chen, L.; Xu, Z.; Xu, D. Stress-Activated Protein Kinase JNK Modulates Depression-like Behaviors in Mice. Mol. Neurobiol. 2023, 60, 2367–2378. [Google Scholar] [CrossRef]
- Maguschak, K.A.; Ressler, K.J. The dynamic role of beta-catenin in synaptic plasticity. Neuropharmacology 2012, 62, 78–88. [Google Scholar] [CrossRef]
- Lv, T.; Wang, M.; Zheng, H.S.; Mao, J.D.; Yang, F.; Yang, L.; Zhao, M.G.; Liu, S.B.; Zhang, K.; Liu, R.; et al. Electroacupuncture alleviates PTSD-like behaviors by modulating hippocampal synaptic plasticity via Wnt/beta-catenin signaling pathway. Brain Res. Bull. 2023, 202, 110734. [Google Scholar] [CrossRef]
- Nakamura, K.; Kosugi, I.; Lee, D.Y.; Hafner, A.; Sinclair, D.A.; Ryo, A.; Lu, K.P. Prolyl isomerase Pin1 regulates neuronal differentiation via beta-catenin. Mol. Cell. Biol. 2012, 32, 2966–2978. [Google Scholar] [CrossRef]
- Varga, A.W.; Yuan, L.L.; Anderson, A.E.; Schrader, L.A.; Wu, G.Y.; Gatchel, J.R.; Johnston, D.; Sweatt, J.D. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J. Neurosci. 2004, 24, 3643–3654. [Google Scholar] [CrossRef]
- Smith, G.D.; Gao, N.; Lugo, J.N. Kv4.2 knockout mice display learning and memory deficits in the Lashley maze. F1000Research 2016, 5, 2456. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, L.; Rong, X.; Wang, W.; Wang, X. Effects of fluoxetine on protein expression of potassium ion channels in the brain of chronic mild stress rats. Acta Pharm. Sin. B 2015, 5, 55–61. [Google Scholar] [CrossRef]
- Gage, P.W. Activation and modulation of neuronal K+ channels by GABA. Trends Neurosci. 1992, 15, 46–51. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Park, H.R.; Yang, E.J. Herbal Neuroprotection Meets Stress-Induced Neuropathology: Bojungikgi-Tang Modulates the Hypothalamic–Pituitary–Adrenal Axis and GABAergic Pathways in Post-Traumatic Stress Disorder. Biomedicines 2025, 13, 2846. https://doi.org/10.3390/biomedicines13122846
Cai M, Park HR, Yang EJ. Herbal Neuroprotection Meets Stress-Induced Neuropathology: Bojungikgi-Tang Modulates the Hypothalamic–Pituitary–Adrenal Axis and GABAergic Pathways in Post-Traumatic Stress Disorder. Biomedicines. 2025; 13(12):2846. https://doi.org/10.3390/biomedicines13122846
Chicago/Turabian StyleCai, Mudan, Hee Ra Park, and Eun Jin Yang. 2025. "Herbal Neuroprotection Meets Stress-Induced Neuropathology: Bojungikgi-Tang Modulates the Hypothalamic–Pituitary–Adrenal Axis and GABAergic Pathways in Post-Traumatic Stress Disorder" Biomedicines 13, no. 12: 2846. https://doi.org/10.3390/biomedicines13122846
APA StyleCai, M., Park, H. R., & Yang, E. J. (2025). Herbal Neuroprotection Meets Stress-Induced Neuropathology: Bojungikgi-Tang Modulates the Hypothalamic–Pituitary–Adrenal Axis and GABAergic Pathways in Post-Traumatic Stress Disorder. Biomedicines, 13(12), 2846. https://doi.org/10.3390/biomedicines13122846





