The Use of Calcaneal Quantitative Ultrasound as a Bone Health Screening Tool Amongst People Living with HIV and Taking Tenofovir-Based Antiretroviral Therapy: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Consideration
2.2. Subjects
2.3. Data Collection
2.4. Bone Health Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet. 2025. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 23 October 2025).
- Starup-Linde, J.; Rosendahl, S.B.; Storgaard, M.; Langdahl, B. Management of Osteoporosis in Patients Living with HIV-A Systematic Review and Meta-analysis. J. Acquir. Immune Defic. Syndr. 2020, 83, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Chan, Y.L.; Pramukti, I.; Ko, N.Y.; Tai, T.W. People with HIV infection had lower bone mineral density and increased fracture risk: A meta-analysis. Arch. Osteoporos. 2021, 16, 47. [Google Scholar] [CrossRef]
- Grant, P.M.; Cotter, A.G. Tenofovir and bone health. Curr. Opin. HIV AIDS 2016, 11, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, I.F.; Pham, L.; Mansky, L.M.; Gopalakrishnan, R.; Mansky, K.C. Tenofovir-associated bone density loss. Ther. Clin. Risk Manag. 2010, 6, 41–47. [Google Scholar] [PubMed]
- Côté, H.C. Mechanisms of antiretroviral therapy-induced mitochondrial dysfunction. Curr. Opin. HIV AIDS 2007, 2, 253–260. [Google Scholar] [CrossRef]
- Kohler, J.J.; Hosseini, S.H.; Hoying-Brandt, A.; Green, E.; Johnson, D.M.; Russ, R.; Tran, D.; Raper, C.M.; Santoianni, R.; Lewis, W. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab. Investig. 2009, 89, 513–519. [Google Scholar] [CrossRef]
- Komatsu, A.; Ikeda, A.; Kikuchi, A.; Minami, C.; Tan, M.; Matsushita, S. Osteoporosis-Related Fractures in HIV-Infected Patients Receiving Long-Term Tenofovir Disoproxil Fumarate: An Observational Cohort Study. Drug Saf. 2018, 41, 843–848. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ng, B.N.; Rostam, M.K.I.; Muhammad Fadzil, N.F.D.; Raman, V.; Mohamed Yunus, F.; Syed Hashim, S.A.; Ekeuku, S.O. A Mini Review on Osteoporosis: From Biology to Pharmacological Management of Bone Loss. J. Clin. Med. 2022, 11, 6434. [Google Scholar] [CrossRef]
- Schinas, G.; Schinas, I.; Ntampanlis, G.; Polyzou, E.; Gogos, C.; Akinosoglou, K. Bone Disease in HIV: Need for Early Diagnosis and Prevention. Life 2024, 14, 522. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Binkley, N.; Morgan, S.L.; Shuhart, C.R.; Camargos, B.M.; Carey, J.J.; Gordon, C.M.; Jankowski, L.G.; Lee, J.-K.; Leslie, W.D. Best Practices for Dual-Energy X-ray Absorptiometry Measurement and Reporting: International Society for Clinical Densitometry Guidance. J. Clin. Densitom. 2016, 19, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, Y.; Amadi-Livingstone, C.; Timamy, A.; Eish, M.; Attia, A.; Panourgia, M.; Mital, D.; Pearce, O.; Ahmed, M.H. Narrative Review on the Management of Neck of Femur Fractures in People Living with HIV: Challenges, Complications, and Long-Term Outcomes. Microorganisms 2025, 13, 1530. [Google Scholar] [CrossRef]
- Ahmed, M.; Mital, D.; Abubaker, N.E.; Panourgia, M.; Owles, H.; Papadaki, I.; Ahmed, M.H. Bone Health in People Living with HIV/AIDS: An Update of Where We Are and Potential Future Strategies. Microorganisms 2023, 11, 789. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. Calcaneal quantitative ultrasound as a determinant of bone health status: What properties of bone does it reflect? Int. J. Med. Sci. 2013, 10, 1778–1783. [Google Scholar] [CrossRef]
- Escobio-Prieto, I.; Blanco-Díaz, M.; Pinero-Pinto, E.; Rodriguez-Rodriguez, A.M.; Ruiz-Dorantes, F.J.; Albornoz-Cabello, M. Quantitative Ultrasound and Bone Health in Elderly People, a Systematic Review. Biomedicines 2023, 11, 1175. [Google Scholar] [CrossRef]
- Laban, M.; Hussain, S.H.; El-Kotb, A.M.; Elghasnawy, F.M.; Hassanin, A.S.; Elsafty, M.S.E. Bone Health Assessed by Calcaneal Quantitative Ultrasound among Cohort of Pregnant Egyptian Women. J. Microsc. Ultrastruct. 2021, 9, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Zadik, Z. Quantitative bone analysis in children: Current methods and recommendations. J. Pediatr. 2006, 149, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Pinzone, M.R.; Castronuovo, D.; Di Gregorio, A.; Celesia, B.M.; Gussio, M.; Borderi, M.; Maggi, P.; Santoro, C.R.; Madeddu, G.; Cacopardo, B.; et al. Heel quantitative ultrasound in HIV-infected patients: A cross-sectional study. Infection 2016, 44, 197–203. [Google Scholar] [CrossRef]
- Segal, E.; Hassoun, G.; Maor, C.; Shahar, E. Quantitative ultrasonometry: An alternative and easy method to evaluate bone quality in people living with human immunodeficiency virus. J. Musculoskelet. Neuronal Interact. 2019, 19, 112–117. [Google Scholar] [PubMed]
- Fantauzzi, A.; Floridia, M.; Ceci, F.; Cacciatore, F.; Vullo, V.; Mezzaroma, I. Usefulness of calcaneal quantitative ultrasound stiffness for the evaluation of bone health in HIV-1-infected subjects: Comparison with dual X-ray absorptiometry. HIV AIDS 2016, 8, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Clò, A.; Gibellini, D.; Damiano, D.; Vescini, F.; Ponti, C.; Morini, S.; Miserocchi, A.; Musumeci, G.; Calza, L.; Colangeli, V.; et al. Calcaneal quantitative ultrasound (QUS) and dual X-ray absorptiometry (DXA) bone analysis in adult HIV-positive patients. New Microbiol. 2015, 38, 345–356. [Google Scholar] [PubMed]
- Krieg, M.A.; Barkmann, R.; Gonnelli, S.; Stewart, A.; Bauer, D.C.; Del Rio Barquero, L.; Kaufman, J.J.; Lorenc, R.; Miller, P.D.; Olszynski, W.P.; et al. Quantitative ultrasound in the management of osteoporosis: The 2007 ISCD Official Positions. J. Clin. Densitom. 2008, 11, 163–187. [Google Scholar] [CrossRef]
- Subramaniam, S.; Chan, C.-Y.; Soelaiman, I.N.; Mohamed, N.; Muhammad, N.; Ahmad, F.; Ng, P.-Y.; Jamil, N.A.; Abd Aziz, N.; Chin, K.-Y. The Performance of a Calcaneal Quantitative Ultrasound Device, CM-200, in Stratifying Osteoporosis Risk among Malaysian Population Aged 40 Years and Above. Diagnostics 2020, 10, 178. [Google Scholar] [CrossRef]
- Malaysian Ministry of Health. The Global AIDS Monitoring Report 2023; Malaysian Ministry of Health: Putrajaya, Malaysia, 2023.
- International Osteoporosis Foundation. Diagnosis of Osteoporosis. Available online: https://www.osteoporosis.foundation/health-professionals/diagnosis (accessed on 24 October 2025).
- Niimi, R.; Chiba, K.; Okazaki, N.; Yonekura, A.; Tomita, M.; Osaki, M. Relationships between QUS and HR-pQCT, DXA, and bone turnover markers. J. Bone Min. Metab. 2022, 40, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.B.; Curwen, C.; Tasker, T.; Zioupos, P. Fracture toughness and compressive properties of cancellous bone at the head of the femur and relationships to non-invasive skeletal assessment measurements. Med. Eng. Phys. 2010, 32, 991–997. [Google Scholar] [CrossRef]
- Carballido-Gamio, J.; Posadzy, M.; Wu, P.H.; Kenny, K.; Saeed, I.; Link, T.M.; Tien, P.C.; Krug, R.; Kazakia, G.J. People living with HIV have low trabecular bone mineral density, high bone marrow adiposity, and poor trabecular bone microarchitecture at the proximal femur. Osteoporos. Int. 2022, 33, 1739–1753. [Google Scholar] [CrossRef]
- Macdonald, H.M.; Maan, E.J.; Berger, C.; Dunn, R.A.; Côté, H.C.F.; Murray, M.C.M.; Pick, N.; Prior, J.C. Deficits in bone strength, density and microarchitecture in women living with HIV: A cross-sectional HR-pQCT study. Bone 2020, 138, 115509. [Google Scholar] [CrossRef]
- Amling, M.; Herden, S.; Pösl, M.; Hahn, M.; Ritzel, H.; Delling, G. Heterogeneity of the skeleton: Comparison of the trabecular microarchitecture of the spine, the iliac crest, the femur, and the calcaneus. J. Bone Min. Res. 1996, 11, 36–45. [Google Scholar] [CrossRef]
- Sheu, A.; Greenfield, J.R.; White, C.P.; Center, J.R. Assessment and treatment of osteoporosis and fractures in type 2 diabetes. Trends Endocrinol. Metab. 2022, 33, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Nimitphong, H.; Sungkanuparph, S.; Saetung, S.; Chailurkit, L.-o.; Sritara, C.; Musikarat, S.; Ongphiphadhanakul, B. A Comparison of Bone Mineral Density and Its Predictors in HIV-Infected and HIV-Uninfected Older Men. Endocr. Pract. 2021, 27, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Amiel, C.; Ostertag, A.; Slama, L.; Baudoin, C.; N’Guyen, T.; Lajeunie, E.; Neit-Ngeilh, L.; Rozenbaum, W.; De Vernejoul, M. BMD Is Reduced in HIV-Infected Men Irrespective of Treatment. J. Bone Min. Res. 2009, 19, 402–409. [Google Scholar] [CrossRef]
- Shaiykova, A.; Pasquet, A.; Goujard, C.; Lion, G.; Durand, E.; Bayan, T.; Lachâtre, M.; Choisy, P.; Ajana, F.; Bourdic, K.; et al. Reduced bone mineral density among HIV-infected, virologically controlled young men: Prevalence and associated factors. AIDS 2018, 32, 2689–2696. [Google Scholar] [CrossRef]
- Casado, J.L.; Santiuste, C.; Vazquez, M.; Bañón, S.; Rosillo, M.; Gomez, A.; Perez-Elías, M.J.; Caballero, C.; Rey, J.M.; Moreno, S. Bone mineral density decline according to renal tubular dysfunction and phosphaturia in tenofovir-exposed HIV-infected patients. AIDS 2016, 30, 1423–1431. [Google Scholar] [CrossRef]
- Wei, M.T.; Le, A.K.; Chang, M.S.; Hsu, H.; Nguyen, P.; Zhang, J.Q.; Wong, C.; Wong, C.; Cheung, R.; Nguyen, M.H. Antiviral therapy and the development of osteopenia/osteoporosis among Asians with chronic hepatitis B. J. Med. Virol. 2019, 91, 1288–1294. [Google Scholar] [CrossRef]
- Quiros Roldan, E.; Brianese, N.; Raffetti, E.; Focà, E.; Pezzoli, M.C.; Bonito, A.; Ferraresi, A.; Lanza, P.; Porcelli, T.; Castelli, F. Comparison between the gold standard DXA with calcaneal quantitative ultrasound based-strategy (QUS) to detect osteoporosis in an HIV infected cohort. Braz. J. Infect. Dis. 2017, 21, 581–586. [Google Scholar] [CrossRef]
- Johansen, A.; Stone, M.D. The effect of ankle oedema on bone ultrasound assessment at the heel. Osteoporos. Int. 1997, 7, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Kotzki, P.O.; Buyck, D.; Hans, D.; Thomas, E.; Bonnel, F.; Favier, F.; Meunier, P.J.; Rossi, M. Influence of fat on ultrasound measurements of the os calcis. Calcif. Tissue Int. 1994, 54, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Chappard, C.; Camus, E.; Lefebvre, F.; Guillot, G.; Bittoun, J.; Berger, G.; Laugier, P. Evaluation of error bounds on calcaneal speed of sound caused by surrounding soft tissue. J. Clin. Densitom. 2000, 3, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Koethe, J.R.; Lagathu, C.; Lake, J.E.; Domingo, P.; Calmy, A.; Falutz, J.; Brown, T.T.; Capeau, J. HIV and antiretroviral therapy-related fat alterations. Nat. Rev. Dis. Primers 2020, 6, 48. [Google Scholar] [CrossRef]
- Arifin, W.N. Sample Size Calculator (Web). Available online: http://wnarifin.github.io (accessed on 15 November 2025).
| Characteristics | Non-HIV-ART | HIV-ART | p-Value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age (years) | 43.0 | 8.9 | 43.0 | 8.0 | 0.991 | |
| Weight (kg) | 73.4 | 10.8 | 73.5 | 13.0 | 0.952 | |
| Height (cm) | 170.1 | 5.9 | 170.7 | 5.3 | 0.590 | |
| BMI (kg/m2) | 25.4 | 3.4 | 25.3 | 4.4 | 0.890 | |
| DXA indices | Lumbar spine BMD (g/cm2) * | 0.99 | 0.14 | 0.97 | 0.13 | 0.376 |
| Femoral neck BMD (g/cm2) * | 0.77 | 0.11 | 0.76 | 0.11 | 0.418 | |
| Lumbar spine T-score * | 0.20 | 1.06 | 0.02 | 1.03 | 0.374 | |
| Femoral neck T-score * | −0.57 | 1.16 | −0.83 | 1.23 | 0.237 | |
| US indices | SOS (m/s) * | 1525.66 | 23.14 | 1521.73 | 20.98 | 0.349 |
| BUA (dB) * | 28.02 | 7.09 | 23.79 | 5.39 | 0.002 | |
| OI * | 46.70 | 5.83 | 45.45 | 4.46 | 0.205 | |
| T-score * | −1.27 | 0.92 | −1.70 | 0.89 | 0.022 | |
| n | % | n | % | p-Value | ||
| Race | Chinese | 16 | 38.1 | 18 | 34.6 | 0.940 |
| Indian | 2 | 4.8 | 3 | 5.8 | ||
| Malay | 24 | 57.1 | 31 | 59.6 | ||
| Cigeratte smoking | Smokers | 34 | 81 | 38 | 73.1 | 0.259 |
| Non-smokers | 8 | 19 | 14 | 26.9 | ||
| Alcohol drinking | Regular alcohol drinkers | 34 | 81 | 44 | 84.6 | 0.421 |
| Non-regular alcohol drinkers | 8 | 19 | 8 | 15.4 | ||
| Viral load | Detectable | n/a | n/a | 10 | 19.2 | n/a |
| Not detectable | n/a | n/a | 42 | 80.8 | ||
| DXA lumbar spine T-score | ≥−1 | 35 | 83.3 | 44 | 84.6 | 0.866 |
| <−1 | 7 | 16.7 | 8 | 15.4 | ||
| DXA femoral neck | ≥−1 | 24 | 57.1 | 29 | 55.8 | 0.894 |
| <−1 | 18 | 42.9 | 23 | 44.2 | ||
| QUS | ≥−1 | 13 | 31 | 9 | 17.3 | 0.120 |
| <−1 | 29 | 69 | 43 | 82.7 | ||
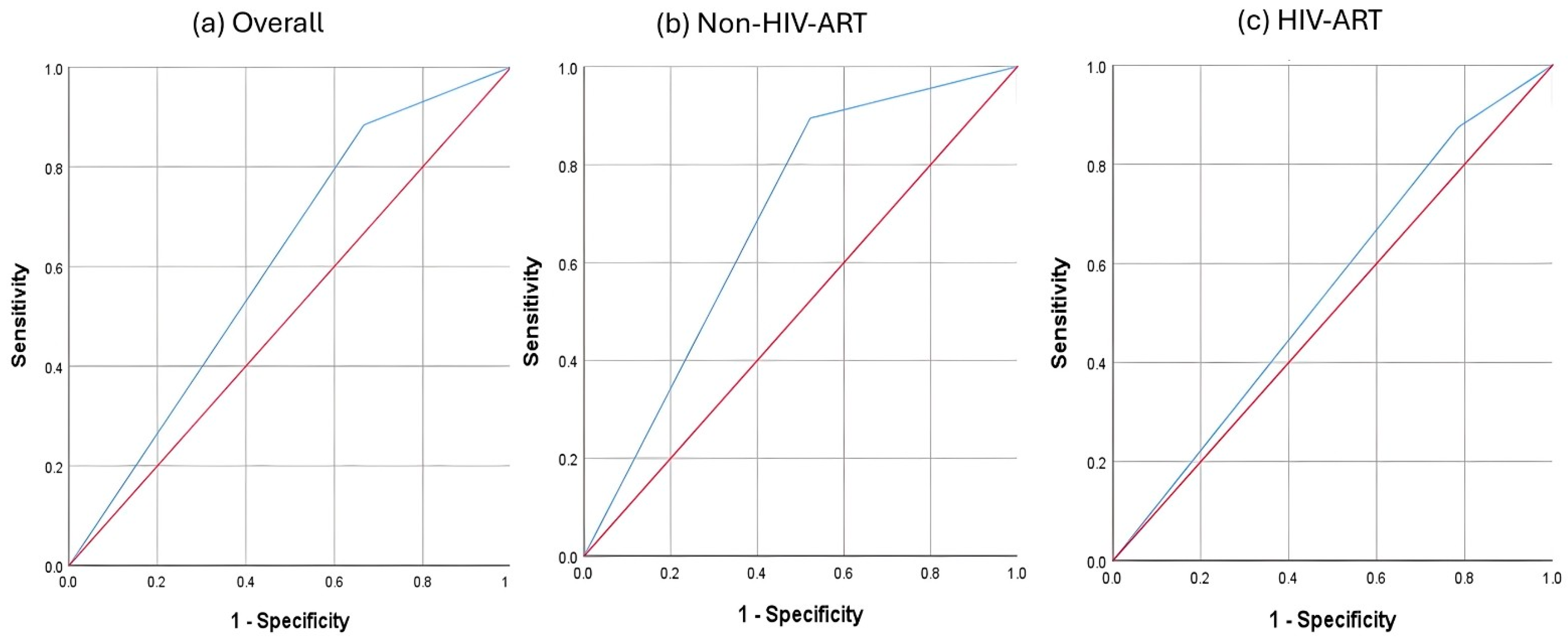
| Group | AUC | SE | p | Asymptotic 95% CI | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||||
| Non-HIV-ART | 0.686 | 0.083 | 0.039 | 0.525 | 0.848 | 0.895 | 0.478 | 0.586 | 0.846 |
| HIV-ART | 0.545 | 0.08 | 0.582 | 0.387 | 0.702 | 0.875 | 0.214 | 0.488 | 0.667 |
| Overall | 0.609 | 0.058 | 0.071 | 0.495 | 0.722 | 0.884 | 0.333 | 0.528 | 0.773 |
| Group | Technique | Bone Health Status | DXA | ||||
|---|---|---|---|---|---|---|---|
| Normal | Low BMD | Total | Kappa | p-Value | |||
| Non-HIV-ART | QUS | Normal | 11 | 2 | 13 | 0.357 | 0.009 |
| Low BMD | 12 | 17 | 29 | ||||
| Total | 23 | 19 | 42 | ||||
| HIV-ART | QUS | Normal | 6 | 3 | 9 | 0.085 | 0.369 |
| Low BMD | 22 | 21 | 43 | ||||
| Total | 28 | 24 | 52 | ||||
| Overall | QUS | Normal | 17 | 5 | 22 | 0.206 | 0.013 |
| Low BMD | 34 | 38 | 72 | ||||
| Total | 51 | 43 | 94 | ||||
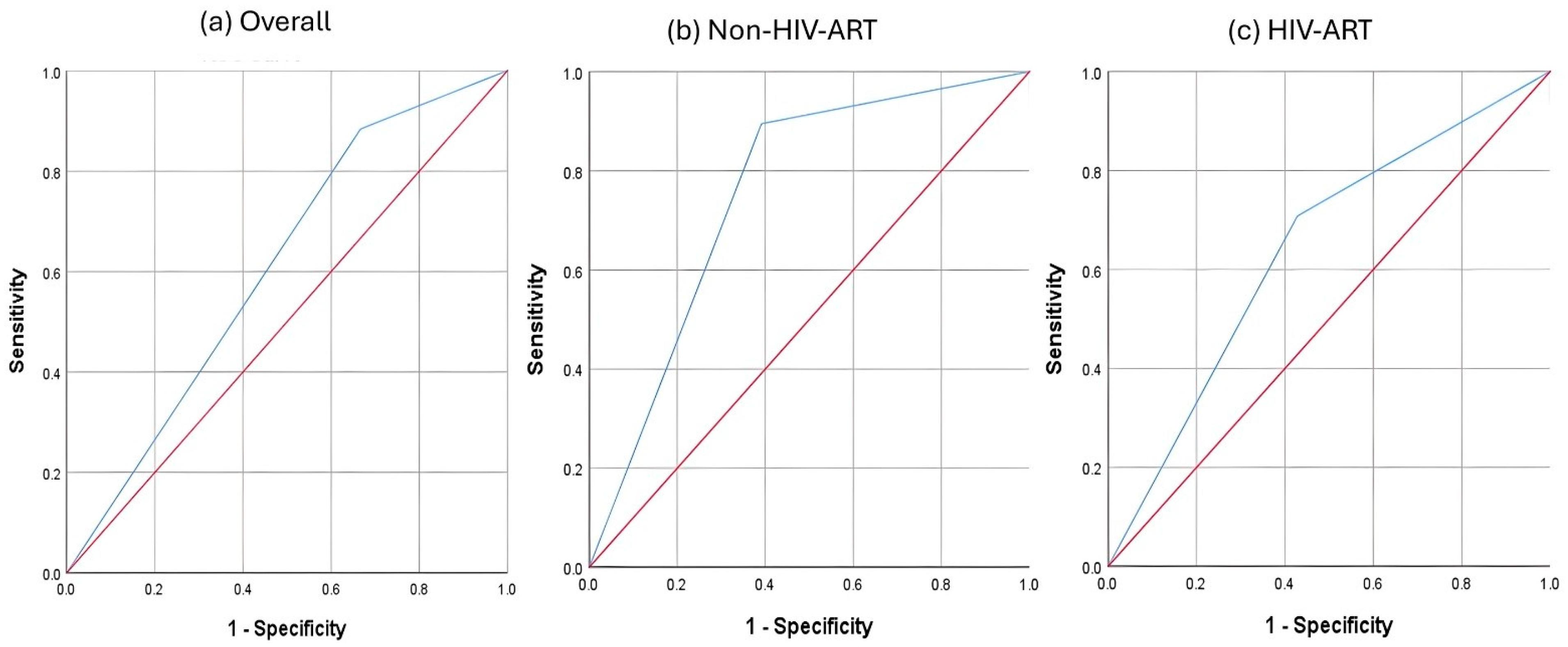
| Group | Cutoff | J-Index | AUC | SE | p | Asymptotic 95% CI | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||||||
| Non-HIV-ART | −1.15 | 0.504 | 0.752 | 0.077 | 0.005 | 0.601 | 0.902 | 0.895 | 0.609 | 0.654 | 0.875 |
| HIV-ART | −1.525 | 0.279 | 0.64 | 0.078 | 0.084 | 0.488 | 0.792 | 0.708 | 0.571 | 0.586 | 0.696 |
| Overall | −0.095 | 0.217 | 0.609 | 0.058 | 0.071 | 0.495 | 0.722 | 0.884 | 0.333 | 0.528 | 0.773 |
| Group | Technique | Bone Health Status | DXA | ||||
|---|---|---|---|---|---|---|---|
| Normal | Low BMD | Total | Kappa | p-Value | |||
| Non-HIV-ART | QUS | Normal | 14 | 2 | 16 | 0.488 | 0.001 |
| Low BMD | 9 | 17 | 26 | ||||
| Total | 23 | 19 | 42 | ||||
| HIV-ART | QUS | Normal | 16 | 7 | 23 | 0.276 | 0.043 |
| Low BMD | 12 | 17 | 29 | ||||
| Total | 28 | 24 | 52 | ||||
| Overall | QUS | Normal | 17 | 5 | 22 | 0.206 | 0.013 |
| Low BMD | 34 | 38 | 72 | ||||
| Total | 51 | 43 | 94 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Daud, M.R.; Tejpal Singh, H.S.; Ekeuku, S.O.; Cheong, X.K.; Kori, N.; Periyasamy, P.; Chin, K.-Y. The Use of Calcaneal Quantitative Ultrasound as a Bone Health Screening Tool Amongst People Living with HIV and Taking Tenofovir-Based Antiretroviral Therapy: A Pilot Study. Biomedicines 2025, 13, 2847. https://doi.org/10.3390/biomedicines13122847
Zhao W, Daud MR, Tejpal Singh HS, Ekeuku SO, Cheong XK, Kori N, Periyasamy P, Chin K-Y. The Use of Calcaneal Quantitative Ultrasound as a Bone Health Screening Tool Amongst People Living with HIV and Taking Tenofovir-Based Antiretroviral Therapy: A Pilot Study. Biomedicines. 2025; 13(12):2847. https://doi.org/10.3390/biomedicines13122847
Chicago/Turabian StyleZhao, Wenjian, Muhamad Riduan Daud, Hashwin Singh Tejpal Singh, Sophia Ogechi Ekeuku, Xiong Khee Cheong, Najma Kori, Petrick Periyasamy, and Kok-Yong Chin. 2025. "The Use of Calcaneal Quantitative Ultrasound as a Bone Health Screening Tool Amongst People Living with HIV and Taking Tenofovir-Based Antiretroviral Therapy: A Pilot Study" Biomedicines 13, no. 12: 2847. https://doi.org/10.3390/biomedicines13122847
APA StyleZhao, W., Daud, M. R., Tejpal Singh, H. S., Ekeuku, S. O., Cheong, X. K., Kori, N., Periyasamy, P., & Chin, K.-Y. (2025). The Use of Calcaneal Quantitative Ultrasound as a Bone Health Screening Tool Amongst People Living with HIV and Taking Tenofovir-Based Antiretroviral Therapy: A Pilot Study. Biomedicines, 13(12), 2847. https://doi.org/10.3390/biomedicines13122847







