Experimental Galactose-1-Phosphate Uridylyltransferase (GALT) mRNA Therapy Improves Motor-Related Phenotypes in a Mouse Model of Classic Galactosemia—A Pilot Study
Abstract
1. Introduction
- Will experimental GALT mRNA therapy improve motor-related phenotypes in a mouse model of classic galactosemia?
- Will the improvement, if any, of motor-related phenotypes seen in the treated animals be sustained after the cessation of the experimental GALT mRNA treatment?
- Does it matter if the GALT mRNA is offered to a younger versus an older mouse?
2. Materials and Methods
2.1. mRNA and Sterile Lipid Nanoparticle Synthesis and Formulation
2.2. Animal Model
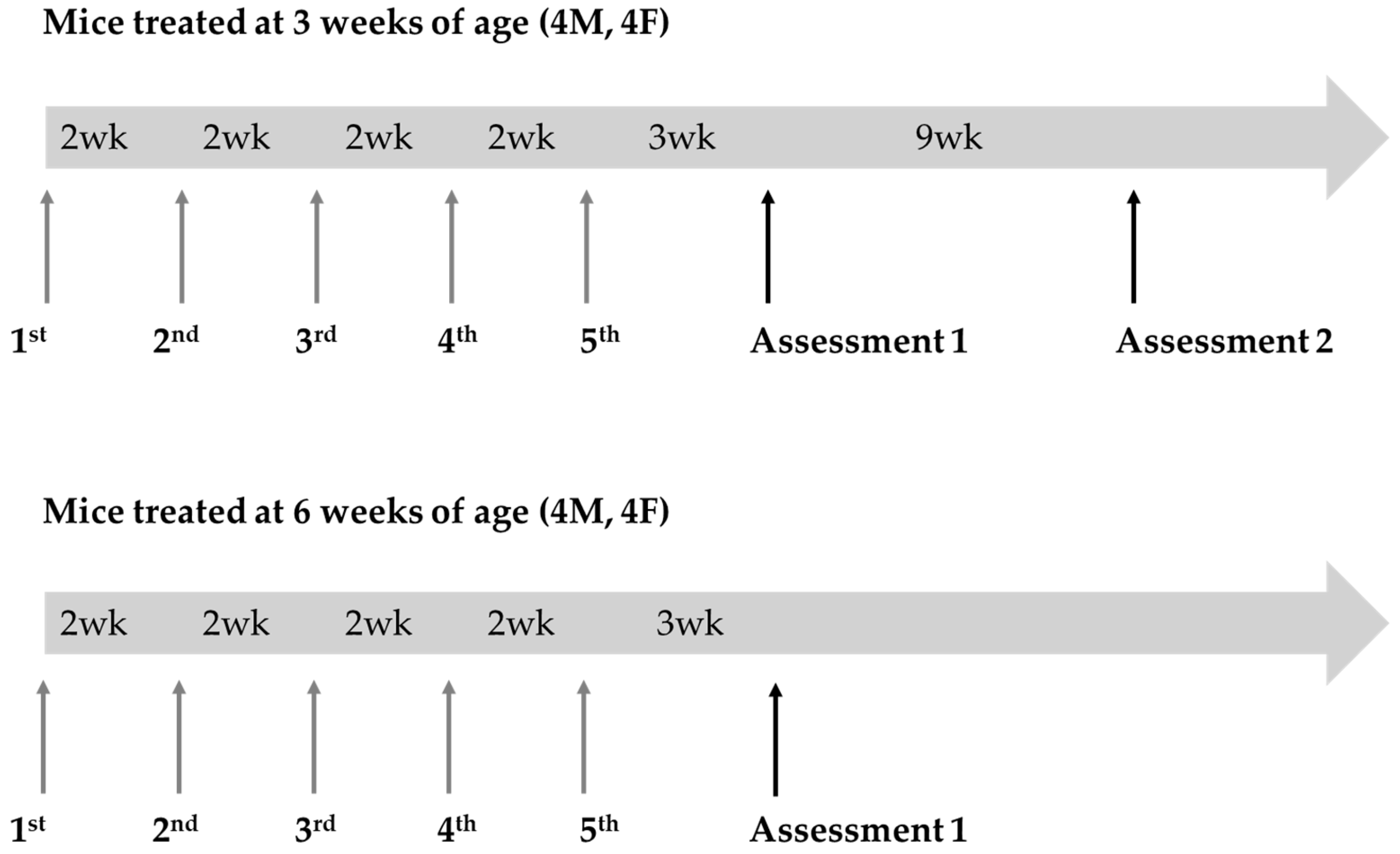
2.3. In Vivo GALT mRNA Administration
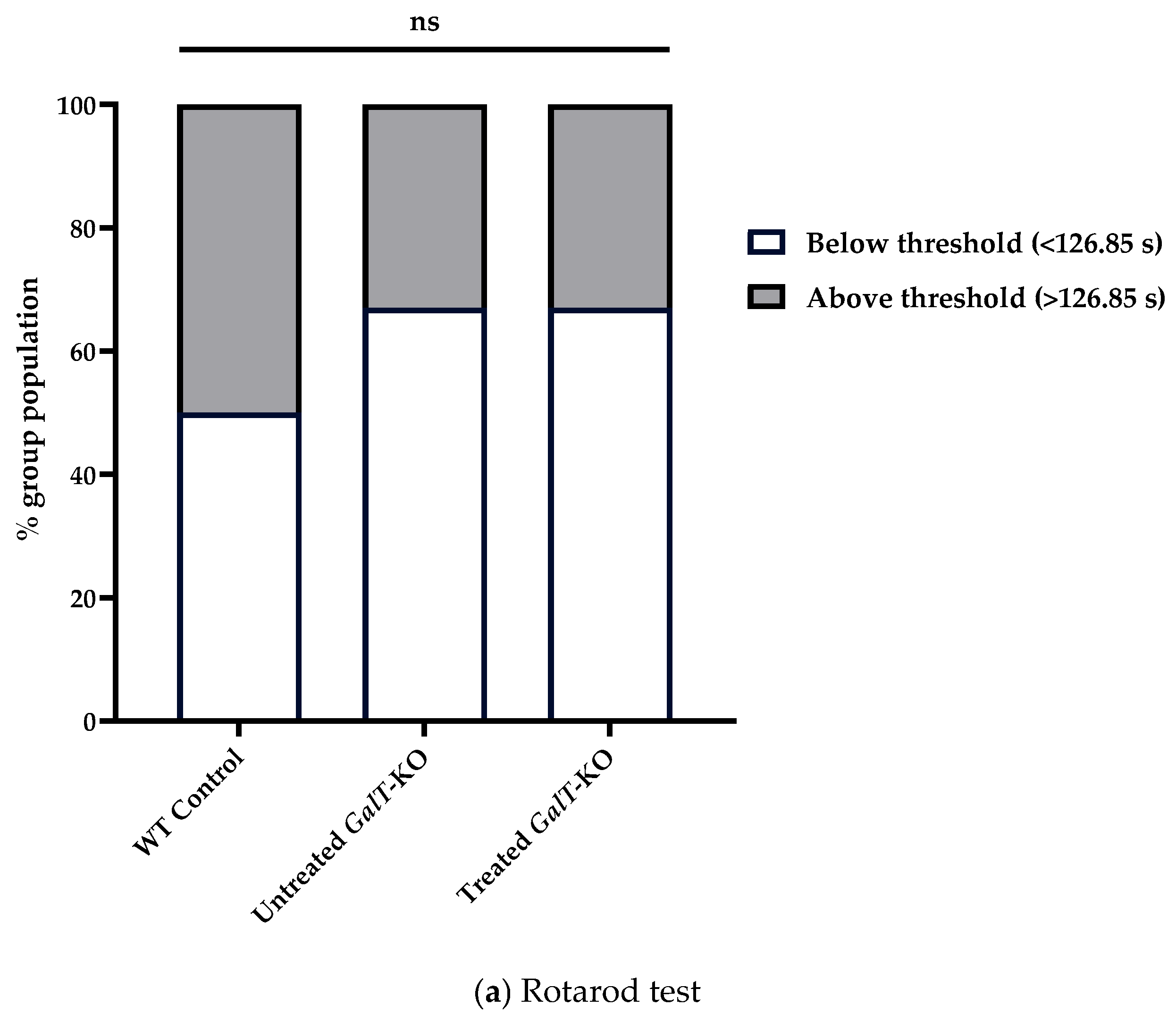
2.4. Rotarod Test
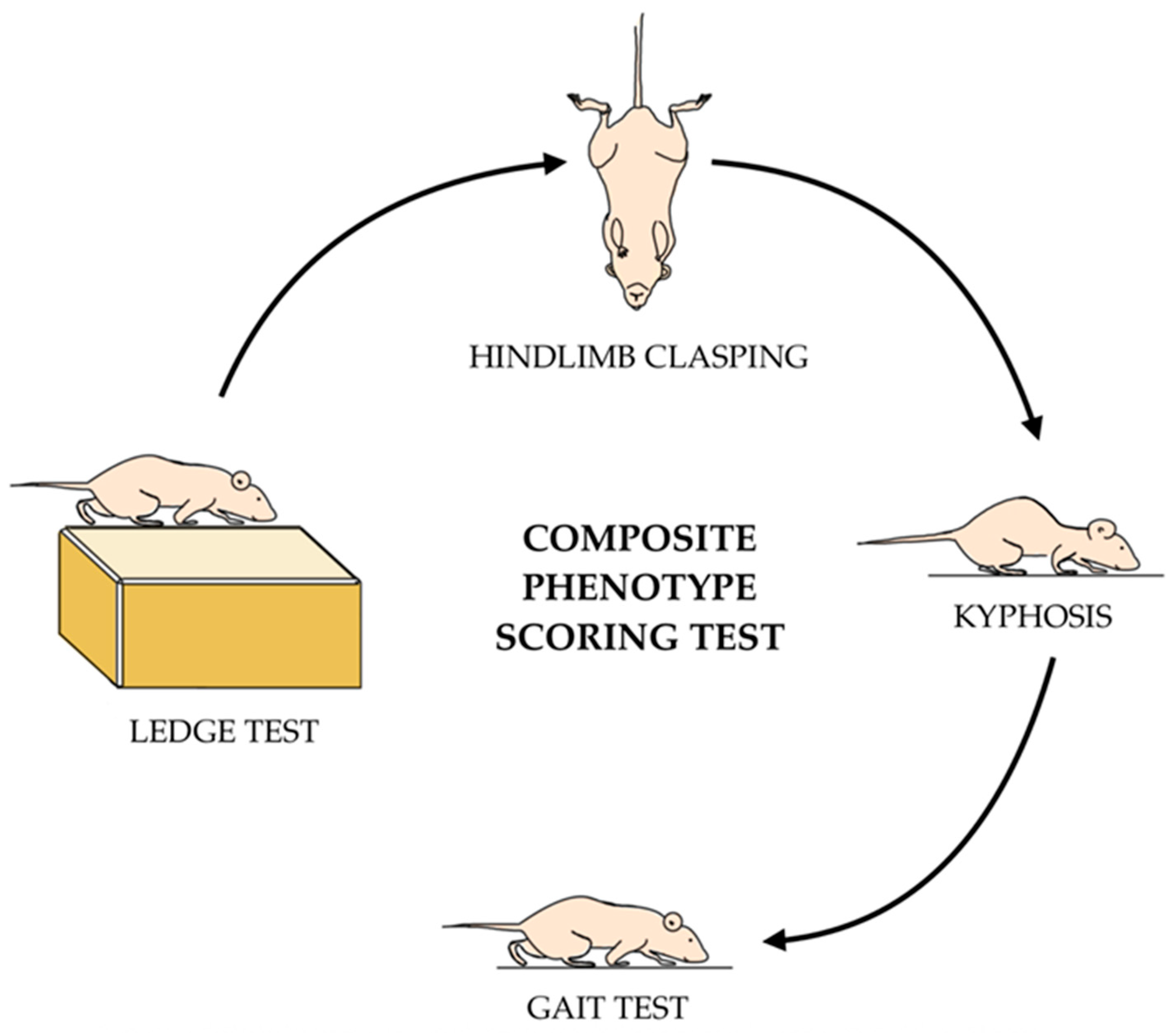
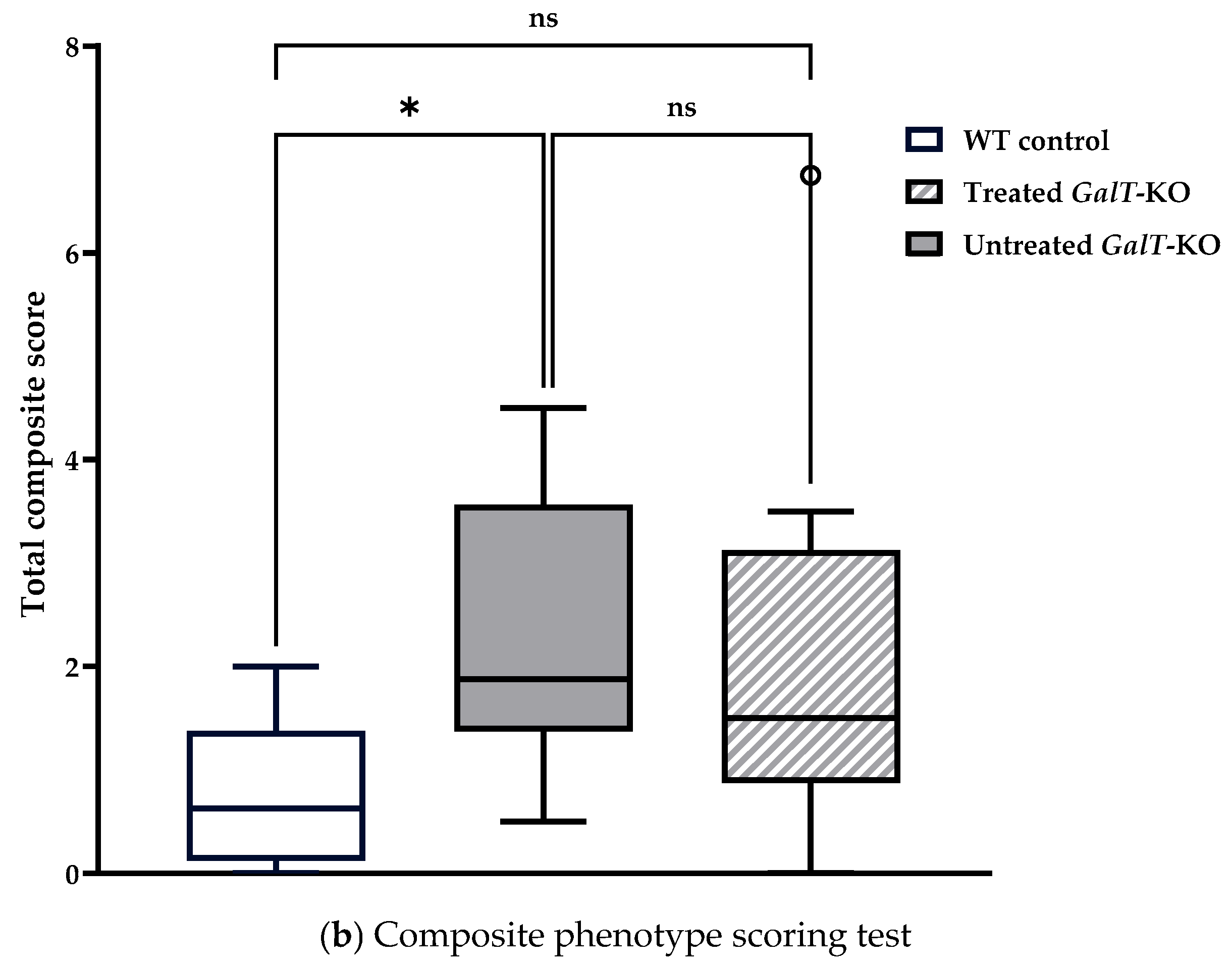
2.5. Composite Phenotype Scoring Test
2.6. Statistical Analysis
3. Results
3.1. Near-Term Improvement in Motor Functions of GALT mRNA-Treated GalT-KO Mice
3.2. Improvement in Motor Functions from a Single Dose of GALT mRNA Therapy Were Not Sustained Far Beyond Three Weeks
3.3. GALT mRNA Treatment Appears to Be More Effective in Early Life
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Berry, G.T.; Segal, S.; Gitzelmann, R. Disorders of Galactose Metabolism. In Inborn Metabolic Diseases—Diagnosis and Treatment, 4th ed.; Fernandes, J., Saudubray, M., van den Berghe, G., Walter, J.H., Eds.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Fridovich-Keil, J.L. Galactosemia: The good, the bad, and the unknown. J. Cell. Physiol. 2006, 209, 701–705. [Google Scholar] [CrossRef]
- Isselbacher, K.J.; Anderson, E.P.; Kurahashi, K.; Kalckar, H.M. Congenital galactosemia, a single enzymatic block in galactose metabolism. Science 1956, 123, 635–636. [Google Scholar] [CrossRef]
- Tyfield, L.; Reichardt, J.; Fridovich-Keil, J.; Croke, D.T.; Elsas, L.J., 2nd; Strobl, W.; Kozak, L.; Coskun, T.; Novelli, G.; Okano, Y.; et al. Classical galactosemia and mutations at the galactose-1-phosphate uridyl transferase (GALT) gene. Hum. Mutat. 1999, 13, 417–430. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lan, L.C.; Yang, X.; Xiao, J.; Liu, H.X.; Shan, Q.W. A case report of classic galactosemia with a GALT gene variant and a literature review. BMC Pediatr. 2024, 24, 352. [Google Scholar] [CrossRef] [PubMed]
- Leloir, L.F. The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Arch. Biochem. 1951, 33, 186–190. [Google Scholar] [CrossRef]
- Gitzelmann, R. Galactose-1-phosphate in the pathophysiology of galactosemia. Eur. J. Pediatr. 1995, 154, S45–S49. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Langley, S.D.; Khwaja, F.W.; Schmitt, E.W.; Elsas, L.J. GALT deficiency causes UDP-hexose deficit in human galactosemic cells. Glycobiology 2003, 13, 285–294. [Google Scholar] [CrossRef]
- Kaye, C.I.; Accurso, F.; La Franchi, S.; Lane, P.A.; Hope, N.; Sonya, P.; S, G.B.; Michele, A.L. Newborn screening fact sheets. Pediatrics 2006, 118, e934–e963. [Google Scholar] [CrossRef]
- Berry, G.T.; Moate, P.J.; Reynolds, R.A.; Yager, C.T.; Ning, C.; Boston, R.C.; Segal, S. The rate of de novo galactose synthesis in patients with galactose-1-phosphate uridyltransferase deficiency. Mol. Genet. Metab. 2004, 81, 22–30. [Google Scholar] [CrossRef]
- Berry, G.T.; Nissim, I.; Lin, Z.; Mazur, A.T.; Gibson, J.B.; Segal, S. Endogenous synthesis of galactose in normal men and patients with hereditary galactosaemia. Lancet 1995, 346, 1073–1074. [Google Scholar] [CrossRef]
- Waggoner, D.; Buist, N.R.M. Long-term complications in treated galactosemia—175 U.S. cases. Int. Pediatr. 1993, 8, 97–100. [Google Scholar]
- Waisbren, S.E.; Potter, N.L.; Gordon, C.M.; Green, R.C.; Greenstein, P.; Gubbels, C.S.; Rubio-Gozalbo, E.; Schomer, D.; Welt, C.; Anastasoaie, V.; et al. The adult galactosemic phenotype. J. Inherit. Metab. Dis. 2012, 35, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Batey, L.A.; Welt, C.K.; Rohr, F.; Wessel, A.; Anastasoaie, V.; Feldman, H.A.; Guo, C.Y.; Rubio-Gozalbo, E.; Berry, G.; Gordon, C.M. Skeletal health in adult patients with classic galactosemia. Osteoporos. Int. 2012, 24, 501–509. [Google Scholar] [CrossRef]
- Panis, B.; Forget, P.P.; van Kroonenburgh, M.J.; Vermeer, C.; Menheere, P.P.; Nieman, F.H.; Rubio-Gozalbo, M.E. Bone metabolism in galactosemia. Bone 2004, 35, 982–987. [Google Scholar] [CrossRef]
- Rubio-Gozalbo, M.E.; Hamming, S.; van Kroonenburgh, M.J.; Bakker, J.A.; Vermeer, C.; Forget, P.P. Bone mineral density in patients with classic galactosaemia. Arch. Dis. Child. 2002, 87, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; An, D.; Nguyen, V.; DeAntonis, C.; Martini, P.G.V.; Lai, K. Novel mRNA-Based Therapy Reduces Toxic Galactose Metabolites and Overcomes Galactose Sensitivity in a Mouse Model of Classic Galactosemia. Mol. Ther. J. Am. Soc. Gene Ther. 2020, 28, 304–312. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Yan, X.; McCue, M.D.; Bellagamba, O.; Guo, A.; Winkler, F.; Thall, J.; Crawford, L.; Dimen, R.; Chen, S.; et al. Whole-body galactose oxidation as a robust functional assay to assess the efficacy of gene-based therapies in a mouse model of Galactosemia. Mol. Ther. Methods Clin. Dev. 2024, 32, 101191. [Google Scholar] [CrossRef]
- Delnoy, B.; Haskovic, M.; Vanoevelen, J.; Steinbusch, L.K.M.; Vos, E.N.; Knoops, K.; Zimmermann, L.J.I.; Noga, M.; Lefeber, D.J.; Martini, P.G.V.; et al. Novel mRNA therapy restores GALT protein and enzyme activity in a zebrafish model of classic galactosemia. J. Inherit. Metab. Dis. 2022, 45, 748–758. [Google Scholar] [CrossRef]
- Bicknell, A.A.; Reid, D.W.; Licata, M.C.; Jones, A.K.; Cheng, Y.M.; Li, M.; Hsiao, C.J.; Pepin, C.S.; Metkar, M.; Levdansky, Y.; et al. Attenuating ribosome load improves protein output from mRNA by limiting translation-dependent mRNA decay. Cell Rep. 2024, 43, 114098. [Google Scholar] [CrossRef]
- Tang, M.; Siddiqi, A.; Witt, B.; Yuzyuk, T.; Johnson, B.; Fraser, N.; Chen, W.; Rascon, R.; Yin, X.; Goli, H.; et al. Subfertility and growth restriction in a new galactose-1 phosphate uridylyltransferase (GALT)—Deficient mouse model. Eur. J. Hum. Genet. 2014, 22, 1172–1179. [Google Scholar] [CrossRef]
- Guyenet, S.J.; Furrer, S.A.; Damian, V.M.; Baughan, T.D.; La Spada, A.R.; Garden, G.A. A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J. Vis. Exp. JoVE 2010, 39, e1787. [Google Scholar] [CrossRef]
- Gama, P.; Cadena-Nava, R.D.; Juarez-Moreno, K.; Perez-Robles, J.; Vazquez-Duhalt, R. Virus-Based Nanoreactors with GALT Activity for Classic Galactosemia Therapy. ChemMedChem 2021, 16, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Grisch-Chan, H.M.; Schlegel, A.; Scherer, T.; Allegri, G.; Heidelberger, R.; Tsikrika, P.; Schmeer, M.; Schleef, M.; Harding, C.O.; Haberle, J.; et al. Low-Dose Gene Therapy for Murine PKU Using Episomal Naked DNA Vectors Expressing PAH from Its Endogenous Liver Promoter. Mol. Ther. Nucleic Acids 2017, 7, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.O. Prospects for Cell-Directed Curative Therapy of Phenylketonuria (PKU). Mol. Front. J. 2019, 3, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, C.G.; Diaz-Trelles, R.; Vega, J.B.; Bao, Y.; Sablad, M.; Limphong, P.; Chikamatsu, S.; Yu, H.; Taylor, W.; Karmali, P.P.; et al. Development of an mRNA replacement therapy for phenylketonuria. Mol. Ther. Nucleic Acids 2022, 28, 87–98. [Google Scholar] [CrossRef]
- Wiedemann, A.; Oussalah, A.; Jeannesson, E.; Gueant, J.L.; Feillet, F. Phenylketonuria, from diet to gene therapy. Med. Sci. 2020, 36, 725–734. [Google Scholar] [CrossRef]
- An, D.; Frassetto, A.; Jacquinet, E.; Eybye, M.; Milano, J.; DeAntonis, C.; Nguyen, V.; Laureano, R.; Milton, J.; Sabnis, S.; et al. Long-term efficacy and safety of mRNA therapy in two murine models of methylmalonic acidemia. EBioMedicine 2019, 45, 519–528. [Google Scholar] [CrossRef]
- An, D.; Schneller, J.L.; Frassetto, A.; Liang, S.; Zhu, X.; Park, J.S.; Theisen, M.; Hong, S.J.; Zhou, J.; Rajendran, R.; et al. Systemic Messenger RNA Therapy as a Treatment for Methylmalonic Acidemia. Cell Rep. 2017, 21, 3548–3558. [Google Scholar] [CrossRef]
- Coughlan, K.A.; Eybye, M.; Henderson, N.; DeAntonis, C.M.; Frassetto, A.; Hanahoe, E.; Ketova, T.; Jacquinet, E.; Presnyak, V.; Jain, R.; et al. Improved therapeutic efficacy in two mouse models of methylmalonic acidemia (MMA) using a second-generation mRNA therapy. Mol. Genet. Metab. 2024, 143, 108560. [Google Scholar] [CrossRef]
- Cao, J.; Choi, M.; Guadagnin, E.; Soty, M.; Silva, M.; Verzieux, V.; Weisser, E.; Markel, A.; Zhuo, J.; Liang, S.; et al. mRNA therapy restores euglycemia and prevents liver tumors in murine model of glycogen storage disease. Nat. Commun. 2021, 12, 3090. [Google Scholar] [CrossRef]
- Chen, W.; Caston, R.; Balakrishnan, B.; Siddiqi, A.; Parmar, K.; Tang, M.; Feng, M.; Lai, K. Assessment of ataxia phenotype in a new mouse model of galactose-1 phosphate uridylyltransferase (GALT) deficiency. J. Inherit. Metab. Dis. 2017, 40, 131–137. [Google Scholar] [CrossRef]
- Brophy, M.L.; Stansfield, J.C.; Ahn, Y.; Cheng, S.H.; Murphy, J.E.; Bell, R.D. AAV-mediated expression of galactose-1-phosphate uridyltransferase corrects defects of galactose metabolism in classic galactosemia patient fibroblasts. J. Inherit. Metab. Dis. 2022, 45, 481–492. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Daenzer, J.M.I.; Fridovich-Keil, J.L. A pilot study of neonatal GALT gene replacement using AAV9 dramatically lowers galactose metabolites in blood, liver, and brain and minimizes cataracts in GALT-null rat pups. J. Inherit. Metab. Dis. 2021, 44, 272–281. [Google Scholar] [CrossRef]

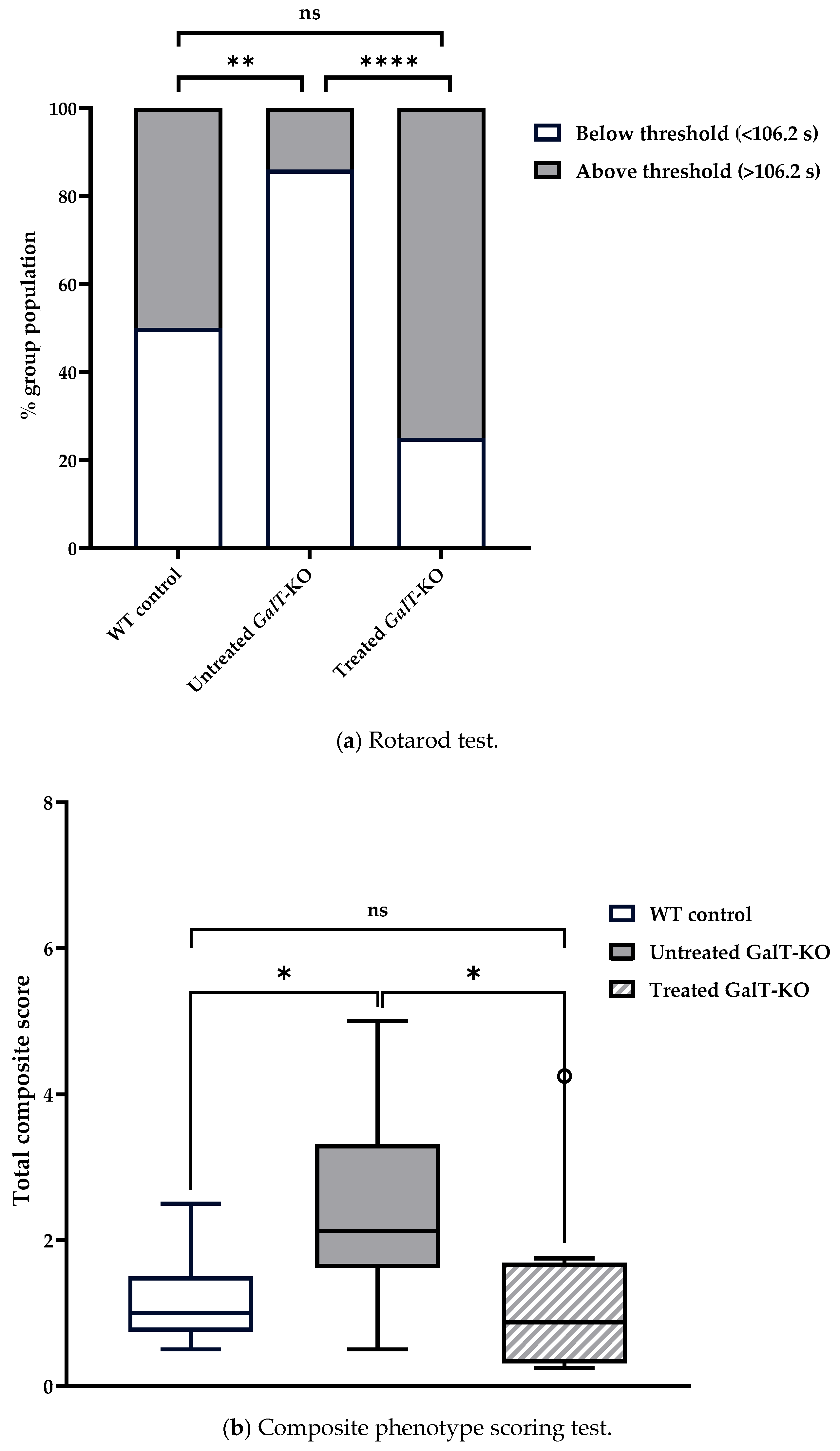
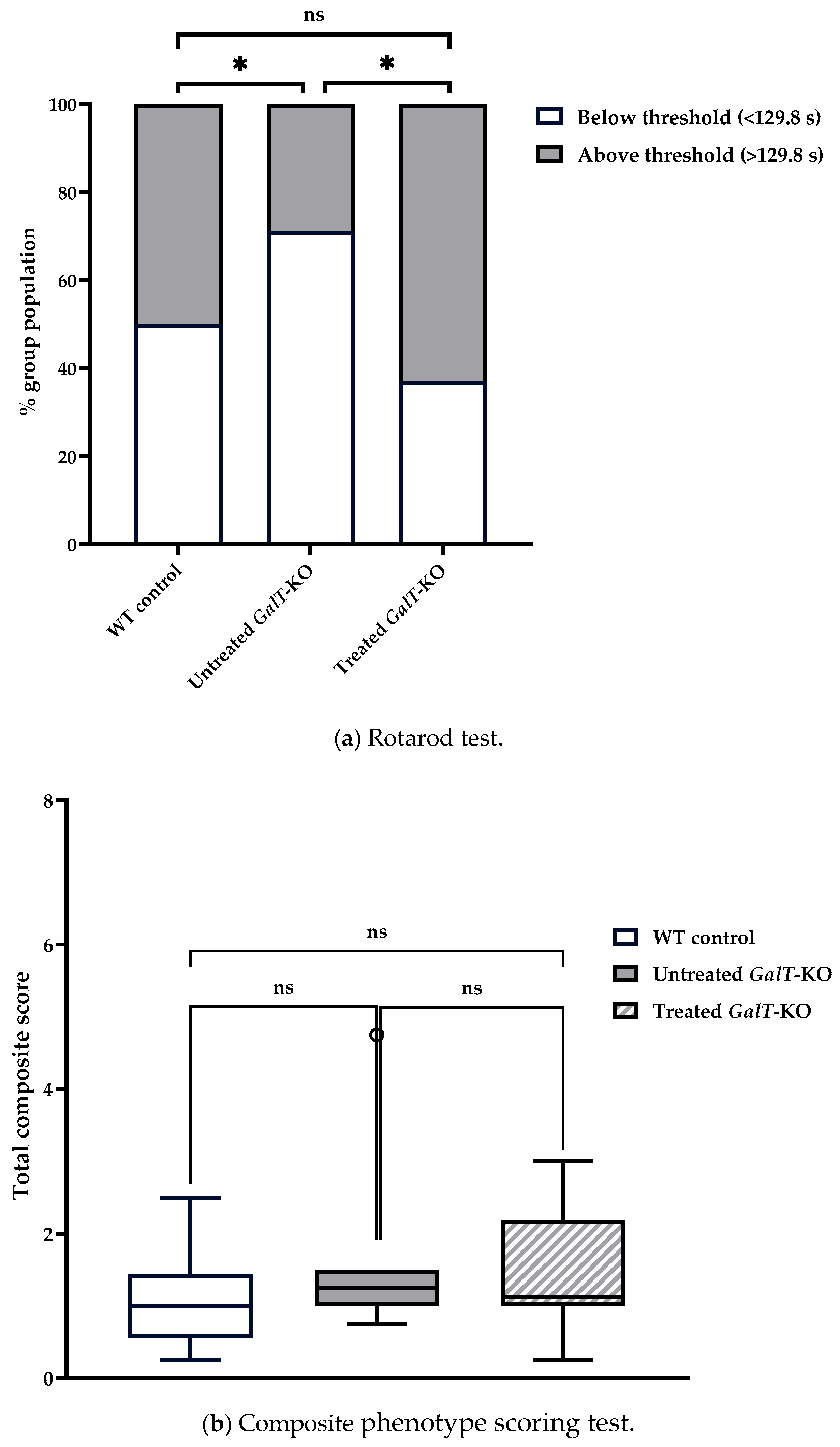
| (a) Descriptive statistics from rotarod assessments. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Group | Treatment Age | Assessment No. | vars | n | Mean | SD | Median | Trimmed | MAD | Min | Max | Range | Skew | Kurtosis | SE |
| WT Control | 3 weeks | 1 | 1 | 24 | 109.0 | 45.7 | 106.2 | 107 | 28.5 | 16.1 | 221 | 205 | 0.477 | 0.334 | 9.33 |
| Untreated GalT-KO | 3 weeks | 1 | 1 | 21 | 72.8 | 37.3 | 64.0 | 68.4 | 25.9 | 20.2 | 164 | 144 | 1.080 | 0.317 | 8.14 |
| Treated GalT-KO | 3 weeks | 1 | 1 | 24 | 122.0 | 40.8 | 134.7 | 124 | 30.2 | 43.9 | 178 | 134 | −0.661 | −0.748 | 8.33 |
| WT Control | 6 weeks | 1 | 1 | 24 | 118.0 | 53.5 | 126.9 | 115 | 73.9 | 46.7 | 244 | 198 | 0.465 | −0.813 | 10.9 |
| Untreated GalT-KO | 6 weeks | 1 | 1 | 24 | 104.0 | 35.3 | 101.2 | 103 | 42.5 | 46.4 | 174 | 127 | 0.202 | −0.987 | 7.2 |
| Treated GalT-KO | 6 weeks | 1 | 1 | 24 | 116.0 | 30.6 | 122.6 | 117 | 20.8 | 46.7 | 162 | 115 | −0.602 | −0.408 | 6.26 |
| WT Control | 3 weeks | 2 | 1 | 24 | 118.0 | 40.1 | 130.0 | 119 | 41.3 | 48.4 | 179 | 131 | −0.202 | −1.240 | 8.19 |
| Untreated GalT-KO | 3 weeks | 2 | 1 | 21 | 104.0 | 40.4 | 95.5 | 100 | 37.8 | 52.5 | 196 | 143 | 0.687 | −0.734 | 8.81 |
| Treated GalT-KO | 3 weeks | 2 | 1 | 24 | 136.0 | 44.3 | 137.5 | 135 | 34.5 | 49 | 268 | 219 | 0.617 | 1.420 | 9.05 |
| (b) Descriptive statistics from composite phenotype scoring assessments. | |||||||||||||||
| Treatment Group | Treatment Age | Assessment No. | vars | n | Mean | SD | Median | Trimmed | MAD | Min | Max | Range | Skew | Kurtosis | SE |
| WT Control | 3 weeks | 1 | 1 | 8 | 1.19 | 0.651 | 1.00 | 1.19 | 0.556 | 0.5 | 2.5 | 2 | 0.79 | −0.693 | 0.23 |
| Untreated GalT-KO | 3 weeks | 1 | 1 | 8 | 2.44 | 1.35 | 2.13 | 2.44 | 0.927 | 0.5 | 5 | 4.5 | 0.492 | −0.797 | 0.479 |
| Treated GalT-KO | 3 weeks | 1 | 1 | 8 | 1.28 | 1.32 | 0.88 | 1.28 | 0.927 | 0.25 | 4.25 | 4 | 1.28 | 0.322 | 0.466 |
| WT Control | 6 weeks | 1 | 1 | 8 | 0.78 | 0.7 | 0.63 | 0.781 | 0.741 | 0 | 2 | 2 | 0.435 | −1.32 | 0.247 |
| Untreated GalT-KO | 6 weeks | 1 | 1 | 8 | 2.25 | 1.35 | 1.88 | 2.25 | 0.741 | 0.5 | 4.5 | 4 | 0.515 | −1.29 | 0.477 |
| Treated GalT-KO | 6 weeks | 1 | 1 | 8 | 2.16 | 2.11 | 1.50 | 2.16 | 0.927 | 0 | 6.75 | 6.75 | 1.13 | −0.0184 | 0.747 |
| WT Control | 3 weeks | 2 | 1 | 8 | 1.09 | 0.706 | 1.00 | 1.09 | 0.556 | 0.25 | 2.5 | 2.25 | 0.697 | −0.691 | 0.25 |
| Untreated GalT-KO | 3 weeks | 2 | 1 | 7 | 1.71 | 1.36 | 1.25 | 1.71 | 0.371 | 0.75 | 4.75 | 4 | 1.49 | 0.546 | 0.516 |
| Treated GalT-KO | 3 weeks | 2 | 1 | 8 | 1.47 | 0.881 | 1.13 | 1.47 | 0.741 | 0.25 | 3 | 2.75 | 0.382 | −1.27 | 0.311 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellagamba, O.; Guo, A.J.; Yan, X.; Sarkis, J.; Balakrishnan, B.; Lai, K. Experimental Galactose-1-Phosphate Uridylyltransferase (GALT) mRNA Therapy Improves Motor-Related Phenotypes in a Mouse Model of Classic Galactosemia—A Pilot Study. Biomedicines 2025, 13, 2848. https://doi.org/10.3390/biomedicines13122848
Bellagamba O, Guo AJ, Yan X, Sarkis J, Balakrishnan B, Lai K. Experimental Galactose-1-Phosphate Uridylyltransferase (GALT) mRNA Therapy Improves Motor-Related Phenotypes in a Mouse Model of Classic Galactosemia—A Pilot Study. Biomedicines. 2025; 13(12):2848. https://doi.org/10.3390/biomedicines13122848
Chicago/Turabian StyleBellagamba, Olivia, Aaron J. Guo, Xinhua Yan, Joe Sarkis, Bijina Balakrishnan, and Kent Lai. 2025. "Experimental Galactose-1-Phosphate Uridylyltransferase (GALT) mRNA Therapy Improves Motor-Related Phenotypes in a Mouse Model of Classic Galactosemia—A Pilot Study" Biomedicines 13, no. 12: 2848. https://doi.org/10.3390/biomedicines13122848
APA StyleBellagamba, O., Guo, A. J., Yan, X., Sarkis, J., Balakrishnan, B., & Lai, K. (2025). Experimental Galactose-1-Phosphate Uridylyltransferase (GALT) mRNA Therapy Improves Motor-Related Phenotypes in a Mouse Model of Classic Galactosemia—A Pilot Study. Biomedicines, 13(12), 2848. https://doi.org/10.3390/biomedicines13122848






