Therapeutic Potential of Gentianaceae Family Plants in the Treatment of Diabetes and Its Complications
Abstract
1. Introduction
2. Plants from Gentianaceae Family: Traditional Use, Phytochemical Composition and Biological Activities
2.1. Traditional Use of Gentianaceae
2.2. Phytochemical Composition and Biological Activity
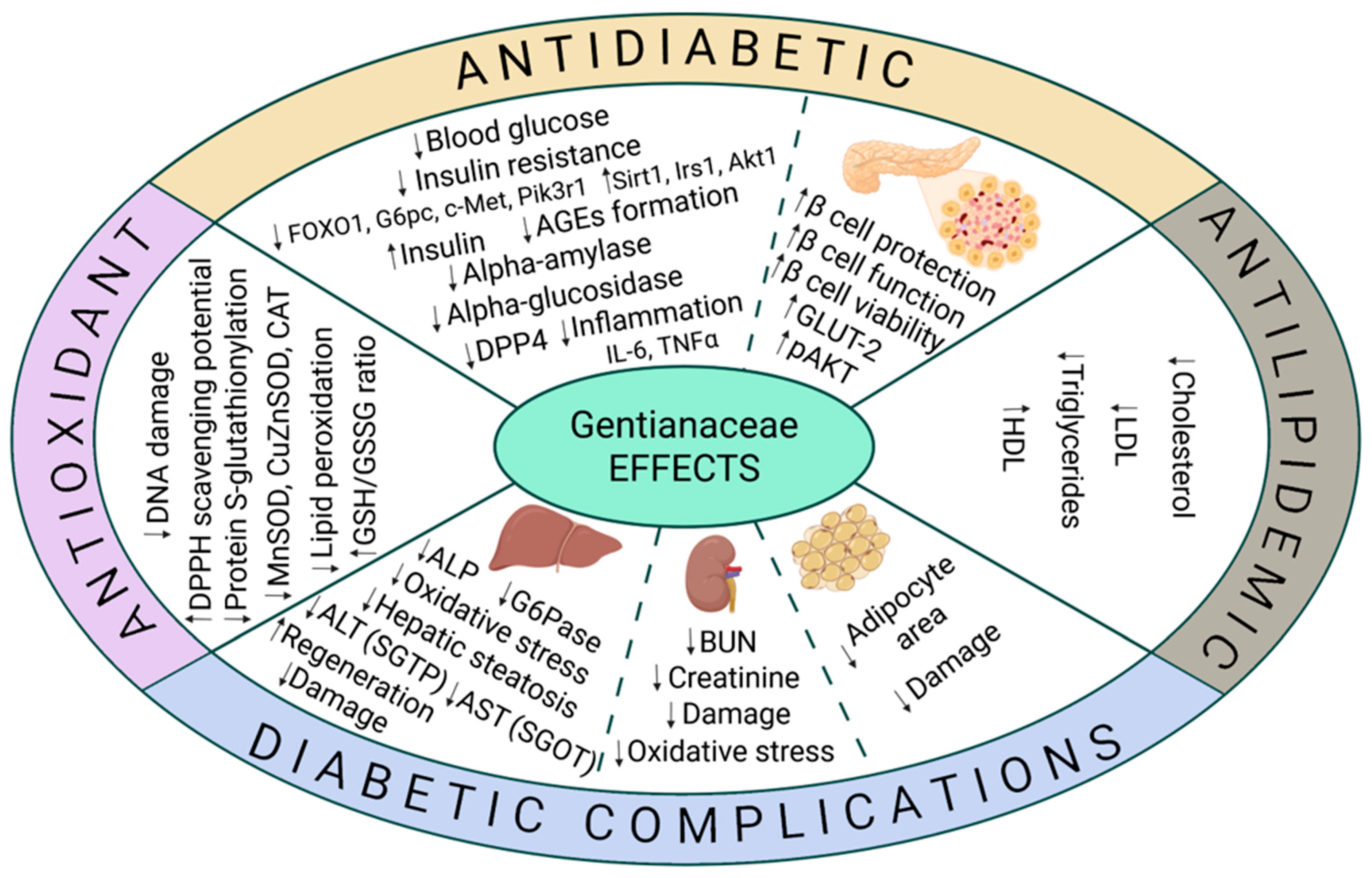
3. Plant Extracts from Gentianaceae Family: Mechanistic Insights into Diabetes and Diabetic Complications
3.1. Antidiabetic Effects
| Species | Plant Part(s) Tested | Pharmacological Activity | Model/Diabetes Induction | Mechanism of Action | Standard Antidiabetic Drug/Comparison with Extract | Reference |
|---|---|---|---|---|---|---|
| Gentiana dinarica | aerial parts of wild growing plants/roots of wild growing plants/shoot culture/vegetative roots/genetically transformed roots | antidiabetic effects | Wistar rats | antihyperglycemic activity in the oral glucose tolerance test | no positive control | [32] |
| Gentiana utriculosa | aerial parts of wild growing plants/roots of wild growing plants/shoot culture/vegetative roots/genetically transformed roots | antidiabetic effects | Wistar rats | antihyperglycemic activity in the oral glucose tolerance test | no positive control | [32] |
| Gentiana olivieri/ Isoorientin | aerial parts | antidiabetic effects | Sprague-Dawley rats/STZ | significant activity in glucose-hyperglycemic rats within 2 h after administration of extract/Isoorientin exhibited significant hypoglycemic and antihyperlipidemic effects | tolbutamide/similar effects of extract as tolbutamide | [45] |
| Veratrilla baillonii | aerial parts | antidiabetic, antioxidant and anti-inflammatory effects | Sprague-Dawley rats/high-sugar and high-fat diet with STZ | improvement of blood glucose and serum insulin levels/suppressed expression of many genes involved in insulin resistance/restored structure of pancreatic islet and the degree of dilatation of the lobular ducts and inflammatory infiltration | metformin/fasting blood glucose and serum insulin better than in metformin group/histology was similar to metformin group | [46] |
| Veratrilla baillonii | aerial parts | antidiabetic effects | Sprague-Dawley rats/high-fat diet | improvement of pathological damage of pancreas/insulin resistance: hypoglycemic effect by relieving insulin resistance | metformin/both extract and metformin significantly promoted weight gain, insulin resistance, ameliorated pathological damage of pancreas | [47] |
| Swertia herbs (swertiamarin, (R)-gentiandiol and (S)-gentiandio) | aerial parts/synthesized | antidiabetic and antihyperlipidemic effects | KKAy mice/High-Fat Diet | improvement of serum levels of total cholesterol, triglyceride, high-density and low-density lipoprotein cholesterol/improvement of histopathology of pancreas/identified 15 endogenous biomarkers, 10 of which were recalled by (R)-gentiandiol; between all biomarkers, glycine was the most effectively recalled by (R)-gentiandiol and regulated eight metabolic pathways | metformin/swertiamarin and (R)-gentiandiol showed similar level of protection as metformin | [28] |
| Gentianella acuta | whole plants/isolated xantones | antidiabetic effects | obese diabetic db/db mice | serum glucose, triglycerides, total cholesterol and free fatty acids were reduced | no positive control | [30] |
| Gentianella bicolor | whole plants | antidiabetic effects | Sprague-Dawley rat/STZ | decrease in blood glucose/pancreatic homogeneous structure with the presence of islets of virtually normal size | glibenclamide/blood glucose level better than glibenclamide | [16] |
| Gentiana quadrifaria | leaf | antidiabetic, antihyperlipidemic and antioxidant effects | Swiss albino mice/STZ | decrease in fasting blood glucose/reduction in serum low-density lipoprotein cholesterol, very-low-density lipoprotein cholesterol, total cholesterol and triglycerides and increase in high-density lipoprotein cholesterol levels/increased antioxidant enzymes catalase, superoxide dismutase, and glutathione reductase, and reduced serum glutamic-pyruvic transaminase, serum glutamic-oxaloacetic transaminase, alkaline phosphatase | ascorbic acid/metformin, glibenclamide, and insulin/similar level of protection of extract as positive controls | [38] |
| Swertia kouitchensis | whole plant | antidiabetic, antihyperlipidemic and antioxidant effects | BALB/c mice/STZ | decrease in blood glucose levels/increase in serum insulin levels/serum malondialdehyde, the activities of superoxidase dismutase and lipid levels (triglycerides, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol levels) | gliclazide/markers of carbohydrate metabolism and serum lipid profiles better than gliclazide/glucose level as gliclazide | [39] |
| Swertia chirata | root | antidiabetic effects | Wistar rats/alloxan | decrease in blood glucose levels | metformin/blood glucose level as in metformin | [43] |
| Swertia chirata | leaf and its pet-ether fraction | antidiabetic effects | Swiss albino mice | decrease in blood glucose level | glibenclamide/pet-ether fraction decreases blood glucose level similar to glibenclamide | [42] |
| Swertia chirata | aerial parts | antidiabetic effects | Swiss albino rats/alloxan | decrease in blood glucose level | glibenclamide/formulation that contained Swertia chirata showed blood glucose reduction similar to glibenclamide | [44] |
| Enicostemma littorale | whole plant | antidiabetic effects | rats/alloxan | decrease in blood glucose level | no positive control | [12] |
| Enicostemma littorale | whole plants | antidiabetic effects | Charles Foster rats/alloxan | improvement of blood glucose level, glycosylated hemoglobin, serum insulin | no positive control | [48] |
| Enicostemma littorale | whole plant | antidiabetic effects | Charles-Foster rats/alloxan/isolated rat pancreatic islets | enhance glucose-induced insulin release/increase in the serum insulin levels from isolated rat pancreatic islets/significant insulinotropic effect | glibenclamide/extract increased the serum insulin levels significantly at 8 h | [49] |
| Enicostemma littorale | whole plant | antidiabetic and antihyperlipidemic effects | human study/diabetic patients | reduced blood glucose and serum insulin levels/reduced urine sugar/serum cholesterol and serum triglycerides were significantly reduced and increase in serum high-density lipoprotein | no positive control | [50] |
| Centaurium erythraea | leaf | antidiabetic and antioxidant effects | Wistar rat/STZ | decrease in blood glucose/improvement of insulin, triglycerides and total cholesterol levels/pancreatic homogeneous structure with the presence of islets virtually normal size/improvement of oxidative stress parameters (malondialdehyde levels, protein carbonyl content, reduced glutathione content and enzymatic activities of superoxide dismutase, catalase and glutathione peroxidase in pancreas) | no positive control | [34] |
| Centaurium erythraea | aerial parts | antidiabetic and antioxidant effects | Wistar rat/STZ | improving the structural and functional properties of pancreatic islets/positive effects of extract on levels of insulin, glucagon, somatostatin, glucose transporter GLUT-2 and p-Akt | no positive control | [36] |
| Centaurium erythraea | aerial parts | antidiabetic and antioxidant effects | Wistar rat/STZ | improvement of serum alanine and aspartate aminotransferase, blood urea nitrogen and creatinine levels | no positive control | [37] |
| Centaurium erythraea | aerial parts | antidiabetic and antihyperlipidemic effects | C57BL/6J mice/high-fat diet | decrease in blood glucose/decreased insulin resistance/antihypercholesterolemic and antihypertriglyceridemic action | no positive control | [35] |
3.2. Antioxidant Properties
| Species | Plant Part(s) Tested | Pharmacological Activity | Model | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| Gentiana dinarica | aerial parts of wild growing plants/roots of wild growing plants/shoot culture/vegetative roots/genetically transformed roots | antioxidant/antidiabetic effects | in vitro testing of extract | scavenge DPPH free radicals/inhibition of intestinal α-glucosidase in vitro/norswertianin-1-O-primeveroside and norswertianin have the highest scavenging potential | [32] |
| Gentiana utriculosa | aerial parts of wild growing plants/roots of wild growing plants/shoot culture/vegetative roots/genetically transformed roots | antioxidant/antidiabetic effects | in vitro testing of extract | scavenge DPPH free radicals/inhibition of intestinal alpha-glucosidase in vitro/mangiferin, decussatin, and decussatin-1-O-primeveroside the highest scavenging potential | [32] |
| Centaurium erythraea | aerial parts | antioxidant/antidiabetic effects | Rin-5F b-cells/STZ | reduced DNA damage, lipid peroxidation, protein S-glutathionylation/improvement of enzyme activity and transcriptional regulation of catalase, Mn superoxide dismutase, CuZn superoxide dismutase, glutathione peroxidase and glutathione reductase enzyme/promoting proliferative and pro-survival pathways in beta-cells: readjustment of the presence and activities of redox-sensitive NFκB-p65, FOXO3A, Sp1 and Nrf-2; fine-tuned modulation of the activities ofAkt, ERK and p38 kinases and of Pdx-1 and MafA regulatory factors | [36] |
| Centaurium erythraea | aerial parts | antioxidant effects | Rin-5F b-cells/H2O2- and SNP-induced oxidative/nitrosative stress | increase cell viability and ameliorate the disturbance of redox homeostasis in H2O2- and SNP-treated cells by decreasing DNA damage, lipid peroxidation and protein S-glutathionylation/adjustment of mRNA, protein levels and activities of glutathione peroxidase, glutathione reductase, Mn superoxide dismutase, CuZn superoxide dismutase and catalase/preventing or slowing down beta-cell damage and dysfunction caused by oxidative/nitrosative stress | [53] |
| Enicostemma littorale | aerial parts | antioxidant/antidiabetic effects | in vitro testing of extract | DPPH radical scavenging activity/hydrogen peroxide scavenging activity/promising activity based on in vitro dipeptidyl peptidase-IV inhibitors | [52] |
| Enicostemma littorale | aerial parts | antidiabetic effects | in vitro testing of extract | alpha-amylase inhibition/in vitro drug release: transdermal delivery of capsulated extract on rat skin | [51] |
| Schenkia spicata | isolated sweroside | antioxidant/antidiabetic effects | in vitro testing of extract | in vitro alpha-amylase and alpha-glucosidase inhibitory activities/in vitro free radical scavenging and antioxidant potential (ABTS, CUPric Reducing Antioxidant Capacity and Ferric Reducing Antioxidant Power assays) | [29] |
| Gentiana quadrifaria | leaf | antioxidant effects | in vitro testing of extract | free radical scavenging capability: DPPH and ABTS assays/total phenolic content/total flavonoid content | [38] |
| Swertia kouitchensis | whole plant | antidiabetic effects | in vitro testing of extract/NIT-1cell | in vitro α-amylase and α-glucosidase activity test/In vitro NIT-1 cell insulin secretion test | [39] |
| Swertia chirata | aerial parts | antioxidant/antidiabetic effects | in vitro testing of extract | DPPH free-radical scavenging activity/in vitro inhibition of alpha-amylase | [44] |
| Species | Plant Part(s) Tested | Model/Diabetes induction | Targeted Organ | Mechanism of Action | Standard Antidiabetic Drug/Comparation with extract | Reference |
|---|---|---|---|---|---|---|
| Veratrilla baillonii | aerial parts | Sprague Dawley/high-sugar and high-fat diet with STZ | liver, kidney, epididymal adipose tissue | inhibited the levels of Foxo1, G6pc, c-Met and Pik3r1 in the liver while the expressions of genes related to metabolism and inflammation, including Sirt1, Irs1, Akt1, were significantly increased/restored structure of pancreatic islet and the degree of dilatation of the lobular ducts and inflammatory infiltration were also alleviated/epididymal adipose tissue showed that the size of adipocytes decreased and the density of adipocytes increased/improvement of kidney histology and fibrosis/α-SMA expression in the liver was significantly reduced and improved liver fibrosis/superoxide dismutase and glutathione peroxidase were increased, malondialdehyde was lowered in the liver/decrease in the indicators of inflammation, IL-6 and TNF-α, in the liver/improved glucose metabolism through modulating the IRS1/PI3K/AKT pathway | metformin/histology and signaling pathways were similar to metformin group | [46] |
| Veratrilla baillonii | aerial parts | Sprague-Dawley rats/high-fat diet | liver | improvement of pathological damage on liver | metformin/both extract and metformin significantly ameliorated pathological damage of liver | [47] |
| Swertia herbs (swertiamarin, (R)-gentiandiol, and (S)-gentiandiol) | aerial parts/synthesized | KKAy mice/High-Fat Diet | kidney | improvement of histopathology of kidney | metformin/swertiamarin and (R)-gentiandiol showed similar level of protection as metformin | [28] |
| Gentianella acuta | whole plants/isolated xantones | obese diabetic db/db mice | liver, epididymal adipose tissue | size and weight of adipose deposits in epididymal adipose tissue/hepatic steatosis was reduced/GeneChip profiling of the liver transcriptome revealed potential activity on stimulating autophagy and regulating mitochondrial function/activity and migration of dynamin-related protein 1 (Drp1) by regulating the orphan nuclear receptor subfamily 4 group A member, thereby attenuating the excessive mitochondrial fission induced by high glucose and fatty acid load | no positive control | [30] |
| Gentiana quadrifaria | leaf | Swiss albino mice/STZ | liver | increased antioxidant enzymes catalase, superoxide dismutase, and glutathione reductase, and reduced serum glutamic-pyruvic transaminase, serum glutamic-oxaloacetic transaminase, alkaline phosphatase and protein carbonyl in liver/improvement of liver histology | ascorbic acid/metformin, glibenclamide and insulin/similar level of protection of extract as positive controls | [38] |
| Swertia chirata | root | Wistar rats/alloxan | liver, kidney | decrease in blood glucose levels/ameliorated the pathological condition of liver and kidney (liver and kidney functioning tests) | metformin/liver and kidney functioning tests similar to the metformin group | [43] |
| Enicostemma littorale | whole plant | rats/alloxan | liver | decrease in the level of glycosylated hemoglobin and glucose-6-phosphatase activity in liver | no positive control | [12] |
| Enicostemma littorale | whole plants | Charles Foster rats/alloxan | liver | improvement of liver glucose 6-phosphatase, catalase, lipid peroxidation and reduced glutathione | no positive control | [48] |
| Enicostemma littorale | whole plant | human study/diabetic patients | kidney, cardiovascular system | improvement of kidney function, lipid profile and blood pressure | no positive control | [50] |
| Centaurium erythraea | aerial parts | Wistar rat/STZ | liver, kidney | reduced DNA damage in the liver and kidney estimated by Comet assay/improvement of liver and kidney catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase enzyme activities and protein levels/decreased level of posttranslational O-GlcNAc modified protein in liver and kidney | no positive control | [37] |
3.3. Improvement of the Lipid Profile
4. Bioavailability Challenges and Toxicity of the Most Abundant Phytoconstituents of Gentianaceae Family
Clinical Evidence on the Therapeutic Application of Plant Extracts from Gentianaceae Family
5. Current Limitations, Research Gaps and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mamdouh, H.B.; Nair, B.; Hussain, H.Y.; Mudawi, M.S.; Alnakhi, W.; Cochinard, S. Cost Analysis of Diabetes Mellitus Treatment in the Public Sector in Dubai, The United Arab Emirates: Analysis Based on a Global Perspective Model. Value Health Reg. 2025, 50, 101156. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/publications/i/item/9789241565257 (accessed on 21 April 2016).
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Corathers, S.D.; Peavie, S.; Salehi, M. Complications of diabetes therapy. Endocrinol. Metab. Clin. N. Am. 2013, 42, 947–970. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Nanoparticles Formulations of Artemisinin and Derivatives as Potential Therapeutics for the Treatment of Cancer, Leishmaniasis and Malaria. Pharmaceutics 2020, 12, 748. [Google Scholar] [CrossRef]
- Salleh, N.H.; Zulkipli, I.N.; Mohd, Y.; Hartini, J.; Fairuzeta, A.; Norhayati, W.A.; Wan Amir Nizam, A.; Siti, R. Systematic Review of Medicinal Plants Used for Treatment of Diabetes in Human Clinical Trials: An ASEAN Perspective. Evid. Based Complement. Alternat. Med. 2021, 2021, 5570939. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Cui, B.W.; Wu, Y.L. Genus Gentiana: A review on phytochemistry, pharmacology and molecular mechanism. J. Ethnopharmacol. 2021, 264, 113391. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, F.; Gao, Q.; Xing, R.; Chen, S. A Review on the Ethnomedicinal Usage, Phytochemistry, and Pharmacological Properties of Gentianeae (Gentianaceae) in Tibetan Medicine. Plants 2021, 10, 2383. [Google Scholar] [CrossRef] [PubMed]
- Vijayvargia, R.; Kumar, M.; Gupta, S. Hypoglycemic effect of aqueous extract of Enicostemma littorale Blume (chhota chirayata) on alloxan induced diabetes mellitus in rats. Indian J. Exp. Biol. 2000, 38, 781–784. [Google Scholar]
- Prokopiak, M.; Mayorova, O.; Hrytsak, L.; Meshko, H.; Drobyk, N. The assessment of the current status of Gentiana lutea L. populations of the Ukrainian Carpathians: Ecological and genetic approaches. Folia Oecologica 2022, 49, 42–50. [Google Scholar] [CrossRef]
- Šavikin, K.; Aljančić, I.S.; Vajs, V.E.; Milosavljević, S.M.; Jadranin, M.; Đorđević, I.; Menković, N.R. Bioactive Secondary Metabolites in Several Genera of Gentianaceae Species from the Central Regions of the Balkan Peninsula. In The Gentianaceae-Volume 2: Biotechnology and Applications; Rybczyński, J., Davey, M., Mikuła, A., Eds.; Springer: Berlin, Heidelberg, 2015; pp. 319–347. [Google Scholar]
- Tovilovic-Kovacevic, G.; Zogovic, N.; Krstic-Milosevic, D. Chapter 19—Secondary metabolites from endangered Gentiana, Gentianella, Centaurium, and Swertia species (Gentianaceae): Promising natural biotherapeutics. In Biodiversity and Biomedicine, 1st ed.; Ozturk, M., Egamberdieva, D., Pešić, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 335–384. [Google Scholar]
- Bermúdez, D.L.; Cuéllar, C.A.; Licham, M.A.; Amparo, M.A.; Saavedra, H.; Jorge, J. Hypoglycemic effect of Gentianella bicolor (Wedd.) Fabris ex J.S. Pringle (Corpus Huay) in Sprague Dowley. Rev. Cubana Plant Med. 2016, 21, 31–41. [Google Scholar]
- Mirzaee, F.; Hosseini, A.; Jouybari, H.B.; Davoodi, A.; Azadbakht, M. Medicinal, biological and phytochemical properties of Gentiana species. J. Tradit. Complement. Med. 2017, 7, 400–408. [Google Scholar] [CrossRef]
- Živković, J.; Ilić, M.; Šavikin, K.; Zdunić, G.; Ilić, A.; Stojković, D. Traditional Use of Medicinal Plants in South-Eastern Serbia (Pčinja District): Ethnopharmacological Investigation on the Current Status and Comparison with Half a Century Old Data. Front. Pharmacol. 2020, 11, 1020. [Google Scholar] [CrossRef]
- Marković, M.; Pljevljakušić, D.; Menković, N.; Matejić, J.; Papović, O.; Stankov-Jovanović, V. Traditional Knowledge on the Medicinal Use of Plants from Genus Gentiana in the Pirot County (Serbia). Lek. Sirov. 2021, 41, 46–53. [Google Scholar] [CrossRef]
- Sobot, A.V.; Savić, J.; Filipović Trčković, J.; Drakulić, D.; Joksić, G. Toxicity Assessment of Gentiana lutea L. Root Extract and Its Monoterpene Compounds. Indian J. Exp. Biol. 2020, 58, 609–616. [Google Scholar] [CrossRef]
- Andryszkiewicz, W.; Chmielewska, M.; Ciecierska, J.; Lenkiewicz, P.; Marciniak, W.; Raczycka, W.; Wojno, A.; Kulbacka, J.; Niewiński, P.; Bieżuńska-Kusiak, K. Gentianaceae Family—Derived Bioactive Compounds—Therapeutic Values and Supporting Role in Inflammation and Detoxification. Nutrients 2025, 17, 2619. [Google Scholar] [CrossRef] [PubMed]
- Šiler, B.; Mišić, D. Chapter 11—Biologically Active Compounds from the Genus Centaurium s.l. (Gentianaceae): Current Knowledge and Future Prospects in Medicine. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 363–397. [Google Scholar]
- Popović, Z.; Krstić-Milošević, D.; Marković, M.; Vidaković, V.; Bojović, S. Gentiana asclepiadea L. from Two High Mountainous Habitats: Inter- and Intrapopulation Variability Based on Species Phytochemistry. Plants 2021, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Phytochemicals of Gentianaceae: A Review of Pharmacological Properties. IJPSN 2008, 1, 33–36. [Google Scholar] [CrossRef]
- Wang, X.; Long, D.; Hu, X.; Guo, N. Gentiopicroside Modulates Glucose Homeostasis in High-Fat-Diet and Streptozotocin-Induced Type 2 Diabetic Mice. Front. Pharmacol. 2023, 14, 1172360. [Google Scholar] [CrossRef]
- Xiao, H.M.; Sun, X.H.; Lin, Z.Y.; Huang, H.Q. Gentiopicroside Targets PAQR3 to Activate the PI3K/AKT Signaling Pathway and Ameliorate Disordered Glucose and Lipid Metabolism. Acta Pharm. Sin. B 2022, 12, 2887–2904. [Google Scholar] [CrossRef]
- Dhanavathy, G. Immunohistochemistry, Histopathology, and Biomarker Studies of Swertiamarin, a Secoiridoid Glycoside, Prevents and Protects Streptozotocin-Induced β-Cell Damage in Wistar Rat Pancreas. J. Endocrinol. Investig. 2015, 38, 669–684. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, J.; Tang, S.; Sun, Y.; Li, H.; Li, P.; Li, X.; Hattori, M.; Wu, X.; Zhang, H.; et al. Anti-diabetes activity of (R)-gentiandiol in KKAy type 2 mice. Sci. Rep. 2025, 15, 15730. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; El-Raey, M.; El-Kashak, W.; Batiha, G.E.; Althumairy, D.; Alamer, S.; Mostafa, N.M.; Eldahshan, O.A. Sweroside: An iridoid glycoside of potential neuroprotective, antidiabetic, and antioxidant activities supported by molecular docking. Amino Acids 2023, 55, 1765–1774. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Chen, Q.; Yu, H.; Liu, M.; Wang, Y.; Zhang, Y.; Wang, T. 7′-Hydroxyl substituted xanthones from Gentianella acuta revert hepatic steatosis in obese diabetic mice through preserving mitochondrial homeostasis. Biochem. Pharmacol. 2025, 236, 116878. [Google Scholar] [CrossRef] [PubMed]
- Sok Yen, F.; Shu Qin, C.; Tan Shi Xuan, S.; Jia Ying, P.; Yi Le, H.; Darmarajan, T.; Gunasekaran, B.; Salvamani, S. Hypoglycemic Effects of Plant Flavonoids: A Review. Evid. Based Complement Alternat. Med. 2021, 8, 2057333. [Google Scholar] [CrossRef]
- Jovanović, J.A.; Krstić-Milošević, D.; Vinterhalter, B.; Dinić, S.; Grdović, N.; Uskoković, A.; Rajić, J.; Ðorđević, M.; Sarić, A.; Vidaković, M.; et al. Evaluation of the Antidiabetic Potential of Xanthone-Rich Extracts from Gentiana dinarica and Gentiana utriculosa. Int. J. Mol. Sci. 2024, 25, 9066. [Google Scholar] [CrossRef] [PubMed]
- El-Hilaly, J.; Hmammouchi, M.; Lyoussi, B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco). J. Ethnopharmacol. 2003, 86, 149–158. [Google Scholar] [CrossRef]
- Sefi, M.; Fetoui, H.; Lachkar, N.; Tahraoui, A.; Badiaa, L.; Boudawara, T.; Zeghal, N. Centaurium erythrea (Gentianaceae) leaf extract alleviates streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. J. Ethnopharmacol. 2011, 135, 243–250. [Google Scholar] [CrossRef]
- Hamza, N.; Berke, B.; Cheze, C.; Garrec, R.L.; Lassalle, R.; Agli, A.-N.; Robinson, P.; Gin, H.; Moore, N. Treatment of high fat diet induced type 2 diabetes in C57BL/6J mice by two medicinal plants used in traditional treatment of diabetes in the east of Algeria. J. Ethnopharmacol. 2011, 133, 931–933. [Google Scholar] [CrossRef]
- Đorđević, M.; Grdović, N.; Mihailović, M.; Arambašić Jovanović, J.; Uskoković, A.; Rajić, J.; Sinadinović, M.; Tolić, A.; Mišić, D.; Šiler, B.; et al. Centaurium erythraea extract improves survival and functionality of pancreatic beta-cells in diabetes through multiple routes of action. J. Ethnopharmacol. 2019, 242, 112043. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, M.; Tolić, A.; Rajić, J.; Mihailović, M.; Arambašić Jovanović, J.; Uskoković, A.; Grdović, N.; Đorđević, M.; Mišić, D.; Šiler, B.; et al. Centaurium erythraea methanol extract improves the functionality of diabetic liver and kidney by mitigating hyperglycemia-induced oxidative stress. J. Funct. Foods 2022, 90, 104975. [Google Scholar] [CrossRef]
- Rymbai, R.; Bora, J.; Nakhro, M.; Donshiew, E.B.; Dey, A.; Lyngdoh, A.R.; Talukdar, N.; Thapliyal, S.; Rustagi, S.; Deshwal, R.K.; et al. Gentiana Quadrifaria Blume. exerts a protective effect on hepatopathy stress in mice model after hyperglycemia induced by streptozotocin. Ann. Med. Surg. 2025, 87, 6352–6364. [Google Scholar] [CrossRef]
- Wan, L.; Chen, C.; Xiao, Z.; Wang, Y.; Min, Q.; Yue, Y.; Chen, J. In vitro and in vivo anti-diabetic activity of Swertia kouitchensis extract. J. Ethnopharmacol. 2013, 147, 622–630. [Google Scholar] [CrossRef]
- Bhargava, S.; Rao, P.S.; Bhargava, P.; Shukla, S. Antipyretic Potential of Swertia chirata Buch Ham. Root Extract. Sci. Pharm. 2009, 77, 617–623. [Google Scholar] [CrossRef]
- Verma, H.; Patil, P.R.; Kolhapure, R.M.; Gopalkrishna, V. Antiviral activity of the Indian medicinal plant extract Swertia chirata against herpes simplex viruses: A study by in-vitro and molecular approach. Indian J. Med. Microbiol. 2008, 26, 322–326. [Google Scholar] [CrossRef]
- Alam, K.D.; Ali, M.S.; Mahjabeen, S.; Hassan, M.R.; Rahman, M.F.; Chowdhury, R. Potential hypoglycemic effect of Swertia chirata an Indian subcontinent herb with important medicinal value. Pharmacol. Online 2011, 2, 642–647. [Google Scholar]
- Tuz-Zohra, F.; Rashid, F.; Jashim, T.; Cruze, L.R.M.D.; Das, R.; Afrin, N. An in vivo assessment of anti-diabetic effect of Swertia chirata on alloxan induced diabetes rat and assess the safety profile. World J. Pharm. Res. 2019, 8, 329–340. [Google Scholar]
- Giri, B.R.; Baral, R.; Bhatt, H.; Khadka, A.; Tamrakar, R.; Timalsina, G.; Gyawali, R. Phytochemical Screening, Free-Radical Scavenging Activity, in vitro Alpha-Amylase Inhibitory Activity, and in vivo Hypoglycemic Activity Studies of Several Crude Drug Formulations Based on Selected Medicinal Plants of Nepal. Pharm. Chem. J. 2023, 56, 1369–1378. [Google Scholar] [CrossRef]
- Sezik, E.; Aslan, M.; Yesilada, E.; Ito, S. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 2005, 76, 1223–1238. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Z.; Ma, L.; Xu, X.; Azhar, M.; Huang, X.; Shi, J.; Li, J. Veratrilla baillonii Franch alleviate the symptoms of diabetes in type 2 diabetic rats induced by high-fat diet and streptozotocin. Food Sci. Hum. Well. 2024, 13, 1378–1389. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Li, J.; Li, J.; Ma, Z.; Huang, X.-J. Veratrilla baillonii Franch Ameliorates Diabetic Liver Injury by Alleviating Insulin Resistance in Rats. Front. Pharmacol. 2021, 12, 775563. [Google Scholar] [CrossRef]
- Maroo, J.; Ghosh, A.; Mathur, R.; Vasu, V.T.; Gupta, S. Antidiabetic Efficacy of Enicostemma littorale Methanol Extract in Alloxan-Induced Diabetic Rats. Pharm. Biol. 2003, 41, 388–391. [Google Scholar] [CrossRef]
- Maroo, J.; Vasu, V.T.; Aalinkeel, R.; Gupta, S. Glucose lowering effect of aqueous extract of Enicostemma littorale Blume in diabetes: A possible mechanism of action. J. Ethnopharmacol. 2002, 81, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, U.M.; Goyal, R.K. Efficacy of Enicostemma littorale in Type 2 Diabetic Patients. Phytother. Res. 2004, 18, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, M.H.; Imran, M.; Foudah, A.I.; Hasmi, S.; Aodah, A.H.; Alam, A. Dissolving Microneedle Transdermal Patch Loaded with Nanoliposomes of for the Management of Type II Diabetes. ACS Omega 2025, 10, 26468–26477. [Google Scholar] [CrossRef]
- Muralidharan, V.; Vaddepalli, P.; Saboo, S.; Vidhya, B.; Jyothi, A.; Gawai, N.; Konatham, T.K.R.; Haque, M.A. In-vitro Antioxidant and DPP-IV Enzyme Assay of Ethyl Acetate Extract of Enicostemma littorale. J. Pharm. Res. Int. 2022, 34, 50–58. [Google Scholar] [CrossRef]
- Đorđević, M.; Grdović, N.; Mihailović, M.; Arambašić Jovanović, J.; Uskoković, A.; Rajić, J.; Đorđević, M.; Tolić, A.; Mišić, D.; Šiler, B.; et al. Centaurium erythraea extract reduces redox imbalance and improves insulin expression and secretion in pancreatic β-cells exposed to oxidative and nitrosative stress. Arch. Biol. Sci. 2020, 72, 117–128. [Google Scholar] [CrossRef]
- Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Jovanović, A.; Grdović, N.; Rajić, J.; Đorđević, M.; Sarić, A.; Bugarski, B.; Vidaković, M.; et al. Liposome Encapsulation Enhances the Antidiabetic Efficacy of Silibinin. Pharmaceutics 2024, 16, 801. [Google Scholar] [CrossRef]
- Antoniadi, L.; Bartnik, M.; Angelis, A.; Wawruszak, A.; Halabalaki, M.; Kukula-Koch, W.; Skaltsounis, L.A. Gentiopicroside—An Insight into Its Pharmacological Significance and Future Perspectives. Cells 2024, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.; Balanč, B.; Volić, M.; Pećinar, I.; Živković, J.; Šavikin, K.P. Rosehip Extract-Loaded Liposomes for Potential Skin Application: Physicochemical Properties of Non- and UV-Irradiated Liposomes. Plants 2023, 12, 3063. [Google Scholar] [CrossRef] [PubMed]
- Raka, F.; Farr, S.; Kelly, J.; Stoianov, A.; Adeli, K. Metabolic Control via Nutrient-Sensing Mechanisms: Role of Taste Receptors and the Gut–Brain Neuroendocrine Axis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E559–E572. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinić, S.; Vidaković, M.; Arambašić Jovanović, J.; Uskoković, A.; Grdović, N.; Đorđević, M.; Rajić, J.; Mihailović, M. Therapeutic Potential of Gentianaceae Family Plants in the Treatment of Diabetes and Its Complications. Biomedicines 2025, 13, 2822. https://doi.org/10.3390/biomedicines13112822
Dinić S, Vidaković M, Arambašić Jovanović J, Uskoković A, Grdović N, Đorđević M, Rajić J, Mihailović M. Therapeutic Potential of Gentianaceae Family Plants in the Treatment of Diabetes and Its Complications. Biomedicines. 2025; 13(11):2822. https://doi.org/10.3390/biomedicines13112822
Chicago/Turabian StyleDinić, Svetlana, Melita Vidaković, Jelena Arambašić Jovanović, Aleksandra Uskoković, Nevena Grdović, Marija Đorđević, Jovana Rajić, and Mirjana Mihailović. 2025. "Therapeutic Potential of Gentianaceae Family Plants in the Treatment of Diabetes and Its Complications" Biomedicines 13, no. 11: 2822. https://doi.org/10.3390/biomedicines13112822
APA StyleDinić, S., Vidaković, M., Arambašić Jovanović, J., Uskoković, A., Grdović, N., Đorđević, M., Rajić, J., & Mihailović, M. (2025). Therapeutic Potential of Gentianaceae Family Plants in the Treatment of Diabetes and Its Complications. Biomedicines, 13(11), 2822. https://doi.org/10.3390/biomedicines13112822








