Melatonin in the Treatment of Female Infertility: Update on Biological and Clinical Findings
Abstract
1. Introduction
2. Different Facets of Melatonin Action
2.1. Melatonin as a Hormone
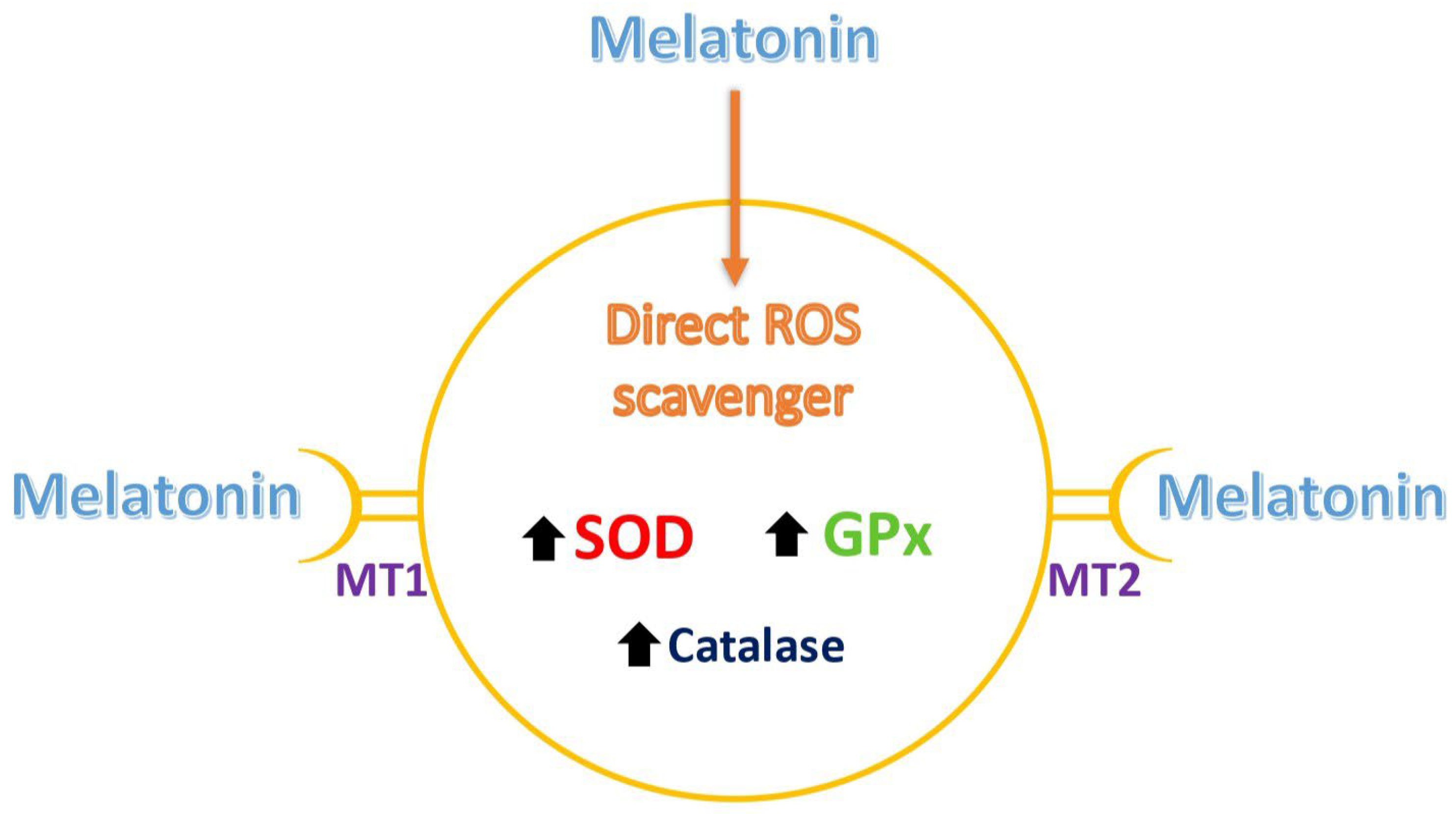
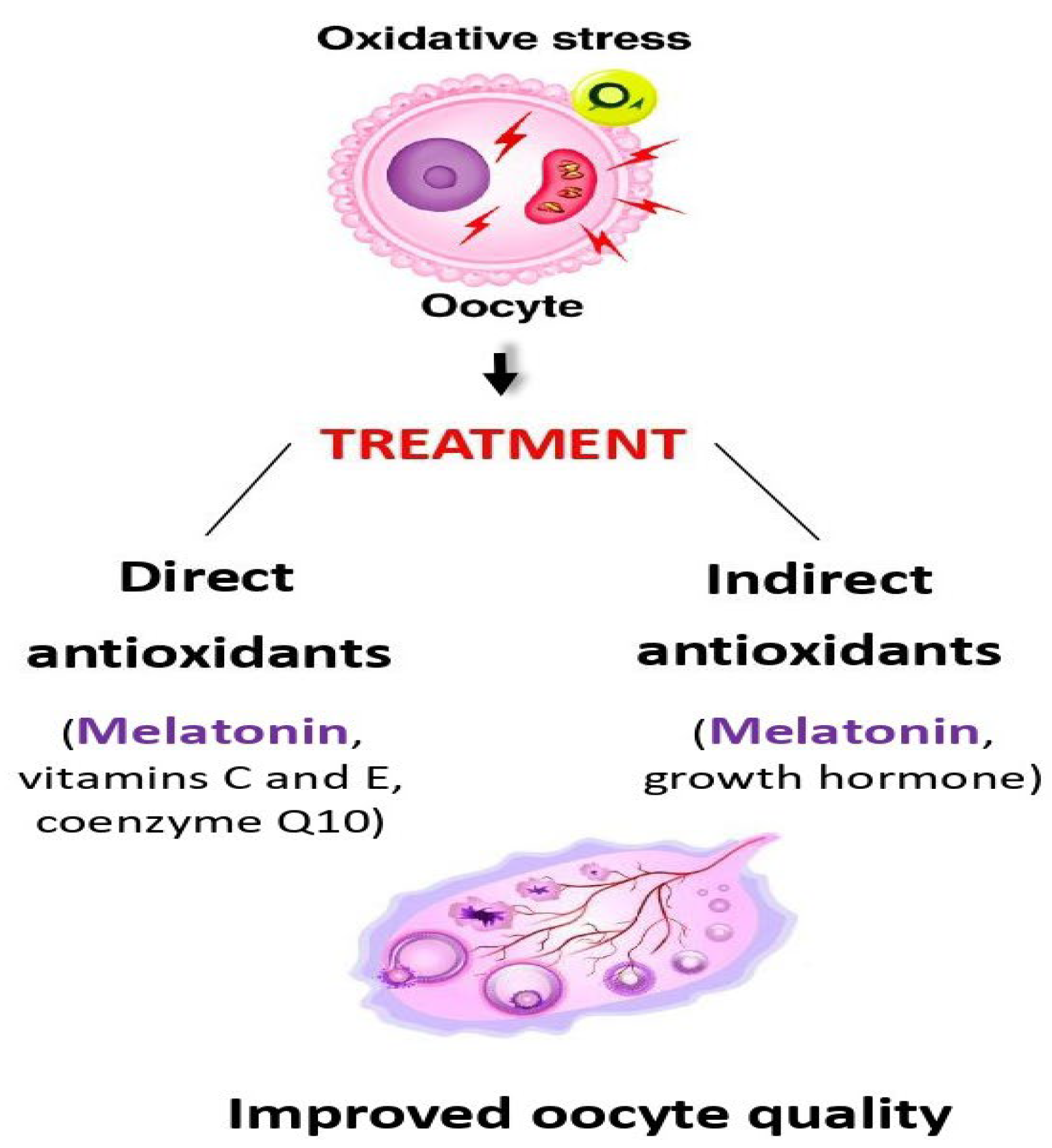
2.2. Melatonin as a Direct Antioxidant
2.3. Melatonin as an Immunomodulator
3. Melatonin Use in Different Types of Female Infertility
3.1. Ovarian Factor
3.1.1. Ovarian Insufficiency
3.1.2. Polycystic Ovary Syndrome
3.2. Uterine Factor
3.3. In Vitro Embryo Development Issues
3.4. Recent Experimental Animal Studies
3.5. Placental Health and Function
3.6. Gynecological Pathologies and Immunological Pregnancy Complications
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Datta, J.; Palmer, M.; Tanton, C.; Gibson, L.; Jones, K.; Macdowall, W.; Glasier, A.; Sonnenberg, P.; Field, N.; Mercer, C.; et al. Prevalence of Infertility and Help Seeking Among 15 000 Women and Men. Hum. Reprod. 2016, 31, 2108–2118. [Google Scholar] [CrossRef]
- Gemzell, C.A.; Diczfalusy, E.; Tillinger, G. Clinical Effect of Human Pituitary Follicle-Stimulating Hormone (FSH). J. Clin. Endocrinol. Metab. 1958, 18, 1333–1348. [Google Scholar] [CrossRef]
- Buxton, C.L.; Herrmann, W. Induction of Ovulation in the Human with Human Gonadotropins. Yale J. Biol. Med. 1960, 33, 145–147. [Google Scholar] [PubMed]
- Kohlberg, K. Die Praxis der Samenübertragung beim Menschen [The Practice of Artificial Insemination in Humans]. Dtsch. Med. Wochenschr. 1953, 78, 835–839. [Google Scholar] [CrossRef]
- Hackelöer, B.J.; Robinson, H.P. Ultraschalldarstellung des wachsenden Follikels und Corpus luteum im normalen physiologischen Zyklus [Ultrasound Examination of the Growing Ovarian Follicle and of the Corpus Luteum During the Normal Physiologie Menstrual Cycle. Geburtshilfe Frauenheilkd. 1978, 38, 163–168. (In German) [Google Scholar]
- Steptoe, P.C.; Edwards, R.G. Birth after Reimplantation of a Human Embryo. Lancet 1978, 2, 366. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after Intracytoplasmic Injection of Single Spermatozoon into an Oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Mendoza, C.; Testart, J. Viable Embryos from Injection of Round Spermatids into Oocytes. N. Engl. J. Med. 1995, 333, 525. [Google Scholar] [CrossRef]
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-Update Overview on Etiology, Diagnosis, Treatment and Future Directions. Reprod. Biol. Endocrinol. 2018, 16, 121. [Google Scholar] [CrossRef]
- Healy, D.L.; Trounson, A.O.; Andersen, A.N. Female Infertility: Causes and Treatment. Lancet 1994, 343, 1539–1544. [Google Scholar] [CrossRef]
- Walker, M.H.; Tobler, K.J. Female Infertility; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556033/ (accessed on 1 January 2025).
- Fernando, S.; Rombauts, L. Melatonin: Shedding Light on Infertility?—A Review of the Recent Literature. J. Ovarian Res. 2014, 7, 98. [Google Scholar] [CrossRef]
- Espino, J.; Macedo, M.; Lozano, G.; Ortiz, Á.; Rodríguez, C.; Rodríguez, A.B.; Bejarano, I. Impact of Melatonin Supplementation in Women with Unexplained Infertility Undergoing Fertility Treatment. Antioxidants 2019, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Ye, X.; Wang, S.; Zhang, D. Melatonin Application in Assisted Reproductive Technology: A Systematic Review and Meta-Analysis of Randomized Trials. Front. Endocrinol. 2020, 11, 160. [Google Scholar] [CrossRef]
- Olcese, J.M. Melatonin and Female Reproduction: An Expanding Universe. Front. Endocrinol. 2020, 11, 85. [Google Scholar] [CrossRef]
- Yang, L.; Xu, H.; Chen, Y.; Miao, C.; Zhao, Y.; Xing, Y.; Zhang, Q. Melatonin: Multi-Target Mechanism Against Diminished Ovarian Reserve Based on Network Pharmacology. Front. Endocrinol. 2021, 12, 630504. [Google Scholar] [CrossRef]
- Li, Y.; Hung, S.W.; Zhang, R.; Man, G.C.; Zhang, T.; Chung, J.P.; Fang, L.; Wang, C.C. Melatonin in Endometriosis: Mechanistic Understanding and Clinical Insight. Nutrients 2022, 14, 4087. [Google Scholar] [CrossRef]
- Tamura, I.; Tamura, H.; Kawamoto-Jozaki, M.; Shirafuta, Y.; Fujimura, T.; Doi-Tanaka, Y.; Mihara, Y.; Taketani, T.; Sugino, N. Effects of Melatonin on the Transcriptome of Human Granulosa Cells, Fertilization and Blastocyst Formation. Int. J. Mol. Sci. 2022, 23, 6731. [Google Scholar] [CrossRef]
- Patel, A.; Dewani, D.; Jaiswal, A.; Yadav, P.; Reddy, L.S. Exploring Melatonin’s Multifaceted Role in Polycystic Ovary Syn-drome Management: A Comprehensive Review. Cureus 2023, 15, e48929. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Romero, A.; Manucha, W.; Tan, D.X.; Zuccari, D.A.P.C.; Chuffa, L.G.A. Aging-Related Ovarian Failure and Infertility: Melatonin to the Rescue. Antioxidants 2023, 12, 695. [Google Scholar] [CrossRef]
- Veiga, E.C.A.; Samama, M.; Ikeda, F.; Cavalcanti, G.S.; Sartor, A.; Parames, S.F.; Baracat, E.C.; Ueno, J.; Junior, J.M.S. Melatonin Improves Fertilization Rate in Assisted Reproduction: Systematic Review and Meta-Analysis. Clinics 2024, 79, 100397. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, S.W.; Kim, H.S.; Kang, M.J.; Kim, S.A.; Han, J.Y.; Kim, H.; Ku, S.Y. Effects of Melatonin, GM-CSF, IGF-1, and LIF in Culture Media on Embryonic Development: Potential Benefits of Individualization. Int. J. Mol. Sci. 2024, 25, 751. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, T.; Chen, J.; Li, B.; Zhang, Q.; Yang, S.; Shao, J.; Guan, W.; Zhang, S. Exploring Melatonin’s Multifaceted Role in Female Reproductive Health: From Follicular Development to Lactation and its Therapeutic Potential in Obstetric Syndromes. J. Adv. Res. 2025, 70, 223–242. [Google Scholar] [CrossRef]
- Okamoto, H.H.; Cecon, E.; Nureki, O.; Rivara, S.; Jockers, R. Melatonin Receptor Structure and Signaling. J. Pineal Res. 2024, 76, e12952. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Cecon, E.; Oishi, A.; Jockers, R. Melatonin Receptors: Molecular Pharmacology and Signalling in the Context of System Bias. Br. J. Pharmacol. 2018, 175, 3263–3280. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous Antioxidants—Double-Eged Swords in Cellular Redox State: Health Beneficial Effects at Physiologic Doses Versus Deleterious Effects at High Doses. Oxid. Med. Cell Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a Mitochondria-Targeted Antioxidant: One of Evolution’s Best Ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Macías, M.; Escames, G.; León, J.; Acuña-Castroviejo, D. Melatonin but not Vitamins C and E Maintains Glutathione Homeostasis in t-Butyl Hydroperoxide-Induced Mitochondrial Oxidative Stress. FASEB J. 2000, 14, 1677–1679. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Simko, F.; Dominguez-Rodriguez, A.; Tesarik, J.; Neel, R.L.; Slominski, A.T.; Kleszczynski, K.; Martin-Gimenez, V.M.; Manucha, W.; et al. Melatonin: Highlighting its Use as a Potential Treatment for SARS-CoV-2 Infection. Cell. Mol. Life Sci. 2022, 79, 143. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T.; et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. Int. J. Mol. Sci. 2020, 21, 1135. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative Stress Impairs Oocyte Quality and Melatonin Protects Oocytes from Free Radical Damage and Improves Fertilization Rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, O.G.; Devran, A.; Sarikaya, E.; Aksakal, F.N.; Mollamahmutoğlu, L.; Cicek, N. Melatonin Improves the Oocyte and the Embryo in IVF Patients with Sleep Disturbances, But Does Not Improve the Sleeping Problems. J. Assist. Reprod. Genet. 2011, 28, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Batıoğlu, A.S.; Sahin, U.; Gürlek, B.; Oztürk, N.; Unsal, E. The Efficacy of Melatonin Administration on Oocyte Quality. Gynecol. Endocrinol. 2012, 28, 91–93. [Google Scholar] [CrossRef]
- Nishihara, T.; Hashimoto, S.; Ito, K.; Nakaoka, Y.; Matsumoto, K.; Hosoi, Y.; Morimoto, Y. Oral Melatonin Supplementation Improves Oocyte and Embryo Quality in Women Undergoing in Vitro Fertilization-Embryo Transfer. Gynecol. Endocrinol. 2014, 30, 359–362. [Google Scholar] [CrossRef]
- Jahromi, B.N.; Sadeghi, S.; Alipour, S.; Parsanezhad, M.E.; Alamdarloo, S.M. Effect of Melatonin on the Outcome of Assisted Reproductive Technique Cycles in Women with Diminished Ovarian Reserve: A Double-Blinded Randomized Clinical Trial. Iran. J. Med. Sci. 2017, 42, 73–78. [Google Scholar]
- Tesarik, J. Endocrinology of Primary Ovarian Insufficiency: Diagnostic and Therapeutic Clues. Endocrines 2025, 6, 18. [Google Scholar] [CrossRef]
- Yie, S.M.; Niles, L.P.; Younglai, E.V. Melatonin Receptors on Human Granulosa Cell Membranes. J. Clin. Endocrinol. Metab. 1995, 80, 1747–1749. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; Antoli, I.; Herrera, F.; Martin, V.; Rodriguez, C. Melatonin Regulation of Antioxidant Enzyme Gene Expression. Cell. Mol. Life Sci. 2002, 59, 1706–1713. [Google Scholar] [CrossRef]
- Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Lopez-Burillo, S.; Reiter, R.J. Oxidative Damage to Catalase Induced by Peroxyl Radicals: Functional Protection by Melatonin and Other Antioxidants. Free Radic. Res. 2003, 37, 543–553. [Google Scholar] [CrossRef]
- Pacchiarotti, A.; Carlomagno, G.; Antonini, G.; Pacchiarotti, A. Effect of Myo-Inositol and Melatonin Versus Myo-Inositol, in a Randomized Controlled Trial, for Improving In Vitro Fertilization of Patients with Polycystic Ovarian Syndrome. Gynecol. Endocrinol. 2016, 32, 69–73. [Google Scholar] [CrossRef]
- Vitale, S.G.; Palumbo, M.; Conde-López, C.; Mendoza, N.; Mendoza-Tesarik, R.; Tesarik, J. Effect of Growth Hormone Ad-ministration on ICSI Outcomes in Patients With Polycystic Ovary Syndrome and Recurrent Implantation Failure: A Retrospective Cross-Over Study. Int. J. Gynaecol. Obstet. 2021, 153, 357–358. [Google Scholar] [CrossRef]
- Schwertner, A.; Conceição Dos Santos, C.C.; Costa, G.D.; Deitos, A.; de Souza, A.; de Souza, I.C.; Torres, I.L.; da Cunha Filho, J.S.; Caumo, W. Efficacy of Melatonin in the Treatment of Endometriosis: A Phase II, Randomized, Double-Blind, Placebo-Controlled Trial. Pain 2013, 154, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Yan, L.; Liu, Z.; Mu, Y.L.; Li, M.; Zhao, X.; Chen, Z.J.; Zhang, H. Melatonin Inhibits 17β-Estradiol-Induced Migration, Invasion and Epithelial-Mesenchymal Transition in Normal and Endometriotic Endometrial Epithelial Cells. Reprod. Biol. Endocrinol. 2018, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Karaoğlan, Ö.; Kuyucu, Y.; Şaker, D.; Dağlıoğlu, G.; Tap, Ö. An Investigation of the Effects of Melatonin and Vitamin D on the Ovaries of a Rat Model of Premature Ovarian Failure Induced by Cyclophosphamide. Int. J. Mol. Sci. 2025, 26, 7772. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.H.; He, Y.F.; Wang, H.L.; Lv, Y.B.; Cong, Y.M.; Sun, Z.L.; Jiang, X.W.; Yu, W.H. Exogenous Melatonin Alleviates Premature Ovarian Failure by Regulating Granulosa Cell Autophagy. NPJ Regen. Med. 2025, 10, 35. [Google Scholar] [CrossRef]
- Valcavi, R.; Zini, M.; Maestroni, G.J.; Conti, A.; Portioli, I. Melatonin Stimulates Growth Hormone Secretion Through Path-ways Other Than the Growth Hormone-Releasing Hormone. Clin. Endocrinol. 1993, 39, 193–199. [Google Scholar] [CrossRef]
- Arık, G.N.; Kaplanoğlu, G.T.; Sağlam, A.S.Y.; Elmazoğlu, Z.; Dinçel, A.S.; Seymen, C.M. Melatonin Effective to Reduce the Microscopic Symptoms of Polycystic Ovary Syndrome-Related Infertility: An Experimental Study. Tissue Cell 2023, 81, 102015. [Google Scholar] [CrossRef]
- Lohrasbi, P.; Karbalay-Doust, S.; Mohammad Bagher Tabei, S.; Azarpira, N.; Alaee, S.; Rafiee, B.; Bahmanpour, S. The Effects of Melatonin and Metformin on Histological Characteristics of the Ovary and Uterus in Letrozole-Induced Polycystic Ovarian Syndrome Mice: A Stereological Study. Int. J. Reprod. Biomed. 2022, 20, 973–988. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Guo, B.; Tao, Y.; Zhang, J.; Wang, J.; Chen, G.; Cheng, M.; Hong, Q.; Cao, Y.; et al. Melatonin Refines Ovarian Mitochondrial Dysfunction in PCOS by Regulating the Circadian Rhythm Gene Clock. Cell. Mol. Life Sci. 2025, 82, 104. [Google Scholar] [CrossRef]
- Blanco-Breindel, M.F.; Singh, M.; Kahn, J. Endometrial Receptivity; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Chuffa, L.G.A.; Lupi, L.A.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Seiva, F.R.F. Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms. Int. J. Mol. Sci. 2019, 21, 300. [Google Scholar] [CrossRef]
- Bao, Z.; Li, G.; Wang, R.; Xue, S.; Zeng, Y.; Deng, S. Melatonin Improves Quality of Repeated-Poor and Frozen-Thawed Embryos in Human, a Prospective Clinical Trial. Front. Endocrinol. 2022, 13, 853999. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wang, K.; Zhang, C.; Chen, B.; Zou, H.; Zou, W.; Xue, R.; Ji, D.; Yu, Z.; Rao, B.; et al. Effect of Melatonin on the Clinical Outcome of Patients with Repeated Cycles after Failed Cycles of in Vitro Fertilization and Intracytoplasmic Sperm Injection. Zygote 2022, 30, 471–479. [Google Scholar] [CrossRef]
- Ma, T.; Tao, J.; Yang, M.; He, C.; Tian, X.; Zhang, X.; Zhang, J.; Deng, S.; Feng, J.; Zhang, Z.; et al. An AANAT/ASMT Transgenic Animal Model Constructed with CRISPR/Cas9 System Serving as the Mammary Gland Bioreactor to Produce Melatonin-Enriched Milk in Sheep. J. Pineal Res. 2017, 63, e12406. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, A.; Li, G.; Wu, H.; Deng, S.; Yang, H.; Ma, W.; Lv, D.; Fu, Y.; Ji, P.; et al. Melatonin Promotes the Development of Sheep Transgenic Cloned Embryos by Protecting Donor and Recipient Cells. Cell Cycle 2022, 21, 1360–1375. [Google Scholar] [CrossRef]
- Afzal, A. Melatonin as a Multifunctional Modulator: Emerging Insights into its Role in Health, Reproductive Efficiency, and Productive Performance in Livestock. Front. Physiol. 2024, 15, 1501334. [Google Scholar] [CrossRef]
- Goleij, P.; Khazeei Tabari, M.A.; Poudineh, M.; Sanaye, P.M.; Khan, H.; Kumar, A.P.; Larsen, D.S.; Daglia, M. Therapeutic Potential of Melatonin-Induced Mitophagy in the Pathogenesis of Alzheimer’s Disease. Inflammopharmacology 2025, 33, 4553–4575. [Google Scholar] [CrossRef]
- Gholami, M.; Davoodian, N.; Kadivar, A.; Shams-Esfandabadi, N.; Nazari, H. The Effect of Melatonin Implants on the Embryo Yield and Oxidative Stress Levels in Superovulated Holstein Heifers. Vet. Res. Commun. 2025, 49, 236. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Liu, M.; Li, J.; Liu, K.; Qi, Q.; Yu, Z.; Samoo, H.A.; Wang, C.; Hou, J. Melatonin Implantation Improves the Reproductive Performance of Estrus-Synchronized Ewes During Seasonal Anestrus and Enhances the Antioxidant and Steroidogenic Capacities of Granulosa and Luteal Cells. Antioxidants 2025, 14, 895. [Google Scholar] [CrossRef] [PubMed]
- Sagrillo-Fagundes, L.; Salustiano, E.M.A.; Ruano, R.; Markus, R.P.; Vaillancourt, C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J. Pineal Res. 2018, 65, e12520. [Google Scholar] [CrossRef] [PubMed]
- Lanoix, D.; Beghdadi, H.; Lafond, J.; Vaillancourt, C. Human Placental Trophoblasts Synthesize Melatonin and Express its Receptors. J. Pineal Res. 2008, 45, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; Lacasse, A.; Lanoix, D.; Sagrillo-Fagundes, L.; Boulard, V.; Vaillancourt, C. Placental Melatonin System is Present Throughout Pregnancy and Regulates Villous Trophoblast Differentiation. J. Pineal Res. 2015, 59, 38–46. [Google Scholar] [CrossRef]
- Pringle, K.G.; Kind, K.L.; Sferruzzi-Perri, A.N.; Thompson, J.G.; Roberts, C.T. Beyond Oxygen: Complex Regulation and Activity of Hypoxia Inducible Factors in Pregnancy. Hum. Reprod. Update 2010, 16, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative Stress in Pregnancy and Reproduction. Obstet. Med. 2016, 9, 113–116. [Google Scholar] [CrossRef]
- Ginsberg, Y.; Khatib, N.; Weiner, Z.; Beloosesky, R. Maternal Inflammation, Fetal Brain Implications and Suggested Neuroprotection: A Summary of 10 Years of Research in Animal Models. Rambam Maimonides Med. J. 2017, 8, e0028. [Google Scholar] [CrossRef]
- Lee, J.Y.; Li, S.; Shin, N.E.; Na, Q.; Dong, J.; Jia, B.; Jones-Beatty, K.; McLane, M.W.; Ozen, M.; Lei, J.; et al. Melatonin for Prevention of Placental Malperfusion and Fetal Compromise Associated with Intrauterine Inflammation-Induced Oxidative Stress in a Mouse Model. J. Pineal Res. 2019, 67, e12591. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, D.; Cai, S.; Yang, L.; Yu, S.; Geng, Q.; Mo, M.; Li, W.; Wei, Y.; Li, Y.; et al. Adenomyosis-Associated Infertility: An Update of the Immunological Perspective. Reprod. Biomed. Online 2025, 50, 104703. [Google Scholar] [CrossRef]
- Guan, X.; Liu, D.; Zhou, H.; Dai, C.; Wang, T.; Fang, Y.; Jia, Y.; Li, K. Melatonin Improves Pregnancy Outcomes in Adenomyosis Mice by Restoring Endometrial Receptivity Via NF-κB/Apoptosis Signaling. Ann. Transl. Med. 2022, 10, 1317. [Google Scholar] [CrossRef]
- Kocadal, N.Ç.; Attar, R.; Yıldırım, G.; Fıçıcıoğlu, C.; Ozkan, F.; Yılmaz, B.; Yesildaglar, N. Melatonin Treatment Results in Regression of Endometriotic Lesions in an Ooferectomized Rat Endometriosis Model. J. Turk. Ger. Gynecol. Assoc. 2013, 14, 81–86. [Google Scholar] [CrossRef]
- Oral, S.; Akpak, Y.K.; Turan, G.; Lafci, D.; Kinci, M.F.; Usta, C.S. Efficacy of Colchicine and Melatonin in the Treatment of Rat Endometriosis Model: An Animal Study. J. Reprod. Immunol. 2024, 165, 104294. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.T.; Schuch, V.; Hossack, D.J.; Chakraborty, R.; Johnson, E.L. Melatonin: The Placental Antioxidant and Anti-Inflammatory. Front. Immunol. 2024, 15, 1339304. [Google Scholar] [CrossRef]
- Andersen, L.P.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza Tesarik, R. Melatonin: The First Noninvasive Causal Therapy for Both Endometriosis and Adenomyosis? J. Gynecol. Women’s Health 2018, 12, 555829. [Google Scholar] [CrossRef]
- Alomari, T.; Al-Abdallat, H.; Hamamreh, R.; Alomari, O.; Hos, B.H.; Reiter, R.J. Assessing the Antiviral Potential of Melatonin: A Comprehensive Systematic Review. Rev. Med. Virol. 2024, 34, e2499. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Choi, W.S. Use of Melatonin in Cancer Treatment: Where Are We? Int. J. Mol. Sci. 2022, 23, 3779. [Google Scholar] [CrossRef]

| Study [Ref] (Type) | Indication | Delivery Route, Dose | Main Outcomes |
|---|---|---|---|
| Tamura et al. [34] (RCT) | Poor oocyte quality in ICSI | Oral, 3 mg/day | Improved fertilization rate |
| Eryilmaz et al. [35] (RCT) | Poor oocyte quality in ICSI | Oral, 3 mg/day | Increased mature oocytes and good embryos |
| Batioglu et al. [36] (RCT) | Poor oocyte quality in ICSI | Oral, 3 mg/day | Increased mature oocytes and good embryos |
| Nishihara et al. [37] (CT) | Poor oocyte quality in ICSI | Oral, 3 mg/day | Increased fertilization rate and good embryos |
| Jahromi et al. [38] (RCT) | Poor oocyte quality in ICSI | Oral, 3 mg/day | Increased mature oocytes and good embryos |
| Espino et al. [13] (RCT) | Poor oocyte quality in ICSI | Oral, 3 or 6 mg/day | Increased total oocytes and good embryos |
| Pacchiarotti et al. [43] (RCT) | PCOS | Oral, 3 mg/day | Enhanced oocyte and embryo quality |
| Vitale et al. [44] (RCOS) | PCOS and RIF | Oral, 6 mg/day | Improved IVF outcomes (pregnancy rate) |
| Schwertner et al. [45] (RCT) | Endometriosis | Oral, 10 mg/day | Reduced pain and dysmenorrhea |
| Qi et al. [46] (HBS) | Endometriosis | In vitro, 1 mmol/L | Inhibition of E2-induced invasiveness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesarik, J.; Tesarik, R.M. Melatonin in the Treatment of Female Infertility: Update on Biological and Clinical Findings. Biomedicines 2025, 13, 2434. https://doi.org/10.3390/biomedicines13102434
Tesarik J, Tesarik RM. Melatonin in the Treatment of Female Infertility: Update on Biological and Clinical Findings. Biomedicines. 2025; 13(10):2434. https://doi.org/10.3390/biomedicines13102434
Chicago/Turabian StyleTesarik, Jan, and Raquel Mendoza Tesarik. 2025. "Melatonin in the Treatment of Female Infertility: Update on Biological and Clinical Findings" Biomedicines 13, no. 10: 2434. https://doi.org/10.3390/biomedicines13102434
APA StyleTesarik, J., & Tesarik, R. M. (2025). Melatonin in the Treatment of Female Infertility: Update on Biological and Clinical Findings. Biomedicines, 13(10), 2434. https://doi.org/10.3390/biomedicines13102434








