Adipose Tissue Insulin Resistance: A Key Driver of Metabolic Syndrome Pathogenesis
Abstract
1. Introduction
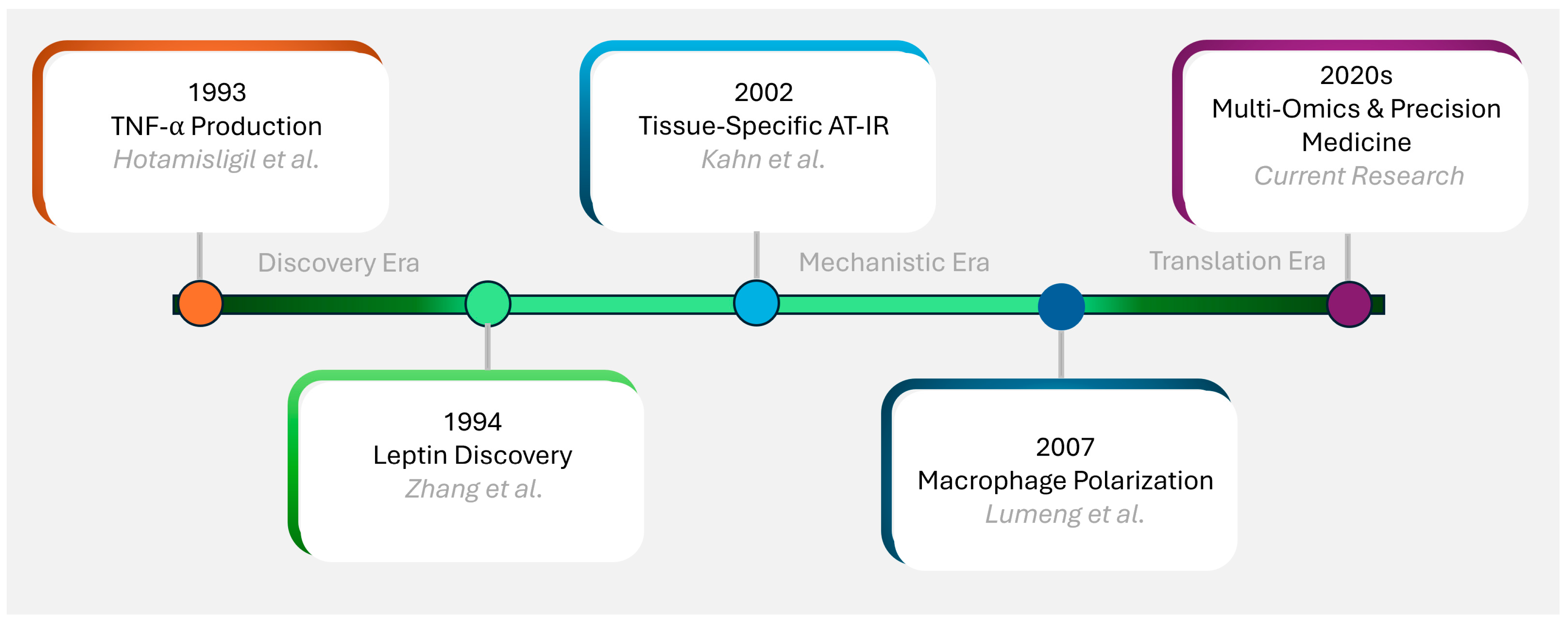
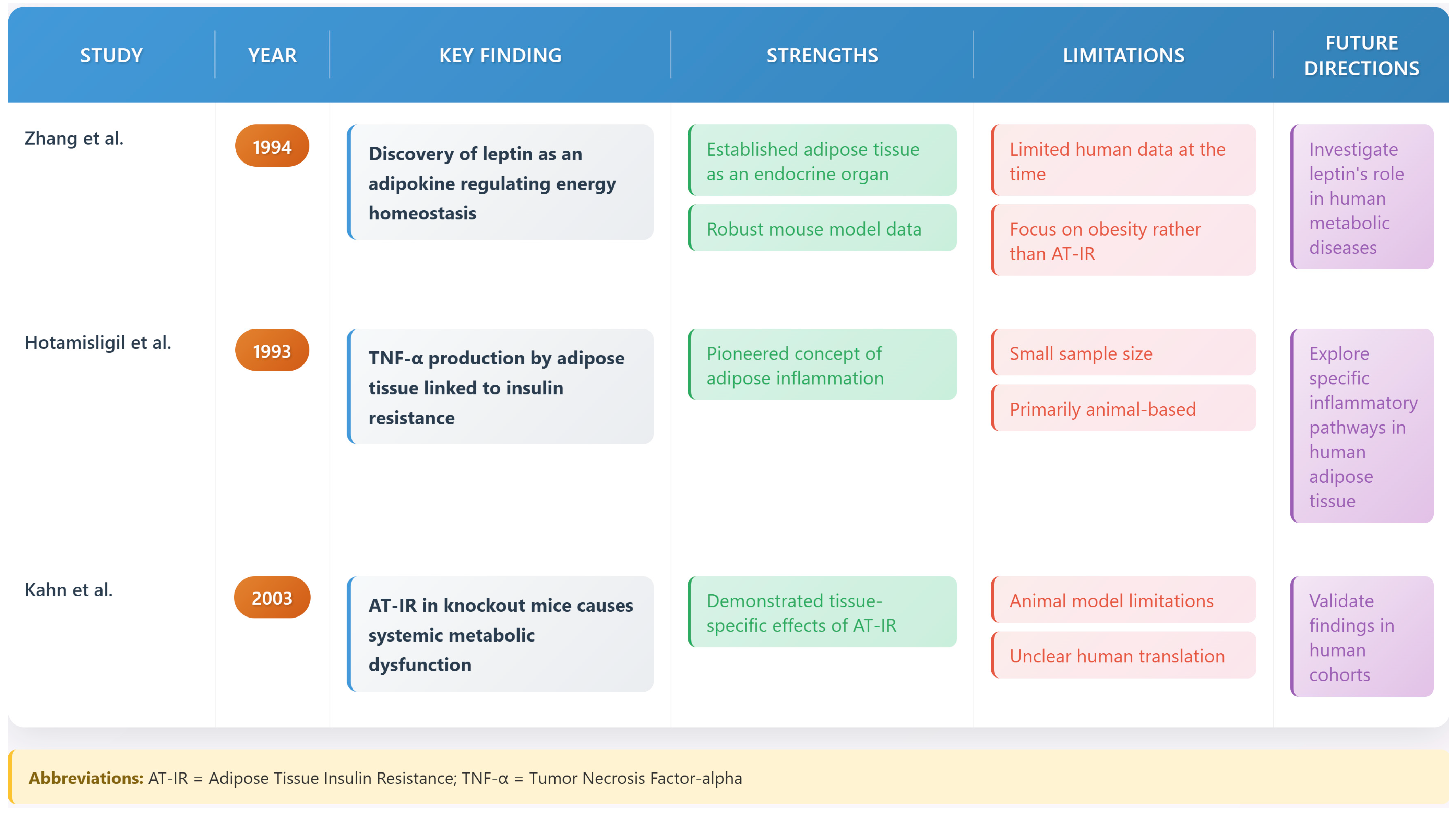
2. Historical Perspective and Evolution of Concepts
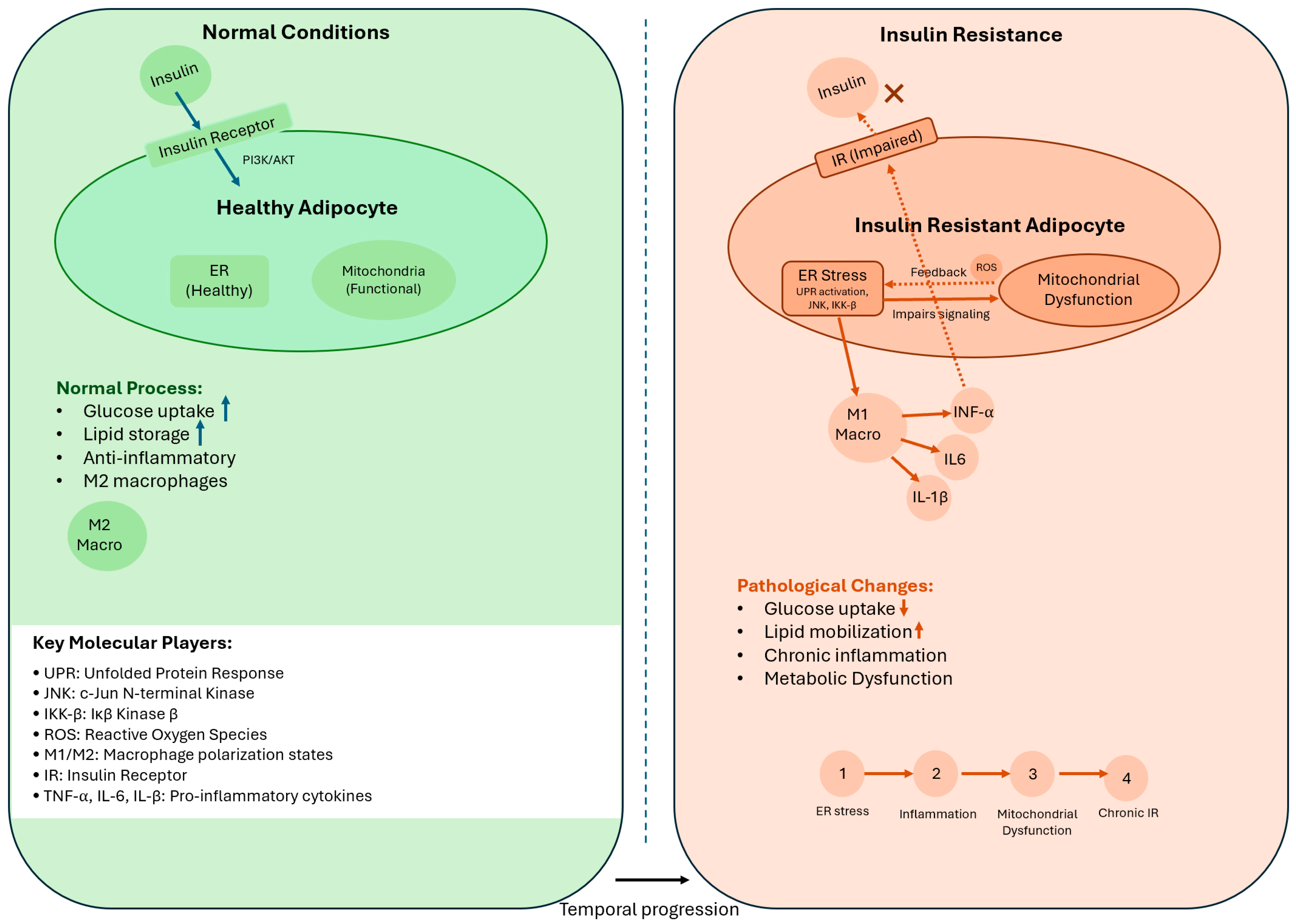
3. Molecular Mechanisms of Adipose Tissue Insulin Resistance
3.1. Insulin Signaling in Healthy Adipose Tissue
3.2. Disruption of Insulin Signaling in AT-IR
3.3. Cellular Stress Pathways
3.3.1. Endoplasmic Reticulum Stress
3.3.2. Mitochondrial Dysfunction
3.4. Integration of Stress Pathways in AT-IR
4. Adipokines and Metabolic Regulation
4.1. The Adipokine Network
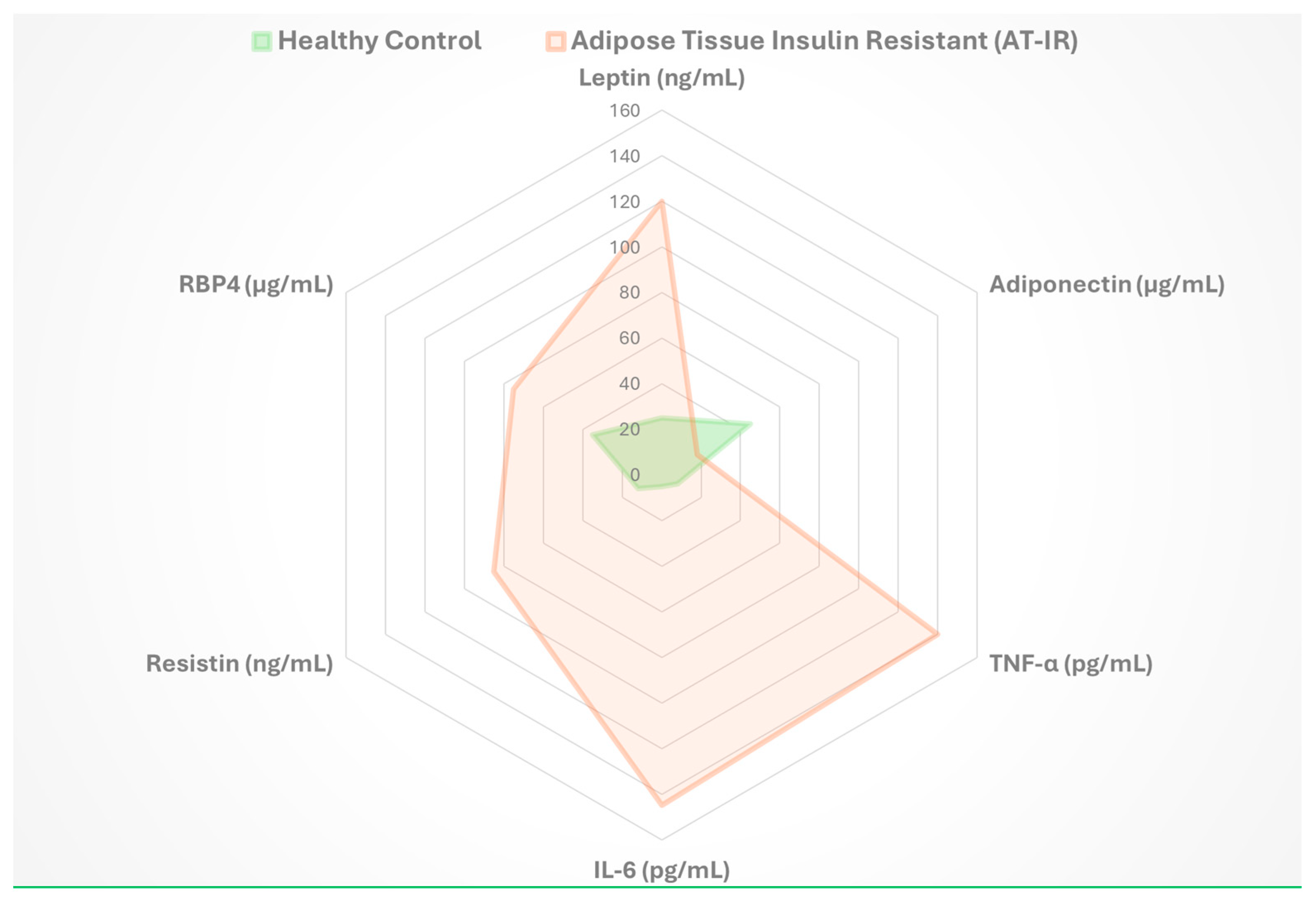
4.2. Key Adipokines in Metabolic Regulation
4.2.1. Leptin
4.2.2. Adiponectin
5. Adipose Tissue Expansion and Remodeling
5.1. Healthy Versus Pathological Expansion
5.2. Extracellular Matrix Dynamics
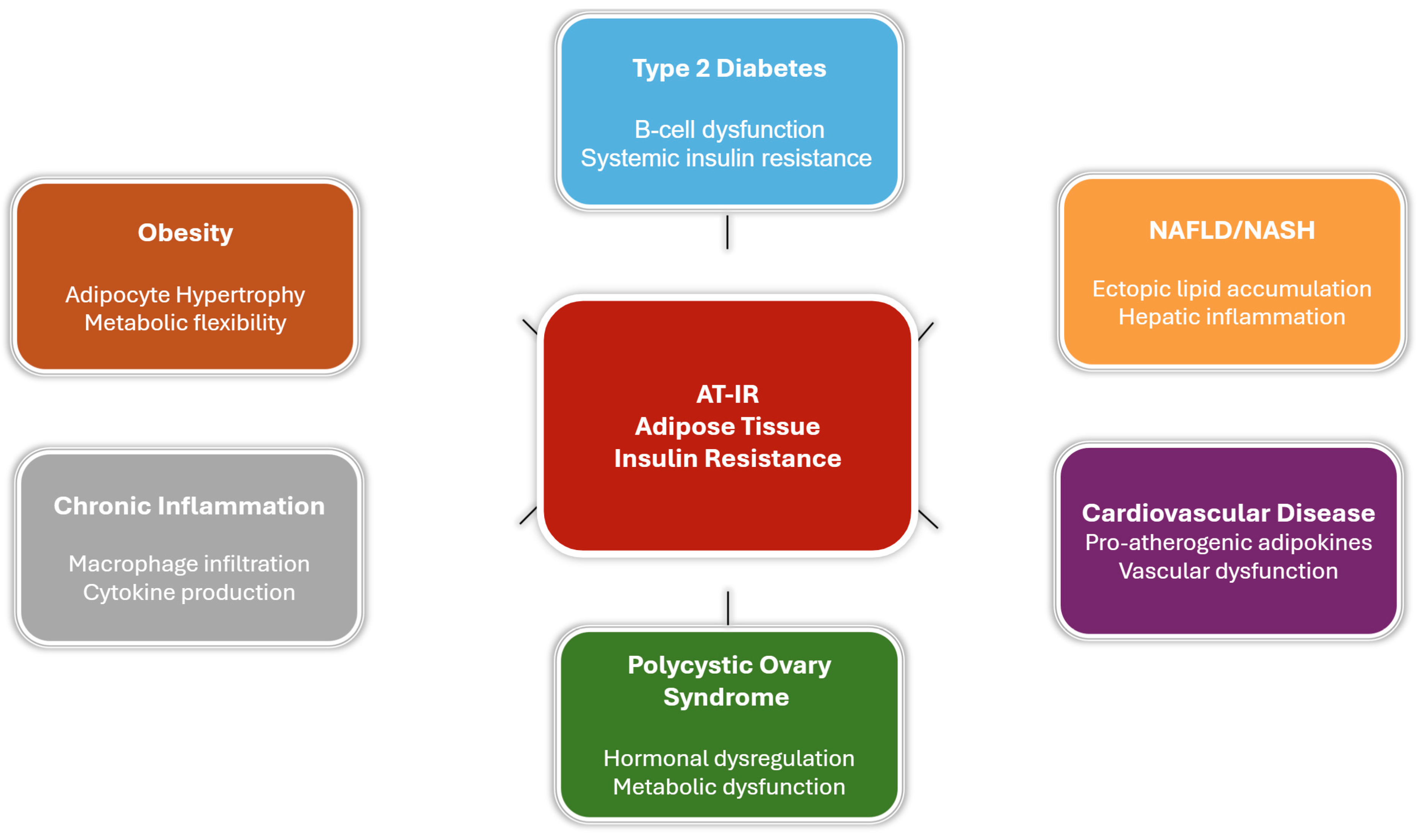
6. Disease States Associated with Adipose Tissue Insulin Resistance
6.1. Obesity and AT-IR
6.2. Type 2 Diabetes Mellitus
6.3. Non-Alcoholic Fatty Liver Disease (NAFLD)
6.4. Cardiovascular Disease
6.5. Polycystic Ovary Syndrome (PCOS)
6.6. Cancer
6.7. Age-Related Metabolic Dysfunction
6.8. Adipose Tissue Inflammation and Systemic Inflammatory Feedback in Insulin Resistance
7. Metabolic Consequences of AT-IR
7.1. Systemic Effects
7.2. Tissue Crosstalk
8. Therapeutic Approaches and Clinical Management
8.1. Current Therapeutic Strategies
8.1.1. Lifestyle Interventions
8.1.2. Pharmacological Interventions
8.2. Emerging Therapeutic Targets
8.2.1. Adipose Tissue Remodeling
8.2.2. Biomarker Development and Clinical Monitoring
8.2.3. Integration of Multi-Omic Approaches
8.2.4. Immune System Modulation in Adipose Tissue
8.3. Clinical Translation and Implementation
8.4. Therapeutic Innovation and Future Directions
9. Conclusions
9.1. Key Findings and Clinical Implications
9.2. Therapeutic Advances
9.3. Future Directions and Challenges
9.4. Controversies and Knowledge Gaps
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AT-IR | Adipose tissue insulin resistance |
| MetS | Metabolic syndrome |
| IRS | Insulin receptor substrate |
| PI3K | Phosphatidylinositol 3-kinase |
| TNF-α | Tumor necrosis factor-alpha |
| ER | Endoplasmic reticulum |
| UPR | Unfolded protein response |
| ISR | Integrated stress response |
| ROS | Reactive oxygen species |
| JNK | c-Jun N-terminal kinase |
| IKK-β | Inhibitor of nuclear factor kappa-B kinase subunit beta |
| ECM | Extracellular matrix |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| CVD | Cardiovascular disease |
| PCOS | Polycystic ovary syndrome |
| T2DM | Type 2 diabetes mellitus |
| FFA | Free fatty acids |
| TZDs | Thiazolidinediones |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| GLP-1 | Glucagon-like peptide-1 |
| GIP | Glucose-dependent insulinotropic polypeptide |
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Frayn, K.N. Adipose tissue and the insulin resistance syndrome. Proc. Nutr. Soc. 2001, 60, 375–380. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Lambadiari, V.; Mitrou, P.; Maratou, E.; Boutati, E.; Panagiotakos, D.B.; Economopoulos, T.; Raptis, S.A. Impaired postprandial blood flow in adipose tissue may be an early marker of insulin resistance in type 2 diabetes. Diabetes Care 2007, 30, 3128–3130. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. S2), S157–S163. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Pedley, A.; Massaro, J.M.; Murabito, J.; Hoffmann, U.; Fox, C.S. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 2012, 55, 2622–2630. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; Thaete, F.L.; Troost, F.; Huwe, T.; Goodpaster, B.H. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E941–E948. [Google Scholar] [CrossRef]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Semnani-Azad, Z.; Connelly, P.W.; Bazinet, R.P.; Retnakaran, R.; Jenkins, D.J.; Harris, S.B.; Zinman, B.; Hanley, A.J. Adipose tissue insulin resistance is longitudinally associated with adipose tissue dysfunction, circulating lipids, and dysglycemia: The promise cohort. Diabetes Care 2021, 44, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Mollica, M.P.; Lombardi, A.; Cavaliere, G.; Gifuni, G.; Barletta, A. From chronic overnutrition to insulin resistance: The role of fat-storing capacity and inflammation. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 146–152. [Google Scholar] [CrossRef] [PubMed]
- da Silva Rosa, S.C.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020, 8, e14607. [Google Scholar] [CrossRef] [PubMed]
- Mlinar, B.; Marc, J. New insights into adipose tissue dysfunction in insulin resistance. cclm 2011, 49, 1925–1935. [Google Scholar] [CrossRef]
- Gastaldelli, A. Role of beta-cell dysfunction, ectopic fat accumulation and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Diabetes Res Clin Pract 2011, 93 (Suppl. S1), S60–S65. [Google Scholar] [CrossRef]
- Calejman, C.M.; Doxsey, W.G.; Fazakerley, D.J.; Guertin, D.A. Integrating adipocyte insulin signaling and metabolism in the multi-omics era. Trends Biochem. Sci. 2022, 47, 531–546. [Google Scholar] [CrossRef]
- Stefan, N.; Machann, J.; Schick, F.; Claussen, C.D.; Thamer, C.; Fritsche, A.; Häring, H.-U. New imaging techniques of fat, muscle and liver within the context of determining insulin sensitivity. Horm. Res. 2005, 64, 38–44. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Gharipour, M.; Nezafati, P.; Sadeghian, L.; Eftekhari, A.; Rothenberg, I.; Jahanfar, S. Precision medicine and metabolic syndrome. ARYA Atheroscler. 2022, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-α: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Kahn, B.B.; Kahn, C.R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 2003, 299, 572–574. [Google Scholar] [CrossRef]
- Blüher, M.; Michael, M.; Peroni, O.D.; Ueki, K.; Carter, N.; Kahn, B.B.; Kahn, C. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell. 2002, 3, 25–38. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Dall’aGnese, A.; Platt, J.M.; Zheng, M.M.; Friesen, M.; Dall’aGnese, G.; Blaise, A.M.; Spinelli, J.B.; Henninger, J.E.; Tevonian, E.N.; Hannett, N.M.; et al. The dynamic clustering of insulin receptor underlies its signaling and is disrupted in insulin resistance. Nat. Commun. 2022, 13, 1–22. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Özcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.-H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Fang, Q.; Liu, M.; Liu, X.; Jia, W.; Dong, L.Q.; Liu, F. Autophagy-mediated insulin receptor down-regulation contributes to endoplasmic reticulum stress-induced insulin resistance. Mol. Pharmacol. 2009, 76, 596–603. [Google Scholar] [CrossRef]
- Boden, G.; Duan, X.; Homko, C.; Molina, E.J.; Song, W.; Perez, O.; Cheung, P.; Merali, S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 2008, 57, 2438–2444. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., III; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Lehr, S.; Hartwig, S.; Sell, H. Adipokines: A treasure trove for the discovery of biomarkers for metabolic disorders. Proteom. Clin. Appl. 2012, 6, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, K.; MacIver, N.J. The Role of the Adipokine Leptin in Immune Cell Function in Health and Disease. Front. Immunol. 2021, 11, 622468. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Beals, J.W.; Smith, G.I.; Shankaran, M.; Fuchs, A.; Schweitzer, G.G.; Yoshino, J.; Field, T.; Matthews, M.; Nyangau, E.; Morozov, D.; et al. Increased Adipose Tissue Fibrogenesis, Not Impaired Expandability, Is Associated With Nonalcoholic Fatty Liver Disease. Hepatology 2021, 74, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Chun, T.H.; Kang, L. Adipose extracellular matrix remodelling in obesity and insulin resistance. Biochem. Pharmacol. 2016, 119, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Korf, H.; Vidal-Puig, A. An adipocentric perspective on the development and progression of non-alcoholic fatty liver disease. J. Hepatol. 2023, 78, 1048–1062. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2020, 320, C375–C391. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Oh, C.M.; Kim, H. The Interplay of Adipokines and Pancreatic Beta Cells in Metabolic Regulation and Diabetes. Biomedicines 2023, 11, 2589. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, J.; Divoux, A.; Prifti, E.; Poitou, C.; Pelloux, V.; Hugol, D.; Basdevant, A.; Bouillot, J.-L.; Chevallier, J.-M.; Bedossa, P.; et al. Structural and inflammatory heterogeneity in subcutaneous adipose tissue: Relation with liver histopathology in morbid obesity. J. Hepatol. 2012, 56, 1152–1158. [Google Scholar] [CrossRef]
- Micu, E.S.; Amzolini, A.M.; Abu-Alhija, A.B.; Forţofoiu, M.C.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Mogoantă, S.Ş.; Crişan, A.E.; Predescu, O.I.; et al. Systemic and adipose tissue inflammation in NASH: Correlations with histopathological aspects. Rom. J. Morphol. Embryol. 2022, 62, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, C.; Sabatini, S.; Gaggini, M.; Carli, F.; Rosso, C.; Positano, V.; Armandi, A.; Caviglia, G.P.; Faletti, R.; Bugianesi, E.; et al. Adipose tissue dysfunction and visceral fat are associated with hepatic insulin resistance and severity of NASH even in lean individuals. Liver Int. 2022, 42, 2418–2427. [Google Scholar] [CrossRef]
- Rodelo, R.G.E.; Porchia, L.M.; Torres-Rasgado, E.; López-Bayghen, E.; Gonzalez-Mejia, M.E. Visceral and subcutaneous abdominal fat is associated with non-alcoholic fatty liver disease while augmenting Metabolic Syndrome’s effect on non-alcoholic fatty liver disease: A cross-sectional study of NHANES 2017–2018. PLoS ONE 2024, 19, e0298662. [Google Scholar] [CrossRef]
- Adachi, Y.; Ueda, K.; Takimoto, E. Perivascular adipose tissue in vascular pathologies—A novel therapeutic target for atherosclerotic disease? Front. Cardiovasc. Med. 2023, 10, 1151717. [Google Scholar] [CrossRef]
- Bril, F.; Ezeh, U.; Amiri, M.; Hatoum, S.; Pace, L.; Chen, Y.-H.; Bertrand, F.; Gower, B.; Azziz, R. Adipose Tissue Dysfunction in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 109, 10–24. [Google Scholar] [CrossRef]
- Spritzer, P.M.; Lecke, S.B.; Satler, F.; Morsch, D.M. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction 2015, 149, R219–R227. [Google Scholar] [CrossRef]
- Su, P.; Chen, C.; Sun, Y. Physiopathology of polycystic ovary syndrome in endocrinology, metabolism and inflammation. J. Ovarian Res. 2025, 18, 34. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, J.H.; Lee, Y.J. The Role of Adipokines in Tumor Progression and Its Association with Obesity. Biomedicines 2024, 12, 97. [Google Scholar] [CrossRef]
- Song, Y.; Na, H.; Lee, S.E.; Kim, Y.M.; Moon, J.; Nam, T.W.; Ji, Y.; Jin, Y.; Park, J.H.; Cho, S.C.; et al. Dysfunctional adipocytes promote tumor progression through YAP/TAZ-dependent cancer-associated adipocyte transformation. Nat. Commun. 2024, 15, 4052. [Google Scholar] [CrossRef]
- Ou, M.Y.; Zhang, H.; Tan, P.C.; Zhou, S.B.; Li, Q.F. Adipose tissue aging: Mechanisms and therapeutic implications. Cell Death Dis. 2022, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Mathioudaki, A.; Fanni, G.; Eriksson, J.W.; Pereira, M.J. Metabolomic Profiling of Adipose Tissue in Type 2 Diabetes: Associations with Obesity and Insulin Resistance. Metabolites 2024, 14, 411. [Google Scholar] [CrossRef]
- Piquet, M.; Martínez, M.C.; Romacho, T. Inter-Organ Crosstalk in the Development of Obesity-Associated Insulin Resistance. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2022; Volume 274. [Google Scholar] [CrossRef]
- Van Pelt, D.W.; Guth, L.M.; Horowitz, J.F. Aerobic exercise elevates markers of angiogenesis and macrophage IL-6 gene expression in the subcutaneous adipose tissue of overweight-to-obese adults. J. Appl. Physiol. 2017, 123, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Sugii, S.; Olson, P.; Sears, D.D.; Saberi, M.; Atkins, A.R.; Barish, G.D.; Hong, S.-H.; Castro, G.L.; Yin, Y.-Q.; Nelson, M.C.; et al. PPARγ activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl. Acad. Sci. USA 2009, 106, 22504–22509. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Park, M.-S.; Choung, J.-S.; Kim, S.-S.; Oh, H.-H.; Choi, C.-S.; Ha, S.-Y.; Kang, Y.; Kim, Y.; Jun, H.-S. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 2012, 55, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Smits, M.M.; Holst, J.J. Endogenous glucagon-like peptide (GLP)-1 as alternative for GLP-1 receptor agonists: Could this work and how? Diabetes Metab. Res. Rev. 2023, 39, e3699. [Google Scholar] [CrossRef]
- Nauck, M.A.; D‘Alessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Méndez-Gutiérrez, A.; Aguilera, C.M.; Plaza-Díaz, J. Extracellular matrix remodeling of adipose tissue in obesity and metabolic diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef]
- Cao, Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013, 18, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Vamvini, M.; Nigro, P.; Caputo, T.; Stanford, K.I.; Hirshman, M.F.; Middelbeek, R.J.; Goodyear, L.J. Exercise training and cold exposure trigger distinct molecular adaptations to inguinal white adipose tissue. Cell. Rep. 2024, 43, 114481. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, C.; Li, C.; Chen, X.; Xu, R.; Chen, M.; Li, Y.; Liu, Y.; Liu, X.; Chen, Y.; et al. Integrating spatial transcriptomics and single-nucleus RNA-seq revealed the specific inhibitory effects of TGF-β on intramuscular fat deposition. Sci. China Life Sci. 2025, 68, 746–763. [Google Scholar] [CrossRef] [PubMed]
- Massier, L.; Jalkanen, J.; Elmastas, M.; Zhong, J.; Wang, T.; Nankam, P.A.N.; Frendo-Cumbo, S.; Bäckdahl, J.; Subramanian, N.; Sekine, T.; et al. An integrated single cell and spatial transcriptomic map of human white adipose tissue. Nat. Commun. 2023, 14, 1–19. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Hunter, D.; Huber, R.; Lemieux, J.; Slaymaker, S.; Vaddi, K.; Charo, I.; Leibel, R.L.; Ferrante, A.W., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Investig. 2006, 116, 115–124. [Google Scholar] [CrossRef]
- Titos, E.; Clària, J. Omega-3-derived mediators counteract obesity-induced adipose tissue inflammation. Prostaglandins Other Lipid Mediat. 2013, 107, 77–84. [Google Scholar] [CrossRef]
- Sibuyi, N.R.S.; Moabelo, K.L.; Meyer, M.; Onani, M.O.; Dube, A.; Madiehe, A.M. Nanotechnology advances towards development of targeted-treatment for obesity. J. Nanobiotechnol. 2019, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Keating, M.F.; Drew, B.G.; Calkin, A.C. Antisense Oligonucleotide Technologies to Combat Obesity and Fatty Liver Disease. Front. Physiol. 2022, 13, 839471. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabiee, A.; Hossain, M.A.; Poojari, A. Adipose Tissue Insulin Resistance: A Key Driver of Metabolic Syndrome Pathogenesis. Biomedicines 2025, 13, 2376. https://doi.org/10.3390/biomedicines13102376
Rabiee A, Hossain MA, Poojari A. Adipose Tissue Insulin Resistance: A Key Driver of Metabolic Syndrome Pathogenesis. Biomedicines. 2025; 13(10):2376. https://doi.org/10.3390/biomedicines13102376
Chicago/Turabian StyleRabiee, Atefeh, Md Arafat Hossain, and Ankita Poojari. 2025. "Adipose Tissue Insulin Resistance: A Key Driver of Metabolic Syndrome Pathogenesis" Biomedicines 13, no. 10: 2376. https://doi.org/10.3390/biomedicines13102376
APA StyleRabiee, A., Hossain, M. A., & Poojari, A. (2025). Adipose Tissue Insulin Resistance: A Key Driver of Metabolic Syndrome Pathogenesis. Biomedicines, 13(10), 2376. https://doi.org/10.3390/biomedicines13102376




