Investigating How Thbs4 Regulates Degeneration and Regeneration of the Peripheral Nerve
Abstract
1. Introduction
2. Methods
2.1. Establishment of Sciatic Nerve Transection Model in Rats
2.2. Quantitative RT-PCR (qRT-PCR) and Western Blot
2.3. Immunofluorescence (IF) Staining
2.4. Primary Cultured DRG Neuron Cells and Transfection
2.5. CCK8 Assay, TUNEL and IF Staining
2.6. Intrathecal Injection of AAV
2.7. Hematoxylin and Eosin (H&E), and IF Staining
2.8. Transmission Electron Microscope (TEM)
2.9. Behavioral Experiment
2.9.1. Footprint Analysis
2.9.2. Assessment of Thermal Hyperalgesia and Mechanical Allodynia
2.10. Electrophysiological Test
2.11. Statistical Analysis
3. Results
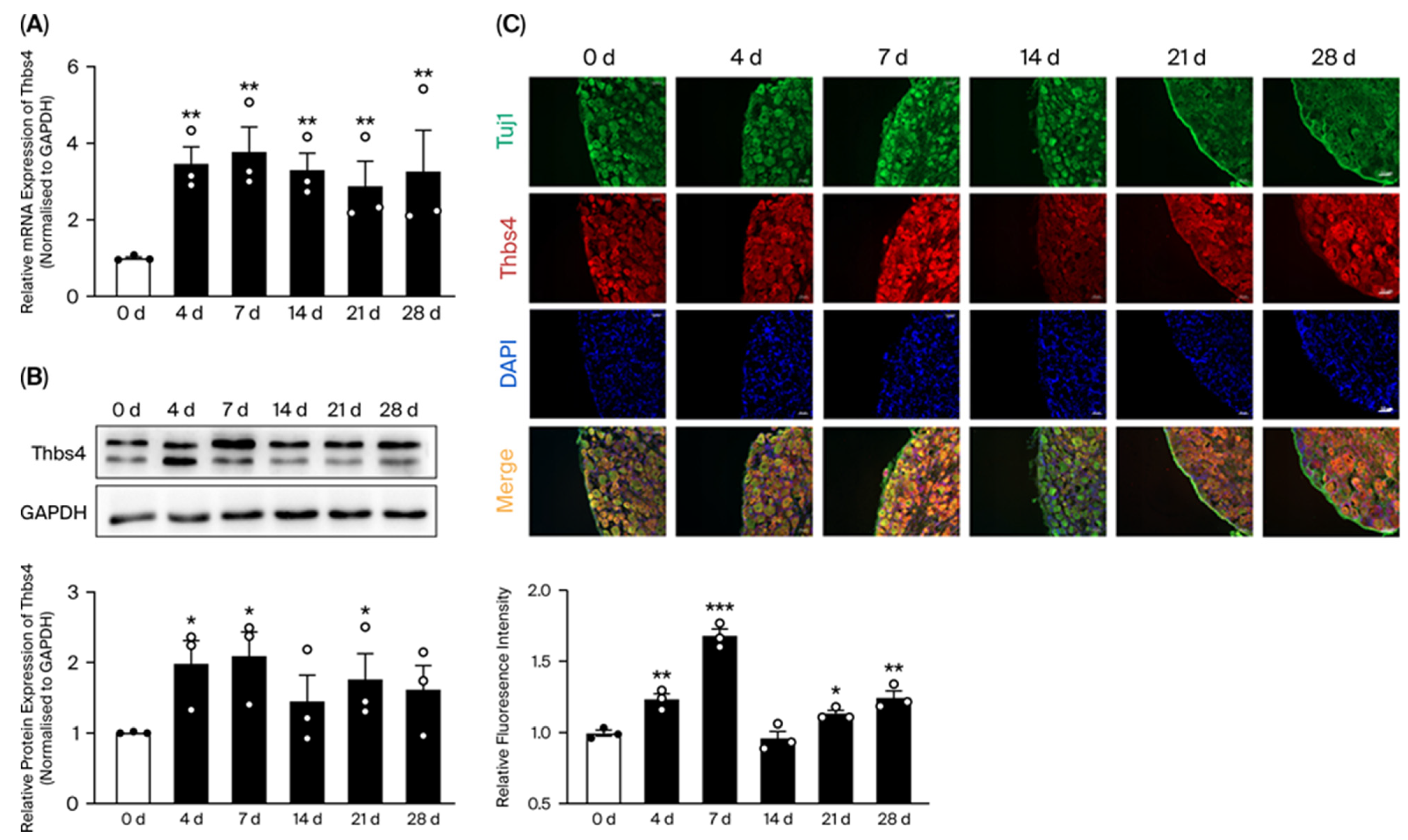
3.1. Time-Course Expressions of Thbs4 in DRG After Sciatic Nerve Transection
3.2. Thbs4 Localization in DRG Tissues and Neuronal Cells
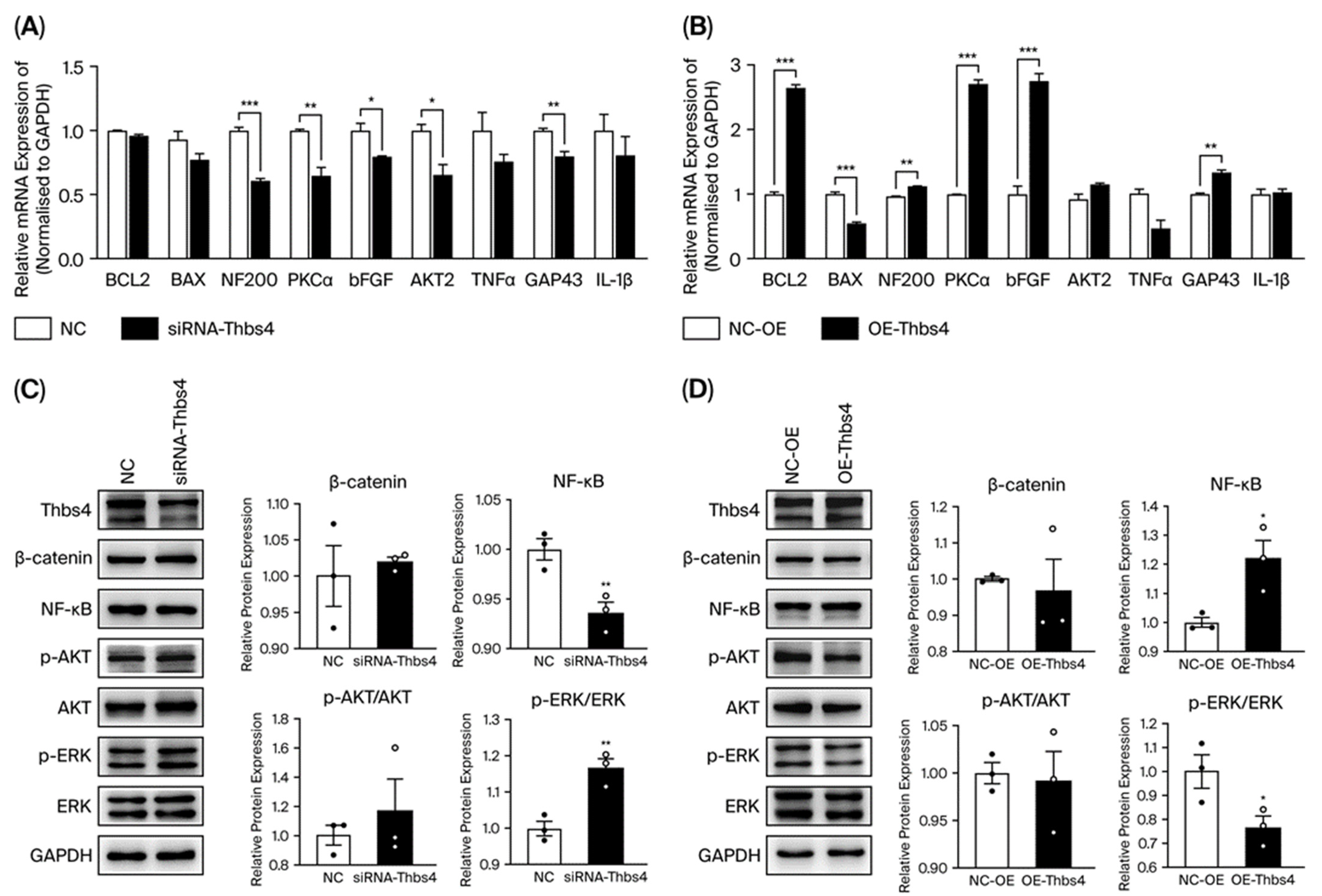
3.3. Effects of Thbs4 on Viability, Neurite Outgrowth and Apoptosis of DRG Neurons
3.4. Effects of Thbs4 on Growth and Apoptosis-Related Factors in DRG Neurons
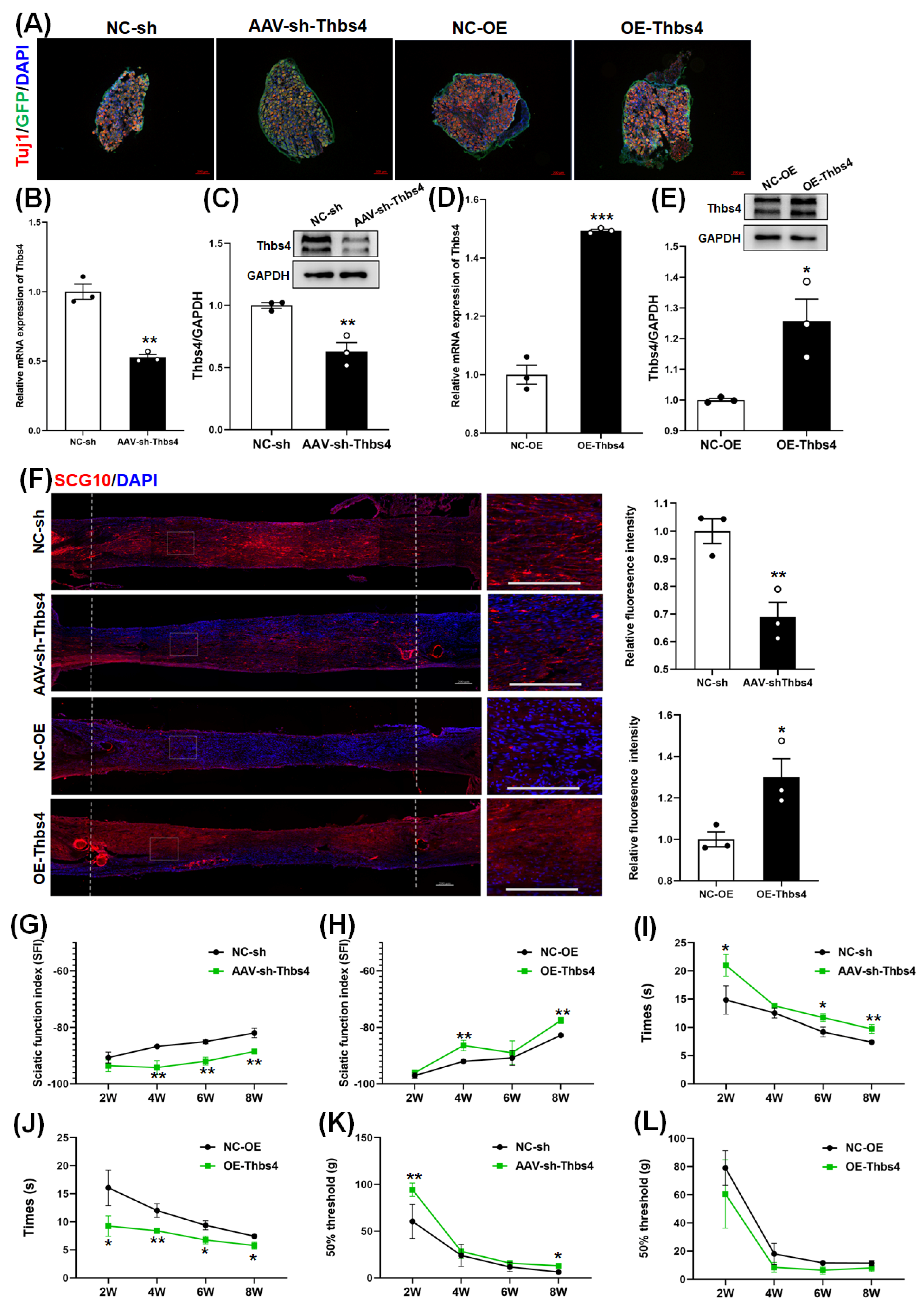
3.5. Effects of Thbs4 on Sciatic Nerve Regeneration In Vivo
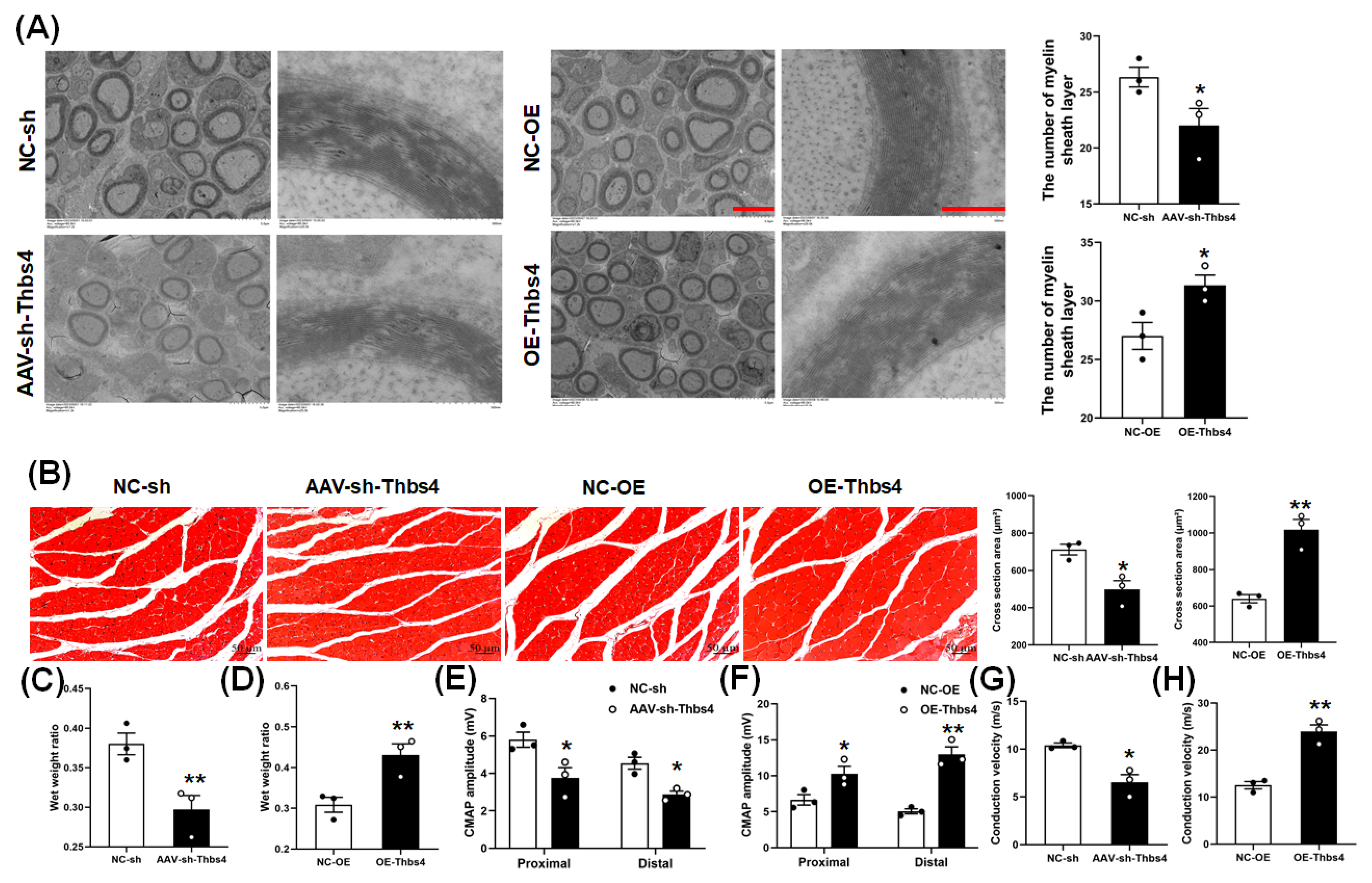
3.6. Effects of Thbs4 on Myelin Sheath and Target Muscle
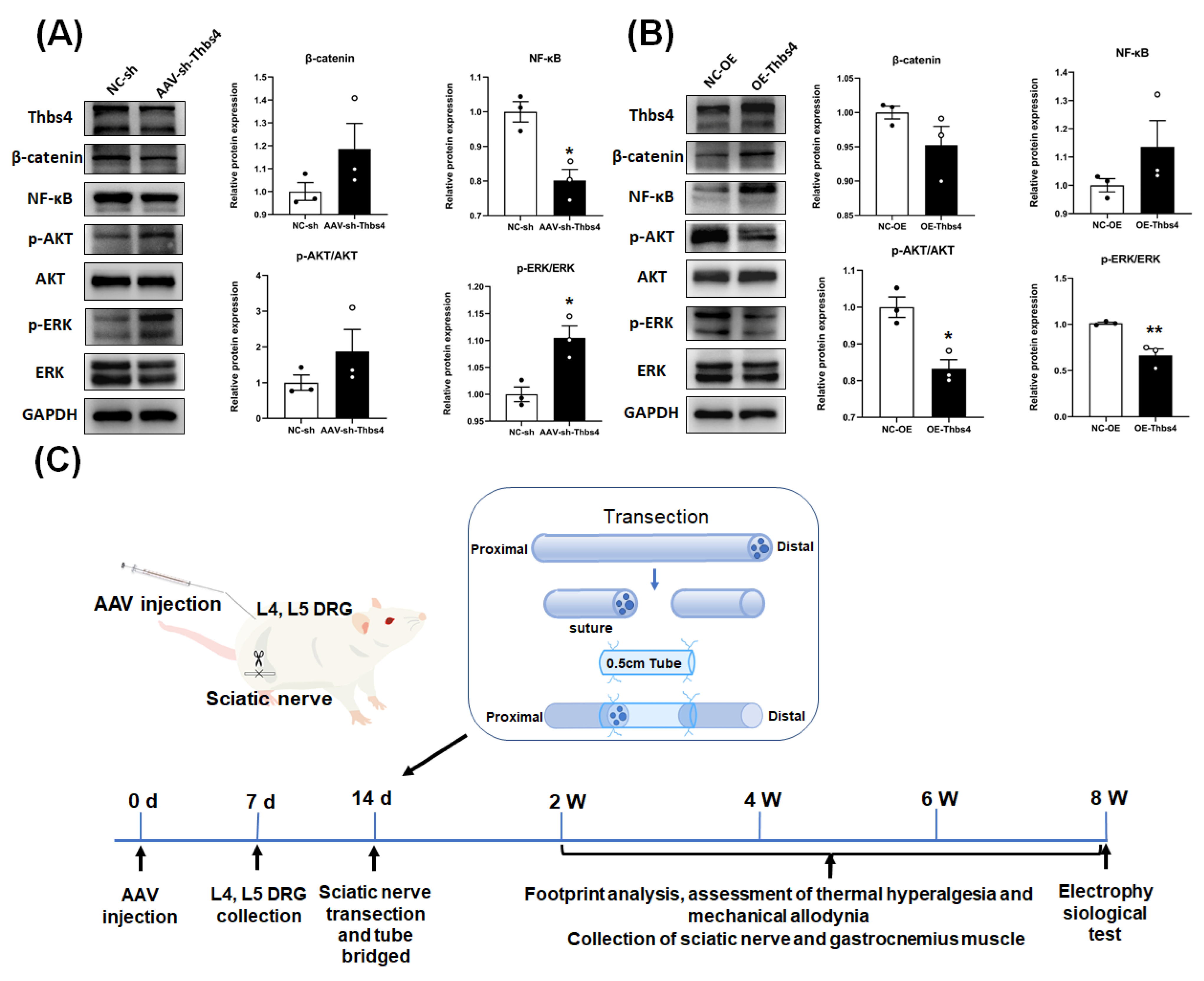
3.7. Effects of Thbs4 on Signal Pathway Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Brien, A.L.; West, J.M.; Saffari, T.M.; Nguyen, M.; Moore, A.M. Promoting Nerve Regeneration: Electrical Stimulation, Gene Therapy, and Beyond. Physiology 2022, 37, 302–310. [Google Scholar] [CrossRef]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shen, W.; Ge, X.; Ao, F.; Zheng, Y.; Wang, Y.; Jia, X.; Mao, Y.; Luo, Y. Advances in Large Gap Peripheral Nerve Injury Repair and Regeneration with Bridging Nerve Guidance Conduits. Macromol. Biosci. 2023, 23, e2300078. [Google Scholar] [CrossRef]
- Mahar, M.; Cavalli, V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018, 19, 323–337. [Google Scholar] [CrossRef]
- Wu, J.; Lu, B.; Yang, R.; Chen, Y.; Chen, X.; Li, Y. EphB2 knockdown decreases the formation of astroglial-fibrotic scars to promote nerve regeneration after spinal cord injury in rats. CNS Neurosci. Ther. 2021, 27, 714–724. [Google Scholar] [CrossRef]
- Krishnan, A.; Verge, V.M.K.; Zochodne, D.W. Hallmarks of peripheral nerve injury and regeneration. Handb. Clin. Neurol. 2024, 201, 1–17. [Google Scholar] [CrossRef]
- Bolandghamat, S.; Behnam-Rassouli, M. Recent Findings on the Effects of Pharmacological Agents on the Nerve Regeneration after Peripheral Nerve Injury. Curr. Neuropharmacol. 2020, 18, 1154–1163. [Google Scholar] [CrossRef]
- Yin, Q.; Kemp, G.J.; Yu, L.G.; Wagstaff, S.C.; Frostick, S.P. Neurotrophin-4 delivered by fibrin glue promotes peripheral nerve regeneration. Muscle Nerve 2015, 24, 345–351. [Google Scholar] [CrossRef]
- Shen, J.; Sun, Y.; Liu, X.; Chai, Y.; Wang, C.; Xu, J. Nerve Regeneration Potential of Antioxidant-Modified Black Phosphorus Quantum Dots in Peripheral Nerve Injury. ACS Nano 2024, 18, 23518–23536. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Q.; Chen, Q.; Xu, L.; Yi, S. Transcriptional Control of Peripheral Nerve Regeneration. Mol. Neurobiol. 2023, 60, 329–341. [Google Scholar] [CrossRef]
- Genaro, K.; Luo, Z.D. Pathophysiological roles of thrombospondin-4 in disease development. Semin. Cell Dev. Biol. 2024, 155, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Muppala, S.; Xiao, R.; Gajeton, J.; Krukovets, I.; Verbovetskiy, D.; Stenina-Adognravi, O. Thrombospondin-4 mediates hyperglycemia- and TGF-beta-induced inflammation in breast cancer. Int. J. Cancer 2021, 148, 2010–2022. [Google Scholar] [CrossRef]
- Dobelmann, V.; Roos, A.; Hentschel, A.; Della Marina, A.; Leo, M.; Schmitt, L.I.; Maggi, L.; Schara-Schmidt, U.; Hagenacker, T.; Ruck, T.; et al. Thrombospondin-4 as potential cerebrospinal fluid biomarker for therapy response in pediatric spinal muscular atrophy. J. Neurol. 2024, 271, 7000–7011. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, M.; Xu, X.; Gao, Y.; Li, X.; Li, Y.; Su, S.; Xie, X.; Yang, Z.; Ke, C. Thrombospondin 4, a mediator and candidate indicator of pain. Eur. J. Cell Biol. 2024, 103, 151395. [Google Scholar] [CrossRef]
- Stenina-Adognravi, O.; Plow, E.F. Thrombospondin-4 in tissue remodeling. Matrix Biol. 2019, 75–76, 300–313. [Google Scholar] [CrossRef]
- Yang, H.J.; Ma, S.P.; Ju, F.; Zhang, Y.P.; Li, Z.C.; Zhang, B.B.; Lian, J.J.; Wang, L.; Cheng, B.F.; Wang, M.; et al. Thrombospondin-4 Promotes Neuronal Differentiation of NG2 Cells via the ERK/MAPK Pathway. J. Mol. Neurosci. 2016, 60, 517–524. [Google Scholar] [CrossRef]
- Zierfuss, B.; Hobaus, C.; Herz, C.T.; Pesau, G.; Koppensteiner, R.; Schernthaner, G.H. Thrombospondin-4 increases with the severity of peripheral arterial disease and is associated with diabetes. Heart Vessels 2020, 35, 52–58. [Google Scholar] [CrossRef]
- Zeng, H.; Lan, B.; Li, B.; Xie, H.; Zhao, E.; Liu, X.; Xue, X.; Sun, J.; Su, L.; Zhang, Y. The role and mechanism of thrombospondin-4 in pulmonary arterial hypertension associated with congenital heart disease. Respir. Res. 2024, 25, 313. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Z.; Wu, H.E.; Luo, Z.D.; Hogan, Q.H.; Pan, B. Increased thrombospondin-4 after nerve injury mediates disruption of intracellular calcium signaling in primary sensory neurons. Neuropharmacology 2017, 117, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Yu, H.; Park, J.; Yu, Y.P.; Luo, Z.D.; Hogan, Q.H. Painful nerve injury upregulates thrombospondin-4 expression in dorsal root ganglia. J. Neurosci. Res. 2015, 93, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.C.; Liu, T.; Gao, Y.J. Chemokines in chronic pain: Cellular and molecular mechanisms and therapeutic potential. Pharmacol. Ther. 2020, 212, 107581. [Google Scholar] [CrossRef]
- Park, J.F.; Yu, Y.P.; Gong, N.; Trinh, V.N.; Luo, Z.D. The EGF-LIKE domain of thrombospondin-4 is a key determinant in the development of pain states due to increased excitatory synaptogenesis. J. Biol. Chem. 2018, 293, 16453–16463. [Google Scholar] [CrossRef]
- Yu, Y.P.; Gong, N.; Kweon, T.D.; Vo, B.; Luo, Z.D. Gabapentin prevents synaptogenesis between sensory and spinal cord neurons induced by thrombospondin-4 acting on pre-synaptic Ca(v)alpha(2)delta(1) subunits and involving T-type Ca(2+) channels. Br. J. Pharmacol. 2018, 175, 2348–2361. [Google Scholar] [CrossRef]
- Yao, D.; Li, M.; Shen, D.; Ding, F.; Lu, S.; Zhao, Q.; Gu, X. Gene expression profiling of the rat sciatic nerve in early Wallerian degeneration after injury. Neural Regen. Res. 2012, 7, 1285–1292. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Feng, Y.M.; Shao, J.; Cai, M.; Zhou, Y.Y.; Yao, Y.; Qian, J.X.; Ding, Z.H.; Jiang, M.R.; Yao, D.B. Long noncoding RNA H19 regulates degeneration and regeneration of injured peripheral nerves. Neural Regen. Res. 2023, 18, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, Y.; Feng, Y.; Qiu, Z.; Luo, S.; Shi, X.; Gu, D.; Jiang, M.; Cai, M.; Yao, D. Fas ligand regulate nerve injury and repair by affecting AKT, beta-catenin, and NF-kappaB pathways. IBRO Neurosci. Rep. 2024, 16, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Zaren, P.; Gawlik, K.I. Thrombospondin-4 deletion does not exacerbate muscular dystrophy in beta-sarcoglycan-deficient and laminin alpha2 chain-deficient mice. Sci. Rep. 2024, 14, 14757. [Google Scholar] [CrossRef]
- Chou, K.Y.; Chang, A.C.; Ho, C.Y.; Tsai, T.F.; Chen, H.E.; Chen, P.C.; Hwang, T.I. Thrombospondin-4 promotes bladder cancer cell migration and invasion via MMP2 production. J. Cell. Mol. Med. 2021, 25, 6046–6055. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Xiu, Y.; Zou, T.; Quan, Y. Sodium ferulate attenuates ischaemic stroke by mediating the upregulation of thrombospondin-4 expression and combined treatment with bone marrow mesenchymal stem cells. Exp. Neurol. 2025, 385, 115124. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Z.; Zhu, T.; Xie, R.; Zhu, J. Downregulation of Thbs4 caused by neurogenic niche changes promotes neuronal regeneration after traumatic brain injury. Neurol. Res. 2020, 42, 703–711. [Google Scholar] [CrossRef]
- Ye, Z.; Zheng, Y.; Li, N.; Zhang, H.; Li, Q.; Wang, X. Repair of spinal cord injury by bone marrow mesenchymal stem cell-derived exosomes: A systematic review and meta-analysis based on rat models. Front. Mol. Neurosci. 2024, 17, 1448777. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, Y.; Gao, Y.; Chang, S.; Kong, R.; Lv, X.; Yu, J.; Jin, Y.; Li, C.; Ma, Y.; et al. PKCalpha inhibitors promote breast cancer immune evasion by maintaining PD-L1 stability. Acta Pharm. Sin. B 2024, 14, 4378–4395. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kumar, S.; Kumar, S.; Shukla, A.; Kumar, N.; Patel, A.K.; Yadav, L.K.; Kaushalendra; Antiwal, M.; Acharya, A. Potential implications of protein kinase Calpha in pathophysiological conditions and therapeutic interventions. Life Sci. 2023, 330, 121999. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Parejo, C.; Deng, C.; Xu, J.; Zhang, Z.; Ren, Z.; Ai, N.; Liu, W.; Ge, W.; Deng, C.; Xu, X.; et al. Protein Kinase C Modulation Determines the Mesoderm/Extraembryonic Fate Under BMP4 Induction From Human Pluripotent Stem Cells. Stem Cells 2023, 41, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Su, L.Y.; Jiao, L.; Liu, Q.; Qiao, X.; Xie, T.; Ma, Z.; Xu, M.; Ye, M.S.; Yang, L.X.; Chen, C.; et al. S-nitrosoglutathione reductase alleviates morphine analgesic tolerance by restricting PKCalpha S-nitrosation. Redox Biol. 2024, 75, 103239. [Google Scholar] [CrossRef]
- Li, H.; Gan, X.; Pan, L.; Zhang, Y.; Hu, X.; Wang, Z. EGF/bFGF promotes survival, migration and differentiation into neurons of GFP-labeled rhesus monkey neural stem cells xenografted into the rat brain. Biochem. Biophys. Res. Commun. 2022, 620, 76–82. [Google Scholar] [CrossRef]
- Chen, X.; Wang, B.; Zhou, Y.; Wu, X.; Du, A.; Al Mamun, A.; Xu, Y.; Wang, S.; Jiang, C.; Xie, L.; et al. Poly (Betulinic Acid) Nanoparticles Loaded with bFGF Improve Functional Recovery After Spinal Cord Injury. Adv. Healthc. Mater. 2024, 13, e2303462. [Google Scholar] [CrossRef]
- Lisek, M.; Tomczak, J.; Boczek, T.; Zylinska, L. Calcium-Associated Proteins in Neuroregeneration. Biomolecules 2024, 14, 183. [Google Scholar] [CrossRef]
- Xie, X.; Wan, J.; Zheng, X.; Pan, W.; Yuan, J.; Hu, B.; Feng, M.; Liu, Z.; Cai, S. Synergistic effects of epigallocatechin gallate and l-theanine in nerve repair and regeneration by anti-amyloid damage, promoting metabolism, and nourishing nerve cells. Front. Nutr. 2022, 9, 951415. [Google Scholar] [CrossRef]
- Williams, L.M.; Gilmore, T.D. Looking Down on NF-kappaB. Mol. Cell. Biol. 2020, 40, e00104-20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Kong, G.; Palmisano, I.; Cencioni, M.T.; Danzi, M.; De Virgiliis, F.; Chadwick, J.S.; Crawford, G.; Yu, Z.; De Winter, F.; et al. Reversible CD8 T cell-neuron cross-talk causes aging-dependent neuronal regenerative decline. Science 2022, 376, eabd5926. [Google Scholar] [CrossRef]
- Kijima, K.; Ono, G.; Kobayakawa, K.; Saiwai, H.; Hara, M.; Yoshizaki, S.; Yokota, K.; Saito, T.; Tamaru, T.; Iura, H.; et al. Zinc deficiency impairs axonal regeneration and functional recovery after spinal cord injury by modulating macrophage polarization via NF-kappaB pathway. Front. Immunol. 2023, 14, 1290100. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, S.; Takeda, K. ERK signalling as a regulator of cell motility. J. Biochem. 2017, 162, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.A.; Roberts, J.L.; Dent, P. The role of cell signaling in the crosstalk between autophagy and apoptosis in the regulation of tumor cell survival in response to sorafenib and neratinib. Semin. Cancer Biol. 2020, 66, 129–139. [Google Scholar] [CrossRef]
- Damasio, M.P.; Marchingo, J.M.; Spinelli, L.; Hukelmann, J.L.; Cantrell, D.A.; Howden, A.J.M. Extracellular signal-regulated kinase (ERK) pathway control of CD8+ T cell differentiation. Biochem. J. 2021, 478, 79–98. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Pei, X.; Zheng, P.; Miao, L.; Li, L.; Luo, C.; Zhang, P.; Jiang, B.; Teng, J.; et al. SCG10 is required for peripheral axon maintenance and regeneration in mice. J. Cell Sci. 2023, 136, jcs260490. [Google Scholar] [CrossRef]
- Baughn, M.W.; Melamed, Z.; Lopez-Erauskin, J.; Beccari, M.S.; Ling, K.; Zuberi, A.; Presa, M.; Gonzalo-Gil, E.; Maimon, R.; Vazquez-Sanchez, S.; et al. Mechanism of STMN2 cryptic splice-polyadenylation and its correction for TDP-43 proteinopathies. Science 2023, 379, 1140–1149. [Google Scholar] [CrossRef]


| Gene | Sequence |
|---|---|
| Thbs4 | F: 5′ GTGCATTGAGGAACGGCAAG 3′ R: 5′ CATCCCCGTCAGTGTCCTTCC 3′ |
| Bcl2 | F: 5′ GCAGAGATGTCCAGTCAGC 3′ R: 5′ CCCACCGAACTCAAAGAAGG 3′ |
| Bax | F: 5′ TGCAGAGGATGATTGCTGAC 3′ R: 5′ GATCAGCTCGGGCACTTTAG 3′ |
| NF200 | F: 5′ GTCAGAGGAGTGGTTCCGAG 3′ R: 5′ GCCGCCGGTACTCAGTTATC 3′ |
| PKCα | F: 5′ GAACACATGATGGACGGGGTCACGAC 3′ R: 5′ CGCTTGGCAGGGTGTTTGGTCATA 3′ |
| bFGF | F: 5′ CCCGCACCCTATCCCTTCACAGC 3′ R: 5′ CACAACGACCAGCCTTCCACCCAAA 3′ |
| AKT2 | F: 5′ CCGGTGAACTCTGACCCTTG 3′ R: 5′ GGCCGCAGCGTCTTCAT 3′ |
| TNFα | F: 5′ ATGGGCTCCCTCTCATCAGT 3′ R: 5′ GCTTGGTGGTTTGCTACGAC 3′ |
| IL-1β | F: 5′ GACCTGTTCTTTGAGGCTGAC 3′ R: 5′ TCCATCTTCTTCTTTGGGTATTGTT 3′ |
| GAP43 | F: 5′ TGCTGTGCTGTATGAGAAGAAC 3′ R: 5′ TTGGTTGCAGCCTTATGAGC 3′ |
| GAPDH | F: 5′ TGGAGTCTACTGGCGTCTT 3′ R: 5′ TGTCATATTTCTCGTGGTTCA 3′ |
| Antibody | Sources | Catalogue Number |
|---|---|---|
| Thbs4 | Santa Cruz | Sc-7657-R |
| GAPDH | Proteintech | 60004-1 |
| β-catenin | Cell signaling | 8480T |
| NF-κB | Cell signaling | 8242T |
| ERK | Abcam | ab201015 |
| p-ERK | Santa Cruz | Sc-7383 |
| AKT | Cell signaling | 4691T |
| p-AKT | Cell signaling | 4060T |
| β-Tubulin III | Abcam | ab78078 |
| SCG10 | Novus | NBP1-4946 |
| NeuN | Abcam | Ab177487 |
| Goat Anti-Mouse IgG HRP | Abways | AB0102 |
| Goat Anti-Rabbit IgG HRP | Abways | AB0101 |
| Cy3-conjugated goat anti-Rabbit | Proteintech | SA00009-2 |
| CoraLite488-conjugated goat anti-Rabbit | Proteintech | SA00013-2 |
| CoraLite488-conjugated goat anti-Mouse | Proteintech | SA00013-1 |
| Product Number | Product Name | Target Sequence |
|---|---|---|
| siG150605090149 | si-r-Thbs4_001 | CCATCCTCCGTTACCTAAA |
| siG150605090215 | si-r-Thbs4_002 | GGATGAGTGTAAATACCAT |
| siG150605090241 | si-r-Thbs4_003 | GGACCAGAGGAACACTGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Zhou, Y.; Zhang, Z.; Huang, Y.; Jiang, T.; Xia, Y.; Gu, D.; Gu, X.; Bai, H.; Jiang, M.; et al. Investigating How Thbs4 Regulates Degeneration and Regeneration of the Peripheral Nerve. Biomedicines 2025, 13, 2375. https://doi.org/10.3390/biomedicines13102375
Yao Y, Zhou Y, Zhang Z, Huang Y, Jiang T, Xia Y, Gu D, Gu X, Bai H, Jiang M, et al. Investigating How Thbs4 Regulates Degeneration and Regeneration of the Peripheral Nerve. Biomedicines. 2025; 13(10):2375. https://doi.org/10.3390/biomedicines13102375
Chicago/Turabian StyleYao, Yi, Yiyue Zhou, Zixu Zhang, Yuxiao Huang, Taoran Jiang, Yiming Xia, Dandan Gu, Xi Gu, Huiyuan Bai, Maorong Jiang, and et al. 2025. "Investigating How Thbs4 Regulates Degeneration and Regeneration of the Peripheral Nerve" Biomedicines 13, no. 10: 2375. https://doi.org/10.3390/biomedicines13102375
APA StyleYao, Y., Zhou, Y., Zhang, Z., Huang, Y., Jiang, T., Xia, Y., Gu, D., Gu, X., Bai, H., Jiang, M., & Yu, C. (2025). Investigating How Thbs4 Regulates Degeneration and Regeneration of the Peripheral Nerve. Biomedicines, 13(10), 2375. https://doi.org/10.3390/biomedicines13102375







