Effects of Platelet-Rich Fibrin on In Vitro Periodontal Ligament Cell Functions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PRF
2.2. Cell Culture
2.3. Real-Time PCR
2.4. ELISA
2.5. In Vitro Wound Healing and Cell Migration
2.6. Cell Viability
2.7. Cell Proliferation
2.8. Statistical Analysis
3. Results
3.1. Dose-Dependent Effects of PRF on the VEGF, BMP2, TNF-α, COX2 and SPP1 Gene Expression in PDL Cells
3.2. PRF-Induced VEGF, BMP2, COX2, TNF-α and SPP1 Gene Expression Under Normal and Inflammatory Conditions
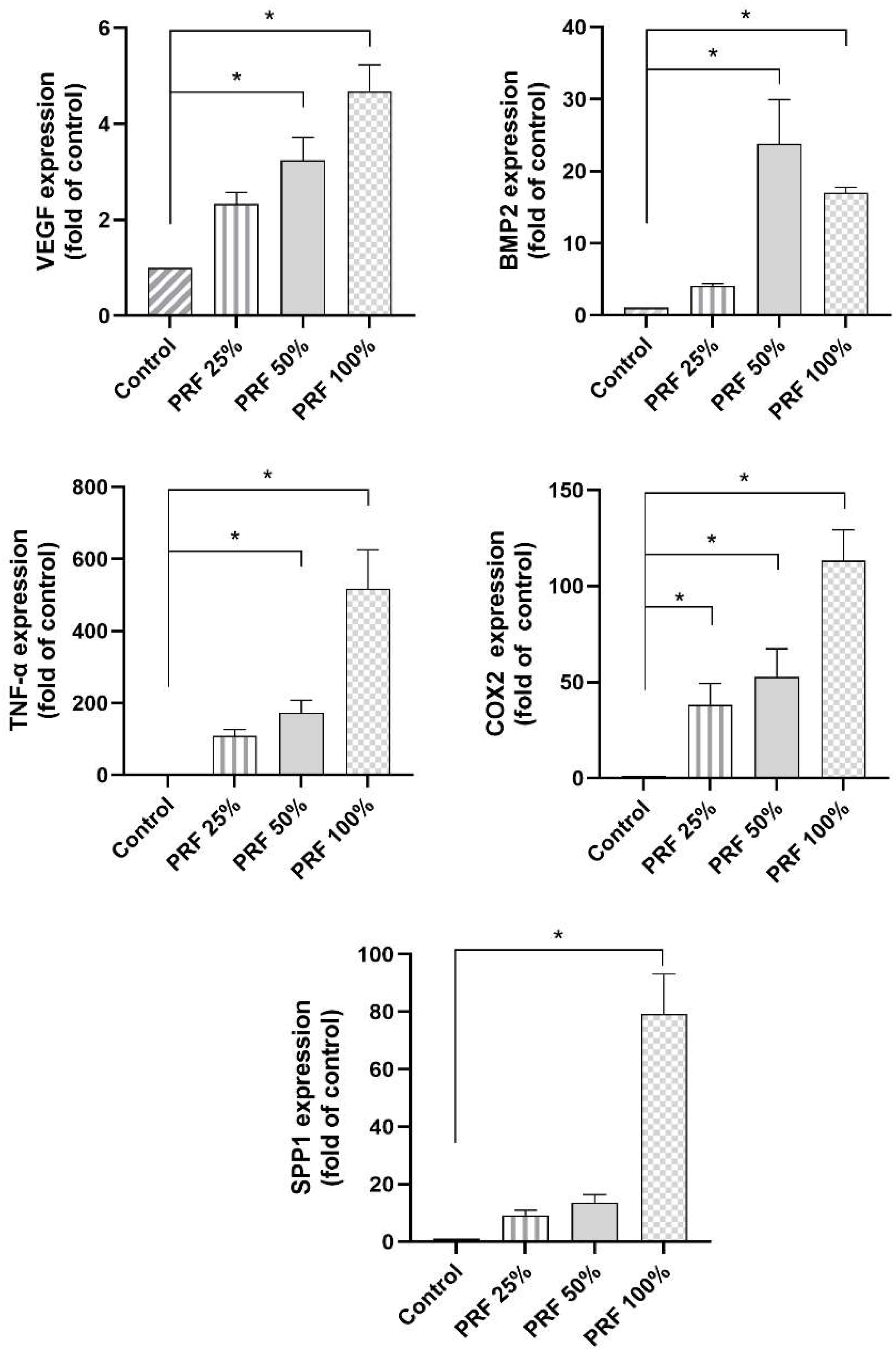
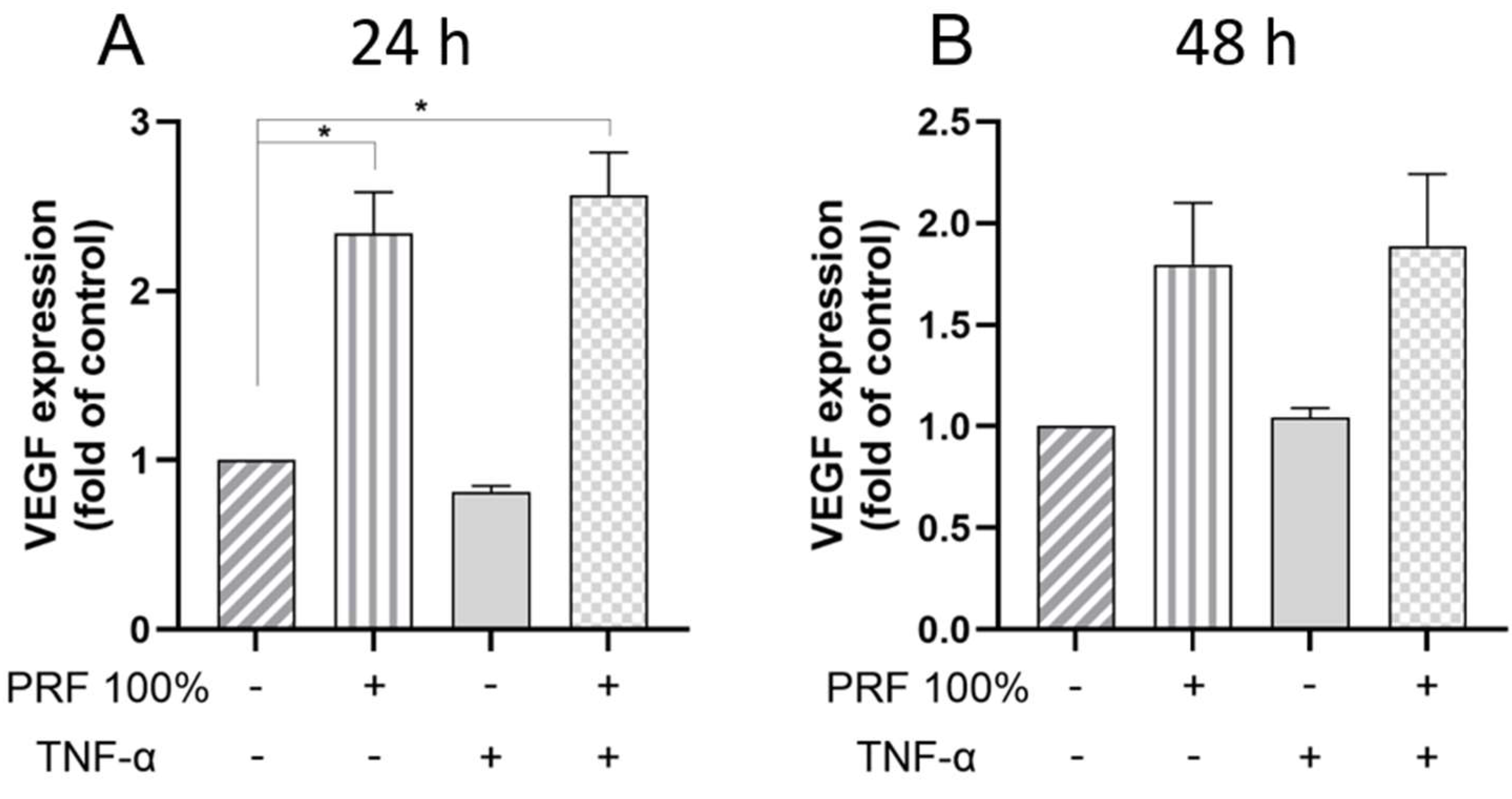
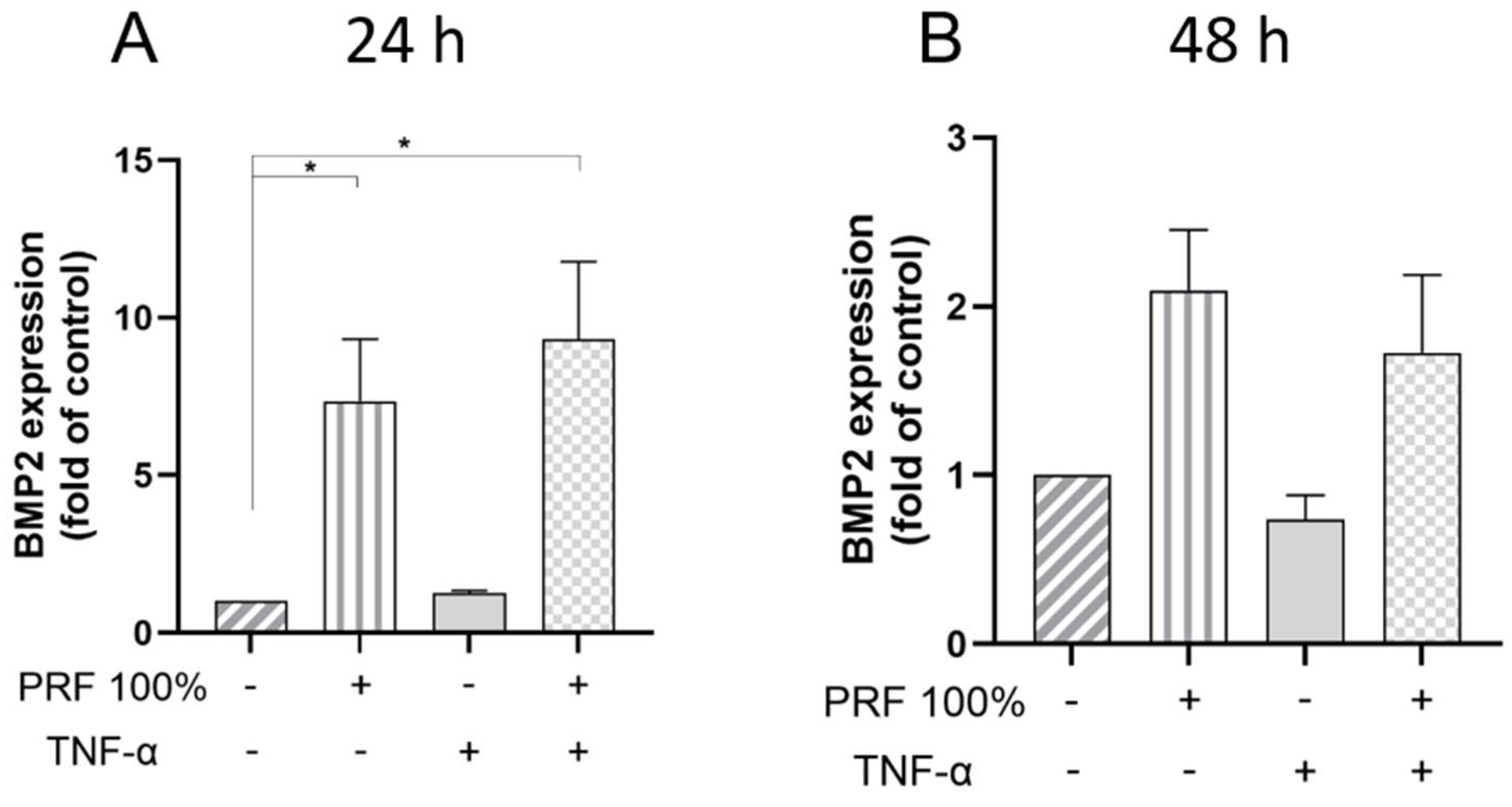
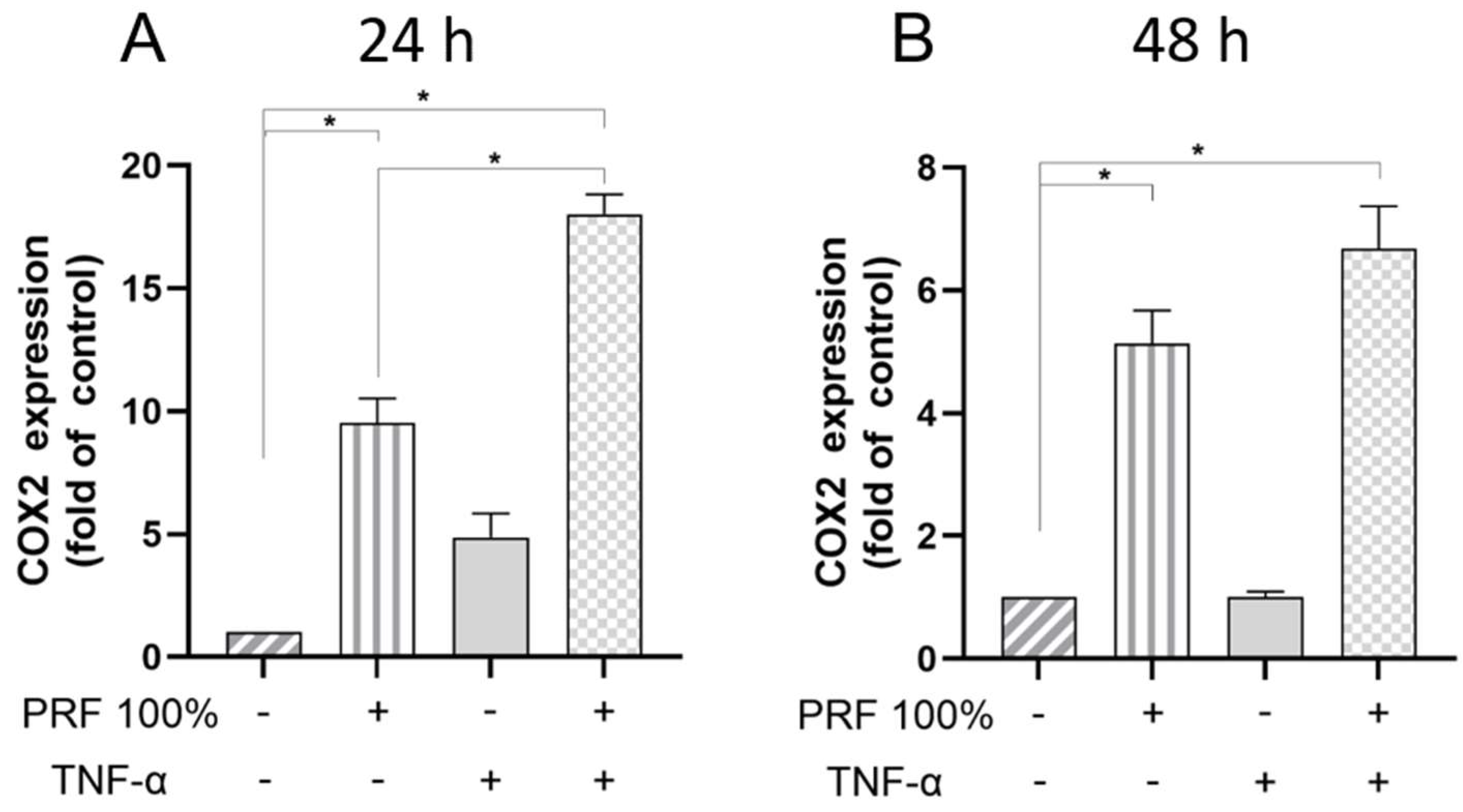
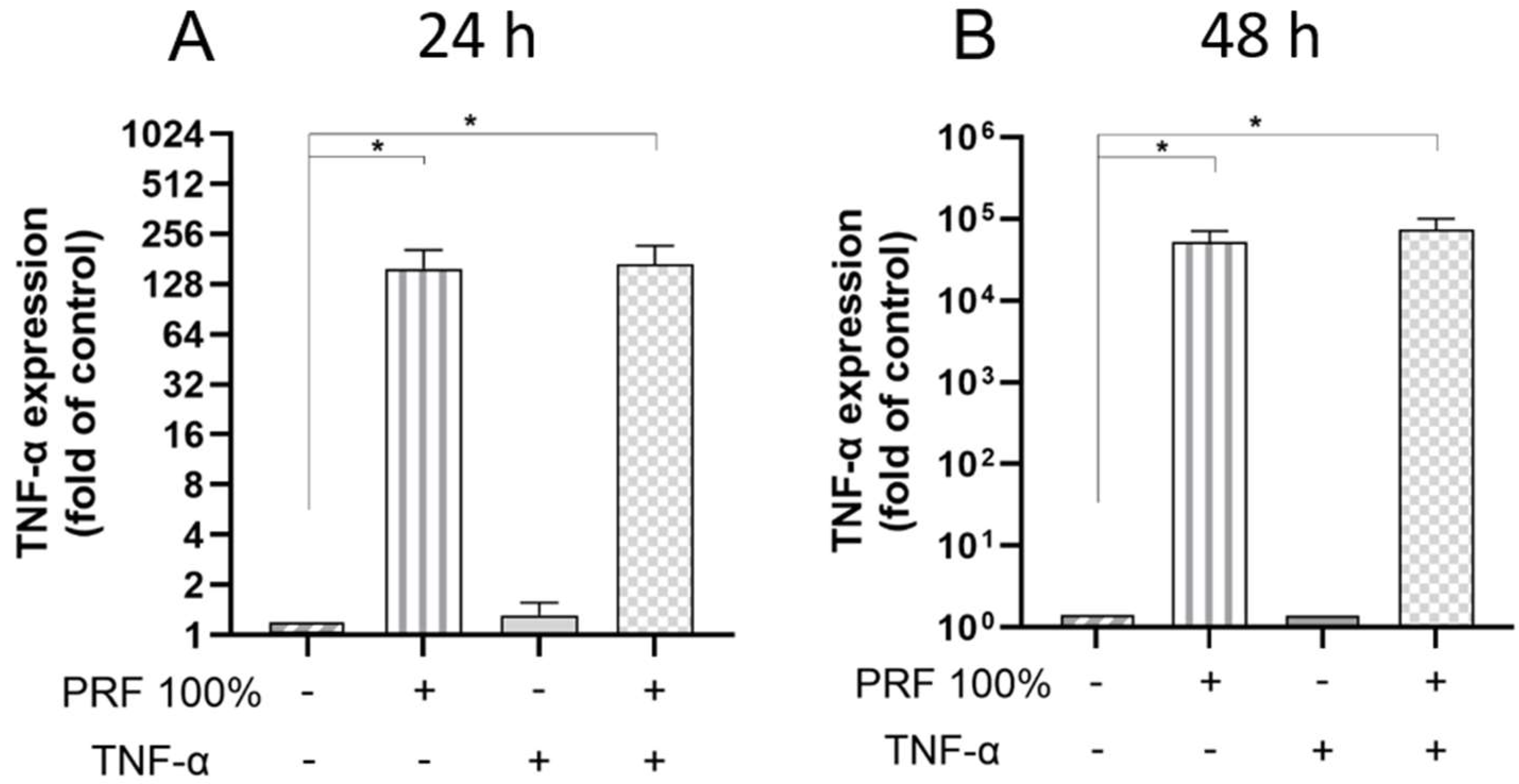
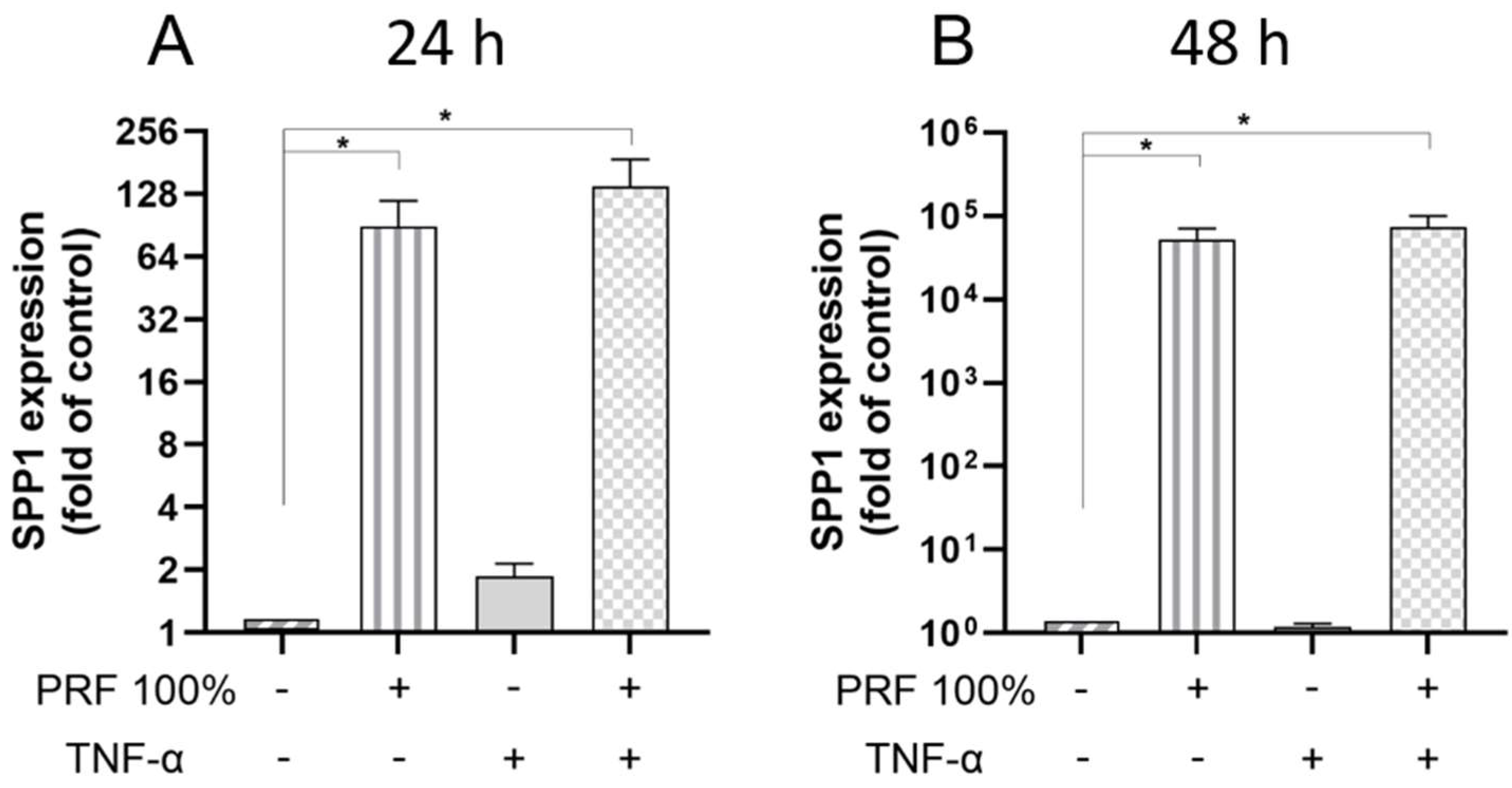
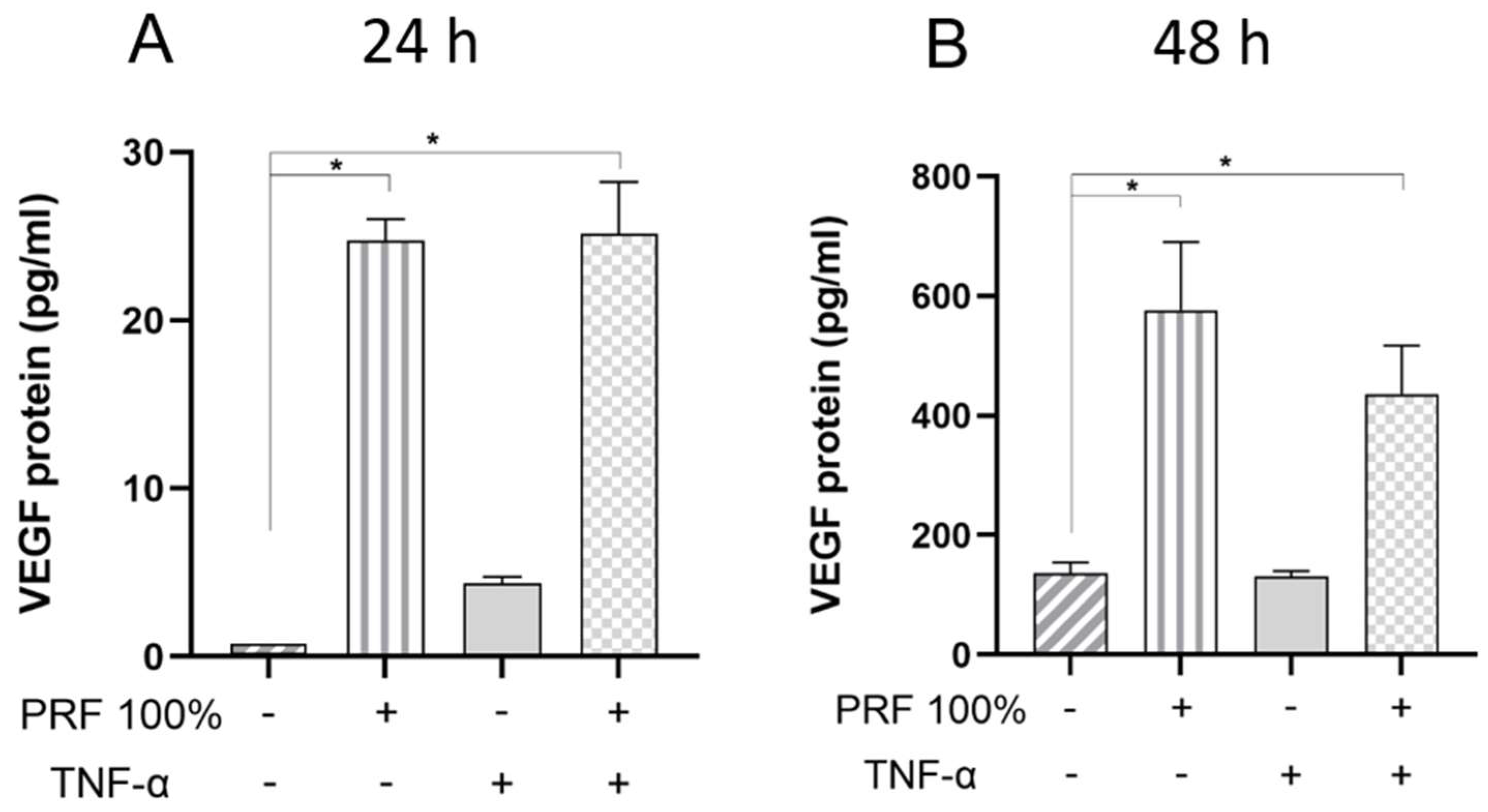
3.3. PRF-Induced VEGF and TNF-α Protein Levels Under Normal and Inflammatory Conditions
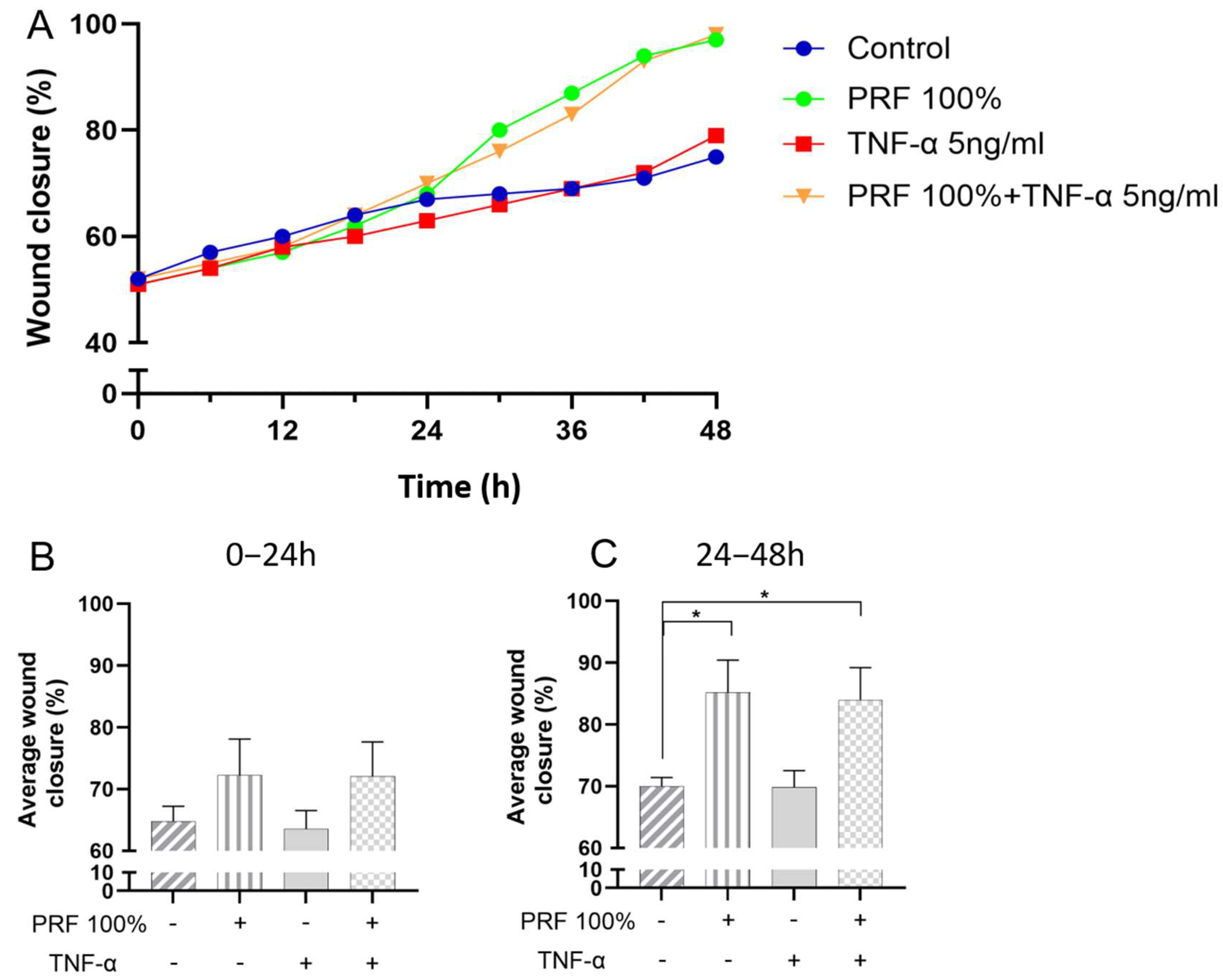
3.4. Effects of PRF on In Vitro Wound Closure Under Normal and Inflammatory Conditions
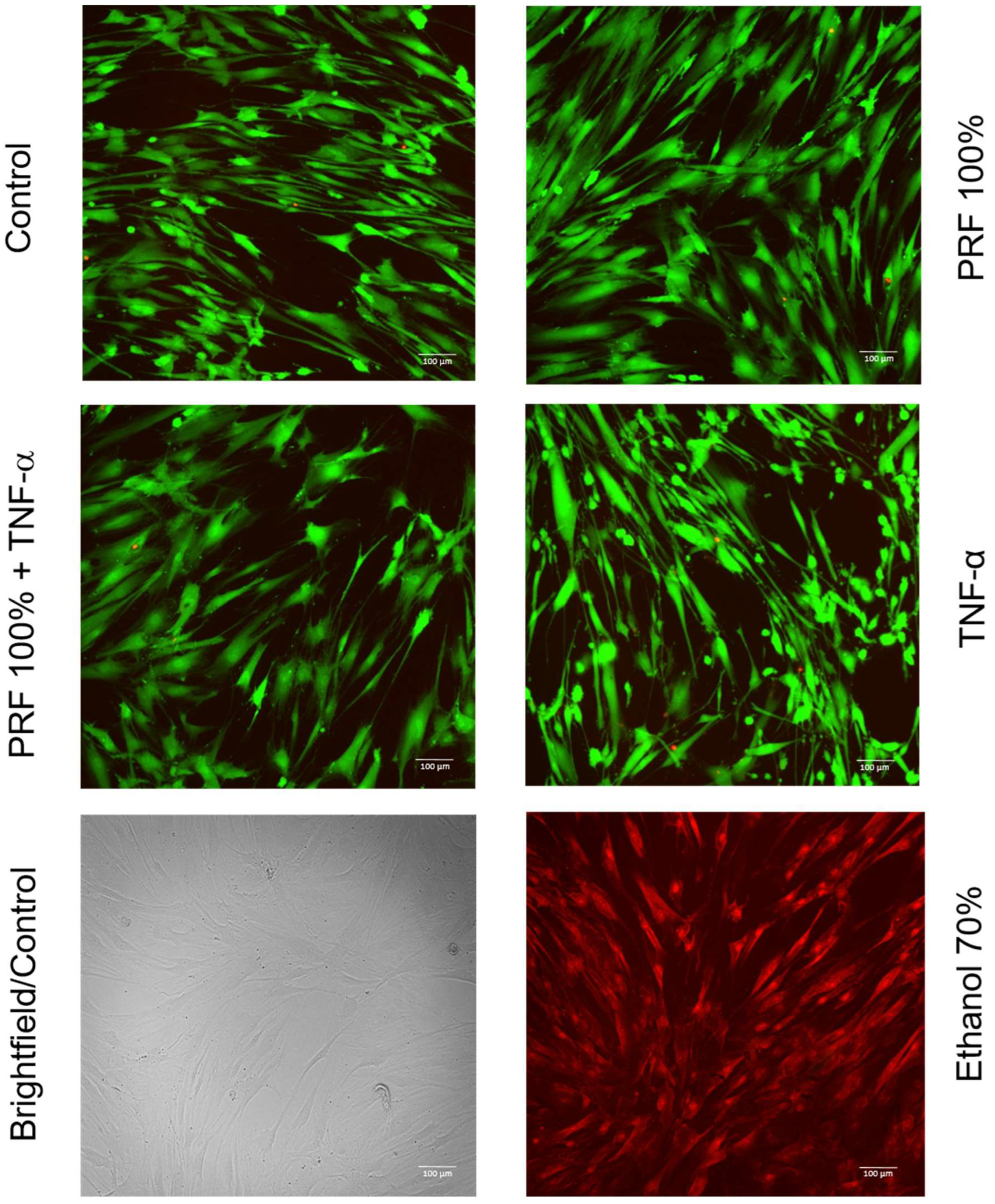
3.5. Effects of PRF on PDL Cell Viability Under Normal and Inflammatory Conditions
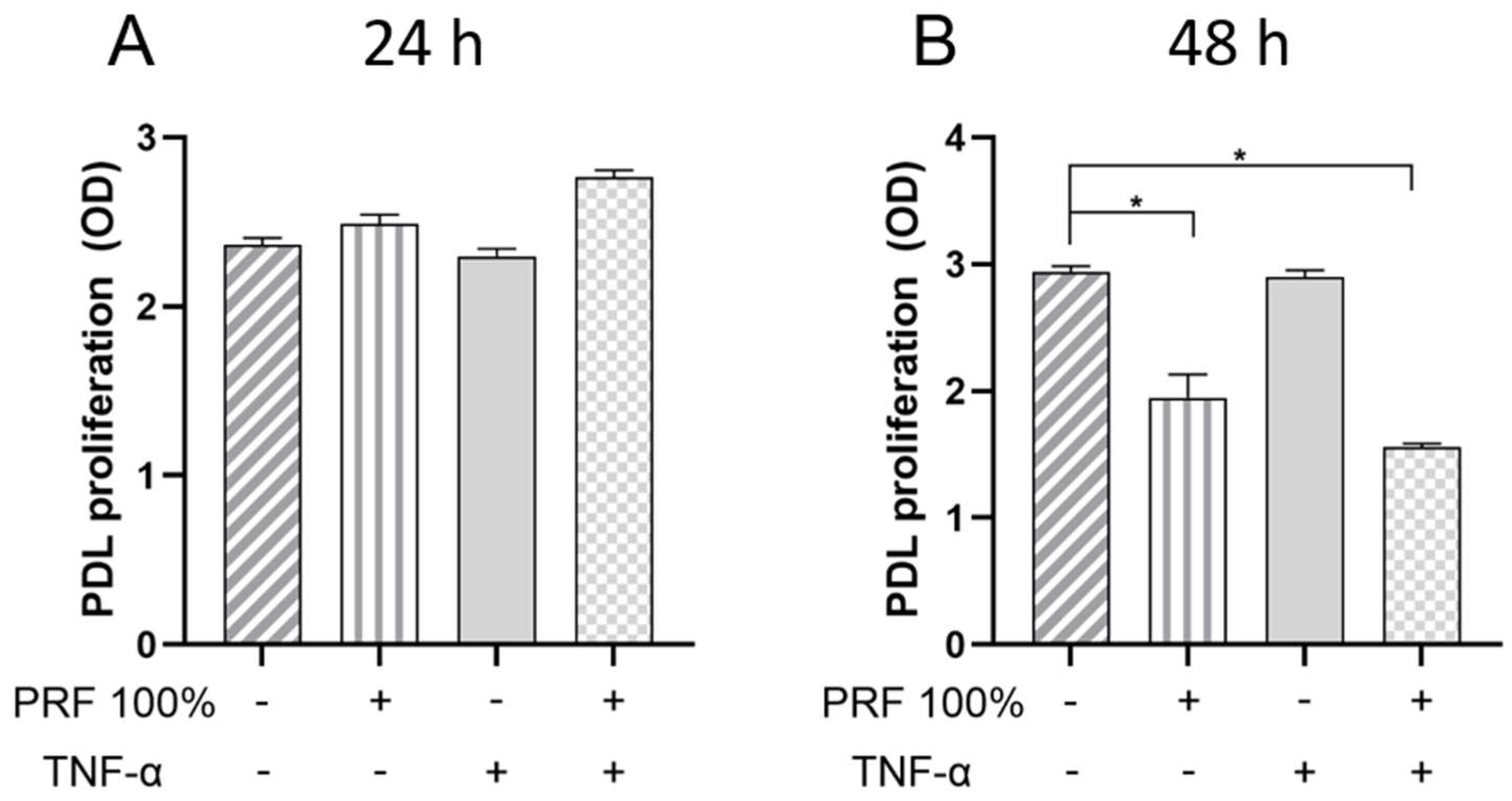
3.6. Effects of PRF on Cell Proliferation of PDL Cells Under Normal and Inflammatory Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRF | Platelet-rich fibrin |
| TNF-α | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
| BMP2 | Bone morphogenetic protein 2 |
| COX2 | Cyclooxygenase 2 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| PDL | Periodontal ligament |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
| ELISA | Enzyme-linked immunosorbent assay |
| PCR | Polymerase chain reaction |
| cDNA | Complementary DNA |
| IL-1β | Interleukin 1 beta |
| LPS | Lipopolysaccharide |
| ANOVA | Analysis of variance |
References
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S1–S8. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef]
- Herrera, D.; Sanz, M.; Kebschull, M.; Jepsen, S.; Sculean, A.; Berglundh, T.; Papapanou, P.N.; Chapple, I.; Tonetti, M.S. Treatment of stage IV periodontitis: The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2022, 49 (Suppl. S24), 4–71. [Google Scholar] [CrossRef] [PubMed]
- Kaldahl, W.B.; Kalkwarf, K.L.; Patil, K.D.; Molvar, M.P.; Dyer, J.K. Long-term evaluation of periodontal therapy: II. Incidence of sites breaking down. J. Periodontol. 1996, 67, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Matuliene, G.; Pjetursson, B.E.; Salvi, G.E.; Schmidlin, K.; Brägger, U.; Zwahlen, M.; Lang, N.P. Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. J. Clin. Periodontol. 2008, 35, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.S. Repair or regeneration following periodontal therapy? J. Clin. Periodontol. 1979, 6, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Wikesjö, U.M.; Selvig, K.A. Periodontal wound healing and regeneration. Periodontol. 2000 1999, 19, 21–39. [Google Scholar] [CrossRef]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35, 87–105. [Google Scholar] [CrossRef]
- Sculean, A.; Donos, N.; Brecx, M.; Karring, T.; Reich, E. Healing of fenestration-type defects following treatment with guided tissue regeneration or enamel matrix proteins. An experimental study in monkeys. Clin. Oral Investig. 2000, 4, 50–56. [Google Scholar] [CrossRef]
- Yukna, R.A.; Mellonig, J.T. Histologic evaluation of periodontal healing in humans following regenerative therapy with enamel matrix derivative. A 10-case series. J. Periodontol. 2000, 71, 752–759. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Winter, J.; Rath, B.; Jäger, A.; Jepsen, S.; Deschner, J. Effects of enamel matrix derivative on periodontal wound healing in an inflammatory environment in vitro. J. Clin. Periodontol. 2011, 38, 479–490. [Google Scholar] [CrossRef]
- Kasaj, A.; Willershausen, B.; Junker, R.; Stratul, S.-I.; Schmidt, M. Human periodontal ligament fibroblasts stimulated by nanocrystalline hydroxyapatite paste or enamel matrix derivative. An in vitro assessment of PDL attachment, migration, and proliferation. Clin. Oral Investig. 2012, 16, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain(R)) for periodontal tissue regeneration in intrabony defects. Cochrane Database Syst. Rev. 2009, 2009, CD003875. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e51–e55. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Moraschini, V.; Estrin, N.; Shibli, J.A.; Cosgarea, R.; Jepsen, K.; Jervøe-Storm, P.-M.; Wang, H.-L.; Sculean, A.; Jepsen, S. Autogenous platelet concentrates for treatment of intrabony defects-A systematic review with meta-analysis. Periodontol. 2000 2025, 97, 153–190. [Google Scholar] [CrossRef]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Kumar, R.V.; Shubhashini, N. Platelet rich fibrin: A new paradigm in periodontal regeneration. Cell Tissue Bank. 2013, 14, 453–463. [Google Scholar] [CrossRef]
- Sharma, A.; Pradeep, A.R. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: A randomized controlled clinical trial. J. Periodontol. 2011, 82, 1705–1712. [Google Scholar] [CrossRef]
- Panda, S.; Jayakumar, N.D.; Sankari, M.; Varghese, S.S.; Kumar, D.S. Platelet rich fibrin and xenograft in treatment of intrabony defect. Contemp. Clin. Dent. 2014, 5, 550–554. [Google Scholar] [CrossRef]
- Lekovic, V.; Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Stankovic, P.; Kenney, E.B.; Camargo, P.M. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J. Periodontal Res. 2012, 47, 409–417. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Rao, N.S.; Agarwal, E.; Bajaj, P.; Kumari, M.; Naik, S.B. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2012, 83, 1499–1507. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.-L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef]
- Pitzurra, L.; Jansen, I.D.C.; de Vries, T.J.; Hoogenkamp, M.A.; Loos, B.G. Effects of L-PRF and A-PRF+ on periodontal fibroblasts in in vitro wound healing experiments. J. Periodontal Res. 2020, 55, 287–295. [Google Scholar] [CrossRef]
- Cores Ziskoven, P.; Nogueira, A.V.B.; Gutierrez, L.S.; Weusmann, J.; Eick, S.; Buduneli, N.; Deschner, J. Apelin Enhances the Effects of Fusobacterium nucleatum on Periodontal Ligament Cells In Vitro. Int. J. Mol. Sci. 2023, 24, 4733. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-S.; Song, W.-H.; Kim, S.-J.; Kim, Y.-G.; Ryu, J.-H. Apelin-APJ axis inhibits TNF-alpha-mediated expression of genes involved in the inflammatory response in periodontal ligament cells. Int. J. Oral Biol. 2019, 44, 182–190. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Doglioli, P.; de Peppo, G.M.; Del Corso, M.; Charrier, J.B. Choukroun’s platelet-rich fibrin (PRF) stimulates in vitro proliferation and differentiation of human oral bone mesenchymal stem cell in a dose-dependent way. Arch. Oral Biol. 2010, 55, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Baca-Gonzalez, L.; Serrano Zamora, R.; Rancan, L.; González Fernández-Tresguerres, F.; Fernández-Tresguerres, I.; López-Pintor, R.M.; López-Quiles, J.; Leco, I.; Torres, J. Plasma rich in growth factors (PRGF) and leukocyte-platelet rich fibrin (L-PRF): Comparative release of growth factors and biological effect on osteoblasts. Int. J. Implant Dent. 2022, 8, 39. [Google Scholar] [CrossRef]
- He, L.; Lin, Y.; Hu, X.; Zhang, Y.; Wu, H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 707–713. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Diss, A.; Odin, G.; Doglioli, P.; Hippolyte, M.P.; Charrier, J.B. In vitro effects of Choukroun’s PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Clipet, F.; Tricot, S.; Alno, N.; Massot, M.; Solhi, H.; Cathelineau, G.; Perez, F.; de Mello, G.; Pellen-Mussi, P. In vitro effects of Choukroun’s platelet-rich fibrin conditioned medium on 3 different cell lines implicated in dental implantology. Implant Dent. 2012, 21, 51–56. [Google Scholar] [CrossRef]
- Rosen, V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009, 20, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Barna, M.; Niswander, L. Visualization of cartilage formation: Insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Dev. Cell 2007, 12, 931–941. [Google Scholar] [CrossRef]
- Polimeni, G.; Xiropaidis, A.V.; Wikesjö, U.M.E. Biology and principles of periodontal wound healing/regeneration. Periodontol. 2000 2006, 41, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.F. The Molecular and Cellular Biology of Wound Repair; Springer: New York, NY, USA, 1988; ISBN 9781489901859. [Google Scholar]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef]
- Futagami, A.; Ishizaki, M.; Fukuda, Y.; Kawana, S.; Yamanaka, N. Wound healing involves induction of cyclooxygenase-2 expression in rat skin. Lab. Investig. 2002, 82, 1503–1513. [Google Scholar] [CrossRef]
- Apostolaki, M.; Armaka, M.; Victoratos, P.; Kollias, G. Cellular Mechanisms of TNF Function in Models of Inflammation and Autoimmunity. In TNF Pathophysiology: Molecular and Cellular Mechanisms; 5 Tables; Kollias, G., Sfikakis, P.P., Eds.; KARGER: Basel, Switzerland; Freiburg, Germany, 2010; pp. 1–26. ISBN 9783805593830. [Google Scholar]
- Xiao, Z.; Liu, Q.; Mao, F.; Wu, J.; Lei, T. TNF-α-induced VEGF and MMP-9 expression promotes hemorrhagic transformation in pituitary adenomas. Int. J. Mol. Sci. 2011, 12, 4165–4179. [Google Scholar] [CrossRef]
- Nagy, J.A.; Benjamin, L.; Zeng, H.; Dvorak, A.M.; Dvorak, H.F. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 2008, 11, 109–119. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Franzén, A.; Heinegård, D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem. J. 1985, 232, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Liaw, L.; Birk, D.E.; Ballas, C.B.; Whitsitt, J.S.; Davidson, J.M.; Hogan, B.L. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J. Clin. Investig. 1998, 101, 1468–1478. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Lopez, C.A.; Rollo, E.E.; Hwang, S.M.; An, X.R.; Walther, S.E. Osteopontin-induced modifications of cellular functions. Ann. N. Y. Acad. Sci. 1995, 760, 127–142. [Google Scholar] [CrossRef]
- Singh, R.P.; Patarca, R.; Schwartz, J.; Singh, P.; Cantor, H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J. Exp. Med. 1990, 171, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Giachelli, C.M.; Schwartz, S.M.; Vracko, R. Macrophages express osteopontin during repair of myocardial necrosis. Am. J. Pathol. 1994, 145, 1450–1462. [Google Scholar]
- Roy, R.; Gopalkrishna, P.; Kabekkodu, S.P. Comparative evaluation of the efficacy of “advanced platelet-rich fibrin plus” and enamel matrix derivative on proliferation and migration of periodontal ligament fibroblasts—An in vitro study. J. Indian Soc. Periodontol. 2025, 29, 49–56. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol. 2000 2020, 84, 14–34. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Deschner, J.; Nokhbehsaim, M. Regulatory effects of inflammatory and biomechanical signals on regenerative periodontal healing. Int. J. Oral Maxillofac. Implant. 2013, 28, e472–e477. [Google Scholar] [CrossRef] [PubMed]
- Kollias, G.; Sfikakis, P.P. (Eds.) TNF Pathophysiology: Molecular and Cellular Mechanisms; 5 Tables; KARGER: Basel, Switzerland; Freiburg, Germany, 2010; ISBN 9783805593830. [Google Scholar]
- Vassalli, P. The pathophysiology of tumor necrosis factors. Annu. Rev. Immunol. 1992, 10, 411–452. [Google Scholar] [CrossRef]
- Gümüş, P.; Nizam, N.; Lappin, D.F.; Buduneli, N. Saliva and serum levels of B-cell activating factors and tumor necrosis factor-α in patients with periodontitis. J. Periodontol. 2014, 85, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Madureira, D.F.; Lucas De Abreu Lima, I.; Costa, G.C.; Lages, E.M.B.; Martins, C.C.; Da Aparecida Silva, T. Tumor Necrosis Factor-alpha in Gingival Crevicular Fluid as a Diagnostic Marker for Periodontal Diseases: A Systematic Review. J. Evid. Based Dent. Pract. 2018, 18, 315–331. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Deschner, B.; Winter, J.; Bourauel, C.; Jäger, A.; Jepsen, S.; Deschner, J. Anti-inflammatory effects of EMD in the presence of biomechanical loading and interleukin-1β in vitro. Clin. Oral Investig. 2012, 16, 275–283. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cores Ziskoven, P.; Nogueira, A.V.B.; Imber, J.-C.; Bani, P.; Hell, C.L.; Weusmann, J.; Deschner, J. Effects of Platelet-Rich Fibrin on In Vitro Periodontal Ligament Cell Functions. Biomedicines 2025, 13, 2360. https://doi.org/10.3390/biomedicines13102360
Cores Ziskoven P, Nogueira AVB, Imber J-C, Bani P, Hell CL, Weusmann J, Deschner J. Effects of Platelet-Rich Fibrin on In Vitro Periodontal Ligament Cell Functions. Biomedicines. 2025; 13(10):2360. https://doi.org/10.3390/biomedicines13102360
Chicago/Turabian StyleCores Ziskoven, Pablo, Andressa Vilas Boas Nogueira, Jean-Claude Imber, Philipp Bani, Charlott Luise Hell, Jens Weusmann, and James Deschner. 2025. "Effects of Platelet-Rich Fibrin on In Vitro Periodontal Ligament Cell Functions" Biomedicines 13, no. 10: 2360. https://doi.org/10.3390/biomedicines13102360
APA StyleCores Ziskoven, P., Nogueira, A. V. B., Imber, J.-C., Bani, P., Hell, C. L., Weusmann, J., & Deschner, J. (2025). Effects of Platelet-Rich Fibrin on In Vitro Periodontal Ligament Cell Functions. Biomedicines, 13(10), 2360. https://doi.org/10.3390/biomedicines13102360





