Structure–Activity Relationships and Therapeutic Applications of Retinoids in View of Potential Benefits from Drug Repurposing Process
Abstract
1. Introduction
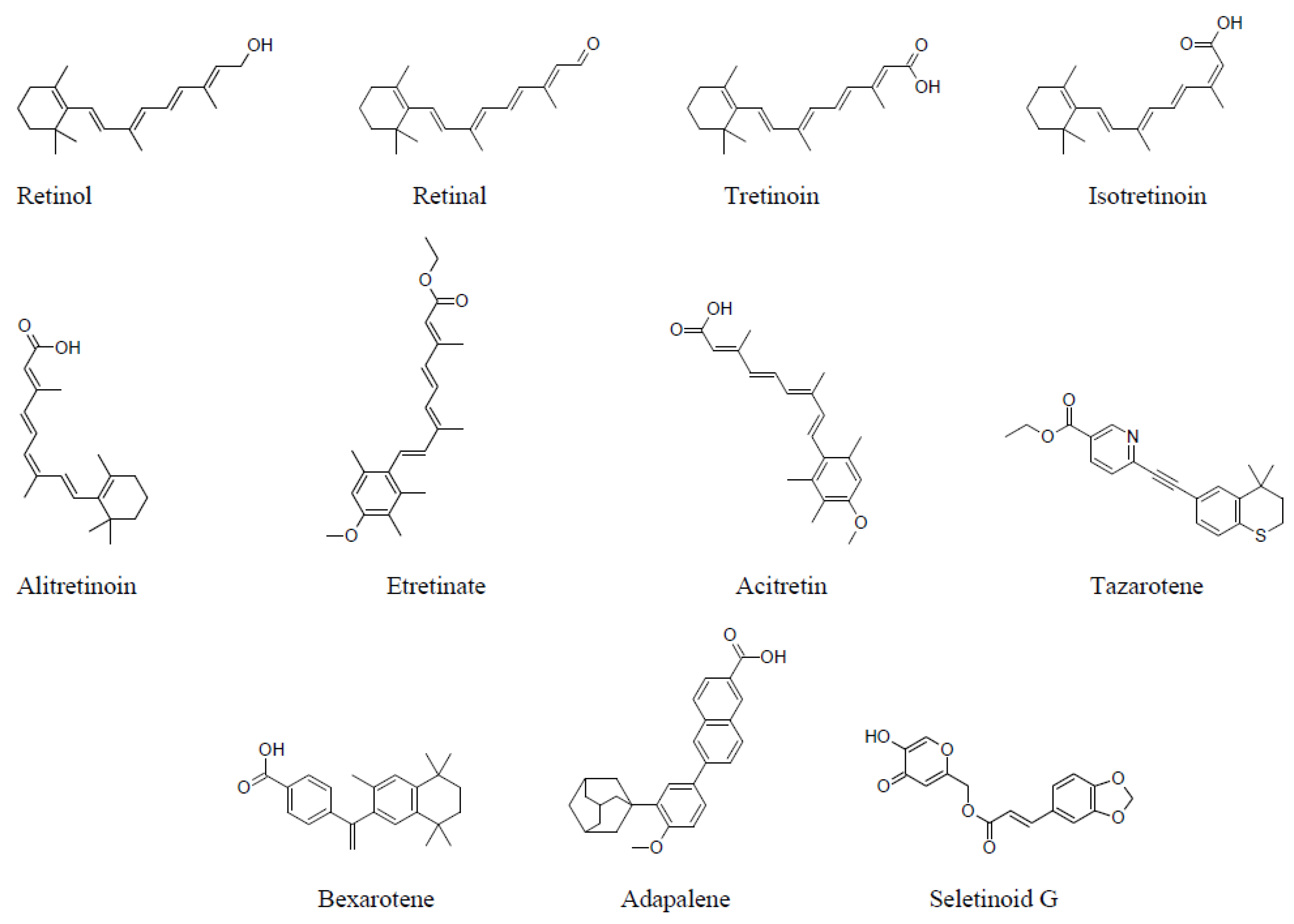
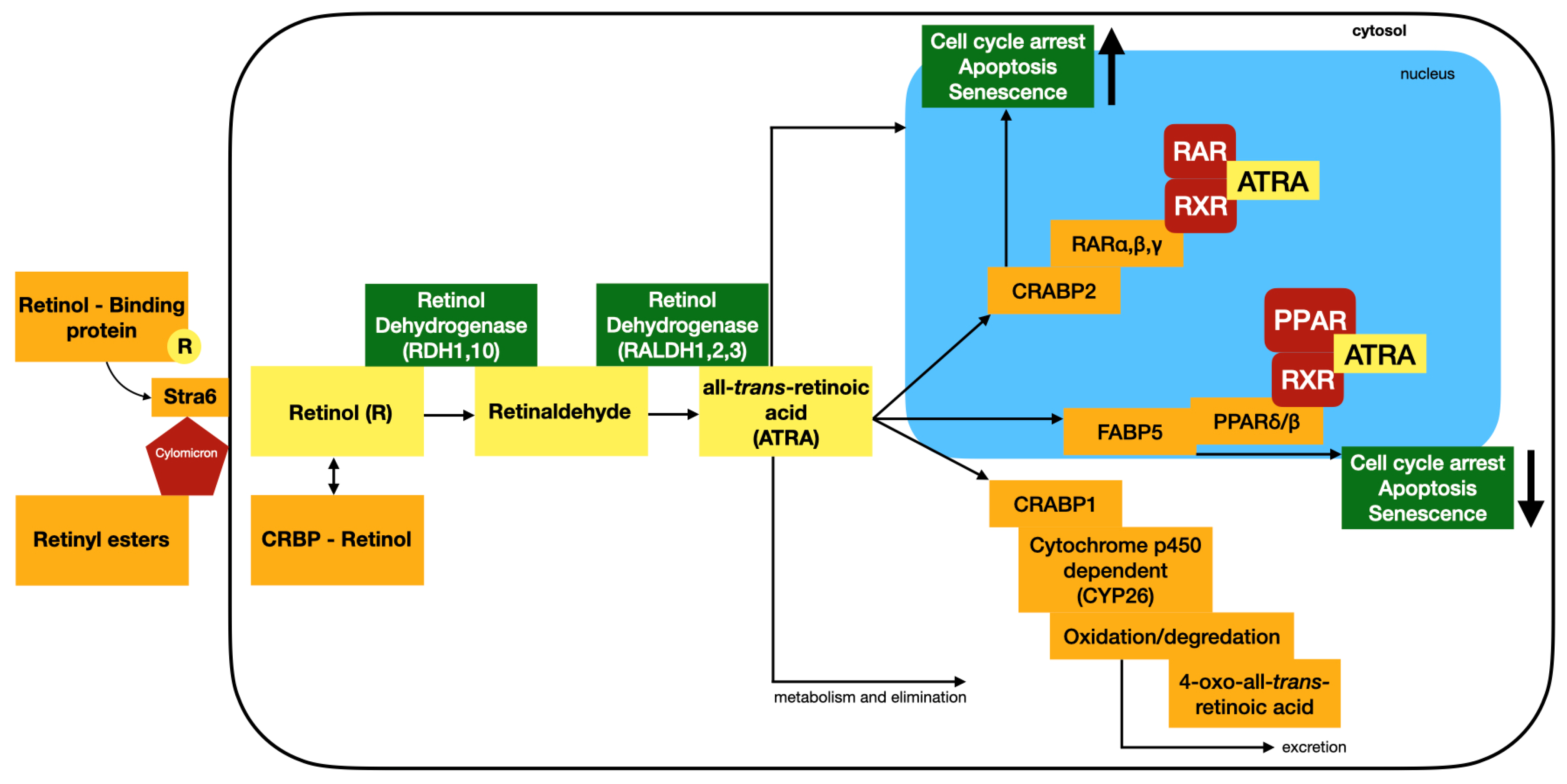
2. Structure–Activity of Natural and Synthetic Retinoids
3. Therapeutic Applications and Drug Repurposing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yurdakok-Dikmen, B.; Filazi, A.; Ince, S. Chapter 27—Retinoids. In Reproductive and Developmental Toxicology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 481–492. [Google Scholar] [CrossRef]
- Thorne-Lyman, A.L.; Fawzi, W.W. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. S1), 36–54. [Google Scholar] [CrossRef]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology: Thematic review series: Fat-soluble vitamins: Vitamin A. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef]
- De Oliveira, M.R. Vitamin A and retinoids as mitochondrial toxicants. Oxid. Med. Cell. Longev. 2015, 2015, 140267. [Google Scholar] [CrossRef]
- IARC. Handbook 1. All-trans-retinoic acid. In Handbook of Cancer Prevention Volume 4; The International Agency for Research on Cancer: Lyon, France, 1999; pp. 95–144. [Google Scholar]
- Rudrapal, M.; Shubham, J.; Khairnar, S.J.; Jadhav, A.G. Drug Repurposing (DR): An Emerging Approach in Drug Discovery. In Drug Repurposing—Hypothesis, Molecular Aspects and Therapeutic Applications; Badria, F.A., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug. Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Roessler, H.I.; Knoers, N.V.A.M.; van Haelst, M.M.; van Haaften, G. Drug Repurposing for Rare Diseases. Trends Pharmacol. Sci. 2021, 42, 255–267. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, H.; Yu, Y.; Yuan, X.; Xiao, L. Informatics on Drug Repurposing for Breast Cancer. Drug Des. Dev. Ther. 2023, 17, 1933–1943. [Google Scholar] [CrossRef]
- Farha, M.A.; Brown, E.D. Drug repurposing for antimicrobial discovery. Nat. Microbiol. 2019, 4, 565–577. [Google Scholar] [CrossRef]
- Mullins, J.G.L. Drug repurposing in silico screening platforms. Biochem. Soc. Trans. 2022, 50, 747–758. [Google Scholar] [CrossRef]
- Schipper, L.J.; Zeverijn, L.J.; Garnett, M.J.; Voest, E.E. Can Drug Repurposing Accelerate Precision Oncology? Cancer Discov. 2022, 12, 1634–1641. [Google Scholar] [CrossRef]
- Singhal, S.; Maheshwari, P.; Krishnamurthy, P.T.; Patil, V.M. Drug Repurposing Strategies for Non-cancer to Cancer Therapeutics. Anticancer Agents Med. Chem. 2022, 22, 2726–2756. [Google Scholar] [CrossRef]
- Fetro, C.; Scherman, D. Drug repurposing in rare diseases: Myths and reality. Therapie 2020, 75, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.; Chehade, A.; Sanghera, R.; Grewal, P. A Clinician’s Guide to Topical Retinoids. J. Cutan. Med. Surg. 2022, 26, 71–78. [Google Scholar] [CrossRef] [PubMed]
- DiKun, K.M.; Gudas, L.J. Vitamin A and retinoid signaling in the kidneys. Pharmacol. Ther. 2023, 248, 108481. [Google Scholar] [CrossRef] [PubMed]
- Honarbakhsh, M.; Ericsson, A.; Zhong, G.; Isoherranen, N.; Zhu, C.; Bromberg, Y.; Van Buiten, C.; Malta, K.; Joseph, L.; Sampath, H.; et al. Impact of vitamin A transport and storage on intestinal retinoid homeostasis and functions. J. Lipid. Res. 2021, 62, 100046. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.R. Vitamins|Vitamin A (Retinoids). In Encyclopedia of Biological Chemistry III, 3rd ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 2, pp. 1088–1096. [Google Scholar] [CrossRef]
- Biyong, E.F.; Tremblay, C.; Leclerc, M.; Caron, V.; Alfos, S.; Helbling, J.-C.; Rodriguez, L.; Pernet, V.; Bennett, D.A.; Pallet, V.; et al. Role of Retinoid X Receptors (RXRs) and dietary vitamin A in Alzheimer’s disease: Evidence from clinicopathological and preclinical studies. Neurobiol. Dis. 2021, 161, 105542. [Google Scholar] [CrossRef] [PubMed]
- Gudas, L.J. Synthetic Retinoids Beyond Cancer Therapy. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 155–175. [Google Scholar] [CrossRef]
- Everts, H.B.; Akuailou, E.N. Retinoids in Cutaneous Squamous Cell Carcinoma. Nutrients 2021, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Jan, N.; Sofi, S.; Qayoom, H.; Haq, B.U.; Shabir, A.; Mir, M.A. Targeting breast cancer stem cells through retinoids: A new hope for treatment. Crit. Rev. Oncol. Hematol. 2023, 192, 104156. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, S.; Zhu, W.; Wu, L.; Chen, X. Retinoids as an Immunity-modulator in Dermatology Disorders. Arch. Immunol. Ther. Exp. 2019, 67, 355–365. [Google Scholar] [CrossRef]
- Hunsu, V.O.; Facey, C.O.B.; Fields, J.Z.; Boman, B.M. Retinoids as Chemo-Preventive and Molecular-Targeted Anti-Cancer Therapies. Int. J. Mol. Sci. 2021, 22, 7731. [Google Scholar] [CrossRef] [PubMed]
- Tratnjek, L.; Jeruc, J.; Romih, R.; Zupančič, D. Vitamin A and Retinoids in Bladder Cancer Chemoprevention and Treatment: A Narrative Review of Current Evidence, Challenges and Future Prospects. Int. J. Mol. Sci. 2021, 22, 3510. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Gibert, Y. Retinoids in Embryonic Development. Biomolecules 2020, 10, 1278. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Tang, X.-H.; Trasino, S.E.; Gudas, L.J. Retinoids in the Pathogenesis and Treatment of Liver Diseases. Nutrients 2022, 14, 1456. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Formulation approaches for improved retinoids delivery in the treatment of several pathologies. Eur. J. Pharm. Biopharm. 2019, 143, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Reay, W.R.; Cairns, M.J. The role of the retinoids in schizophrenia: Genomic and clinical perspectives. Mol. Psychiatry 2020, 25, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Barnard, J.H.; Collings, J.C.; Whiting, A.; Przyborski, S.A.; Marder, T.B. Synthetic Retinoids: Structure–Activity Relationships. Chemistry 2009, 15, 11430–11442. [Google Scholar] [CrossRef] [PubMed]
- Frickel, F. Chemistry and physical properties of retinoids. In The Retinoids, 2nd ed.; Sporn, M.B., Roberts, A.B., Goodman, D.S., Eds.; Academic Press: Orlando, FL, USA, 1984; pp. 8–145. [Google Scholar] [CrossRef]
- Ross, S.A.; McCaffery, P.J.; Drager, U.C.; De Luca, L.M. Retinoids in Embryonal Development. Physiol. Rev. 2000, 80, 1021–1054. [Google Scholar] [CrossRef]
- Napoli, J.L. Biochemical pathways of retinoid transport, metabolism, and signal transduction. Clin. Immunol. Immunopathol. 1996, 80, S52–S62. [Google Scholar] [CrossRef]
- Lowe, N.; Marks, R. Retinoids: A Clinicians Guide, 2nd ed.; Informa Health Care: London, UK, 1998; p. 6. [Google Scholar]
- Soprano, D.R.; Qin, P.; Soprano, K.J. Retinoic acid receptors and cancers. Annu. Rev. Nutr. 2004, 24, 201–221. [Google Scholar] [CrossRef]
- Vahlquist, A.; Rollman, O. Clinical pharmacology of 3 generations of retinoids. Dermatologica 1987, 175 (Suppl. S1), 20–27. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R. Three generations of retinoids: Basic pharmacologicdata, mode of action, and effect on keratinocyte proliferation and differentiation. In Pharmacology of the Skin II; Greaves, M.W., Shuster, S., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 329–358. [Google Scholar] [CrossRef]
- Murayama, A.; Suzuki, T.; Matsui, M. Photoisomerization of retinoic acids under room light: A warning for cell biological study of geometrical isomers of retinoids. J. Nutr. Sci. Vitaminol. 1997, 43, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kunchala, S.R.; Matsui, M.; Murayama, A. Molecular Flexibility of Retinoic Acid under White Fluorescent Ligh. J. Nutr. Sci. Vitaminol. 1998, 44, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Christie, V.B.; Barnard, J.H.; Batsanov, A.S.; Bridgens, C.E.; Cartmell, E.B.; Collings, J.C.; Maltman, D.J.; Redfern, C.P.F.; Marder, T.B.; Przyborski, S.A.; et al. Synthesis and evaluation of synthetic retinoid derivatives as inducers of stem cell differentiation. Org. Biomol. Chem. 2008, 6, 3497–3507. [Google Scholar] [CrossRef]
- Maltman, D.J.; Christie, V.B.; Collings, J.C.; Barnard, J.H.; Fenyk, S.; Marder, T.B.; Whiting, A.; Przyborski, S.A. Proteomic profiling of the stem cellresponse to retinoic acid and synthetic retinoid analogues: Identification of major retinoid-inducible proteins. Mol. BioSyst. 2009, 5, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Quadro, L.; Hamberger, L.; Colantuoni, V.; Gottesman, M.E.; Blaner, W.S. Understanding the physiological role of retinol-binding protein in vitamin A metabolism using transgenic and knockout mouse models. Mol. Asp. Med. 2003, 24, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Blaner, W.S.; Olson, J.A. Retinol and retinoic acid metabolism. In The Retinoids, Biology, Chemistry and Medicine; Sporn, M.B., Roberts, A.B., Goodman, D.S., Eds.; Raven: New York, NY, USA, 1994; pp. 229–256. [Google Scholar] [CrossRef]
- Chen, H.; Howald, W.N.; Juchau, M.R. Biosynthesis of all-trans-retinoic acid from all-trans-retinol: Catalysis of all-trans-retinol oxidation by human P-450 cytochromes. Drug Metab. Dispos. 2000, 28, 315–322. [Google Scholar] [PubMed]
- Dong, D.; Ruuska, S.E.; Levinthal, D.J.; Noy, N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J. Biol. Chem. 1999, 274, 23695–23698. [Google Scholar] [CrossRef] [PubMed]
- Marill, J.; Capron, C.C.; Idres, N.; Chabot, G.G. Human cytochrome P450s involved in the metabolism of 9-cis- and 13-cis-retinoic acids. Biochem. Pharmacol. 2002, 63, 933–943. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schtz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Clamon, G.H.; Sporn, M.B.; Smith, J.M.; Saffiotti, U. α- and β retinyl acetate reverese metaplasia of vitamin A deficiency in hamster tracheal cell culture. Nature 1974, 250, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Clamon, G.H.; Dunlop, N.M.; Newton, D.L.; Smith, J.M.; Saffiotti, U. Activity of vitamin A analogues in cell cultures of mouse epidermidis and organ cultures of hamster trachea. Nature 1975, 253, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Dunlop, N.M.; Newton, D.L.; Henderson, W.R. Relationships between structure and activity of retinoids. Nature 1976, 263, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Sussman, F.; de Lera, A.R. Ligand recognition by RAR and RXR receptors: Binding and selectivity. J. Med. Chem. 2005, 48, 6212–6219. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.T.; Klein, E.S.; Gillett, S.J.; Wang, L.; Song, T.K.; Pino, M.E.; Chandraratna, R.A.S. Synthesis and characterization of a highly potent and effective antagonist of retinoic acid receptors. J. Med. Chem. 1995, 38, 4764–4767. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.T.; Wang, L.; Gillett, S.J.; Chandraratna, R.A.S. High affinity retinoic acid receptor antagonists: Analogs of AGN 193109. Bioorg. Med. Chem. Lett. 1999, 9, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.S.; Pino, M.E.; Johnson, A.T.; Davies, P.J.A.; Nagpal, S.; Thacher, S.M.; Krasinski, G.; Chandraratna, R.A.S. Identification and functional separation of retinoic acid receptor neutral antagonists and inverse agonists. J. Biol. Chem. 1996, 271, 22692–22696. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.C.-K.; Cooney, A.J. Retinoid Receptors. In The Nuclear Receptors and Genetic Disease; Burris, T.P., McCabe, E.R.B., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 245–295. [Google Scholar]
- Chambon, P. The retinoid signaling pathway: Molecular and genetic analyses. Semin. Cell Biol. 1994, 5, 115–125. [Google Scholar] [CrossRef]
- Peck, G.L.; Olsen, T.G.; Yoder, F.W.; Strauss, J.S.; Downing, D.T. Prolonged Remissions of Cystic and Conglobate Acne with 13-cis-Retinoic Acid. N. Engl. J. Med. 1979, 300, 329–333. [Google Scholar] [CrossRef]
- Sardana, K.; Garg, V.; Sehgal, V.; Mahajan, S.; Bhushan, P. Efficacy of Fixed low-dose Isotretinoin (20 mg, Alternate days) with Topical Clindamycin Gel in Moderately Severe Acne Vulgaris. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 556–560. [Google Scholar] [CrossRef]
- Bagatin, E.; Costa, C.S. The Use of Isotretinoin for Acne—An Update on Optimal dosing, surveillance, and Adverse Effects. Expert Rev. Clin. Pharmacol. 2020, 13, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Sanjel, K.; Zhang, X.M. Progress of different treatment modalities to limit the use of antibiotics in the treatment of acne. Our Dermatol. Online 2022, 13, 92–97. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Fedorowicz, Z. Low-Dose Isotretinoin: An Option for Difficult-to-Treat Papulopustular Rosacea. J. Investig. Dermatol. 2016, 136, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Luo, Q.; Li, X.-M.; Zhang, X.-B. Off-label uses of retinoids in dermatology. Our Dermatol. Online 2012, 3 (Suppl. S1), 259–278. [Google Scholar]
- Boer, J. Oral Retinoids for Hidradenitis Suppurativa; Springer eBooks: Berlin/Heidelberg, Germany, 2006; pp. 128–135. [Google Scholar] [CrossRef]
- Al Soufi, L.; Fawal, H.; Kassam, L.; Al-Shehabi, Z. PAPASH syndrome: The first case report from Syria. Our Dermatol. Online 2023, 14, 201–203. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Levy, M.L.; Stefanko, N.S.; Benjamin, L.T.; Bruckner, A.L. Consensus Recommendations for the Use of Retinoids in Ichthyosis and Other Disorders of Cornification in Children and Adolescents. Pediatr. Dermatol. 2021, 38, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Paichitrojjana, A.; Paichitrojjana, A. Oral Isotretinoin and Its Uses in Dermatology: A Review. Drug Des. Dev. Ther. 2023, 17, 2573–2591. [Google Scholar] [CrossRef] [PubMed]
- Gopal Anoop, D.S.; Samayam, A.; Bijina, K.D. Ichthyoses: Case series. Our Dermatol. Online 2018, 9, 190–193. [Google Scholar] [CrossRef]
- Levine, N.; Moon, T.E.; Cartmel, B.; Bangert, J.L. Trial of Retinol and Isotretinoin in Skin Cancer prevention: A randomized, double-blind, Controlled trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiol. Biomarkers Prev. 1997, 6, 957–961. [Google Scholar]
- Lippman, S.M.; Parkinson, D.; Itri, L.M.; Weber, R.S.; Schantz, S.P. 13-cis-Retinoic Acid and Interferon -2a: Effective Combination. Therapy for Advanced Squamous Cell Carcinoma of the Skin. J. Natl. Cancer Inst. 1992, 84, 235–241. [Google Scholar] [CrossRef]
- Wong, W.Y.; Kolbusz, R.V.; Goldberg, L.H.; Guana, A. Treatment of a recurrent keratoacanthoma with oral isotretinoin. Int. J. Dermatol. 1994, 33, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.C.; White, C.R. Treatment of Multiple Keratoacanthomas with Oral Isotretinoin. J. Am. Acad. Dermatol. 1986, 15, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, H.; Khezri, S.; Hosseini, H.; Khezri, F.; Vasigh, M. A Single Blind Randomized Clinical study: The Efficacy of Isotretinoin plus Narrow Band Ultraviolet B in the Treatment of Psoriasis Vulgaris. Photodermatol. Photoimmunol. Photomed. 2011, 27, 159–161. [Google Scholar] [CrossRef]
- Sofen, H.L.; Moy, R.L.; Lowe, N.J. Treatment of generalised pustular psoriasis with isotretinoin. Lancet 1984, 323, 40. [Google Scholar] [CrossRef] [PubMed]
- Topal, I.O.; Otunctemur, A. An investigation of the effects of acitretin on erectile function. Our Dermatol. Online 2020, 11 (Suppl. S3), 1–5. [Google Scholar] [CrossRef]
- Scarisbrick, J.J.; Kim, Y.H.; Whittaker, S.J.; Wood, G.S.; Vermeer, M.H.; Prince, H.M.; Quaglino, P. Prognostic factors, prognostic indices and staging in mycosis fungoides and Sézary syndrome: Where are we now? Br. J. Dermatol. 2014, 170, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.A.; Whittaker, S.J.; Morris, S.L.; Russell-Jones, R.; Hung, T.; Bashir, S.J.; Scarisbrick, J.J. Bexarotene therapy for mycosis fungoides and Sézary syndrome. Br. J. Dermatol. 2009, 160, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Cisoń, H.; Wożniak, Z.; Białynicki-Birula, R. Usefulness of ultrasonography in the assessment of skin lesions of cutaneous t-cell lymphoma. Our Dermatol. Online 2024, 15, 150–153. [Google Scholar]
- Su, M.; Alonso, S.; Jones, J.W.; Yu, J.; Kane, M.A.; Jones, R.J.; Ghiaur, G. All-Trans Retinoic Acid Activity in Acute Myeloid Leukemia: Role of Cytochrome P450 Enzyme Expression by the Microenvironment. PLoS ONE 2015, 10, e0127790. [Google Scholar] [CrossRef]
- Estey, E.; Dohner, H. Acute myeloid leukaemia. Lancet 2006, 368, 1894–1907. [Google Scholar]
- Dos Santos, G.A.; Kats, L.; Pandolfi, P.P. Synergy against PML-RARa: Targeting transcription, proteolysis, differentiation, and self-renewal in acute promyelocytic leukemia. J. Exp. Med. 2013, 210, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Theodosiou, M.; Laudet, V.; Schubert, M. From carrot to clinic: An overview of the retinoic acid signaling pathway. Cell. Mol. Life Sci. 2010, 67, 1423–1445. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.J.; Bain, G.; Yao, M.; Gottlieb, D.I. CYP26, a novel mammalian cytochrome P450, is induced by retinoic acid and defines a new family. J. Biol. Chem. 1997, 272, 18702–18708. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Knight, D.; Smith, C.; Wilhelm, D.; Richman, J.; Mamiya, S.; Yashiro, K.; Chawengsaksophak, K.; Wilson, M.J.; Rossant, J.; et al. Retinoid signaling determines germ cell fate in mice. Science 2006, 312, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Kantarjian, H.; O’Brien, S.; Beran, M.; Estey, E.; Keating, M.; Talpaz, M. A pilot study of all-trans retinoic acid in patients with Philadelphia chromosome-positive chronic myelogenous leukemia. Leukemia 1997, 11, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Hoting, E.; Paul, E.; Plewig, G. Treatment of rosacea with isotretinoin. Int. J. Dermatol. 1986, 25, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Baden, H.P.; Buxman, M.M.; Weinstein, G.D.; Yoder, F.W. Treatment of ichthyosis with isotretinoin. J. Am. Acad. Dermatol. 1982, 6, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, B.; Hapa, A.; Mutlu, E. Isotretinoin treatment for folliculitis decalvans: A retrospective case–series study. Int. J. Dermatol. 2018, 57, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Badaoui, A.; Reygagne, P.; Cavelier-Balloy, B.; Pinquier, L.; Deschamps, L.; Crickx, B.; Descamps, V. Dissecting cellulitis of the scalp: A retrospective study of 51 patients and review of literature. Br. J. Dermatol. 2016, 174, 421–423. [Google Scholar] [CrossRef]
- Vena, G.A.; Coviello, C.; Angelini, G. Use of oral isotretinoin in the treatment of cutaneous lupus erythematosus. G. Ital. Dermatol. Venereol. 1989, 124, 311–315. [Google Scholar]
- Muthu, S.K.; Narang, T.; Saikia, U.N.; Kanwar, A.J.; Parsad, D.; Dogra, S. Low–dose oral isotretinoin therapy in lichen planus pigmentosus: An open–label non–randomized prospective pilot study. Int. J. Dermatol. 2016, 55, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.C.; Hogan, D.J. Improvement of Chronic Generalized Granuloma Annulare with Isotretinoin. Arch. Dermatol. 2002, 138, 1518–1519. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Batsakis, J.G.; Toth, B.B.; Weber, R.S.; Lee, J.J.; Martin, J.W.; Hays, G.L.; Goepfert, H.; Hong, W.K. Comparison of low–dose isotretinoin with beta carotene to prevent oral carcinogenesis. N. Engl. J. Med. 1993, 328, 15–20. [Google Scholar] [CrossRef]
- Sherman, C.; Michelle, L.; Ekelem, C.; Sung, C.T.; Rojek, N.; Mesinkovska, N.A. Oral isotretinoin for the treatment of dermatologic conditions other than acne: A systematic review and discussion of future directions. Arch. Dermatol. Res. 2021, 313, 391–430. [Google Scholar] [CrossRef]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.-G.; Nemer, G.; Kurban, M. Retinoids: A journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J. Dermatol. Treat. 2017, 28, 684–696. [Google Scholar] [CrossRef] [PubMed]
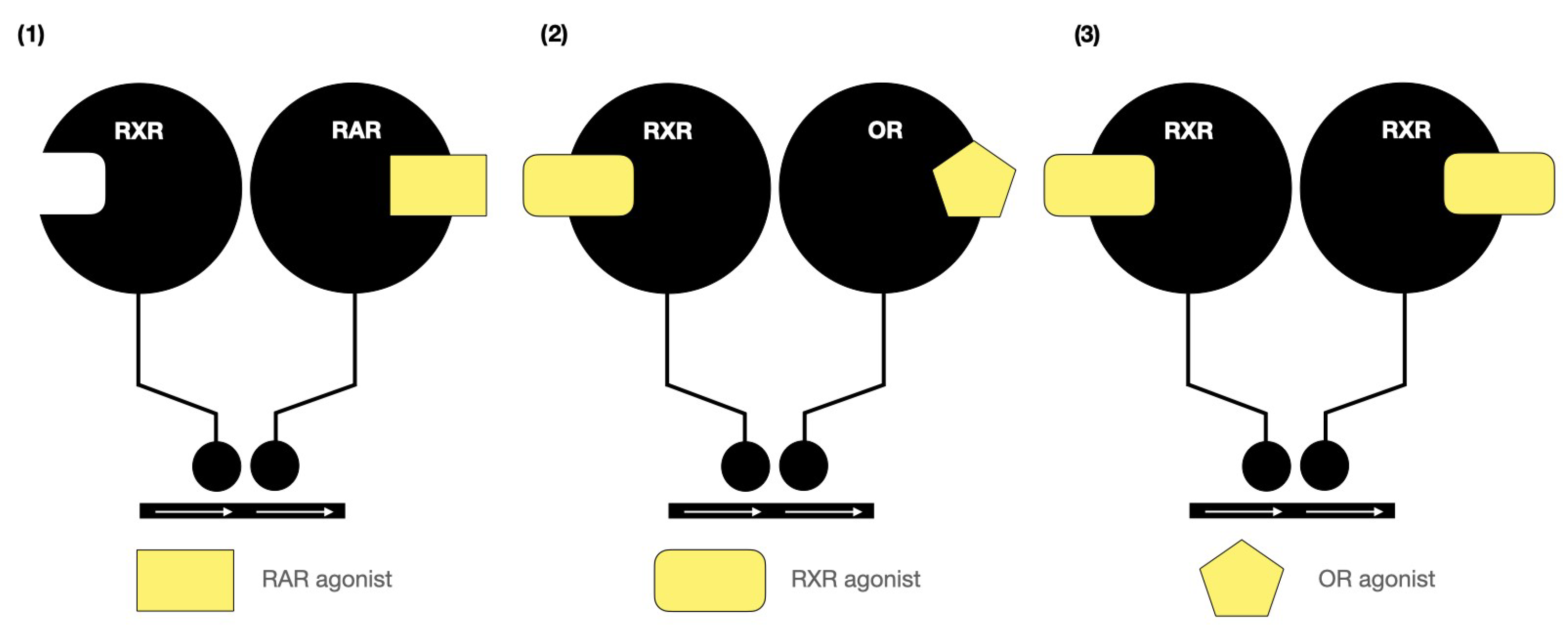
| Helices | RARα | RARβ | RARγ |
|---|---|---|---|
| H3 | Ser232 | Arg225 | Arg234 |
| H5 | Ile270 | Ile263 | Met272 |
| H11 | Val395 | Val388 | Ala397 |
| Disease Unit | Medication | Dosage | Results | Ref. |
|---|---|---|---|---|
| Acne Vulgaris | Isotretinoin | 0.5–1.0 mg/kg/day; mean of 38.4 mg/kg cumulative dose | n = 305; remission: 87.64% (267), no effect: 12.46% (38) | [59] |
| Rosacea | Isotretinoin | 0.5–1.0 mg/kg/day; mean of 33.3 mg/kg cumulative dose | n= 70 patients; full effect 34% (24), partial effect 57% (40) | [86] |
| Hidradenitis Suppurativa | Isotretinoin | 0.45 ± 0.20 mg/kg/day (range: 0.14–0.95) | n = 25; 36% (9/25) complete responses; 32% (8/26) partial responses; 32% (8/25) no responses | [63] |
| Ichthyosis | Isotretinoin | 1.83–2.05 mg/kg/day | n = 18; visible improvement: 60% (11/18) | [87] |
| SCC | IFNα–2a + Isotretinoin | mean of 1.0 mg/kg/day | n = 32; overall response rate: 68%, complete response rate: 25% | [70] |
| Keratoacanthoma | Isotretinoin | mean of 1.0 mg/kg/day | n = 1 CASE REPORT | [71] |
| Psorasis | narrow band ultraviolet B (NBUVB) + Isotretinoin | 0.5 mg/kg/day | n = 17; 82% (n = 14) complete clearing of psorasis plaques | [73] |
| Mycosis Fungoides and Sézary syndrome | Bexarotene | 150–300 mg/day | n = 66; 9% (n = 6) complete response, 35% (n = 23) partial response, 23% (n = 15) stabilized disease | [77] |
| Folliculitis Decalvans | Isotretinoin | 0.1–1.02 mg/kg/day | n = 39; 82% full remission, 66% never relapsed | [88] |
| Dissecting Cellulitis | Isotretinoin | 0.5–0.8 mg/kg/day | n = 51; 92% temporary remissions | [89] |
| Cutaneous Lupus Erythematosus | Isotretinoin | 0.15–0.5 mg/kg/day | n = 24; 86.9% major clinical improvement or full clearing of lesions | [90] |
| Lichen Planus | Isotretinoin | 20 mg/day | n = 27; 21.8% (n = 7) good response, 55.7% (n = 15), moderate improvement | [91] |
| Granuloma Annulare | Isotretinoin | 40–80 mg/day (for 1 year) | n = 1 CASE REPORT | [92] |
| Leucoplakia | Isotretinoin | 0.5 mg/kg/day | n = 53; 92% (n = 22) clinical response or stabilization of lesions | [93] |
| Darier’s Disease | Isotretinoin | 0.5–4 mg/kg/day | n = 119 (metanalysis); 75–100% lesionclearance at 1st week, 80–100%lesion relapse within 7 days to 6 months post-treatment | [94] |
| Topical | Adverse Effects |
|---|---|
| Skin | irritation, dryness, peeling, erythema and pruritus |
| System | Adverse Effects |
| Mucocutaneous | cheilitis, dryness of the oral mucosa, epistaxis, xerophthalmia, xerosis, fingertip fissuring, hair loss, nail fragility, periungual granuloma, paronychia |
| Musculoskeletal | myalgias, arthralgias, bony pain, premature fusion of the epiphyses, skeletal hyperostosis, calcification of tendons and ligaments |
| Neurologic | headaches, pseudotumor cerebri |
| Ophthalmologic | nyctalopia |
| Gastrointestinal/Metabolic | nausea, abdominal pain, diarrhea, elevation in liver function tests, elevation in serum triglycerides and cholesterol |
| Teratogenicity | abnormalities of the central nervous system, face, heart, and thymus |
| Psychiatric | depression, irritability/aggression, suicidality, sleep disturbances, mania, psychosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawczak, P.; Feszak, I.; Brzeziński, P.; Bączek, T. Structure–Activity Relationships and Therapeutic Applications of Retinoids in View of Potential Benefits from Drug Repurposing Process. Biomedicines 2024, 12, 1059. https://doi.org/10.3390/biomedicines12051059
Kawczak P, Feszak I, Brzeziński P, Bączek T. Structure–Activity Relationships and Therapeutic Applications of Retinoids in View of Potential Benefits from Drug Repurposing Process. Biomedicines. 2024; 12(5):1059. https://doi.org/10.3390/biomedicines12051059
Chicago/Turabian StyleKawczak, Piotr, Igor Feszak, Piotr Brzeziński, and Tomasz Bączek. 2024. "Structure–Activity Relationships and Therapeutic Applications of Retinoids in View of Potential Benefits from Drug Repurposing Process" Biomedicines 12, no. 5: 1059. https://doi.org/10.3390/biomedicines12051059
APA StyleKawczak, P., Feszak, I., Brzeziński, P., & Bączek, T. (2024). Structure–Activity Relationships and Therapeutic Applications of Retinoids in View of Potential Benefits from Drug Repurposing Process. Biomedicines, 12(5), 1059. https://doi.org/10.3390/biomedicines12051059






