Potential Immunohistochemical Biomarkers for Grading Oral Dysplasia: A Literature Review
Abstract
1. Introduction
2. Discussion of Potential Immunohistochemical Biomarkers in Grading of Oral Epithelial Dysplasia
2.1. Biomarkers Related to Cell Division and Proliferation
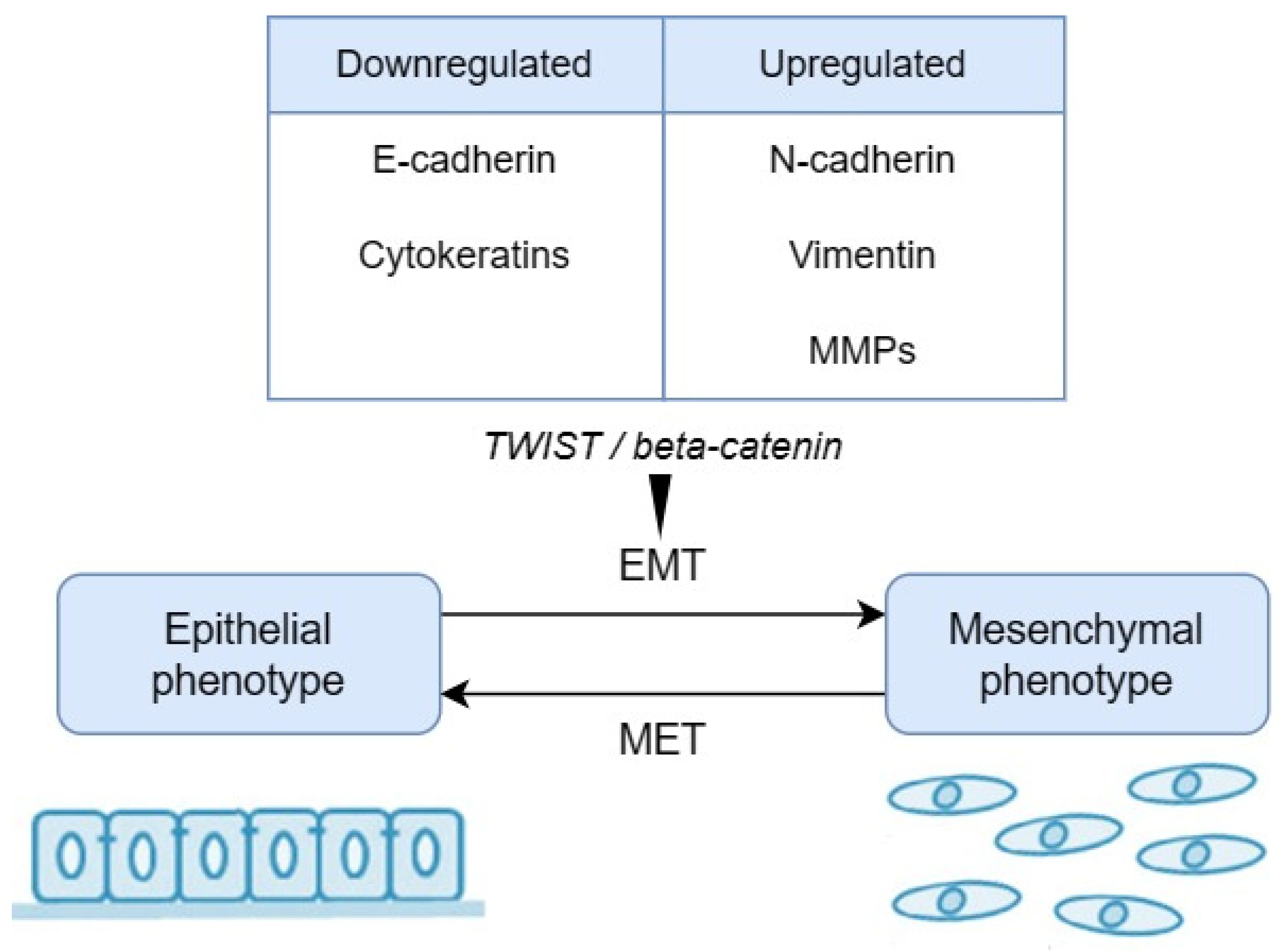
2.2. Biomarkers Related to Epithelial–Mesenchymal Transition (EMT)
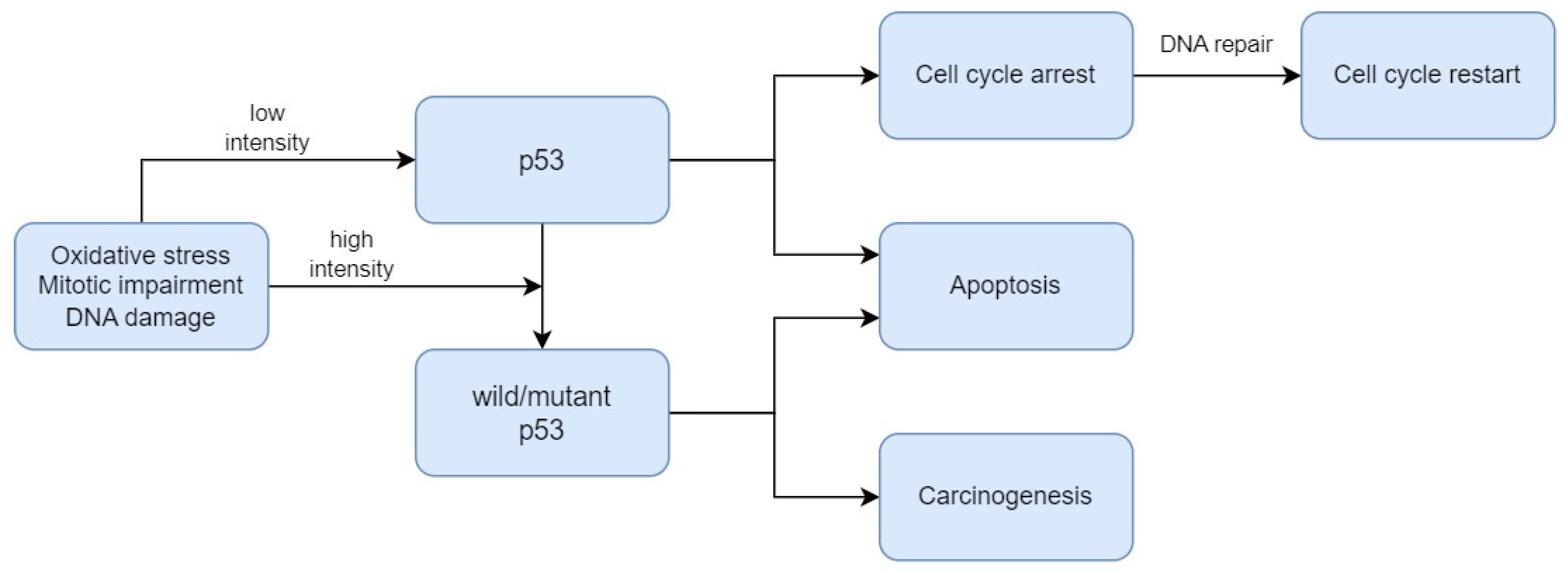
2.3. Biomarkers Related to Cell Death Regulation
2.4. Biomarkers Related to Cellular Metabolism
2.5. Biomarkers Related to Extracellular Signalling Pathways
2.6. Limitations and Challenges
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, N.; Gupta, R.; Acharya, A.K.; Patthi, B.; Goud, V.; Reddy, S.; Garg, A.; Singla, A. Changing Trends in Oral Cancer—A Global Scenario. Nepal J. Epidemiol. 2016, 6, 613–619. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Nijakowski, K.; Gruszczyński, D.; Kopała, D.; Surdacka, A. Salivary Metabolomics for Oral Squamous Cell Carcinoma Diagnosis: A Systematic Review. Metabolites 2022, 12, 294. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2010.
- Yang, Y.; Zhou, M.; Zeng, X.; Wang, C. The Burden of Oral Cancer in China, 1990–2017: An Analysis for the Global Burden of Disease, Injuries, and Risk Factors Study 2017. BMC Oral Health 2021, 21, 44. [Google Scholar] [CrossRef]
- Edirisinghe, S.T.; Weerasekera, M.; De Silva, D.K.; Liyanage, I.; Niluka, M.; Madushika, K.; Deegodagamage, S.; Wijesundara, C.; Rich, A.M.; De Silva, H.; et al. The Risk of Oral Cancer among Different Categories of Exposure to Tobacco Smoking in Sri Lanka. Asian Pac. J. Cancer Prev. APJCP 2022, 23, 2929–2935. [Google Scholar] [CrossRef]
- Bouaoud, J.; Bossi, P.; Elkabets, M.; Schmitz, S.; van Kempen, L.C.; Martinez, P.; Jagadeeshan, S.; Breuskin, I.; Puppels, G.J.; Hoffmann, C.; et al. Unmet Needs and Perspectives in Oral Cancer Prevention. Cancers 2022, 14, 1815. [Google Scholar] [CrossRef]
- Gupta, S.; Jawanda, M.K.; Madhushankari, G. Current Challenges and the Diagnostic Pitfalls in the Grading of Epithelial Dysplasia in Oral Potentially Malignant Disorders: A Review. J. Oral Biol. Craniofacial Res. 2020, 10, 788–799. [Google Scholar] [CrossRef]
- Rich, A.M.; Hussaini, H.M.; Nizar, M.A.M.; Gavidi, R.O.; Tauati-Williams, E.; Yakin, M.; Seo, B. Diagnosis of Oral Potentially Malignant Disorders: Overview and Experience in Oceania. Front. Oral Health 2023, 4, 1122497. [Google Scholar] [CrossRef]
- Muller, S.; Tilakaratne, W.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumours of the Oral Cavity and Mobile Tongue. Head Neck Pathol. 2022, 16, 54–62. [Google Scholar] [CrossRef]
- Bernard, C.; Zhang, J.Z.; Klieb, H.; Blanas, N.; Xu, W.; Magalhaes, M. Clinical Outcomes of Oral Epithelial Dysplasia Managed by Observation versus Excision. Head Neck 2023, 45, 3096–3106. [Google Scholar] [CrossRef]
- Mukherjee, A.; Ferrao, T.; Spadigam, A.E.; Dhupar, A.; Mukherjee, A.; Ferrao, T.; Spadigam, A.E.; Dhupar, A. Oral Epithelial Dysplasia in Tobacco Non-Habitués: A Case Report and Review of Literature. Cureus 2023, 15, 47362. [Google Scholar] [CrossRef]
- Küffer, R.; Lombardi, T. Premalignant Lesions of the Oral Mucosa. A Discussion about the Place of Oral Intraepithelial Neoplasia (OIN). Oral Oncol. 2002, 38, 125–130. [Google Scholar] [CrossRef]
- Ranganathan, K.; Kavitha, L. Oral Epithelial Dysplasia: Classifications and Clinical Relevance in Risk Assessment of Oral Potentially Malignant Disorders. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 19–27. [Google Scholar] [CrossRef]
- Izumo, T. Oral Premalignant Lesions: From the Pathological Viewpoint. Int. J. Clin. Oncol. 2011, 16, 15–26. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Reibel, J.; Bouquot, J.; Dabelsteen, E. Oral Epithelial Dysplasia Classification Systems: Predictive Value, Utility, Weaknesses and Scope for Improvement. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2008, 37, 127–133. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and Classification of Potentially Malignant Disorders of the Oral Mucosa. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Napier, S.S.; Speight, P.M. Natural History of Potentially Malignant Oral Lesions and Conditions: An Overview of the Literature. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2008, 37, 1–10. [Google Scholar] [CrossRef]
- Hankinson, P.; Mahmood, H.; Walsh, H.; Speight, P.M.; Khurram, S.A. Demystifying Oral Epithelial Dysplasia: A Histological Guide. Pathology 2023, 56, 11–23. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Head and Neck Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2022.
- Kujan, O.; Khattab, A.; Oliver, R.J.; Roberts, S.A.; Thakker, N.; Sloan, P. Why Oral Histopathology Suffers Inter-Observer Variability on Grading Oral Epithelial Dysplasia: An Attempt to Understand the Sources of Variation. Oral Oncol. 2007, 43, 224–231. [Google Scholar] [CrossRef]
- Krishnan, L.; Karpagaselvi, K.; Kumarswamy, J.; Sudheendra, U.S.; Santosh, K.V.; Patil, A. Inter- and Intra-Observer Variability in Three Grading Systems for Oral Epithelial Dysplasia. J. Oral Maxillofac. Pathol. JOMFP 2016, 20, 261–268. [Google Scholar] [CrossRef]
- Odell, E.; Kujan, O.; Warnakulasuriya, S.; Sloan, P. Oral Epithelial Dysplasia: Recognition, Grading and Clinical Significance. Oral Dis. 2021, 27, 1947–1976. [Google Scholar] [CrossRef]
- Mahmood, H.; Bradburn, M.; Rajpoot, N.; Islam, N.M.; Kujan, O.; Khurram, S.A. Prediction of Malignant Transformation and Recurrence of Oral Epithelial Dysplasia Using Architectural and Cytological Feature Specific Prognostic Models. Mod. Pathol. 2022, 35, 1151–1159. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What Are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Wu, Y. Recent Advances in Nanotechnology-Enabled Biosensors for Detection of Exosomes as New Cancer Liquid Biopsy. Exp. Biol. Med. 2022, 247, 2152–2172. [Google Scholar] [CrossRef]
- Kourou, K.; Exarchos, T.P.; Exarchos, K.P.; Karamouzis, M.V.; Fotiadis, D.I. Machine Learning Applications in Cancer Prognosis and Prediction. Comput. Struct. Biotechnol. J. 2015, 13, 8–17. [Google Scholar] [CrossRef]
- Kamel, H.F.M.; Al-Amodi, H.S.A.B. Exploitation of Gene Expression and Cancer Biomarkers in Paving the Path to Era of Personalized Medicine. Genom. Proteom. Bioinform. 2017, 15, 220–235. [Google Scholar] [CrossRef]
- de Vicente, J.C.; Rodríguez-Santamarta, T.; Rodrigo, J.P.; Allonca, E.; Vallina, A.; Singhania, A.; Donate-Pérez Del Molino, P.; García-Pedrero, J.M. The Emerging Role of NANOG as an Early Cancer Risk Biomarker in Patients with Oral Potentially Malignant Disorders. J. Clin. Med. 2019, 8, 1376. [Google Scholar] [CrossRef]
- Grubelnik, G.; Boštjančič, E.; Aničin, A.; Dovšak, T.; Zidar, N. MicroRNAs and Long Non-Coding RNAs as Regulators of NANOG Expression in the Development of Oral Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 579053. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, W.-C.; Su, C.-W.; Hsu, C.-W.; Yuan, S.-S.; Chen, Y.-K. Expression of Orai1 and STIM1 in Human Oral Squamous Cell Carcinogenesis. J. Dent. Sci. 2022, 17, 78–88. [Google Scholar] [CrossRef]
- Lunz, V.; Romanin, C.; Frischauf, I. STIM1 Activation of Orai1. Cell Calcium 2019, 77, 29–38. [Google Scholar] [CrossRef]
- Wolgemuth, D.J. Function of Cyclins in Regulating the Mitotic and Meiotic Cell Cycles in Male Germ Cells. Cell Cycle Georget. 2008, 7, 3509–3513. [Google Scholar] [CrossRef]
- Wang, Z. Cell Cycle Progression and Synchronization: An Overview. Methods Mol. Biol. 2022, 2579, 3–23. [Google Scholar] [CrossRef]
- Loyer, P.; Trembley, J.H. Roles of CDK/Cyclin Complexes in Transcription and Pre-mRNA Splicing: Cyclins L and CDK11 at the Cross-Roads of Cell Cycle and Regulation of Gene Expression. Semin. Cell Dev. Biol. 2020, 107, 36–45. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Jang, H.; Nussinov, R. Cell Cycle Progression Mechanisms: Slower Cyclin-D/CDK4 Activation and Faster Cyclin-E/CDK2. BioRxiv Prepr. Serv. Biol. 2023. [Google Scholar] [CrossRef]
- Steurer, S.; Riemann, C.; Büscheck, F.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Höflmayer, D.; Weidemann, S.; Fraune, C.; et al. P63 Expression in Human Tumors and Normal Tissues: A Tissue Microarray Study on 10,200 Tumors. Biomark. Res. 2021, 9, 7. [Google Scholar] [CrossRef]
- Bavle, R.M.; Paremala, K.; Venugopal, R.; Rudramuni, A.S.; Khan, N.; Hosthor, S.S. Grading of Oral Leukoplakia: Can It Be Improvised Using Immunohistochemical Markers P63 and CD31. Contemp. Clin. Dent. 2021, 12, 37–43. [Google Scholar] [CrossRef]
- Truong, A.B.; Kretz, M.; Ridky, T.W.; Kimmel, R.; Khavari, P.A. P63 Regulates Proliferation and Differentiation of Developmentally Mature Keratinocytes. Genes Dev. 2006, 20, 3185–3197. [Google Scholar] [CrossRef]
- Bergholz, J.; Xiao, Z.-X. Role of P63 in Development, Tumorigenesis and Cancer Progression. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2012, 5, 311–322. [Google Scholar] [CrossRef]
- Patel, S.B.; Manjunatha, B.S.; Shah, V.; Soni, N.; Sutariya, R. Immunohistochemical Evaluation of P63 and Cyclin D1 in Oral Squamous Cell Carcinoma and Leukoplakia. J. Korean Assoc. Oral Maxillofac. Surg. 2017, 43, 324–330. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, V.; Tyagi, N.; Vij, R.; Vij, H.; Sharma, E. Analysis of Role of Angiogenesis in Epithelial Dysplasia: An Immunohistochemical Study. J. Clin. Diagn. Res. 2017, 11, EC29–EC34. [Google Scholar] [CrossRef]
- Tawara, M.; Suzuki, H.; Goto, N.; Tanaka, T.; Kaneko, M.K.; Kato, Y. A Novel Anti-CD44 Variant 9 Monoclonal Antibody C44Mab-1 Was Developed for Immunohistochemical Analyses against Colorectal Cancers. Curr. Issues Mol. Biol. 2023, 45, 3658–3673. [Google Scholar] [CrossRef]
- Venkat Naga, S.K.S.; Shekar, P.C.; Kattappagari, K.K.; Prakash Chandra, K.L.; Reddy, G.S.; Ramana Reddy, B.V. Expression of Cluster Differentiation-44 Stem Cell Marker in Grades of Oral Epithelial Dysplasia: A Preliminary Study. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 203–207. [Google Scholar] [CrossRef]
- Aravind, T.; Janardhanan, M.; Rakesh, S.; Savithri, V.; Unnikrishnan, U.G. Immunolocalization of Osteopontin in Dysplasias and Squamous Cell Carcinomas Arising from Oral Epithelium. J. Oral Maxillofac. Pathol. JOMFP 2017, 21, 18–23. [Google Scholar] [CrossRef]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2000, 11, 279–303. [Google Scholar] [CrossRef]
- Mrochem, J.; Bartnik, W. Osteopontin—A New Marker in Neoplastic Diseases. Contemp. Oncol. Onkol. 2008, 12, 349–353. [Google Scholar]
- Ozaki, T.; Nakagawara, A. Role of P53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef]
- Williams, A.B.; Schumacher, B. P53 in the DNA-Damage-Repair Process. Cold Spring Harb. Perspect. Med. 2016, 6, a026070. [Google Scholar] [CrossRef]
- Babamohamadi, M.; Babaei, E.; Ahmed Salih, B.; Babamohammadi, M.; Jalal Azeez, H.; Othman, G. Recent Findings on the Role of Wild-Type and Mutant P53 in Cancer Development and Therapy. Front. Mol. Biosci. 2022, 9, 903075. [Google Scholar] [CrossRef]
- Borrero, L.J.H.; El-Deiry, W.S. Tumor Suppressor P53: Biology, Signaling Pathways, and Therapeutic Targeting. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef]
- Pandya, J.A.; Boaz, K.; Natarajan, S.; Manaktala, N.; Nandita, K.P.; Lewis, A.J. A Correlation of Immunohistochemical Expression of TP53 and CDKN1A in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. J. Cancer Res. Ther. 2018, 14, 666–670. [Google Scholar] [CrossRef]
- Patil, S.; Gawande, M.; Chaudhari, M.; Sharma, P.; Hande, A.; Sonone, A. Prognostic Significance of P53 Expression in Various Grades of Epithelial Dysplasia. J. Datta Meghe Inst. Med. Sci. Univ. 2022, 17, 306–310. [Google Scholar] [CrossRef]
- Sawada, K.; Momose, S.; Kawano, R.; Kohda, M.; Irié, T.; Mishima, K.; Kaneko, T.; Horie, N.; Okazaki, Y.; Higashi, M.; et al. Immunohistochemical Staining Patterns of P53 Predict the Mutational Status of TP53 in Oral Epithelial Dysplasia. Mod. Pathol. Off. J. US Can. Acad. Pathol. Inc. 2022, 35, 177–185. [Google Scholar] [CrossRef]
- Imaizumi, T.; Matsuda, K.; Tanaka, K.; Kondo, H.; Ueki, N.; Kurohama, H.; Otsubo, C.; Matsuoka, Y.; Akazawa, Y.; Miura, S.; et al. Detection of Endogenous DNA Double-Strand Breaks in Oral Squamous Epithelial Lesions by P53-Binding Protein 1. Anticancer Res. 2021, 41, 4771–4779. [Google Scholar] [CrossRef]
- Napoli, M.; Wu, S.J.; Gore, B.L.; Abbas, H.A.; Lee, K.; Checker, R.; Dhar, S.; Rajapakshe, K.; Tan, A.C.; Lee, M.G.; et al. ΔNp63 Regulates a Common Landscape of Enhancer Associated Genes in Non-Small Cell Lung Cancer. Nat. Commun. 2022, 13, 614. [Google Scholar] [CrossRef]
- Abylkassov, R.; Xie, Y. Role of Yes-Associated Protein in Cancer: An Update. Oncol. Lett. 2016, 12, 2277–2282. [Google Scholar] [CrossRef]
- Ono, S.; Nakano, K.; Takabatake, K.; Kawai, H.; Nagatsuka, H. Immunohistochemistry of YAP and dNp63 and Survival Analysis of Patients Bearing Precancerous Lesion and Oral Squamous Cell Carcinoma. Int. J. Med. Sci. 2019, 16, 766–773. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a Proliferation Marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Booth, D.G.; Takagi, M.; Sanchez-Pulido, L.; Petfalski, E.; Vargiu, G.; Samejima, K.; Imamoto, N.; Ponting, C.P.; Tollervey, D.; Earnshaw, W.C.; et al. Ki-67 Is a PP1-Interacting Protein That Organises the Mitotic Chromosome Periphery. eLife 2014, 3, e01641. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, C.; Li, F.; Nie, G.; Zhang, H. The Role of Contactin 1 in Cancers: What We Know So Far. Front. Oncol. 2020, 10, 574208. [Google Scholar] [CrossRef]
- Iqbal, A.; Tamgadge, S.; Tamgadge, A.; Pereira, T.; Kumar, S.; Acharya, S.; Jadhav, A. Evaluation of Ki-67 Expression in Oral Submucous Fibrosis and Its Correlation with Clinical and Histopathological Features. J. Microsc. Ultrastruct. 2019, 8, 20–24. [Google Scholar] [CrossRef]
- Kim, C.-H.; Lee, H.S.; Park, J.-H.; Choi, J.-H.; Jang, S.-H.; Park, Y.-B.; Lee, M.G.; Hyun, I.G.; Kim, K.I.; Kim, H.S.; et al. Prognostic Role of P53 and Ki-67 Immunohistochemical Expression in Patients with Surgically Resected Lung Adenocarcinoma: A Retrospective Study. J. Thorac. Dis. 2015, 7, 822–833. [Google Scholar] [CrossRef]
- Lalkota, B.P.; Srinivasa, B.J.; Swamy, M.V.; Hazarika, D.; Jeet, B.M.; Jyothi, K.; Ghosh, M.; Sayeed, S.M.; Nasiruddin, M.; Naik, R. The Role of P53 and Ki67 in Predicting Clinical Outcome in Breast Cancer Patients. J. Cancer Res. Ther. 2023, 19, 208. [Google Scholar] [CrossRef]
- Humayun, S.; Prasad, V.R. Expression of P53 Protein and Ki-67 Antigen in Oral Premalignant Lesions and Oral Squamous Cell Carcinomas: An Immunohistochemical Study. Natl. J. Maxillofac. Surg. 2011, 2, 38–46. [Google Scholar] [CrossRef]
- Kumar, P.; Kane, S.; Rathod, G.P. Coexpression of P53 and Ki 67 and Lack of C-erbB2 Expression in Oral Leukoplakias in India. Braz. Oral Res. 2012, 26, 228–234. [Google Scholar] [CrossRef]
- Kamala, K.A.; Kanetkar, S.R.; Datkhile, K.D.; Sankethguddad, S. Expression of Ki67 Biomarker in Oral Submucous Fibrosis with Clinico-Pathological Correlations: A Prospective Study. Asian Pac. J. Cancer Prev. APJCP 2022, 23, 253–259. [Google Scholar] [CrossRef]
- Dash, K.C.; Mahapatra, N.; Bhuyan, L.; Panda, A.; Behura, S.S.; Mishra, P. An Immunohistochemical Study Showing Ki-67 as an Analytical Marker in Oral Malignant and Premalignant Lesions. J. Pharm. Bioallied Sci. 2020, 12, S274–S278. [Google Scholar] [CrossRef]
- Mondal, K.; Mandal, R.; Sarkar, B.C. Importance of Ki-67 Labeling in Oral Leukoplakia with Features of Dysplasia and Carcinomatous Transformation: An Observational Study over 4 Years. S. A. J. Cancer 2020, 9, 99–104. [Google Scholar] [CrossRef]
- Takkem, A.; Barakat, C.; Zakaraia, S.; Zaid, K.; Najmeh, J.; Ayoub, M.; Seirawan, M.Y. Ki-67 Prognostic Value in Different Histological Grades of Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 3279–3286. [Google Scholar] [CrossRef]
- Swain, S.; Nishat, R.; Ramachandran, S.; Raghuvanshi, M.; Behura, S.S.; Kumar, H. Comparative Evaluation of Immunohistochemical Expression of MCM2 and Ki67 in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. J. Cancer Res. Ther. 2022, 18, 997–1002. [Google Scholar] [CrossRef]
- Gadbail, A.R.; Chaudhary, M.; Sarode, S.C.; Gondivkar, S.; Tekade, S.A.; Zade, P.; Hande, A.; Sarode, G.S.; Patil, S. Ki67, CD105, and α-SMA Expression Supports the Transformation Relevant Dysplastic Features in the Atrophic Epithelium of Oral Submucous Fibrosis. PLoS ONE 2018, 13, e0200171. [Google Scholar] [CrossRef]
- Gadbail, A.R.; Chaudhary, M.S.; Sarode, S.C.; Gawande, M.; Korde, S.; Tekade, S.A.; Gondivkar, S.; Hande, A.; Maladhari, R. Ki67, CD105, and α-SMA Expressions Better Relate the Binary Oral Epithelial Dysplasia Grading System of World Health Organization. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2017, 46, 921–927. [Google Scholar] [CrossRef]
- Suwasini, S.; Chatterjee, K.; Purkait, S.K.; Samaddar, D.; Chatterjee, A.; Kumar, M. Expression of P53 Protein and Ki-67 Antigen in Oral Leukoplakia with Different Histopathological Grades of Epithelial Dysplasia. J. Int. Soc. Prev. Community Dent. 2018, 8, 513–522. [Google Scholar] [CrossRef]
- Leung, E.Y.; McMahon, J.D.; McLellan, D.R.; Syyed, N.; McCarthy, C.E.; Nixon, C.; Orange, C.; Brock, C.; Hunter, K.D.; Adams, P.D. DNA Damage Marker Phosphorylated Histone H2AX Is a Potential Predictive Marker for Progression of Epithelial Dysplasia of the Oral Cavity. Histopathology 2017, 71, 522–528. [Google Scholar] [CrossRef]
- Monteiro, L.; Silva, P.; Delgado, L.; Amaral, B.; Garcês, F.; Salazar, F.; Pacheco, J.-J.; Lopes, C.; Bousbaa, H.; Warnakulasuriya, S. Expression of Spindle Assembly Checkpoint Proteins BubR1 and Mad2 Expression as Potential Biomarkers of Malignant Transformation of Oral Leukoplakia: An Observational Cohort Study. Med. Oral Patol. Oral Cirugia Bucal 2021, 26, e719–e728. [Google Scholar] [CrossRef]
- Rubin, C.I.; Atweh, G.F. The Role of Stathmin in the Regulation of the Cell Cycle. J. Cell. Biochem. 2004, 93, 242–250. [Google Scholar] [CrossRef]
- Feng, S.; Song, Y.; Shen, M.; Xie, S.; Li, W.; Lu, Y.; Yang, Y.; Ou, G.; Zhou, J.; Wang, F.; et al. Microtubule-Binding Protein FOR20 Promotes Microtubule Depolymerization and Cell Migration. Cell Discov. 2017, 3, 17032. [Google Scholar] [CrossRef]
- Vadla, P.; Deepthi, G.; Kumar, C.A.; Bashamalla, R.; Syeda, N.; Naramala, S. Immunohistochemical Expression of Stathmin in Oral Dysplasia: An Original Study with an Insight of Its Action on Microtubules. J. Oral Maxillofac. Pathol. JOMFP 2021, 25, 247–252. [Google Scholar] [CrossRef]
- Blanpain, C.; Horsley, V.; Fuchs, E. Epithelial Stem Cells: Turning over New Leaves. Cell 2007, 128, 445–458. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial-Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Chen, T.; You, Y.; Jiang, H.; Wang, Z.Z. Epithelial-Mesenchymal Transition (EMT): A Biological Process in the Development, Stem Cell Differentiation, and Tumorigenesis. J. Cell. Physiol. 2017, 232, 3261–3272. [Google Scholar] [CrossRef]
- Toriumi, K.; Berto, S.; Koike, S.; Usui, N.; Dan, T.; Suzuki, K.; Miyashita, M.; Horiuchi, Y.; Yoshikawa, A.; Asakura, M.; et al. Combined Glyoxalase 1 Dysfunction and Vitamin B6 Deficiency in a Schizophrenia Model System Causes Mitochondrial Dysfunction in the Prefrontal Cortex. Redox Biol. 2021, 45, 102057. [Google Scholar] [CrossRef]
- Krisanaprakornkit, S.; Iamaroon, A. Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma. ISRN Oncol. 2012, 2012, 681469. [Google Scholar] [CrossRef]
- Ling, Z.; Cheng, B.; Tao, X. Epithelial-to-Mesenchymal Transition in Oral Squamous Cell Carcinoma: Challenges and Opportunities. Int. J. Cancer 2021, 148, 1548–1561. [Google Scholar] [CrossRef]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Radisky, E.S.; Radisky, D.C. Matrix Metalloproteinase-Induced Epithelial-Mesenchymal Transition in Breast Cancer. J. Mammary Gland Biol. Neoplasia 2010, 15, 201–212. [Google Scholar] [CrossRef]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.-T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Fedele, M.; Sgarra, R.; Battista, S.; Cerchia, L.; Manfioletti, G. The Epithelial–Mesenchymal Transition at the Crossroads between Metabolism and Tumor Progression. Int. J. Mol. Sci. 2022, 23, 800. [Google Scholar] [CrossRef]
- Herrmann, H.; Bär, H.; Kreplak, L.; Strelkov, S.V.; Aebi, U. Intermediate Filaments: From Cell Architecture to Nanomechanics. Nat. Rev. Mol. Cell Biol. 2007, 8, 562–573. [Google Scholar] [CrossRef]
- Batool, S.; Fahim, A.; Qureshi, A.; Jabeen, H.; Ali, S.N.; Khoso, M.Y. Role Of Alteration Of Ck5\6 Profile In Dysplastic Progression Of Oral Mucosa In Tobacco Users. J. Ayub Med. Coll. Abbottabad JAMC 2020, 32, 527–530. [Google Scholar]
- Lindberg, K.; Rheinwald, J.G. Suprabasal 40 Kd Keratin (K19) Expression as an Immunohistologic Marker of Premalignancy in Oral Epithelium. Am. J. Pathol. 1989, 134, 89–98. [Google Scholar]
- Rajeswari, P.; Janardhanan, M.; Suresh, R.; Savithri, V.; Aravind, T.; Raveendran, G.C. Expression of CK 19 as a Biomarker in Early Detection of Oral Squamous Cell Carcinoma. J. Oral Maxillofac. Pathol. JOMFP 2020, 24, 523–529. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.H.; Zheng, G. E-Cadherin/β-Catenin Complex and the Epithelial Barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Regulation of Cadherin-Mediated Adhesion in Morphogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 622–634. [Google Scholar] [CrossRef]
- Hulpiau, P.; van Roy, F. Molecular Evolution of the Cadherin Superfamily. Int. J. Biochem. Cell Biol. 2009, 41, 349–369. [Google Scholar] [CrossRef]
- Yuksel, H.; Ocalan, M.; Yilmaz, O. E-Cadherin: An Important Functional Molecule at Respiratory Barrier Between Defence and Dysfunction. Front. Physiol. 2021, 12, 720227. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nagamalini, B.R.; Singh, J.; Ashwini, B.K.; Sharada; Swaminathan, U. Expression of β-Catenin in Oral Leukoplakia and Oral Submucous Fibrosis: An Immunohistochemical Study. J. Oral Maxillofac. Pathol. JOMFP 2021, 25, 124–130. [Google Scholar] [CrossRef]
- Prgomet, Z.; Andersson, T.; Lindberg, P. Higher Expression of WNT5A Protein in Oral Squamous Cell Carcinoma Compared with Dysplasia and Oral Mucosa with a Normal Appearance. Eur. J. Oral Sci. 2017, 125, 237–246. [Google Scholar] [CrossRef]
- Sharada, P.; Swaminathan, U.; Nagamalini, B.R.; Kumar, K.V.; Ashwini, B.K.; Lavanya, V. Coalition of E-Cadherin and Vascular Endothelial Growth Factor Expression in Predicting Malignant Transformation in Common Oral Potentially Malignant Disorders. J. Oral Maxillofac. Pathol. JOMFP 2018, 22, 40–47. [Google Scholar] [CrossRef]
- Sharma, J.; Bhargava, M.; Aggarwal, S.; Aggarwal, A.; Varshney, A.; Chopra, D. Immunohistochemical Evaluation of E-Cadherin in Oral Epithelial Dysplasia and Squamous Cell Carcinoma. Indian J. Pathol. Microbiol. 2022, 65, 755–760. [Google Scholar] [CrossRef]
- Puneeta, N.; Santosh, T.; Mishra, I.; Gaikwad, P.; Sahu, A. Evaluation of E-Cadherin and Vimentin Expression for Different Grades of Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma—An Immunohistochemical Study. J. Oral Maxillofac. Pathol. JOMFP 2022, 26, 285–286. [Google Scholar] [CrossRef]
- Miguel, A.F.P.; Embaló, B.; Alves Dias, H.B.; Rivero, E.R.C. Immunohistochemical Expression of MMP-9, TIMP-1, and Vimentin and Its Correlation With Inflammatory Reaction and Clinical Parameters in Oral Epithelial Dysplasia. Appl. Immunohistochem. Mol. Morphol. AIMM 2021, 29, 382–389. [Google Scholar] [CrossRef]
- Chandolia, B.; Rajliwal, J.P.; Bajpai, M.; Arora, M. Prognostic Potential of N-Cadherin in Oral Squamous Cell Carcinoma via Immunohistochemical Methods. J. Coll. Physicians Surg.-Pak. JCPSP 2017, 27, 475–478. [Google Scholar]
- Nelson, W.J.; Nusse, R. Convergence of Wnt, β-Catenin, and Cadherin Pathways. Science 2004, 303, 1483–1487. [Google Scholar] [CrossRef]
- Qahtani, M.S.; El-Deeb, A.M.; Metwaly, H.A.M. Evaluation of Immunohistochemical Expression of TWIST in Oral Epithelial Dysplasia and Squamous Cell Carcinoma. Int. J. Health Sci. 2020, 14, 33–39. [Google Scholar]
- Quintanilla, M.; Montero-Montero, L.; Renart, J.; Martín-Villar, E. Podoplanin in Inflammation and Cancer. Int. J. Mol. Sci. 2019, 20, 707. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Y.; Wei, Z.; Tai, B.; Jiang, H.; Du, M. The Prevalence and Risk Indicators of Tooth Wear in 12- and 15-Year-Old Adolescents in Central China. BMC Oral Health 2015, 15, 120. [Google Scholar] [CrossRef]
- Ward, L.S.C.; Sheriff, L.; Marshall, J.L.; Manning, J.E.; Brill, A.; Nash, G.B.; McGettrick, H.M. Podoplanin Regulates the Migration of Mesenchymal Stromal Cells and Their Interaction with Platelets. J. Cell Sci. 2019, 132, jcs222067. [Google Scholar] [CrossRef]
- Karunagaran, M.; Murali, P.; Palaniappan, V.; Sivapathasundharam, B. Expression and Distribution Pattern of Podoplanin in Oral Submucous Fibrosis with Varying Degrees of Dysplasia—An Immunohistochemical Study. J. Histotechnol. 2019, 42, 80–86. [Google Scholar] [CrossRef]
- Lunawat, S.D.; Prakash, N.; Pradeep, G.L.; Chaware, S.J.; Chaudhari, N.R.; Salunkhe, V.P. Assessment of Podoplanin Lymphatic Vessel Density in Oral Epithelial Dysplasia. J. Oral Maxillofac. Pathol. JOMFP 2021, 25, 548. [Google Scholar] [CrossRef]
- Monteiro, L.; do Amaral, B.; Delgado, L.; Garcês, F.; Salazar, F.; Pacheco, J.J.; Lopes, C.; Warnakulasuriya, S. Podoplanin Expression Independently and Jointly with Oral Epithelial Dysplasia Grade Acts as a Potential Biomarker of Malignant Transformation in Oral Leukoplakia. Biomolecules 2022, 12, 606. [Google Scholar] [CrossRef]
- Abidullah, M.; Nahar, P.; Ahmed, S.A. Expression of MUC4 in Oral Dysplastic Epithelium. Int. J. Pharm. Investig. 2019, 9, 85–88. [Google Scholar] [CrossRef]
- Carraway, K.L.; Theodoropoulos, G.; Kozloski, G.A.; Carothers Carraway, C.A. Muc4/MUC4 Functions and Regulation in Cancer. Future Oncol. 2009, 5, 1631–1640. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef]
- Chao, D.T.; Korsmeyer, S.J. BCL-2 Family: Regulators of Cell Death. Annu. Rev. Immunol. 1998, 16, 395–419. [Google Scholar] [CrossRef]
- Hua, C.; Zorn, S.; Jensen, J.P.; Coupland, R.W.; Ko, H.S.; Wright, J.J.; Bakhshi, A. Consequences of the t(14;18) Chromosomal Translocation in Follicular Lymphoma: Deregulated Expression of a Chimeric and Mutated BCL-2 Gene. Oncogene Res. 1988, 2, 263–275. [Google Scholar]
- Pathak, A.; Shetty, D.C.; Dhanapal, R.; Kaur, G. To Analyse the Mitotic and Keratinisation Correlation with Bcl-2 Expression in Varying Grades of Oral Epithelial Dysplasia and Squamous Cell Carcinoma. J. Oral Maxillofac. Pathol. JOMFP 2022, 26, 316–321. [Google Scholar] [CrossRef]
- Pallavi, N.; Nalabolu, G.R.K.; Hiremath, S.K.S. Bcl-2 and c-Myc Expression in Oral Dysplasia and Oral Squamous Cell Carcinoma: An Immunohistochemical Study to Assess Tumor Progression. J. Oral Maxillofac. Pathol. JOMFP 2018, 22, 325–331. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Kujan, O.; Agag, M.; Smaga, M.; Vaishnaw, Y.; Idrees, M.; Shearston, K.; Farah, C.S. PD-1/PD-L1, Treg-Related Proteins, and Tumour-Infiltrating Lymphocytes Are Associated with the Development of Oral Squamous Cell Carcinoma. Pathology 2022, 54, 409–416. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.-S. The Role of Pdcd4 in Tumor Suppression and Protein Translation. Biol. Cell 2018, 110, 169–177. [Google Scholar] [CrossRef]
- Desai, K.M.; Kale, A.D. Immunoexpression of Programmed Cell Death 4 Protein in Normal Oral Mucosa, Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. J. Oral Maxillofac. Pathol. JOMFP 2017, 21, 462. [Google Scholar] [CrossRef]
- Ferns, G.; Shams, S.; Shafi, S. Heat Shock Protein 27: Its Potential Role in Vascular Disease. Int. J. Exp. Pathol. 2006, 87, 253–274. [Google Scholar] [CrossRef]
- Vidyasagar, A.; Wilson, N.A.; Djamali, A. Heat Shock Protein 27 (HSP27): Biomarker of Disease and Therapeutic Target. Fibrogenesis Tissue Repair 2012, 5, 7. [Google Scholar] [CrossRef]
- Karri, R.L.; Subramanyam, R.V.; Venigella, A.; Babburi, S.; Pinisetti, S.; Rudraraju, A. Differential Expression of Heat Shock Protein 27 in Oral Epithelial Dysplasias and Squamous Cell Carcinoma. J. Microsc. Ultrastruct. 2020, 8, 62–68. [Google Scholar] [CrossRef]
- Yagui-Beltran, A.; Craig, A.L.; Lawrie, L.; Thompson, D.; Pospisilova, S.; Johnston, D.; Kernohan, N.; Hopwood, D.; Dillon, J.F.; Hupp, T.R. The Human Oesophageal Squamous Epithelium Exhibits a Novel Type of Heat Shock Protein Response. Eur. J. Biochem. 2001, 268, 5343–5355. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Dai, Y.; Li, J.; Qin, Y.; Zhu, Y.; Zeng, T.; Ban, X.; Fu, L.; Guan, X.-Y. Characterization of Tumor Suppressive Function of Cornulin in Esophageal Squamous Cell Carcinoma. PLoS ONE 2013, 8, e68838. [Google Scholar] [CrossRef]
- Santosh, N.; McNamara, K.K.; Beck, F.M.; Kalmar, J.R. Expression of Cornulin in Oral Premalignant Lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 526–534. [Google Scholar] [CrossRef]
- Lee, J.-W.; Bae, S.-H.; Jeong, J.-W.; Kim, S.-H.; Kim, K.-W. Hypoxia-Inducible Factor (HIF-1)Alpha: Its Protein Stability and Biological Functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef]
- Patel, N.R.; Jain, L.; Mahajan, A.M.; Hiray, P.V.; Shinde, S.S.; Patel, P.A. An Immunohistochemical Study of HIF-1 Alpha in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Indian J. Otolaryngol. Head Neck Surg. Off. Publ. Assoc. Otolaryngol. India 2019, 71, 435–441. [Google Scholar] [CrossRef]
- Kleinert, H.; Forstermann, U. Inducible Nitric Oxide Synthase. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–12. ISBN 978-0-08-055232-3. [Google Scholar]
- Singh, D.N.; Srivastava, K.C.; Potsangbam, A.D.; Shrivastava, D.; Nandini, D.B.; Singh, W.T.; Singh, K.S. A Case-Control Study Comparing and Correlating iNOS Expression among Various Clinicopathological Variants of Oral Leukoplakia and Oral Squamous Cell Carcinoma: A Immunohistochemistry Study. J. Pharm. Bioallied Sci. 2020, 12, S324–S331. [Google Scholar] [CrossRef]
- Turini, M.E.; DuBois, R.N. Cyclooxygenase-2: A Therapeutic Target. Annu. Rev. Med. 2002, 53, 35–57. [Google Scholar] [CrossRef]
- Sharada, P.; Swaminathan, U.; Nagamalini, B.; Vinod Kumar, K.; Ashwini, B. Histoscore and Discontinuity Score—A Novel Scoring System to Evaluate Immunohistochemical Expression of COX-2 and Type IV Collagen in Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. J. Orofac. Sci. 2021, 13, 96–104. [Google Scholar] [CrossRef]
- Schaller, M.D. Paxillin: A Focal Adhesion-Associated Adaptor Protein. Oncogene 2001, 20, 6459–6472. [Google Scholar] [CrossRef]
- Alam, S.; Astekar, M.S.; Sapra, G.; Agarwal, A.; Agarwal, A.M.; Vishnu Rao, S.G. Immunohistochemical Expression of Paxillin in Potentially Malignant Disorders and Squamous Cell Carcinoma Patients. J. Oral Maxillofac. Pathol. JOMFP 2022, 26, 322–329. [Google Scholar] [CrossRef]
- Soonthornthum, T.; Arias-Pulido, H.; Joste, N.; Lomo, L.; Muller, C.; Rutledge, T.; Verschraegen, C. Epidermal Growth Factor Receptor as a Biomarker for Cervical Cancer. Ann. Oncol. 2011, 22, 2166–2178. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, Y.T.; Kim, D.K.; Song, C.H.; Lee, J.W. Expression of Epidermal Growth Factor Receptor in Carcinoma of the Cervix. Gynecol. Oncol. 1996, 60, 283–287. [Google Scholar] [CrossRef]
- Fakurnejad, S.; van Keulen, S.; Nishio, N.; Engelen, M.; van den Berg, N.S.; Lu, G.; Birkeland, A.; Baik, F.; Colevas, A.D.; Rosenthal, E.L.; et al. Fluorescence Molecular Imaging for Identification of High-Grade Dysplasia in Patients with Head and Neck Cancer. Oral Oncol. 2019, 97, 50–55. [Google Scholar] [CrossRef]
- Kawai, R.; Sugita, Y.; Suzumura, T.; Hattori, T.; Yoshida, W.; Kubo, K.; Maeda, H. Melanoma Inhibitory Activity and Melanoma Inhibitory Activity 2 as Novel Immunohistochemical Markers of Oral Epithelial Dysplasia. J. Clin. Med. 2021, 10, 3661. [Google Scholar] [CrossRef]
- Ekblom, P.; Lonai, P.; Talts, J.F. Expression and Biological Role of Laminin-1. Matrix Biol. J. Int. Soc. Matrix Biol. 2003, 22, 35–47. [Google Scholar] [CrossRef]
- Vageli, D.; Doukas, P.G.; Zacharouli, K.; Kakanis, V.; Strataki, M.; Zioga, A.; Skoulakis, C.; Koukoulis, G.; Ioannou, M. Laminin Immunostaining in Biopsies as a Useful Biomarker of Early Invasion in Actinic Cheilitis and Differential Diagnosis Between Actinic Cheilitis and Lip Cancer: New Insights. Head Neck Pathol. 2022, 17, 331–338. [Google Scholar] [CrossRef]
- Nguyen, C.T.K.; Okamura, T.; Morita, K.-I.; Yamaguchi, S.; Harada, H.; Miki, Y.; Izumo, T.; Kayamori, K.; Yamaguchi, A.; Sakamoto, K. LAMC2 Is a Predictive Marker for the Malignant Progression of Leukoplakia. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2017, 46, 223–231. [Google Scholar] [CrossRef]
- Debta, P.; Sarode, G.; Siddhartha, S.; Sarode, S.; Debta, F.M.; Swain, S.K.; Sahu, M.C.; Patro, S.; Patil, S. GLUT-1 Expression: An Aid in Complementing the WHO Oral Epithelial Dysplasia Grading System. J. Contemp. Dent. Pract. 2020, 21, 951–955. [Google Scholar]
- Patlolla, P.; Shyam, N.D.V.; Kumar, G.K.; Narayen, V.; Konda, P.; Mudududla, P. Evaluation of Glucose Transporter-1 Expression in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma: An Immunohistochemical Study. J. Oral Maxillofac. Pathol. JOMFP 2020, 24, 578. [Google Scholar] [CrossRef]
- Udompatanakorn, C.; Taebunpakul, P. The Expression of Methyltransferase-Like 3 in Oral Precancerous Lesions and Oral Squamous Cell Carcinoma. Eur. J. Dent. 2022, 17, 349–356. [Google Scholar] [CrossRef]
- Singh, D.; Nishi, K.; Khambata, K.; Balasinor, N.H. Introduction to Epigenetics: Basic Concepts and Advancements in the Field. In Epigenetics and Reproductive Health; Tollefsbol, T., Ed.; Translational Epigenetics; Academic Press: Cambridge, MA, USA, 2020; Volume 21, pp. xxv–xliv. [Google Scholar]
- Takisawa, H.; Mimura, S.; Kubota, Y. Eukaryotic DNA Replication: From Pre-Replication Complex to Initiation Complex. Curr. Opin. Cell Biol. 2000, 12, 690–696. [Google Scholar] [CrossRef]
- Zakaria, S.H.; Farag, H.A.; Khater, D.S. Immunohistochemical Expression of MCM-2 in Oral Epithelial Dysplasias. Appl. Immunohistochem. Mol. Morphol. AIMM 2018, 26, 509–513. [Google Scholar] [CrossRef]
- da Silva Barros, C.C.; de Almeida Freitas, R.; da Costa Miguel, M.C.; da Silveira, É.J.D. DNA Damage through Oxidative Stress Is an Important Event in Oral Leukoplakia. Arch. Oral Biol. 2022, 135, 105359. [Google Scholar] [CrossRef]
| Architectural Features | Cytological Features |
|---|---|
| Irregular epithelial stratification | Abnormal variation in nuclear size |
| Loss of polarity of basal cells | Abnormal variation in nuclear shape |
| Drop-shaped rete ridges | Abnormal variation in cell size |
| Mitoses high in epithelium | Abnormal variation in cell shape |
| Generalised premature keratinisation | Increased N/C ratio |
| Keratin pearls within rete ridges | Atypical mitotic figures |
| Loss of epithelial cell cohesion | Increased number and size of nucleoli |
| Altered keratin pattern for oral sub-site | Hyperchromasia |
| Verrucous or papillary architecture | Increased number of mitotic figures |
| Extension of changes along minor gland ducts | Single-cell keratinisation |
| Sharply defined margin of changes | Apoptotic mitoses |
| Multiple different patterns of dysplasia | Increased nuclear size |
| Multifocal or skip lesions | |
| Expanded proliferative compartment | |
| Basal cell clustering/nesting |
| Relation of Biomarkers | Examples of Biomarkers for Grading of Oral Dysplasia |
|---|---|
| Cell division and proliferation | p53 [53,54,55,75,76,117], Ki-67 [56,68,69,70,71,72,73,74,75,76], CD105 [73,74], p63 [39,42,117], CD31 [39], CD34 [43], cycD1 [42], VEGF [43], YAP, Np63 [59], stathmin [80], CDKN1A [53] |
| Epithelial–mesenchymal transition | CK5\6 [96], CK19 [98], β-catenin [103,104], N-cadherin [109], E-cadherin [104,105,106,107], TWIST [111], VIM [107,108], PDPN [115,117], MMP-9 [108] |
| Cell death regulation | Bcl-2 [123,124], PDCD4 [128], HSP27 [131], cornulin [134] |
| Cellular metabolism | HIF-1a [136], iNOS [138], COX-2 [140] |
| Extracellular signalling pathways | Paxillin [142], EGFR [145], MIA, MIA2 [146], laminin [148], LAMC2 [149], GLUT-1 [150,151], METTL3 [152], MCM2 [155], 8-OHdG, Ref-1, XRCC-1 [156], Orai1, STIM1 [32], NANOG [30,31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdrojewski, J.; Nowak, M.; Nijakowski, K.; Jankowski, J.; Scribante, A.; Gallo, S.; Pascadopoli, M.; Surdacka, A. Potential Immunohistochemical Biomarkers for Grading Oral Dysplasia: A Literature Review. Biomedicines 2024, 12, 577. https://doi.org/10.3390/biomedicines12030577
Zdrojewski J, Nowak M, Nijakowski K, Jankowski J, Scribante A, Gallo S, Pascadopoli M, Surdacka A. Potential Immunohistochemical Biomarkers for Grading Oral Dysplasia: A Literature Review. Biomedicines. 2024; 12(3):577. https://doi.org/10.3390/biomedicines12030577
Chicago/Turabian StyleZdrojewski, Jakub, Monika Nowak, Kacper Nijakowski, Jakub Jankowski, Andrea Scribante, Simone Gallo, Maurizio Pascadopoli, and Anna Surdacka. 2024. "Potential Immunohistochemical Biomarkers for Grading Oral Dysplasia: A Literature Review" Biomedicines 12, no. 3: 577. https://doi.org/10.3390/biomedicines12030577
APA StyleZdrojewski, J., Nowak, M., Nijakowski, K., Jankowski, J., Scribante, A., Gallo, S., Pascadopoli, M., & Surdacka, A. (2024). Potential Immunohistochemical Biomarkers for Grading Oral Dysplasia: A Literature Review. Biomedicines, 12(3), 577. https://doi.org/10.3390/biomedicines12030577










