Genetic Mutations Associated with Inflammatory Response Caused by HPV Integration in Oropharyngeal Squamous Cell Carcinoma
Abstract
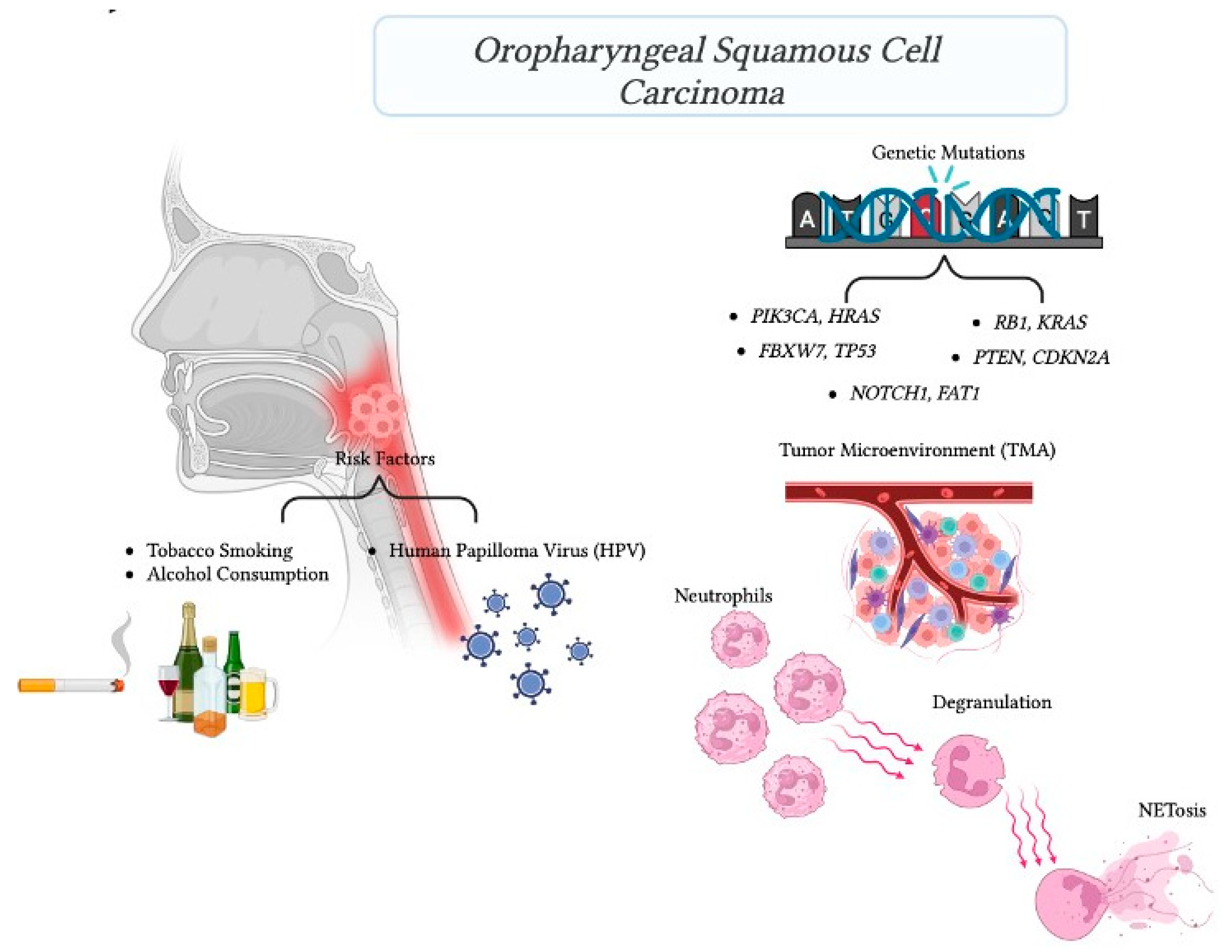
:1. Introduction
2. Materials and Methods
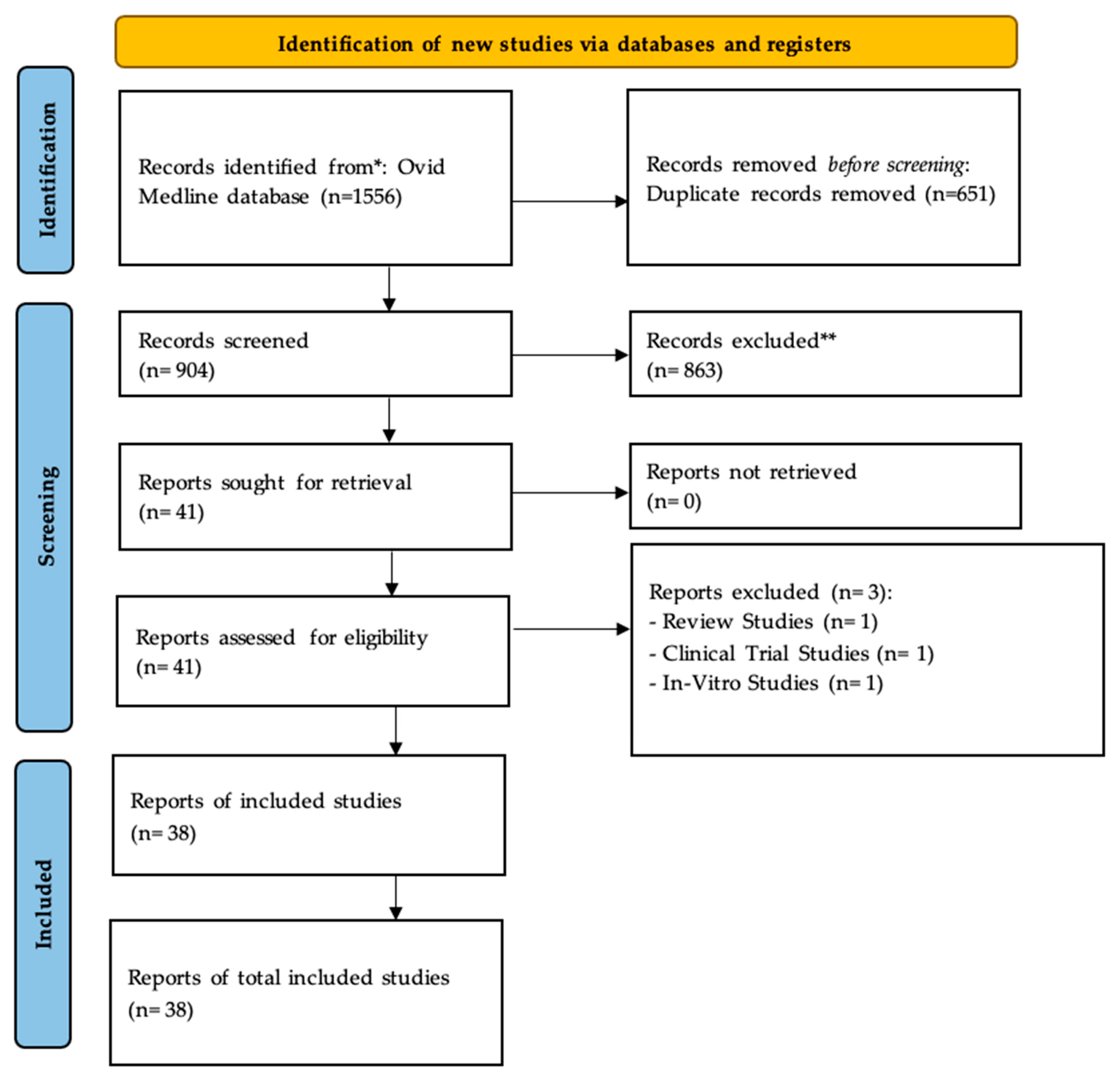
2.1. Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Technical Validation in Public Database
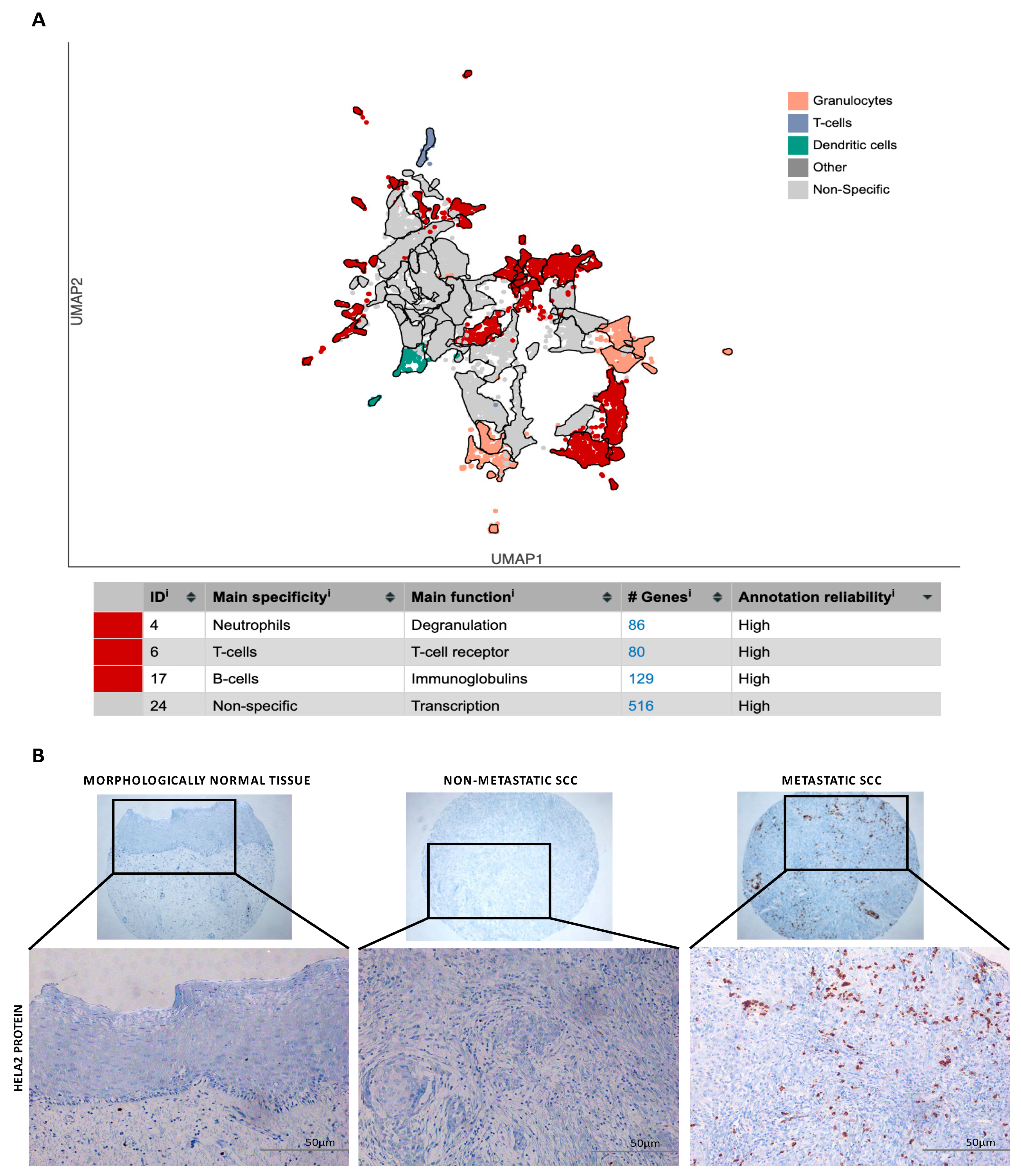
2.5. Experimental Validation in Patients’ Samples
Ethics and Patient Cohort
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. Overview of the Included Studies
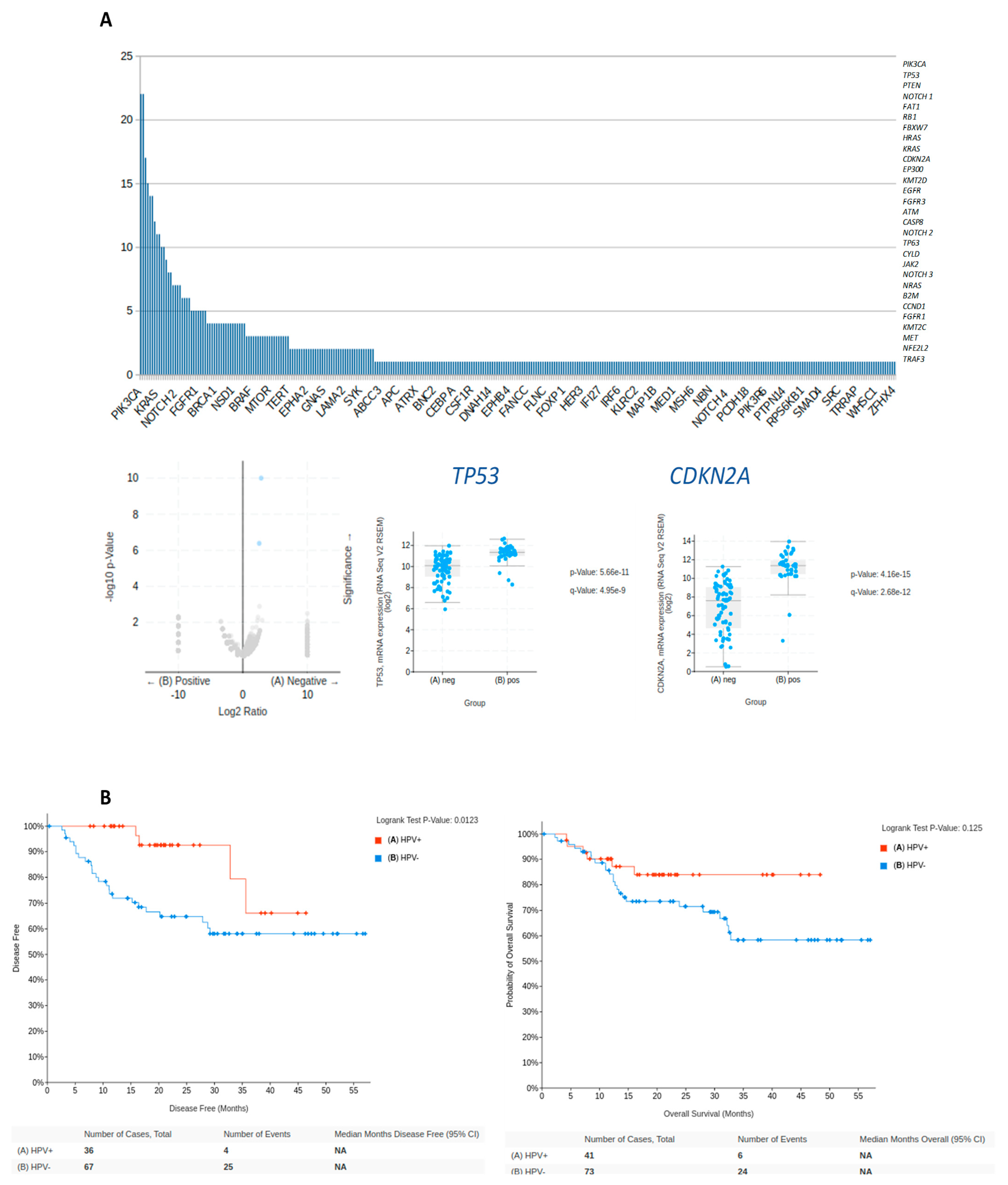
3.2. Technical Validation—Common Gene Mutations in HPV-Positive HNC
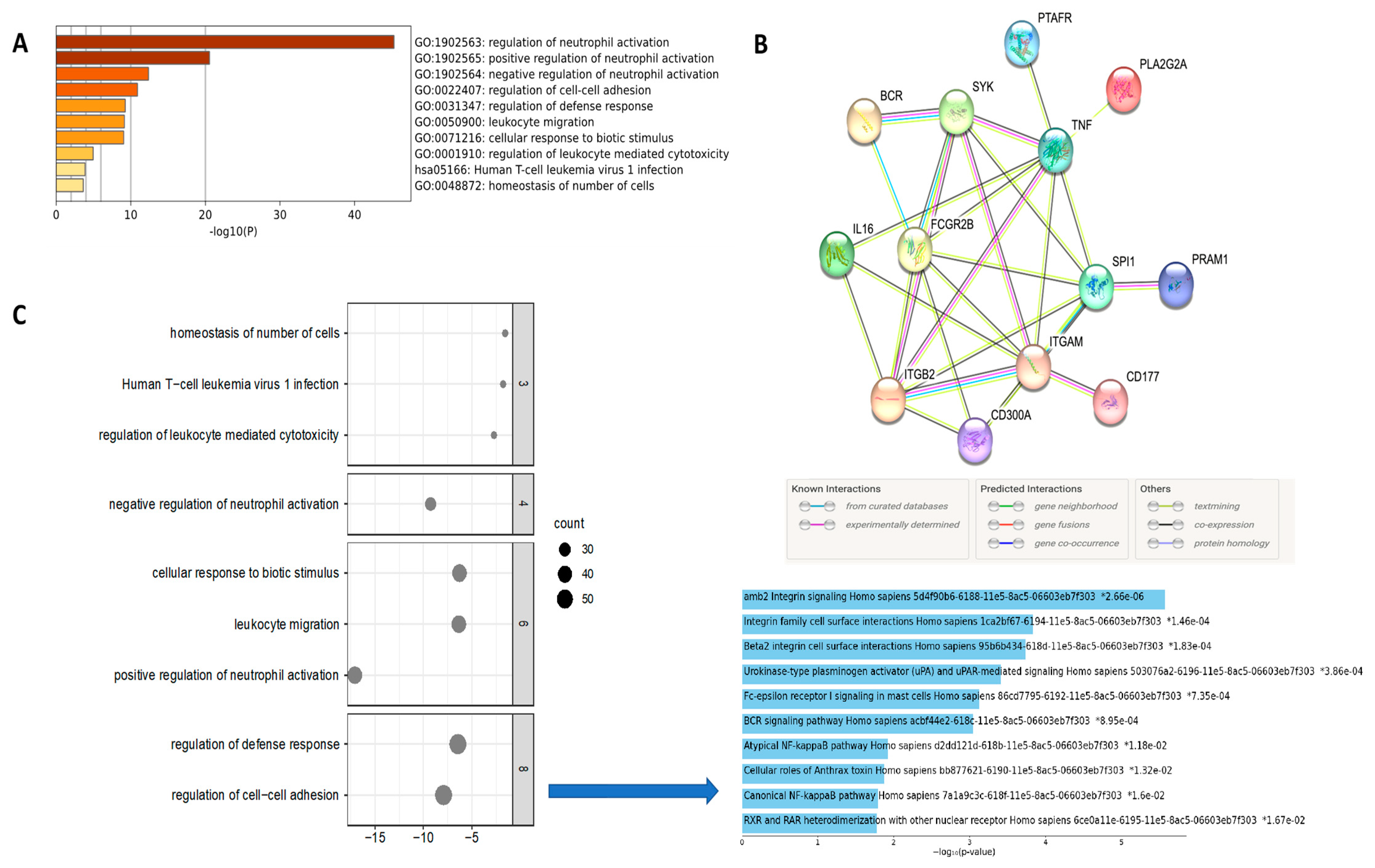
3.3. Enriched Analysis of Mutated Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the Epidemiology of Head and Neck Cancer: Definitions, Trends and Risk Factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.D.; Chung, C.H. Biology of Human Papillomavirus–Related Oropharyngeal Cancer. Semin. Radiat. Oncol. 2012, 22, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.G.; Bensumaidea, S.H.; Alshammari, F.D.; Alenazi, F.; Almutlaq, B.A.; Alturkstani, M.Z.; Aladani, I.A. Prevalence of Human Papillomavirus Subtypes 16 and 18 among Yemeni Patients with Cervical Cancer. PubMed 2017, 18, 1543–1548. [Google Scholar]
- Morgan, I.M.; DiNardo, L.J.; Windle, B. Integration of Human Papillomavirus Genomes in Head and Neck Cancer: Is It Time to Consider a Paradigm Shift? Viruses 2017, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Thierry, F. Transcriptional Regulation of the Papillomavirus Oncogenes by Cellular and Viral Transcription Factors in Cervical Carcinoma. Virology 2009, 384, 375–379. [Google Scholar] [CrossRef]
- LaBarge, B.; Hennessy, M.; Zhang, L.; Goldrich, D.; Chartrand, S.; Purnell, C.; Wright, S.; Goldenberg, D.; Broach, J.R. Human Papillomavirus Integration Strictly Correlates with Global Genome Instability in Head and Neck Cancer. Mol. Cancer Res. 2022, 20, 1420–1428. [Google Scholar] [CrossRef]
- Sinha, P.; Logan, H.L.; Mendenhall, W.M. Human Papillomavirus, Smoking, and Head and Neck Cancer. Am. J. Otolaryngol. 2012, 33, 130–136. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human Papillomavirus as a Driver of Head and Neck Cancers. Br. J. Cancer 2019, 122, 306–314. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Wei, J.; Meng, L.; Zhang, Q.; Qu, C.; Xin, Y.; Jiang, X. Molecular Mechanisms Underlying Increased Radiosensitivity in Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma. Int. J. Biol. Sci. 2020, 16, 1035–1043. [Google Scholar] [CrossRef]
- Powell, S.; Vu, L.; Spanos, W.C.; Pyeon, D. The Key Differences between Human Papillomavirus-Positive and -Negative Head and Neck Cancers: Biological and Clinical Implications. Cancers 2021, 13, 5206. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Thorstad, W.L.; Chernock, R.D.; Haughey, B.H.; Yip, J.H.; Zhang, Q.; El-Mofty, S.K. P16 Positive Oropharyngeal Squamous Cell Carcinoma:An Entity With a Favorable Prognosis Regardless of Tumor HPV Status. Am. J. Surg. Pathol. 2010, 34, 1088–1096. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hisamatsu, K.; Suzui, N.; Hara, A.; Tomita, H.; Miyazaki, T. A Review of HPV-Related Head and Neck Cancer. J. Clin. Med. 2018, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Zhang, Q.; Nguyen-Tân, P.F.; Rosenthal, D.I.; El-Naggar, A.K.; Garden, A.S.; Soulières, D.; Trotti, A.; Avizonis, V.N.; Ridge, J.A.; et al. Human Papillomavirus and Overall Survival after Progression of Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 2014, 32, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.; Bucchi, L.; Micheletti, L. Four-decade trends in lymph node status of patients with vulvar squamous cell carcinoma in northern Italy. Sci. Rep. 2021, 11, 5661. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Machczynski, P.; Majchrzak, E.; Niewinski, P.; Marchlewska, J.; Golusiński, W. A Review of the 8th Edition of the AJCC Staging System for Oropharyngeal Cancer According to HPV Status. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2407–2412. [Google Scholar] [CrossRef]
- Van Gysen, K.; Stevens, M.; Guo, L.; Jayamanne, D.; Veivers, D.; Wignall, A.; Pang, L.; Guminski, A.; Lee, A.V.; Hruby, G.; et al. Validation of the 8th Edition UICC/AJCC TNM Staging System for HPV Associated Oropharyngeal Cancer Patients Managed with Contemporary Chemo-Radiotherapy. BMC Cancer 2019, 19, 674. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Zuo, Z.; Keck, M.K.; Khattri, A.; Pedamallu, C.S.; Stricker, T.; Brown, C.D.; Pugh, T.J.; Stojanov, P.; Cho, J.; et al. Integrative and Comparative Genomic Analysis of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinomas. Clin. Cancer Res. 2015, 21, 632–641. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.M.; Fedorowicz, Z.; Elmagarmid, A.K. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.; Akl, E.A.; Brennan, S.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Harbison, R.A.; Kubik, M.; Konnick, E.Q.; Zhang, Q.; Lee, S.-G.; Park, H.; Zhang, J.; Carlson, C.S.; Chu, C.; Schwartz, S.M.; et al. The Mutational Landscape of Recurrent versus Nonrecurrent Human Papillomavirus–Related Oropharyngeal Cancer. JCI Insight 2018, 3, e99327. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Guthrie, V.B.; Masica, D.L.; Tokheim, C.; Kang, H.; Richmon, J.D.; Agrawal, N.; Fakhry, C.; Quon, H.; Subramaniam, R.M.; et al. Genomic Alterations in Head and Neck Squamous Cell Carcinoma Determined by Cancer Gene-Targeted Sequencing. Ann. Oncol. 2015, 26, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Doerstling, S.S.; Winski, D.; Katsoulakis, E.; Agarwal, P.K.; Poonnen, P.; Snowdon, J.L.; Jackson, G.P.; Weeraratne, D.; Kelley, M.J.; Vashistha, V. Mutational Profiles of Head and Neck Squamous Cell Carcinomas Based upon Human Papillomavirus Status in the Veterans Affairs National Precision Oncology Program. J. Cancer Res. Clin. Oncol. 2022, 149, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Doğan, S.; Xu, B.; Middha, S.; Vanderbilt, C.; Bowman, A.S.; Migliacci, J.; Morris, L.G.T.; Seshan, V.; Ganly, I. Identification of Prognostic Molecular Biomarkers in 157 HPV-positive and HPV-negative Squamous Cell Carcinomas of the Oropharynx. Int. J. Cancer 2019, 145, 3152–3162. [Google Scholar] [CrossRef]
- Dubot, C.; Bernard, V.; Sablin, M.; Vacher, S.; Chemlali, W.; Schnitzler, A.; Pierron, G.; Raïs, K.A.; Bessoltane, N.; Jeannot, E.; et al. Comprehensive Genomic Profiling of Head and Neck Squamous Cell Carcinoma Reveals FGFR1 Amplifications and Tumour Genomic Alterations Burden as Prognostic Biomarkers of Survival. Eur. J. Cancer 2018, 91, 47–55. [Google Scholar] [CrossRef]
- Gleber-Netto, F.O.; Zhao, M.; Trivedi, S.; Wang, J.; Jasser, S.A.; McDowell, C.; Kadara, H.; Zhang, J.; Wang, J.; William, W.N.; et al. Distinct Pattern of TP53 Mutations in Human Immunodeficiency Virus-Related Head and Neck Squamous Cell Carcinoma. Cancer 2017, 124, 84–94. [Google Scholar] [CrossRef]
- Larsen, C.G.; Jensen, D.H.; Agander, T.K.; Kiss, K.; Høgdall, E.; Specht, L.; Bagger, F.O.; Nielsen, F.C. Deep Sequencing of Human Papillomavirus Positive Loco-Regionally Advanced Oropharyngeal Squamous Cell Carcinomas Reveals Novel Mutational Signature. BMC Cancer 2018, 18, 640. [Google Scholar]
- Haft, S.; Ren, S.; Xu, G.; Mark, A.; Fisch, K.; Guo, T.W.; Khan, Z.; Pang, J.; Ando, M.; Liu, C.; et al. Mutation of Chromatin Regulators and Focal Hotspot Alterations Characterize Human Papillomavirus–Positive Oropharyngeal Squamous Cell Carcinoma. Cancer 2019, 125, 2423–2434. [Google Scholar] [CrossRef]
- Koncar, R.; Feldman, R.; Bahassi, E.M.; Sadraei, N.H. Comparative Molecular Profiling of HPV-Induced Squamous Cell Carcinomas. Cancer Med. 2017, 6, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Cho, S.; Hwang, I.G.; Choi, J.W.; Chang, H.; Ahn, M.; Park, K.U.; Kim, J.; Ko, Y.H.; Ahn, H.K.; et al. Investigating the Feasibility of Targeted Next-Generation Sequencing to Guide the Treatment of Head and Neck Squamous Cell Carcinoma. Cancer Res. Treat. 2019, 51, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Zhang, Y.; Zarins, K.R.; Jones, T.R.; Virani, S.; Peterson, L.A.; McHugh, J.B.; Chepeha, D.; Wolf, G.T.; Rozek, L.S.; et al. Expressed HNSCC Variants by HPV-Status in a Well-Characterized Michigan Cohort. Sci. Rep. 2018, 8, 11458. [Google Scholar] [CrossRef] [PubMed]
- Reder, H.; Wagner, S.; Gamerdinger, U.; Sandmann, S.; Wuerdemann, N.; Braeuninger, A.; Dugas, M.; Gattenloehner, S.; Klußmann, J.P.; Wittekindt, C. Genetic Alterations in Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma of Patients with Treatment Failure. Oral Oncol. 2019, 93, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Reder, H.; Wagner, S.; Wuerdemann, N.; Langer, C.; Sandmann, S.; Braeuninger, A.; Dugas, M.; Gattenloehner, S.; Wittekindt, C.; Klußmann, J.P. Mutation Patterns in Recurrent and/or Metastatic Oropharyngeal Squamous Cell Carcinomas in Relation to Human Papillomavirus Status. Cancer Med. 2021, 10, 1347–1356. [Google Scholar] [CrossRef]
- Saba, N.F.; Dinasarapu, A.R.; Magliocca, K.R.; Dwivedi, B.; Seby, S.; Qin, Z.S.; Patel, M.R.; Griffith, C.C.; Wang, X.; El-Deiry, M.; et al. Signatures of Somatic Mutations and Gene Expression from P16INK4A Positive Head and Neck Squamous Cell Carcinomas (HNSCC). PLoS ONE 2020, 15, e0238497. [Google Scholar] [CrossRef]
- Wahle, B.; Zolkind, P.; Ramirez, R.; Skidmore, Z.L.; Anderson, S.R.; Mazul, A.L.; Hayes, D.N.; Sandulache, V.C.; Thorstad, W.L.; Adkins, D.R.; et al. Integrative Genomic Analysis Reveals Low T-Cell Infiltration as the Primary Feature of Tobacco Use in HPV-Positive Oropharyngeal Cancer. iScience 2022, 25, 104216. [Google Scholar] [CrossRef]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The Mutational Landscape of Head and Neck Squamous Cell Carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef]
- Williams, E.; Montesion, M.; Alexander, B.M.; Ramkissoon, S.; Elvin, J.A.; Ross, J.S.; Williams, K.J.; Glomski, K.; Bledsoe, J.R.; Tse, J.Y.; et al. CYLD Mutation Characterizes a Subset of HPV-Positive Head and Neck Squamous Cell Carcinomas with Distinctive Genomics and Frequent Cylindroma-like Histologic Features. Mod. Pathol. 2021, 34, 358–370. [Google Scholar] [CrossRef]
- Antonsson, A.; Law, M.H.; Neale, R.E.; Coman, W.B.; Pryor, D.; Porceddu, S.; Whiteman, D.C. Variants of EVER1 and EVER2 (TMC6 and TMC8) and Human Papillomavirus Status in Patients with Mucosal Squamous Cell Carcinoma of the Head and Neck. Cancer Causes Control 2016, 27, 809–815. [Google Scholar] [CrossRef]
- Barten, M.; Ostwald, C.; Wukasch, Y.; Müller, P.; Löning, T.; Milde-Langosch, K. HPV DNA and P53 Alterations in Oropharyngeal Carcinomas. Virchows Arch. 1995, 427, 153–157. [Google Scholar] [CrossRef]
- Benzerdjeb, N.; Tantot, J.; Blanchet, C.; Philouze, P.; Mekki, Y.; Lopez, J.; Devouassoux-Shisheboran, M. Oropharyngeal Squamous Cell Carcinoma: P16/P53 Immunohistochemistry as a Strong Predictor of HPV Tumour Status. Histopathology 2021, 79, 381–390. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Chen, J.; Wang, D.; Tu, J.; Van Waes, C.; Saba, N.F.; Chen, Z.G.; Chen, Z. The Proteomic Landscape of Growth Factor Signaling Networks Associated with FAT1Mutations in Head and Neck Cancers. Cancer Res. 2021, 81, 4402–4416. [Google Scholar] [CrossRef]
- Chiosea, S.I.; Grandis, J.R.; Lui, V.W.Y.; Diergaarde, B.; Maxwell, J.H.; Ferris, R.L.; Kim, S.W.; Luvison, A.; Miller, M.A.; Nikiforova, M.N. PIK3CA, HRAS and PTEN in Human Papillomavirus Positive Oropharyngeal Squamous Cell Carcinoma. BMC Cancer 2013, 13, 602. [Google Scholar] [CrossRef]
- Ekalaksananan, T.; Wongjampa, W.; Phusingha, P.; Chuerduangphui, J.; Vatanasapt, P.; Promthet, S.; Patarapadungkit, N.; Pientong, C. Comprehensive Data of P53 R282 Gene Mutation with Human Papillomaviruses (HPV)-Associated Oral Squamous Cell Carcinoma (OSCC). Pathol. Oncol. Res. 2020, 26, 1191–1199. [Google Scholar] [CrossRef]
- Fallai, C.; Perrone, F.; Licitra, L.; Pilotti, S.; Locati, L.D.; Bossi, P.; Orlandi, E.; Palazzi, M.; Olmi, P. Oropharyngeal Squamous Cell Carcinoma Treated with Radiotherapy or Radiochemotherapy: Prognostic Role of TP53 and HPV Status. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1053–1059. [Google Scholar] [CrossRef]
- Farnebo, L.; Stjernström, A.; Fredrikson, M.; Ansell, A.; Garvin, S.; Thunell, L.K. DNA Repair Genes XPC, XPD, XRCC1, and XRCC3 Are Associated with Risk and Survival of Squamous Cell Carcinoma of the Head and Neck. DNA Repair 2015, 31, 64–72. [Google Scholar] [CrossRef]
- Hong, A.; Zhang, X.; Jones, D.B.; Veillard, A.-S.; Zhang, M.; Martin, A.; Lyons, J.G.; Lee, C.S.; Rose, B. Relationships between P53 Mutation, HPV Status and Outcome in Oropharyngeal Squamous Cell Carcinoma. Radiother. Oncol. 2016, 118, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Cortelazzi, B.; Verderio, P.; Ciniselli, C.M.; Pizzamiglio, S.; Bossi, P.; Gloghini, A.; Gualeni, A.V.; Volpi, C.C.; Locati, L.D.; Pierotti, M.A.; et al. Receptor Tyrosine Kinase Profiles and Human Papillomavirus Status in Oropharyngeal Squamous Cell Carcinoma. J. Oral Pathol. Med. 2014, 44, 734–745. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, A.C.; Melendez, M.E.; Da Silva Sábato, C.; Palmero, E.I.; Arantes, L.M.R.B.; Neto, C.S. Clinical and Molecular Characterization of Surgically Treated Oropharynx Squamous Cell Carcinoma Samples. Pathol. Oncol. Res. 2018, 25, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Friedland, P.; Thomas, A.; Naran, A.; Amanuel, B.; Grieu-Iacopetta, F.; Carrello, A.; Harnett, G.B.; Meyer, C.; Phillips, M. Human Papillomavirus and Gene Mutations in Head and Neck Squamous Carcinomas. Anz J. Surg. 2011, 82, 362–366. [Google Scholar] [CrossRef]
- Ghosh, A.; Maiti, G.P.; Bandopadhyay, M.N.; Chakraborty, J.; Biswas, J.; Roychoudhury, S.; Panda, C.K. Inactivation of 9q22.3 tumor suppressor genes predict outcome for patients with head and neck squamous cell carcinoma. Anticancer Res. 2013, 33, 1215–1220. [Google Scholar]
- Gross, A.M.; Orosco, R.K.; Shen, J.P.; Egloff, A.M.; Carter, H.; Hofree, M.; Choueiri, M.; Coffey, C.S.; Lippman, S.M.; Hayes, D.N.; et al. Multi-Tiered Genomic Analysis of Head and Neck Cancer Ties TP53 Mutation to 3p Loss. Nat. Genet. 2014, 46, 939–943. [Google Scholar] [CrossRef]
- Chao, H.; Cintra, M.B.; Brennan, K.; Zhou, M.; Colevas, A.D.; Fischbein, N.J.; Zhu, S.; Gevaert, O. Development and Validation of Radiomic Signatures of Head and Neck Squamous Cell Carcinoma Molecular Features and Subtypes. EBioMedicine 2019, 45, 70–80. [Google Scholar]
- Licitra, L.; Perrone, F.; Bossi, P.; Suardi, S.; Mariani, L.; Artusi, R.; Oggionni, M.; Rossini, C.; Cantu, G.; Squadrelli, M.; et al. High-Risk Human Papillomavirus Affects Prognosis in Patients with Surgically Treated Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 2006, 24, 5630–5636. [Google Scholar] [CrossRef]
- Mazurek, A.; Rutkowski, T.; Fiszer-Kierzkowska, A.; Małusecka, E.; Składowski, K. Assessment of the Total CfDNA and HPV16/18 Detection in Plasma Samples of Head and Neck Squamous Cell Carcinoma Patients. Oral Oncol. 2016, 54, 36–41. [Google Scholar] [CrossRef]
- Saba, N.F.; Wilson, M.M.; Doho, G.; DaSilva, J.; Isett, R.B.; Newman, S.; Chen, Z.G.; Magliocca, K.R.; Rossi, M.R. Mutation and Transcriptional Profiling of Formalin-Fixed Paraffin Embedded Specimens as Companion Methods to Immunohistochemistry for Determining Therapeutic Targets in Oropharyngeal Squamous Cell Carcinoma (OPSCC): A Pilot of Proof of Principle. Head Neck Pathol. 2014, 9, 223–235. [Google Scholar] [CrossRef]
- Sewell, A.; Brown, B.; Biktasova, A.; Mills, G.B.; Lu, Y.; Tyson, D.R.; Issaeva, N.; Yarbrough, W.G. Reverse-Phase Protein Array Profiling of Oropharyngeal Cancer and Significance of PIK3CA Mutations in HPV-Associated Head and Neck Cancer. Clin. Cancer Res. 2014, 20, 2300–2311. [Google Scholar] [CrossRef]
- Shaikh, H.; McGrath, J.; Hughes, B.N.; Xiu, J.; Brodskiy, P.; Sukari, A.; Darabi, S.; Ikpeazu, C.; Nabhan, C.; Korn, W.M.; et al. Genomic and Molecular Profiling of Human Papillomavirus Associated Head and Neck Squamous Cell Carcinoma Treated with Immune Checkpoint Blockade Compared to Survival Outcomes. Cancers 2021, 13, 6309. [Google Scholar] [CrossRef]
- Timbang, M.R.; Sim, M.W.; Bewley, A.F.; Farwell, D.G.; Mantravadi, A.V.; Moore, M.G. HPV-Related Oropharyngeal Cancer: A Review on Burden of the Disease and Opportunities for Prevention and Early Detection. Hum. Vaccines Immunother. 2019, 15, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Johnson, D.E.; Grandis, J.R. Therapeutic Implications of the Genetic Landscape of Head and Neck Cancer. Semin. Radiat. Oncol. 2018, 28, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mateos, J.; Pérez-García, J.; Seijas-Tamayo, R.; MesíA, R.; Rubió-Casadevall, J.; García-Girón, C.; Iglesias, L.; Maseda, A.C.; Klain, J.C.A.; Cequier, Á.; et al. Oncogenic Driver Mutations Predict Outcome in a Cohort of Head and Neck Squamous Cell Carcinoma (HNSCC) Patients within a Clinical Trial. Sci. Rep. 2020, 10, 16634. [Google Scholar] [CrossRef] [PubMed]
- German, S.; Aslam, H.M.; Saleem, S.; Raees, A.; Anum, T.; Alvi, A.A.; Haseeb, A. Carcinogenesis of PIK3CA. Hered. Cancer Clin. Pract. 2013, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the P53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. P53 Signaling in Cancer Progression and Therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef]
- Vogt, P.K.; Hart, J.R.; Gymnopoulos, M.; Jiang, H.; Kang, S.; Bader, A.G.; Zhao, L.; Denley, A. Phosphatidylinositol 3-Kinase: The Oncoprotein. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 79–104. [Google Scholar]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in Cancer: Mechanisms and Advances in Clinical Trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Ligresti, G.; Militello, L.; Steelman, L.S.; Cavallaro, A.; Burzotta, F.; Nicoletti, F.; Stivala, F.; McCubrey, J.A.; Libra, M. PIK3CA Mutations in Human Solid Tumors: Role in Sensitivity to Various Therapeutic Approaches. Cell Cycle 2009, 8, 1352–1358. [Google Scholar] [CrossRef]
- Whiteside, T.L. The Tumor Microenvironment and Its Role in Promoting Tumor Growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Lechien, J.R.; Descamps, G.; Seminerio, I.; Furgiuele, S.; Dequanter, D.; Mouawad, F.; Badoual, C.; Journé, F.; Saussez, S. HPV Involvement in the Tumor Microenvironment and Immune Treatment in Head and Neck Squamous Cell Carcinomas. Cancers 2020, 12, 1060. [Google Scholar] [CrossRef]
- Julian, R.; Savani, M.; Bauman, J.E. Immunotherapy Approaches in HPV-Associated Head and Neck Cancer. Cancers 2021, 13, 5889. [Google Scholar] [CrossRef]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil Diversity and Plasticity in Tumour Progression and Therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V. Leveling Up the Controversial Role of Neutrophils in Cancer: When the Complexity Becomes Entangled. Cells 2021, 10, 2486. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Bhagat, S.; Paul, S.; Katz, J.P.; Sengupta, D.N.; Bhargava, D. Neutrophils in Cancer and Potential Therapeutic Strategies Using Neutrophil-Derived Exosomes. Vaccines 2023, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Ohms, M.; Möller, S.; Laskay, T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in Vitro. Front. Immunol. 2020, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Aarts, C.E.M.; Hiemstra, I.H.; Béguin, E.P.; Hoogendijk, A.J.; Bouchmal, S.; Van Houdt, M.; Tool, A.T.J.; Mul, E.; Jansen, M.H.; Janssen, H.; et al. Activated Neutrophils Exert Myeloid-Derived Suppressor Cell Activity Damaging T Cells beyond Repair. Blood Adv. 2019, 3, 3562–3574. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, E.K.; Flynn, J.; Panageas, K.S.; Ferraro, R.; Cruz, J.; Postow, M.A.; Coit, D.G.; Ariyan, C.E. High Neutrophil-to-lymphocyte Ratio (NLR) Is Associated with Treatment Failure and Death in Patients Who Have Melanoma Treated with PD-1 Inhibitor Monotherapy. Cancer 2019, 126, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Gago-Domínguez, M.; Matabuena, M.; Redondo, C.M.; Patel, S.P.; Carracedo, Á.; Ponte, S.M.; MartíNez, M.E.; Castelao, J.E. Neutrophil to Lymphocyte Ratio and Breast Cancer Risk: Analysis by Subtype and Potential Interactions. Sci. Rep. 2020, 10, 13203. [Google Scholar] [CrossRef]
- Howard, R.; Kanetsky, P.A.; Egan, K.M. Exploring the Prognostic Value of the Neutrophil-to-Lymphocyte Ratio in Cancer. Sci. Rep. 2019, 9, 19673. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Malech, H.L.; DeLeo, F.R.; Quinn, M.T. The Role of Neutrophils in the Immune System: An Overview. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–10. [Google Scholar]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, H.; Yan, A.; Wang, H.; Li, X.; Liu, J.; Li, W. Pretreatment Neutrophil to Lymphocyte Ratio in Determining the Prognosis of Head and Neck Cancer: A Meta-Analysis. BMC Cancer 2018, 18, 383. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in Cancer Carcinogenesis and Metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef]
- Al-Sahaf, S.; Hendawi, N.; Ollington, B.; Bolt, R.J.; Ottewell, P.D.; Hunter, K.D.; Murdoch, C. Increased Abundance of Tumour-Associated Neutrophils in HPV-Negative Compared to HPV-Positive Oropharyngeal Squamous Cell Carcinoma Is Mediated by IL-1R Signalling. Front. Oral Health 2021, 2, 604565. [Google Scholar] [CrossRef]
- Ocaña, A.; Nieto-Jiménez, C.; Pandiella, A.; Templeton, A.J. Neutrophils in Cancer: Prognostic Role and Therapeutic Strategies. Mol. Cancer 2017, 16, 137. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in Inflammation, Autoimmunity and Cancer. Int. Immunol. 2020, 33, 127–148. [Google Scholar] [CrossRef]
- Raftopoulou, S.; Valadez-Cosmes, P.; Mihalic, Z.N.; Schicho, R.; Kargl, J. Tumor-Mediated Neutrophil Polarization and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 3218. [Google Scholar] [CrossRef]
| Author, Year | Journal Impact Factor | Country | Sample Size | Study Type | Molecular Techniques * |
|---|---|---|---|---|---|
| Harbison et al., 2018 [23] | 19.477 | USA | 84 | Cross-sectional | WGS, NGS |
| Chung et al., 2015 [24] | 32.976 | USA | 252 | Multicenter | NGS, ISH, IHC |
| Doerstling et al., 2023 [25] | 4.322 | USA | 79 | Retrospective | IHC, NGS |
| Dogan et al., 2019 [26] | 7.316 | USA | 157 | Retrospective | Target sequencing |
| Dubot et al., 2018 [27] | 10.002 | FRANCE | 122 | Retrospective | NGS |
| Gleber-Netto et al., 2018 [28] | 6.921 | USA | 52 | Retrospective | NGS, PCR, IHC |
| Gronhoj et al., 2018 [29] | 4.638 | DENMARK | 114 | Retrospective | NGS |
| Haft et al., 2019 [30] | 6.921 | USA | 46 | Retrospective | NGS |
| Koncar et al., 2017 [31] | 4.711 | USA | 743 | Retrospective | IHC, ISH, NG |
| Labarge et al., 2022 [8] | 6.333 | USA | 12 | Retrospective | WGS, OGM |
| Lim et al., 2019 [32] | 13.312 | KOREA | 93 | Multicenter | NGS |
| Qin et al., 2018 [33] | 4.997 | USA | 36 | Rettrospective | NGS. |
| Reder et al., 2019 [34] | 5.972 | GERMANY | 24 | Retrospective | NGS. |
| Reder et al., 2021 [35] | 4.711 | GERMANY | 139 | Retrospective | NGS. |
| Saba et al., 2020 [36] | 3.240 | USA | 35 | Retrospective | NGS |
| Wahle et al., 2022 [37] | 5.08 | USA | 47 | Retrospective | WGS, ISH, IHC |
| Stransky et al., 2011 [38] | 63.832 | USA | 92 | Retrospective | WGS |
| Williams et al., 2021 [39] | 8.209 | USA | 703 | Retrospective | NGS |
| Antonsson et al., 2016 [40] | 2.532 | AUSTRALIA | 219 | Case-control | NGS |
| Barten et al., 1995 [41] | 4.548 | GERMANY | 37 | Retrospective | PCR, IHC |
| Benzerdjeb et al., 2021 [42] | 7.778 | FRANCE | 110 | Cross-sectional | PCR, NGS |
| Chen et al., 2021 [43] | 13.312 | USA | 489 | Retrospective | ELISA |
| Chiosea et al., 2013 [44] | 4.638 | USA | 75 | Retrospective | NGS |
| Ekalaksananan et al., 2020 [45] | 2.874 | THAILAND | 106 | Case-control | PCR |
| Fallai et al., 2009 [46] | 8.013 | ITALY | 78 | Prospective | NGS, PCR |
| Farnebo et al., 2015 [47] | 4.354 | SWEDAN | 169 | Case-control | PCR–RFLP. |
| Hong et al., 2016 [48] | 6.901 | AUSTRALIA | 202 | Retrospective | Pyrosequencing |
| Cortelazzi et al., 2015 [49] | 3.539 | ITALY | 76 | Cross-sectional | PCR |
| De Carvalho et al., 2019 [50] | 2.874 | BRAZIL | 25 | Retrospective | PCR, WGS |
| Friedland et al., 2012 [51] | 2.025 | AUSTRALIA | 60 | Retrospective | PCR |
| Ghosh et al., 2013 [52] | 2.435 | INDIA | 84 | Prospective | NGS |
| Gross et al., 2014 [53] | 41.376 | USA | 376 | Prospective | PCR |
| Huang et al., 2019 [54] | 11.205 | USA | 113 | Retrospective | ISH, IHC, WGS |
| Licitra et al., 2006 [55] | 50.739 | ITALY | 100 | Retrospective | NGS, PCR, IHC |
| Mazurek et al., 2016 [56] | 5.972 | POLAND | 200 | Case-control | PCR |
| Saba et al., 2015 [57] | 2.031 | USA | 8 | Proof of concept | NGS |
| Sewell et al., 2014 [58] | 13.801 | USA | 49 | Prospective | RPPA |
| Shaikh et al., 2021 [59] | 6.575 | USA | 2905 | Retrospective | WGS, NGS, IHC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atique, M.; Muniz, I.; Farshadi, F.; Hier, M.; Mlynarek, A.; Macarella, M.; Maschietto, M.; Nicolau, B.; Alaoui-Jamali, M.A.; da Silva, S.D. Genetic Mutations Associated with Inflammatory Response Caused by HPV Integration in Oropharyngeal Squamous Cell Carcinoma. Biomedicines 2024, 12, 24. https://doi.org/10.3390/biomedicines12010024
Atique M, Muniz I, Farshadi F, Hier M, Mlynarek A, Macarella M, Maschietto M, Nicolau B, Alaoui-Jamali MA, da Silva SD. Genetic Mutations Associated with Inflammatory Response Caused by HPV Integration in Oropharyngeal Squamous Cell Carcinoma. Biomedicines. 2024; 12(1):24. https://doi.org/10.3390/biomedicines12010024
Chicago/Turabian StyleAtique, Mai, Isis Muniz, Fatemeh Farshadi, Michael Hier, Alex Mlynarek, Marco Macarella, Mariana Maschietto, Belinda Nicolau, Moulay A. Alaoui-Jamali, and Sabrina Daniela da Silva. 2024. "Genetic Mutations Associated with Inflammatory Response Caused by HPV Integration in Oropharyngeal Squamous Cell Carcinoma" Biomedicines 12, no. 1: 24. https://doi.org/10.3390/biomedicines12010024
APA StyleAtique, M., Muniz, I., Farshadi, F., Hier, M., Mlynarek, A., Macarella, M., Maschietto, M., Nicolau, B., Alaoui-Jamali, M. A., & da Silva, S. D. (2024). Genetic Mutations Associated with Inflammatory Response Caused by HPV Integration in Oropharyngeal Squamous Cell Carcinoma. Biomedicines, 12(1), 24. https://doi.org/10.3390/biomedicines12010024











