Gene-Expression Patterns of Tumor and Peritumor Tissues of Smoking and Non-Smoking HPV-Negative Patients with Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characterization and Ethical Approval
2.2. Testing the HPV Status of the Patients
2.3. Histopathologic and Immunohistochemistry Analysis
2.4. Determination of Gene Expression Levels
2.5. Statistical Analysis
3. Results
3.1. Verification of Patients’ HPV Status
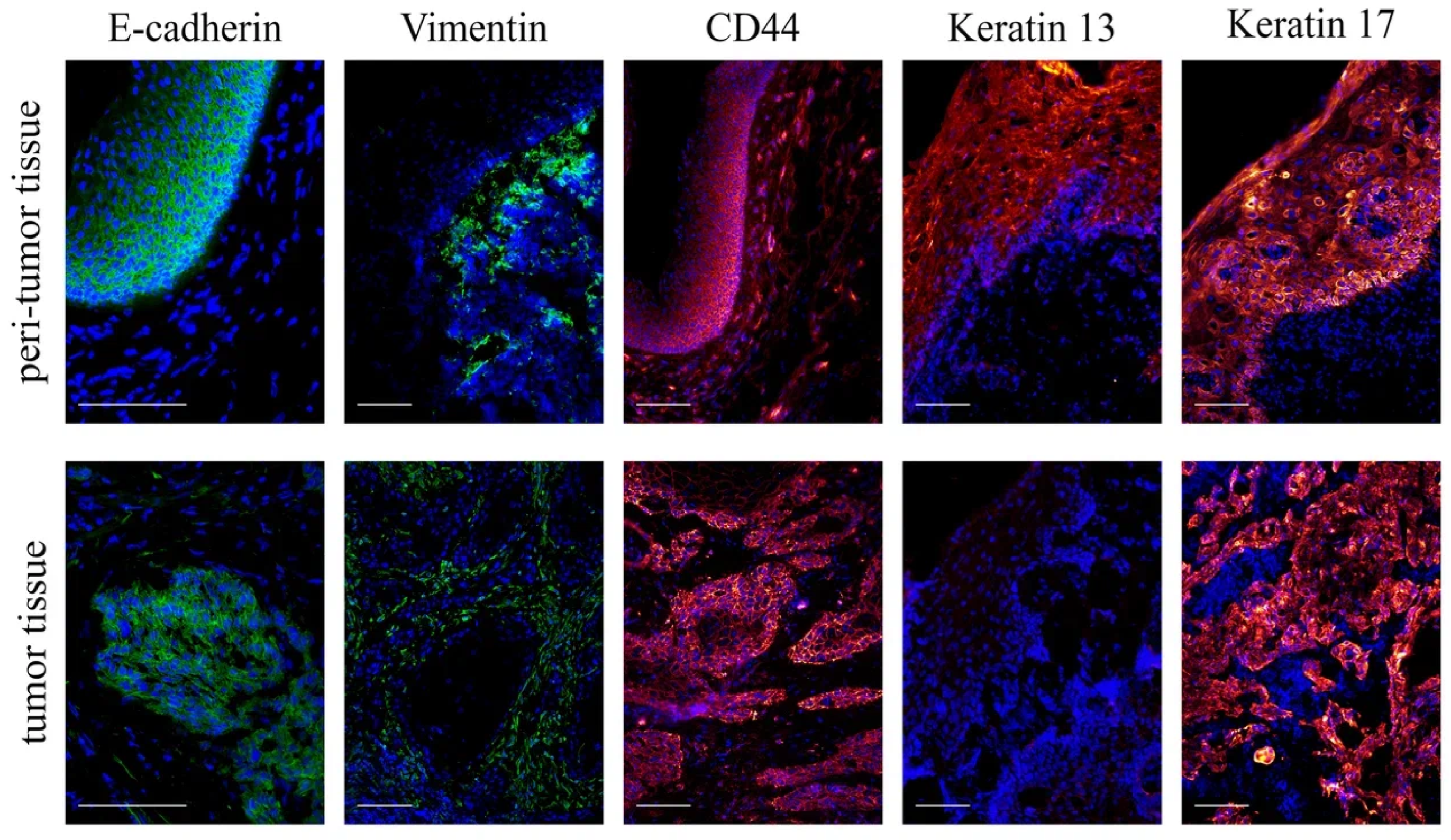
3.2. Histological Examination of Tumor and Peritumor Tissues
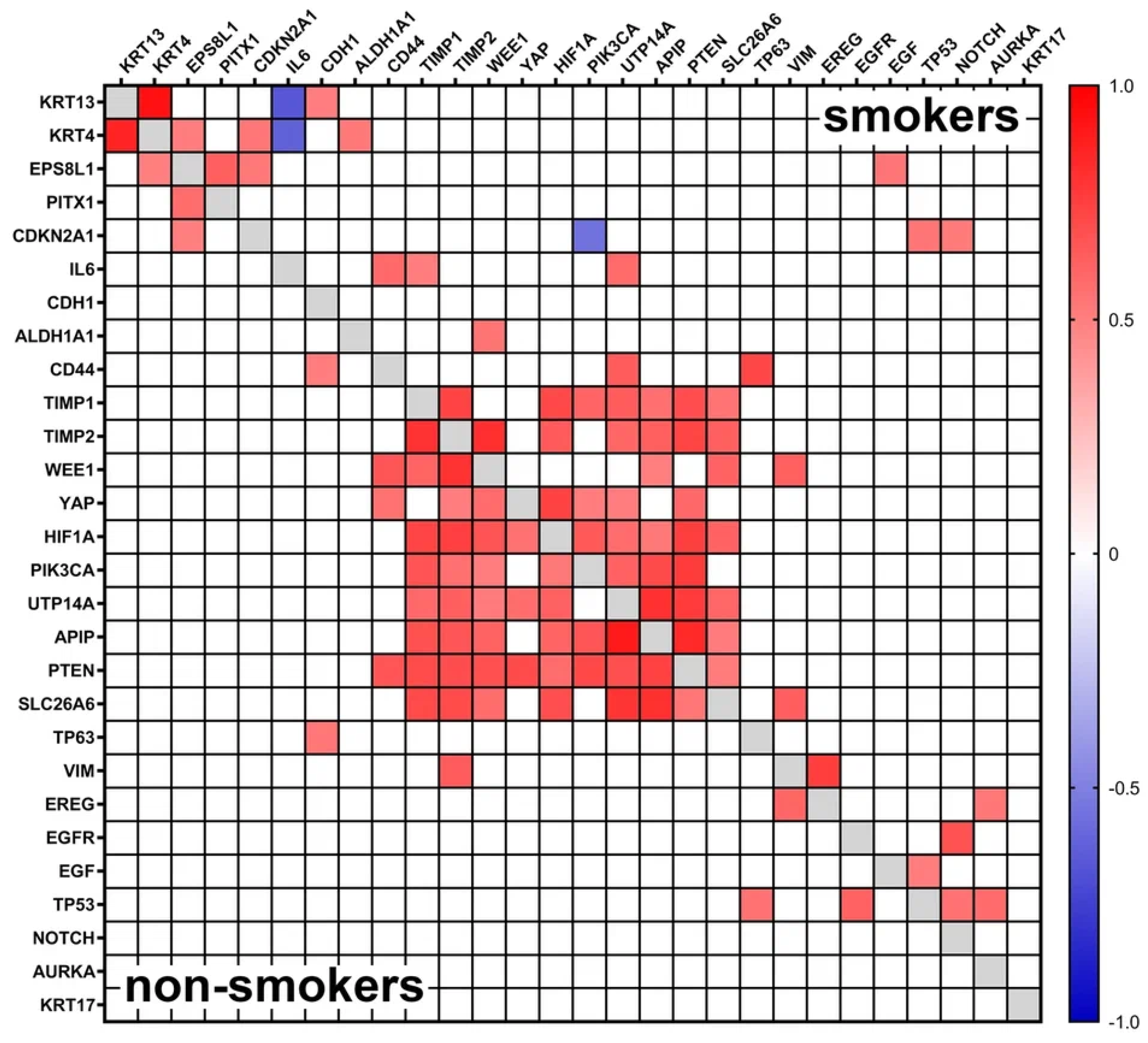
3.3. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Canning, M.; Guo, G.; Yu, M.; Myint, C.; Groves, M.W.; Byrd, J.K.; Cui, Y. Heterogeneity of the head and neck squamous cell carcinoma immune landscape and its impact on immunotherapy. Front. Cell Dev. Biol. 2019, 7, 52. [Google Scholar] [CrossRef]
- Powell, S.F.; Vu, L.; Spanos, W.C.; Pyeon, D. The key differences between human papillomavirus-positive and -negative head and neck cancers: Biological and clinical implications. Cancers 2021, 13, 5206. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Burtness, B. Oropharyngeal squamous cell carcinoma treatment: Current standards and future directions. Curr. Opin. Oncol. 2014, 26, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S. Molecular landscape of head and neck cancer and implications for therapy. Ann. Transl. Med. 2021, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Khariwala, S.S.; Ma, B.; Ruszczak, C.; Carmella, S.G.; Lindgren, B.; Hatsukami, D.K.; Hecht, S.S.; Stepanov, I. High level of tobacco carcinogen-derived DNA damage in oral cells is an independent predictor of oral/head and neck cancer risk in smokers. Cancer Prev. Res. 2017, 10, 507–513. [Google Scholar] [CrossRef]
- Hecht, S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 2003, 3, 733–744. [Google Scholar] [CrossRef]
- Furrukh, M. Tobacco smoking and lung cancer: Perception-changing facts. Sultan Qaboos Univ. Med. J. 2013, 13, 345–358. [Google Scholar] [CrossRef]
- Veyrat-Follet, C.; Bruno, R.; Olivares, R.; Rhodes, G.R.; Chaikin, P. Clinical trial simulation of docetaxel in patients with cancer as a tool for dosage optimization. Clin. Pharmacol. Ther. 2000, 68, 677–687. [Google Scholar] [CrossRef]
- Zevallos, J.P.; Mallen, M.J.; Lam, C.Y.; Karam-Hage, M.; Blalock, J.; Wetter, D.W.; Garden, A.S.; Sturgis, E.M.; Cinciripini, P.M. Complications of radiotherapy in laryngopharyngeal cancer. Cancer 2009, 115, 4636–4644. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Chen, L.M.; Vaughan, A.; Sreeraman, R.; Farwell, D.G.; Luu, Q.; Lau, D.H.; Stuart, K.; Purdy, J.A.; Vijayakumar, S. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Zhang, L.; Zhao, X.C.; Gao, S.H.; Qu, L.W.; Yu, H.; Fang, W.F.; Zhou, Y.C.; Liang, F.; Zhang, C.; et al. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat. Commun. 2019, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Y.; Du, Q.; Peng, X.; Jin, J.; Guo, H.; Li, Y.; Li, Q. Effects of Clinicopathological Characteristics on the Survival of Patients Treated with PD-1/PD-L1 Inhibitor Monotherapy or Combination Therapy for Advanced Cancer: A Systemic Review and Meta-Analysis. J. Immunol. Res. 2020, 2020, 5269787. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Sobus, S.L.; Warren, G.W. The biologic effects of cigarette smoke on cancer cells. Cancer 2014, 120, 3617–3626. [Google Scholar] [CrossRef]
- Gislon, L.C.; Curado, M.P.; López, R.V.M.; de Oliveira, J.C.; de Podestá, J.R.V.; von Zeidler, S.V.; Brennan, P.; Kowalski, L.P. Risk factors associated with head and neck cancer in former smokers: A Brazilian multicentric study. Cancer Epidemiol. 2022, 78, 102143. [Google Scholar] [CrossRef] [PubMed]
- Kiani, M.N.; Asif, M.; Ansari, F.M.; Ara, N.; Ishaque, M.; Khan, A.R. Diagnostic utility of Cytokeratin 13 and Cytokeratin 17 in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Biol. 2020, 5, 153–158. [Google Scholar] [CrossRef]
- Liu, S.; Ye, D.; Xu, D.; Liao, Y.; Zhang, L.; Liu, L.; Yu, W.; Wang, Y.; He, Y.; Hu, J.; et al. Autocrine epiregulin activates EGFR pathway for lung metastasis via EMT in salivary adenoid cystic carcinoma. Oncotarget 2016, 7, 25251–25263. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Kim, J. The potential role of YAP in head and neck squamous cell carcinoma. Exp. Mol. Med. 2020, 52, 1264–1274. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Yi, C.H.; Zhai, Q.; Wang, B.Y. Updates on immunohistochemical and molecular markers in selected head and neck diagnostic problems. Arch. Pathol. Lab. Med. 2017, 141, 1214–1235. [Google Scholar] [CrossRef]
- Caruntu, A.; Moraru, L.; Lupu, M.; Ciubotaru, D.A.; Dumitrescu, M.; Eftimie, L.; Hertzog, R.; Zurac, S.; Caruntu, C.; Voinea, O.C. Assessment of histological features in squamous cell carcinoma involving head and neck skin and mucosa. J. Clin. Med. 2021, 10, 2343. [Google Scholar] [CrossRef]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef]
- Sakamoto, K.; Aragaki, T.; Morita, K.I.; Kawachi, H.; Kayamori, K.; Nakanishi, S.; Omura, K.; Miki, Y.; Okada, N.; Katsube, K.I.; et al. Down-regulation of keratin 4 and keratin 13 expression in oral squamous cell carcinoma and epithelial dysplasia: A clue for histopathogenesis. Histopathology 2011, 58, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Søland, T.M.; Bjerkli, I.H.; Sand, L.P.; Petersen, F.C.; Costea, D.E.; Senguven, B.; Sapkota, D. Combined loss of expression of involucrin and cytokeratin 13 is associated with poor prognosis in squamous cell carcinoma of mobile tongue. Head Neck 2021, 43, 3374–3385. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, R.; Toyoshima, T.; Tanaka, H.; Kawano, S.; Kiyosue, T.; Matsubara, R.; Goto, Y.; Hirano, M.; Oobu, K.; Nakamura, S. Association of cytokeratin 17 expression with differentiation in oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2012, 138, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Khanom, R.; Nguyen, C.T.K.; Kayamori, K.; Zhao, X.; Morita, K.; Miki, Y.; Katsube, K.I.; Yamaguchi, A.; Sakamoto, K. Keratin 17 is induced in oral cancer and facilitates tumor growth. PLoS ONE 2016, 11, e0161163. [Google Scholar] [CrossRef]
- Liu, C.Y.; Lin, H.H.; Tang, M.J.; Wang, Y.K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966–15983. [Google Scholar] [CrossRef] [PubMed]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.T.; Waseem, A. Vimentin is at the heart of epithelial mesenchymal transition (EMT) mediated metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Gupta, S.K.; Agarwal, S.; Singh, S.N.; Sehra, R.; Jat, P.S.; Singhal, P. Role of Vimentin and E-cadherin Expression in Premalignant and Malignant Lesions of Oral Cavity. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 350–355. [Google Scholar] [CrossRef]
- Kuburich, N.A.; den Hollander, P.; Pietz, J.T.; Mani, S.A. Vimentin and cytokeratin: Good alone, bad together. Semin. Cancer Biol. 2022, 86, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Nijkamp, M.M.; Span, P.N.; Hoogsteen, I.J.; Van Der Kogel, A.J.; Kaanders, J.H.A.M.; Bussink, J. Expression of E-cadherin and vimentin correlates with metastasis formation in head and neck squamous cell carcinoma patients. Radiother. Oncol. 2011, 99, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.S.; Ceausu, A.R.; Comsa, S.; Raica, M. Loss of E-Cadherin Expression Correlates With Ki-67 in Head and Neck Squamous Cell Carcinoma. In Vivo 2022, 36, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, J.; Lin, X.; Wang, X. E-cadherin expression and prognosis of head and neck squamous cell carcinoma: Evidence from 19 published investigations. OncoTargets Ther. 2016, 9, 2447–2453. [Google Scholar] [CrossRef]
- Lin, C.W.; Yang, W.E.; Su, C.W.; Lu, H.J.; Su, S.C.; Yang, S.F. IGF2BP2 promotes cell invasion and epithelial-mesenchymal transition through Src-mediated upregulation of EREG in oral cancer. Int. J. Biol. Sci. 2024, 20, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, J.; Lu, J.; Xiong, H.; Shi, X.; Gong, L. Significance of CD44 expression in head and neck cancer: A systemic review and meta-analysis. BMC Cancer 2014, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Osman, A.A.; Takahashi, Y.; Lindemann, A.; Patel, A.A.; Zhao, M.; Takahashi, H.; Myers, J.N. Head and neck cancer organoids established by modification of the CTOS method can be used to predict in vivo drug sensitivity. Oral Oncol. 2018, 87, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, M.; Raudenska, M.; Gumulec, J.; Balvan, J.; Fojtu, M.; Kratochvilova, M.; Polanska, H.; Horakova, Z.; Kostrica, R.; Babula, P.; et al. Establishment of oral squamous cell carcinoma cell line and magnetic bead-based isolation and characterization of its CD90/CD44 subpopulations. Oncotarget 2017, 8, 66254–66269. [Google Scholar] [CrossRef][Green Version]
- Zhou, C.; Sun, B. The prognostic role of the cancer stem cell marker aldehyde dehydrogenase 1 in head and neck squamous cell carcinomas: A meta-analysis. Oral Oncol. 2014, 50, 1144–1148. [Google Scholar] [CrossRef]
- Qian, X.; Coordes, A.; Kaufmann, A.M.; Albers, A.E. Expression of aldehyde dehydrogenase family 1 member A1 and high mobility group box 1 in oropharyngeal squamous cell carcinoma in association with survival time. Oncol. Lett. 2016, 12, 3429–3434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Španko, M.; Strnadová, K.; Pavlíček, A.J.; Szabo, P.; Kodet, O.; Valach, J.; Dvořánková, B.; Smetana, K.; Lacina, L. IL-6 in the ecosystem of head and neck cancer: Possible therapeutic perspectives. Int. J. Mol. Sci. 2021, 22, 11027. [Google Scholar] [CrossRef] [PubMed]
- Kita, H.; Shiraishi, Y.; Watanabe, K.; Suda, K.; Ohtsuka, K.; Koshiishi, Y.; Goya, T. Does postoperative serum interleukin-6 influence early recurrence after curative pulmonary resection of lung cancer? Ann. Thorac. Cardiovasc. Surg. 2011, 17, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.M.; France, T.J.; Teknos, T.N.; Kumar, P. Interleukin-6 role in head and neck squamous cell carcinoma progression. World J. Otorhinolaryngol.-Head Neck Surg. 2016, 2, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Tartour, E.; Pere, H.; Maillere, B.; Terme, M.; Merillon, N.; Taieb, J.; Sandoval, F.; Quintin-Colonna, F.; Lacerda, K.; Karadimou, A.; et al. Angiogenesis and immunity: A bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011, 30, 83–95. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.; Van Der Plaat, D.A.; Vonk, J.M.; Boezen, H.M. No association between DNA methylation and COPD in never and current smokers. BMJ Open Respir. Res. 2018, 5, e000282. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, O.; Byrum, S.; Wolfe, A.R. Discovery of the inhibitor of DNA binding 1 as a novel marker for radioresistance in pancreatic cancer using genome-wide RNA-seq. Cancer Drug Resist. 2022, 5, 926–938. [Google Scholar] [CrossRef]
- Takenobu, M.; Osaki, M.; Fujiwara, K.; Fukuhara, T.; Kitano, H.; Kugoh, H.; Okada, F. PITX1 is a novel predictor of the response to chemotherapy in head and neck squamous cell carcinoma. Mol. Clin. Oncol. 2016, 5, 89–94. [Google Scholar] [CrossRef]
- Libório, T.N.; Acquafreda, T.; Matizonkas-Antonio, L.F.; Silva-Valenzuela, M.G.; Ferraz, A.R.; Nunes, F.D. In situ hybridization detection of homeobox genes reveals distinct expression patterns in oral squamous cell carcinomas. Histopathology 2011, 58, 225–233. [Google Scholar] [CrossRef]
- Qiao, F.; Gong, P.; Song, Y.; Shen, X.; Su, X.; Li, Y.; Wu, H.; Zhao, Z.; Fan, H. Downregulated PITX1 modulated by MiR-19a-3p Promotes Cell Malignancy and Predicts a Poor Prognosis of Gastric Cancer by Affecting Transcriptionally Activated PDCD5. Cell. Physiol. Biochem. 2018, 46, 2215–2231. [Google Scholar] [CrossRef]
- Chen, Y.; Knösel, T.; Ye, F.; Pacyna-Gengelbach, M.; Deutschmann, N.; Petersen, I. Decreased PITX1 homeobox gene expression in human lung cancer. Lung Cancer 2007, 55, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Knösel, T.; Chen, Y.; Hotovy, S.; Settmacher, U.; Altendorf-Hofmann, A.; Petersen, I. Loss of desmocollin 1-3 and homeobox genes PITX1 and CDX2 are associated with tumor progression and survival in colorectal carcinoma. Int. J. Color. Dis. 2012, 27, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Stender, J.D.; Stossi, F.; Funk, C.C.; Charn, T.H.; Barnett, D.H.; Katzenellenbogen, B.S. The estrogen-regulated transcription factor PITX1 coordinates gene-specific regulation by estrogen receptor-alpha in breast cancer cells. Mol. Endocrinol. 2011, 25, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, S.; Gan, L.; Zhuang, Z. Bioinformatics analysis of prognostic value of PITX1 gene in breast cancer. Biosci. Rep. 2020, 40, BSR20202537. [Google Scholar] [CrossRef]
- Poos, A.M.; Schroeder, C.; Jaishankar, N.; Röll, D.; Oswald, M.; Meiners, J.; Braun, D.M.; Knotz, C.; Frank, L.; Gunkel, M.; et al. PITX1 Is a Regulator of TERT Expression in Prostate Cancer with Prognostic Power. Cancers 2022, 14, 1267. [Google Scholar] [CrossRef]
- Song, X.; Zhao, C.; Jiang, L.; Lin, S.; Bi, J.; Wei, Q.; Yu, L.; Zhao, L.; Wei, M. High PITX1 expression in lung adenocarcinoma patients is associated with DNA methylation and poor prognosis. Pathol. Res. Pract. 2018, 214, 2046–2053. [Google Scholar] [CrossRef]
- Jeong, A.R.; Forbes, K.; Orosco, R.K.; Cohen, E.E.W. Hereditary oral squamous cell carcinoma associated with CDKN2A germline mutation: A case report. J. Otolaryngol.-Head Neck Surg. 2022, 51, 5. [Google Scholar] [CrossRef]
- Xue, L.; Tang, W.; Zhou, J.; Xue, J.; Li, Q.; Ge, X.; Lin, F.; Zhao, W.; Guo, Y. Next-generation sequencing identifies CDKN2A alterations as prognostic biomarkers in recurrent or metastatic head and neck squamous cell carcinoma predominantly receiving immune checkpoint inhibitors. Front. Oncol. 2023, 13, 1276009. [Google Scholar] [CrossRef]
- Shah, P.A.; Huang, C.; Li, Q.; Kazi, S.A.; Byers, L.A.; Wang, J.; Johnson, F.M.; Frederick, M.J. NOTCH1 Signaling in Head and Neck Squamous Cell Carcinoma. Cells 2020, 9, 2677. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, S.M.; Ellisen, L.W. The molecular pathogenesis of head and neck squamous cell carcinoma. J. Clin. Investig. 2012, 122, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Foy, J.P.; Bazire, L.; Ortiz-Cuaran, S.; Deneuve, S.; Kielbassa, J.; Thomas, E.; Viari, A.; Puisieux, A.; Goudot, P.; Bertolus, C.; et al. A 13-gene expression-based radioresistance score highlights the heterogeneity in the response to radiation therapy across HPV-negative HNSCC molecular subtypes. BMC Med. 2017, 15, 165. [Google Scholar] [CrossRef]
- Huang, C.; Chen, L.; Savage, S.R.; Eguez, R.V.; Dou, Y.; Li, Y.; da Veiga Leprevost, F.; Jaehnig, E.J.; Lei, J.T.; Wen, B.; et al. Proteogenomic insights into the biology and treatment of HPV-negative head and neck squamous cell carcinoma. Cancer Cell 2021, 39, 361–379.e16. [Google Scholar] [CrossRef] [PubMed]
- Rehmani, H.S.; Issaeva, N. EGFR in head and neck squamous cell carcinoma: Exploring possibilities of novel drug combinations. Ann. Transl. Med. 2020, 8, 813. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ulekleiv, C.H.; Halstensen, T.S. Epidermal growth factor (EGF) receptor-ligand based molecular staging predicts prognosis in head and neck squamous cell carcinoma partly due to deregulated EGF- induced amphiregulin expression. J. Exp. Clin. Cancer Res. 2016, 35, 151. [Google Scholar] [CrossRef] [PubMed]
- Steurer, S.; Riemann, C.; Büscheck, F.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Höflmayer, D.; Weidemann, S.; Fraune, C.; et al. p63 expression in human tumors and normal tissues: A tissue microarray study on 10,200 tumors. Biomark. Res. 2021, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Compagnone, M.; Gatti, V.; Presutti, D.; Ruberti, G.; Fierro, C.; Markert, E.K.; Vousden, K.H.; Zhou, H.; Mauriello, A.; Anemone, L.; et al. ΔNp63-mediated regulation of hyaluronic acid metabolism and signaling supports HNSCC tumorigenesis. Proc. Natl. Acad. Sci. USA 2017, 114, 13254–13259. [Google Scholar] [CrossRef] [PubMed]
- Carpén, T.; Sorsa, T.; Jouhi, L.; Tervahartiala, T.; Haglund, C.; Syrjänen, S.; Tarkkanen, J.; Mohamed, H.; Mäkitie, A.; Hagström, J.; et al. High levels of tissue inhibitor of metalloproteinase-1 (TIMP-1) in the serum are associated with poor prognosis in HPV-negative squamous cell oropharyngeal cancer. Cancer Immunol. Immunother. 2019, 68, 1263–1272. [Google Scholar] [CrossRef]
- Yuan, M.L.; Li, P.; Xing, Z.H.; Di, J.M.; Liu, H.; Yang, A.K.; Lin, X.J.; Jiang, Q.W.; Yang, Y.; Huang, J.R.; et al. Inhibition of WEE1 Suppresses the Tumor Growth in Laryngeal Squamous Cell Carcinoma. Front. Pharmacol. 2018, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Parsons, J.L. The radiobiology of HPV-positive and HPV-negative head and neck squamous cell carcinoma. Expert Rev. Mol. Med. 2020, 22, e3. [Google Scholar] [CrossRef]
- Aggarwal, N.; Yadav, J.; Thakur, K.; Bibban, R.; Chhokar, A.; Tripathi, T.; Bhat, A.; Singh, T.; Jadli, M.; Singh, U.; et al. Human Papillomavirus Infection in Head and Neck Squamous Cell Carcinomas: Transcriptional Triggers and Changed Disease Patterns. Front. Cell. Infect. Microbiol. 2020, 10, 537650. [Google Scholar] [CrossRef]
- Santos-De-Frutos, K.; Segrelles, C.; Lorz, C. Hippo pathway and YAP signaling alterations in squamous cancer of the head and neck. J. Clin. Med. 2019, 8, 2131. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lin, S.; Lu, Y.; Mao, L.; Li, S.; Li, Z. C12orf59 Promotes Esophageal Squamous Cell Carcinoma Progression via YAP-Mediated Epithelial-Mesenchymal Transition. Front. Oncol. 2022, 12, 927249. [Google Scholar] [CrossRef]
- Ahmad, U.S.; Parkinson, E.K.; Wan, H. Desmoglein-3 induces YAP phosphorylation and inactivation during collective migration of oral carcinoma cells. Mol. Oncol. 2022, 16, 1625–1649. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Mao, C.Y.; Ma, Q.; Bao, T.; Wang, Y.J.; Guo, W.; Zhao, X.L. U three protein 14a (UTP14A) promotes tumour proliferation and metastasis via the PERK/eIF2a/GRP78 signalling pathway in oesophageal squamous cell carcinoma. J. Cancer 2021, 12, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.X.; Inoue, J.; Harada, H.; Inazawa, J. Potential for reversing miR-634-mediated cytoprotective processes to improve efficacy of chemotherapy against oral squamous cell carcinoma. Mol. Ther. Oncolytics 2022, 24, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, S.; Liu, S.; Liao, X.; Yan, S.; Liu, Q. SLC26 family: A new insight for kidney stone disease. Front. Physiol. 2023, 14, 1118342. [Google Scholar] [CrossRef]
- Cao, J.; Wang, P.; Chen, J.; He, X. Systemic characterization of the SLC family genes reveals SLC26A6 as a novel oncogene in hepatocellular carcinoma. Transl. Cancer Res. 2021, 10, 2882–2894. [Google Scholar] [CrossRef]
- Lee, D.; Lee, P.C.W.; Hong, J.H.; Shin, D.M. Estrogen treatment reduced oxalate transporting activity and enhanced migration through the involvement of SLC26A6 in lung cancer cells. Toxicol. In Vitro 2022, 82, 105373. [Google Scholar] [CrossRef]



| Characteristics | Nonsmokers, n (%) | Smokers, n (%) | Total, n (%) |
|---|---|---|---|
| Smoking status | 17 (65.3%) | 9 (34.7%) | 26 (100.0%) |
| Sex | |||
| Males | 8 (47.1%) | 7 (77.8%) | 15 (57.7%) |
| Females | 9 (52.9%) | 2 (22.2%) | 11 (42.3%) |
| Age (in years) | |||
| Median | 59 | 63 | 60,5 |
| Range | 19–82 | 50–64 | 19–82 |
| T stage | |||
| cT1 | 1 (5.9%) | 1 (11.1%) | 2 (7.7%) |
| cT2 | 7 (41.2%) | 1 (11.1%) | 8 (30.8%) |
| cT3 | 4 (23.5%) | 2 (22.2%) | 6 (23.1%) |
| cT4 | 5 (29.4%) | 5 (55.6%) | 10 (38.4%) |
| N stage | |||
| cN0 | 16 (94.1%) | 7 (77.8%) | 23(88.5%) |
| cN1 | 0 (0%) | 1 (11.1%) | 1 (3.8%) |
| cN2 | 1 (5.9%) | 1 (11.1%) | 2 (7.7%) |
| Differentiation | |||
| Well | 11 (64.7%) | 6 (66.7%) | 17 (65.4%) |
| Moderate | 5 (29.4%) | 2 (22.2%) | 7 (26.9%) |
| Poor | 1 (5.9%) | 1 (11.1%) | 2 (7.7%) |
| Keratinization | |||
| Keratinizing | 11 (64.7%) | 7 (77.8%) | 18 (69.2%) |
| Non-keratinizing | 6 (35.3%) | 2 (22.2%) | 8 (30.8%) |
| Location | |||
| alveolar ridge | 2 (11.8%) | 2 (22.2%) | 4 (15.4%) |
| larynx | 2 (11.8%) | 3 (33.3%) | 5 (19.2%) |
| oral cavity | 6 (35.3%) | 1 (11.1%) | 7 (26.9%) |
| mobile tongue | 7 (41.1%) | 3 (33.3%) | 10 (38.5%) |
| Gene | (5′ to 3′) | (3′ to 5′) |
|---|---|---|
| ALDH1A1 | GATTGGATCCCCGTGGCGTA | GATTGGATCCCCGTGGCGTA |
| APIP | CGCGCAGGACAAGGAGCAT | CTTCGATGGCGAAGGTCCAC |
| AURKA | AGTGGCGGAGCGTCAAGTC | AGTGGCGGAGCGTCAAGTC |
| CDH1 | ACTGATGCTGATGCCCCCAA | ACTGATGCTGATGCCCCCAA |
| CDKN2A | CTGCCCAACGCACCGAATAG | CTGCCCAACGCACCGAATAG |
| CD44 | AGGAGCAGCACTTCAGGAGG | AGGAGCAGCACTTCAGGAGG |
| EGF | TTCACTGTCTTGACTCTACTCCACC | TTCACTGTCTTGACTCTACTCCACC |
| EGFR | CCCCCTGACTCCGTCCAGTA | CCCCCTGACTCCGTCCAGTA |
| EPS8L1 | GGAAGGGAAAGGACAGCGGA | CTCACCCAGGCAGAACGTCA |
| EREG | TGCTCTGCCTGGGTTTCCAT | TGCTCTGCCTGGGTTTCCAT |
| HIF1A | GCCCATTCCGCGTCTGAGT | GCCCATTCCGCGTCTGAGT |
| IL-6 | GGTATACCTAGAGTACCTCCA | GGTATACCTAGAGTACCTCCA |
| KRT4 | GATCGCCACCTACCGCAAAC | GATCGCCACCTACCGCAAAC |
| KRT13 | GGGACTCATCAGCAGCATCG | GGGACTCATCAGCAGCATCG |
| KRT17 | AGATTGCCACCTACCGCCG | AGATTGCCACCTACCGCCG |
| NOTCH1 | CCCACTCATTCTGGTTGTCG | CCCACTCATTCTGGTTGTCG |
| PITX1 | AACCGCTACCCCGACATGAG | CTGCACTAGGCCGCTGAACT |
| PIK3CA | TTCCGGGGGATTGTAGGCTC | TTCCGGGGGATTGTAGGCTC |
| PTEN | CCCAGTCAGAGGCGCTATGT | CCCAGTCAGAGGCGCTATGT |
| SLC26A6 | AGACAGCCAGAGATGCTGCC | GTAGGTGACCACGAAGCCGA |
| TIMP1 | CCTTCCAGGTGTTTCCCTGTT | CCTTCCAGGTGTTTCCCTGTT |
| TIMP2 | GACCCACAAGGAGATTGGGG | GACCCACAAGGAGATTGGGG |
| TP53 | GTGCTTTCCACGACGGTGAC | GTGCTTTCCACGACGGTGAC |
| TP63 | GTGTTGGAGGGATGAACCGC | GTGTTGGAGGGATGAACCGC |
| UTP14 | TCTGGCTTTGAGCCAACAGG | GACCTCTCAGCCAATTTCCGC |
| VIM | TCAATCGGCGGGACAGCAG | TCAATCGGCGGGACAGCAG |
| WEE1 | AACAATGGGCCTCGTCTGGA | AACAATGGGCCTCGTCTGGA |
| YAP | CAGCAACTCCAACCAGCAGC | CAGCAACTCCAACCAGCAGC |
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soboleva, A.; Arutyunyan, I.; Jumaniyazova, E.; Vishnyakova, P.; Zarubina, D.; Nimatov, E.; Elchaninov, A.; Fatkhudinov, T. Gene-Expression Patterns of Tumor and Peritumor Tissues of Smoking and Non-Smoking HPV-Negative Patients with Head and Neck Squamous Cell Carcinoma. Biomedicines 2024, 12, 696. https://doi.org/10.3390/biomedicines12030696
Soboleva A, Arutyunyan I, Jumaniyazova E, Vishnyakova P, Zarubina D, Nimatov E, Elchaninov A, Fatkhudinov T. Gene-Expression Patterns of Tumor and Peritumor Tissues of Smoking and Non-Smoking HPV-Negative Patients with Head and Neck Squamous Cell Carcinoma. Biomedicines. 2024; 12(3):696. https://doi.org/10.3390/biomedicines12030696
Chicago/Turabian StyleSoboleva, Anna, Irina Arutyunyan, Enar Jumaniyazova, Polina Vishnyakova, Daria Zarubina, Eldar Nimatov, Andrey Elchaninov, and Timur Fatkhudinov. 2024. "Gene-Expression Patterns of Tumor and Peritumor Tissues of Smoking and Non-Smoking HPV-Negative Patients with Head and Neck Squamous Cell Carcinoma" Biomedicines 12, no. 3: 696. https://doi.org/10.3390/biomedicines12030696
APA StyleSoboleva, A., Arutyunyan, I., Jumaniyazova, E., Vishnyakova, P., Zarubina, D., Nimatov, E., Elchaninov, A., & Fatkhudinov, T. (2024). Gene-Expression Patterns of Tumor and Peritumor Tissues of Smoking and Non-Smoking HPV-Negative Patients with Head and Neck Squamous Cell Carcinoma. Biomedicines, 12(3), 696. https://doi.org/10.3390/biomedicines12030696










