The State-of-the-Art Mechanisms and Antitumor Effects of Somatostatin in Colorectal Cancer: A Review
Abstract
1. Introduction
2. Cellular Sources and Function of Somatostatin in Normal Colon
3. Somatostatin and the Histological Spectrum of CRC
4. Antitumor Effects of Somatostatin in CRC
4.1. Inhibition of Cell Proliferation
4.1.1. Evidence from In Vivo Studies
4.1.2. Evidence from In Vitro and Animal Model Studies
4.1.3. Mechanisms of the Antiproliferative Action of SST
4.2. Pro-Apoptotic Effects of Somatostatin
4.2.1. Evidence from In Vivo Studies
4.2.2. Evidence from In Vitro and Animal Model Studies
4.2.3. Mechanisms of the Pro-Apoptotic Action of SST
4.3. Antiangiogenic Effects of Somatostatin
4.3.1. Evidence from In Vivo Studies
4.3.2. Evidence from In Vitro and Animal Model Studies
4.4. Anti-Inflammatory Effects of Somatostatin
4.4.1. Evidence from In Vivo Studies
4.4.2. Evidence from In Vitro and Animal Model Studies
4.5. Somatostatin and Colorectal Cancer’s Colon Microbiome (Microbiota)
5. Epigenetic Alterations of SST Gene in CRC and Implications for Anticancer Effects
6. Somatostatin—Implications for Cancer Therapy
6.1. Somatostatin versus Somatostatin Analogues: Structural Characteristics, Pharmacokinetics, Pharmacodynamics and Bioavailability
6.2. Somatostatin Analogues in the Therapy of Colon Neuroendocrine Tumors
6.3. Somatostatin Analogues in the Therapy of Colorectal Cancer (CRC)
6.4. Somatostatin Analogues in the Therapy of Selected Non-Endocrine Cancers beyond CRC
6.4.1. Breast Cancer (BC)
6.4.2. Prostate Cancer (PC)
6.4.3. Lung Cancer (LC)
6.4.4. Hepatocellular Carcinoma (HCC)
7. Concluding Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALDH | aldehyde dehydrogenase |
| AKT | serine/threonine-protein kinase or protein kinase B (PKB) |
| APC | adenomatous polyposis coli |
| CGRP | calcitonin gene-related peptide |
| CD | Crohn’s disease |
| c-Met | tyrosine-protein kinase Met or hepatocyte growth factor receptor |
| CRC | colorectal cancer |
| CSCs | cancer stem cells |
| DSS | dextran sodium sulfate |
| ECL | enterochromaffin-like |
| EECs | enteroendocrine cells |
| ENS | enteric nervous system |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| GH | growth hormone |
| GPCR | G protein-coupled receptor |
| 5-HT | 5-hydroxytryptamine (serotonin) |
| HNPCC | hereditary non-polyposis CRC |
| IBD | inflammatory bowel disease |
| IC50 | half-maximal inhibitory concentration |
| IHC | immunohistochemistry |
| IL-6/12 | interleukin-6/12 |
| IFN-γ | interferon γ |
| ISH | in situ hybridization |
| MANEC/MiNENs | mixed adenoendocrine carcinomas |
| MAP | mitogen-activated protein kinase (originally called ERK) |
| MIN/MSI | microsatellite instability |
| NCs | neuroendocrine cells |
| NEN | neuroendocrine neoplasm |
| NET | neuroendocrine tumor |
| OCT | octreotide |
| OS | overall survival |
| PTP | protein phosphotyrosine phosphatase |
| SCs | stem cells |
| SH2 | Src homology 2 |
| SHP1/2 | Src homology 2 domain phosphatase 1/2 |
| SRCC | signet-ring cell carcinoma |
| SRIF/SRIH/SST | somatotropin-release inhibitory factor/SRI hormone/somatostatin |
| SSAs | somatostatin analogues |
| SSTRs | somatostatin receptors |
| TNBS | trinitrobenzene sulfonic acid |
| UC | ulcerative colitis |
| VIP | vasoactive intestinal peptide |
| Wnt | gene wingless + integrated or int-1 |
References
- World Health Organization. Tenth Revision. In International Statistical Classification of Diseases and Related Health Problems; WHO: Geneva, Switzerland, 2013; Volume 2. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer. 2021, 157, 308–347. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Vogelstein, B.; Fearon, E.R.; Hamilton, S.R.; Kern, S.E.; Preisinger, A.C.; Leppert, M.; Nakamura, Y.; White, R.; Smits, A.M.; Bos, J.L. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988, 319, 525–532. [Google Scholar] [CrossRef]
- Senga, S.S.; Grose, R.P. Hallmarks of cancer—The new testament. Open Biol. 2021, 11, 200358. [Google Scholar] [CrossRef]
- Karin, M.; Shalapour, S. Regulation of antitumor immunity by inflammation-induced epigenetic alterations. Cell Mol. Immunol. 2022, 19, 59–66. [Google Scholar] [CrossRef]
- Park, C.H.; Eun, C.S.; Han, D.S. Intestinal microbiota, chronic inflammation, and colorectal cancer. Intest. Res. 2018, 16, 338–345. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Geng, C.; Guo, Y.; Wang, C. Somatostatin receptor 5 is critical for protecting intestinal barrier function in vivo and in vitro. Mol. Cell. Endocrinol. 2021, 535, 111390. [Google Scholar] [CrossRef]
- Overacre-Delgoffe, A.E.; Bumgarner, H.J.; Cillo, A.R.; Burr, A.H.P.; Tometich, J.T.; Bhattacharjee, A.; Bruno, T.C.; Vignali, D.A.A.; Hand, T.W. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity 2021, 54, 2812–2824.e4. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Xie, Y.; Xie, F.; Zhou, X.; Zhang, L.; Yang, B.; Huang, J.; Wang, F.; Yan, H.; Zeng, L.; Zhang, L.; et al. Microbiota in Tumors: From Understanding to Application. Adv. Sci. 2022, 9, e2200470. [Google Scholar] [CrossRef]
- Krulich, L.; Dhariwal, A.P.; McCann, S.M. Stimulatory and inhibitory effects of purified hypothalamic extracts on growth hormone release from rat pituitary in vitro. Endocrinology 1968, 83, 783–790. [Google Scholar] [CrossRef]
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef]
- Florio, T. Somatostatin/somatostatin receptor signalling: Phosphotyrosine phosphatases. Mol. Cell. Endocrinol. 2008, 286, 40–48. [Google Scholar] [CrossRef]
- Vanetti, M.; Kouba, M.; Wang, X.; Vogt, G.; Höllt, V. Cloning and expression of a novel mouse somatostatin receptor (SSTR2B). FEBS Lett. 1992, 311, 290–294. [Google Scholar] [CrossRef]
- Schindler, M.; Kidd, E.J.; Carruthers, A.M.; Wyatt, M.A.; Jarvie, E.M.; Sellers, L.A.; Feniuk, W.; Humphrey, P.P. Molecular cloning and functional characterization of a rat somatostatin sst2(b) receptor splice variant. Br. J. Pharmacol. 1998, 125, 209–217. [Google Scholar] [CrossRef][Green Version]
- Benali, N.; Ferjoux, G.; Puente, E.; Buscail, L.; Susini, C. Somatostatin receptors. Digestion 2000, 62 (Suppl. S1), 27–32. [Google Scholar] [CrossRef]
- Lahlou, H.; Guillermet, J.; Hortala, M.; Vernejoul, F.; Pyronnet, S.; Bousquet, C.; Susini, C. Molecular signaling of somatostatin receptors. Ann. N. Y. Acad. Sci. 2004, 1014, 121–131. [Google Scholar] [CrossRef]
- Barbieri, F.; Bajetto, A.; Pattarozzi, A.; Gatti, M.; Würth, R.; Thellung, S.; Corsaro, A.; Villa, V.; Nizzari, M.; Florio, T. Peptide receptor targeting in cancer: The somatostatin paradigm. Int. J. Pept. 2013, 2013, 926295. [Google Scholar] [CrossRef]
- Gomes-Porras, M.; Cárdenas-Salas, J.; Álvarez-Escolá, C. Somatostatin Analogs in Clinical Practice: A Review. Int. J. Mol. Sci. 2020, 21, 1682. [Google Scholar] [CrossRef]
- Kumar, U. Somatostatin and Somatostatin Receptors in Tumour Biology. Int. J. Mol. Sci. 2023, 25, 436. [Google Scholar] [CrossRef]
- Laczmanska, I.; Sasiadek, M.M. Tyrosine phosphatases as a superfamily of tumor suppressors in colorectal cancer. Acta Biochim. Pol. 2011, 58, 467–470. [Google Scholar]
- Gatto, F.; Barbieri, F.; Arvigo, M.; Thellung, S.; Amarù, J.; Albertelli, M.; Ferone, D.; Florio, T. Biological and Biochemical Basis of the Differential Efficacy of First and Second Generation Somatostatin Receptor Ligands in Neuroendocrine Neoplasms. Int. J. Mol. Sci. 2019, 20, 3940. [Google Scholar] [CrossRef]
- Ferjoux, G.; Bousquet, C.; Cordelier, P.; Benali, N.; Lopez, F.; Rochaix, P.; Buscail, L.; Susini, C. Signal transduction of somatostatin receptors negatively controlling cell proliferation. J. Physiol. Paris 2000, 94, 205–210. [Google Scholar] [CrossRef]
- Pyronnet, S.; Bousquet, C.; Najib, S.; Azar, R.; Laklai, H.; Susini, C. Antitumor effects of somatostatin. Mol. Cell. Endocrinol. 2008, 286, 230–237. [Google Scholar] [CrossRef]
- Keskin, O.; Yalcin, S. A review of the use of somatostatin analogs in oncology. OncoTargets Ther. 2013, 6, 471–483. [Google Scholar] [CrossRef][Green Version]
- Ramírez-Perdomo, A.; Márquez-Barrios, G.; Gutiérrez-Castañeda, L.D.; Parra-Medina, R. Neuroendocrine Peptides in the pathogenesis of colorectal carcinoma. Exp. Oncol. 2023, 45, 3–16. [Google Scholar] [CrossRef]
- Strosberg, J.; Kvols, L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J. Gastroenterol. 2010, 16, 2963–2970. [Google Scholar] [CrossRef]
- Baldelli, R.; Barnabei, A.; Rizza, L.; Isidori, A.M.; Rota, F.; Di Giacinto, P.; Paoloni, A.; Torino, F.; Corsello, S.M.; Lenzi, A.; et al. Somatostatin analogs therapy in gastroenteropancreatic neuroendocrine tumors: Current aspects and new perspectives. Front. Endocrinol. 2014, 5, 7. [Google Scholar] [CrossRef]
- Ohmori, T.; Okada, K.; Tabei, R. Immunoreactivity to neurohormonal polypeptide in colorectal carcinomas and tumor-neighboring mucosa, and its significance. Arch. Pathol. Lab. Med. 1996, 120, 560–568. [Google Scholar]
- Ohmori, T.; Asahi, S.; Sato, C.; Maki, F.; Masumoto, A.; Okada, K. Bcl-2 protein expression and gut neurohormonal polypeptide/amine production in colorectal carcinomas and tumor-neighboring mucosa, which closely correlate to the occurrence of tumor. Histol. Histopathol. 1999, 14, 37–44. [Google Scholar] [CrossRef]
- Swatek, J.; Chibowski, D. Endocrine cells in colorectal carcinomas. Immunohistochemical study. Pol. J. Pathol. 2000, 51, 127–136. [Google Scholar]
- Jesinghaus, M.; Konukiewitz, B.; Keller, G.; Kloor, M.; Steiger, K.; Reiche, M.; Penzel, R.; Endris, V.; Arsenic, R.; Hermann, G.; et al. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod. Pathol. 2017, 30, 610–619. [Google Scholar] [CrossRef]
- Ogimi, T.; Sadahiro, S.; Kamei, Y.; Chan, L.F.; Miyakita, H.; Saito, G.; Okada, K.; Suzuki, T.; Kajiwara, H. Distribution of Neuroendocrine Marker-Positive Cells in Colorectal Cancer Tissue and Normal Mucosal Tissue: Consideration of Histogenesis of Neuroendocrine Cancer. Oncology 2019, 97, 294–300. [Google Scholar] [CrossRef]
- Modarai, S.R.; Opdenaker, L.M.; Viswanathan, V.; Fields, J.Z.; Boman, B.M. Somatostatin signaling via SSTR1 contributes to the quiescence of colon cancer stem cells. BMC Cancer 2016, 16, 941. [Google Scholar] [CrossRef]
- Zhang, T.; Ahn, K.; Emerick, B.; Modarai, S.R.; Opdenaker, L.M.; Palazzo, J.; Schleiniger, G.; Fields, J.Z.; Boman, B.M. APC mutations in human colon lead to decreased neuroendocrine maturation of ALDH+ stem cells that alters GLP-2 and SST feedback signaling: Clue to a link between WNT and retinoic acid signalling in colon cancer development. PLoS ONE 2020, 15, e0239601. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Sun, K.; Xu, Y.; Hu, P.; Li, X.; Xu, F. Identification of biomarkers associated with diagnosis and prognosis of colorectal cancer patients based on integrated bioinformatics analysis. Gene 2019, 692, 119–125. [Google Scholar] [CrossRef]
- Payer, J., Jr.; Huorka, M.; Duris, I.; Mikulecky, M.; Kratochvilovà, H.; Ondrejka, P. Somatostatin and large bowel polyps. Hepatogastroenterology 1995, 42, 775–777. [Google Scholar]
- Payer, J., Jr.; Huorka, M.; Duris, I.; Ondrejka, P.; Kratochvílova, H.; Ilkova, M.; Holéczy, P. Circadian rhythmicity of plasma somatostatin, gastrin and cortisol in colon cancer patients. Hepatogastroenterology 1997, 44, 72–77. [Google Scholar]
- Hejna, M.; Schmidinger, M.; Raderer, M. The clinical role of somatostatin analogues as antineoplastic agents: Much ado about nothing? Ann. Oncol. 2002, 13, 653–668. [Google Scholar] [CrossRef]
- Rai, U.; Thrimawithana, T.R.; Valery, C.; Young, S.A. Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacol. Ther. 2015, 152, 98–110. [Google Scholar] [CrossRef]
- Diamantopoulos, L.N.; Laskaratos, F.M.; Kalligeros, M.; Shah, R.; Navalkissoor, S.; Gnanasegaran, G.; Banks, J.; Smith, J.; Jacobs, B.; Galanopoulos, M.; et al. Antiproliferative Effect of Above-Label Doses of Somatostatin Analogs for the Management of Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology 2021, 111, 650–659. [Google Scholar] [CrossRef]
- Rinzivillo, M.; De Felice, I.; Magi, L.; Annibale, B.; Panzuto, F. Octreotide long-acting release (LAR) in combination with other therapies for treatment of neuroendocrine neoplasia: A systematic review. J. Gastrointest. Oncol. 2021, 12, 845–855. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, Z.; Wang, F.; Qin, Y.; Xu, X.; Yu, X.; Ji, S. Role of Somatostatin Receptor in Pancreatic Neuroendocrine Tumor Development, Diagnosis, and Therapy. Front. Endocrinol. 2021, 12, 679000. [Google Scholar] [CrossRef]
- Buffa, R.; Capella, C.; Fontana, P.; Usellini, L.; Solcia, E. Types of endocrine cells in the human colon and rectum. Cell Tissue Res. 1978, 192, 227–240. [Google Scholar] [CrossRef]
- El-Salhy, M.; Mazzawi, T.; Umezawa, K.; Gilja, O.H. Enteroendocrine cells, stem cells and differentiation progenitors in rats with TNBS-induced colitis. Int. J. Mol. Med. 2016, 38, 1743–1751. [Google Scholar] [CrossRef]
- Martins, P.; Fakhry, J.; de Oliveira, E.C.; Hunne, B.; Fothergill, L.J.; Ringuet, M.; Reis, D.D.; Rehfeld, J.F.; Callaghan, B.; Furness, J.B. Analysis of enteroendocrine cell populations in the human colon. Cell Tissue Res. 2017, 367, 161–168. [Google Scholar] [CrossRef]
- Billing, L.J.; Larraufie, P.; Lewis, J.; Leiter, A.; Li, J.; Lam, B.; Yeo, G.S.; Goldspink, D.A.; Kay, R.G.; Gribble, F.M.; et al. Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice—Identification of selective stimuli for insulin-like peptide-5 and glucagon-like peptide-1 co-expressing cells. Mol. Metab. 2019, 29, 158–169. [Google Scholar] [CrossRef]
- Weidmann, S.; Schrödl, F.; Neuhuber, W.; Brehmer, A. Quantitative estimation of putative primary afferent neurons in the myenteric plexus of human small intestine. Histochem. Cell Biol. 2007, 128, 399–407. [Google Scholar] [CrossRef]
- Gonkowski, S.; Rytel, L. Somatostatin as an Active Substance in the Mammalian Enteric Nervous System. Int. J. Mol. Sci. 2019, 20, 4461. [Google Scholar] [CrossRef]
- Brehmer, A. Classification of human enteric neurons. Histochem. Cell Biol. 2021, 156, 95–108. [Google Scholar] [CrossRef]
- O’Briain, D.S.; Dayal, Y.; DeLellis, R.A.; Tischler, A.S.; Bendon, R.; Wolfe, H.J. Rectal carcinoids as tumors of the hindgut endocrine cells: A morphological and immunohistochemical analysis. Am. J. Surg. Pathol. 1982, 6, 131–142. [Google Scholar] [CrossRef]
- De Fontgalland, D.; Wattchow, D.A.; Costa, M.; Brookes, S.J. Immunohistochemical characterization of the innervation of human colonic mesenteric and submucosal blood vessels. Neurogastroenterol. Motil. 2008, 20, 1212–1226. [Google Scholar] [CrossRef]
- Corleto, V.D. Somatostatin and the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 63–68. [Google Scholar] [CrossRef]
- Günther, T.; Tulipano, G.; Dournaud, P.; Bousquet, C.; Csaba, Z.; Kreienkamp, H.J.; Lupp, A.; Korbonits, M.; Castaño, J.P.; Wester, H.J.; et al. International Union of Basic and Clinical Pharmacology. CV. Somatostatin Receptors: Structure, Function, Ligands, and New Nomenclature. Pharmacol. Rev. 2018, 70, 763–835. [Google Scholar] [CrossRef]
- Naitou, K.; Shiina, T.; Nakamori, H.; Sano, Y.; Shimaoka, H.; Shimizu, Y. Colokinetic effect of somatostatin in the spinal defecation center in rats. J. Physiol. Sci. 2018, 68, 243–251. [Google Scholar] [CrossRef]
- Warhurst, G.; Higgs, N.B.; Fakhoury, H.; Warhurst, A.C.; Garde, J.; Coy, D.H. Somatostatin receptor subtype 2 mediates somatostatin inhibition of ion secretion in rat distal colon. Gastroenterology 1996, 111, 325–333. [Google Scholar] [CrossRef]
- Warhurst, G.; Barbezat, G.O.; Higgs, N.B.; Reyl-Desmars, F.; Lewin, M.J.; Coy, D.H.; Ross, I.; Grigor, M.R. Expression of somatostatin receptor genes and their role in inhibiting Cl-secretion in HT-29cl.19A colonocytes. Am. J. Physiol. 1995, 269, G729–G736. [Google Scholar] [CrossRef]
- Song, S.; Li, X.; Geng, C.; Li, Y.; Wang, C. Somatostatin stimulates colonic MUC2 expression through SSTR5-Notch-Hes1 signaling pathway. Biochem. Biophys. Res. Commun. 2020, 521, 1070–1076. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Gut clock: Implication of circadian rhythms in the gastrointestinal tract. J. Physiol. Pharmacol. 2011, 62, 139–150. [Google Scholar]
- Kim, K.; Choe, H.K. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech. Ageing Dev. 2019, 177, 74–79. [Google Scholar] [CrossRef]
- Schimmack, S.; Svejda, B.; Lawrence, B.; Kidd, M.; Modlin, I.M. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch. Surg. 2011, 396, 273–298. [Google Scholar] [CrossRef]
- Kleist, B.; Poetsch, M. Neuroendocrine differentiation: The mysterious fellow of colorectal cancer. World J. Gastroenterol. 2015, 21, 11740–11747. [Google Scholar] [CrossRef]
- Jansson, D.; Gould, V.E.; Gooch, G.T.; Rittenhouse, H.G.; Shin, S.S.; Manderino, G.L.; Tomita, J.T.; Staren, E.D. Immunohistochemical analysis of colon carcinomas applying exocrine and neuroendocrine markers. APMIS 1988, 96, 1129–1139. [Google Scholar] [CrossRef]
- de Bruïne, A.P.; Wiggers, T.; Beek, C.; Volovics, A.; von Meyenfeldt, M.; Arends, J.W.; Bosman, F.T. Endocrine cells in colorectal adenocarcinomas: Incidence, hormone profile and prognostic relevance. Int. J. Cancer 1993, 54, 765–771. [Google Scholar] [CrossRef]
- Godlewski, J.; Kaleczyc, J. Somatostatin, substance P and calcitonin gene-related peptide-positive intramural nerve structures of the human large intestine affected by carcinoma. Folia Histochem. Cytobiol. 2010, 48, 475–483. [Google Scholar] [CrossRef]
- Yao, G.Y.; Zhou, J.L.; Lai, M.D.; Chen, X.Q.; Chen, P.H. Neuroendocrine markers in adenocarcinomas: An investigation of 356 cases. World J. Gastroenterol. 2003, 9, 858–861. [Google Scholar] [CrossRef]
- Hamilton, S.R.; Aaltonen, L.A. (Eds.) World Health Organization Classification of Tumours. In Pathology and Genetics of Tumours of the Digestive System, 3rd ed.; IARC Press: Lyon, France, 2000; Volume 2, pp. 103–143. [Google Scholar]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. (Eds.) World Health Organization Classification of Tumors. In WHO Classification of Tumors of the Digestive System, 4th ed.; IARC Press: Lyon, France, 2000; pp. 13–14. [Google Scholar]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Assarzadegan, N.; Montgomery, E. What is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch. Pathol. Lab. Med. 2021, 145, 664–677. [Google Scholar] [CrossRef]
- Ramage, J.K.; Valle, J.W.; Nieveen van Dijkum, E.J.M.; Sundin, A.; Pascher, A.; Couvelard, A.; Kloeppel, G.; The ENETS 2016 Munich Advisory Board Participants. Colorectal Neuroendocrine Neoplasms: Areas of Unmet Need. Neuroendocrinology 2019, 108, 45–53. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Schroeder, G.; Bauman, M.D.; Krook, J.E.; Jin, L.; Goldberg, R.M.; Farr, G.H., Jr. Prevalence and Prognostic Significance of Neuroendocrine Differentiation in Colorectal Carcinomas. Endocr. Pathol. 1998, 9, 35–42. [Google Scholar] [CrossRef]
- Grabowski, P.; Schönfelder, J.; Ahnert-Hilger, G.; Foss, H.D.; Heine, B.; Schindler, I.; Stein, H.; Berger, G.; Zeitz, M.; Scherübl, H. Expression of neuroendocrine markers: A signature of human undifferentiated carcinoma of the colon and rectum. Virchows Arch. 2002, 441, 256–263. [Google Scholar] [CrossRef]
- Bosman, F.; Yan, P. Molecular pathology of colorectal cancer. Pol. J. Pathol. 2014, 65, 257–266. [Google Scholar] [CrossRef]
- Munro, M.J.; Wickremesekera, S.K.; Peng, L.; Tan, S.T.; Itinteang, T. Cancer stem cells in colorectal cancer: A review. J. Clin. Pathol. 2018, 71, 110–116. [Google Scholar] [CrossRef]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153–173. [Google Scholar] [CrossRef]
- Sun, M.H. Neuroendocrine differentiation in sporadic CRC and hereditary nonpolyosis colorectal cancer. Dis. Markers. 2004, 20, 283–288. [Google Scholar] [CrossRef]
- Li, Y.; Yau, A.; Schaeffer, D.; Magliocco, A.; Gui, X.; Urbanski, S.; Waghray, R.; Owen, D.; Gao, Z.H. Colorectal glandular-neuroendocrine mixed tumor: Pathologic spectrum and clinical implications. Am. J. Surg. Pathol. 2011, 35, 413–425. [Google Scholar] [CrossRef]
- Hamada, Y.; Oishi, A.; Shoji, T.; Takada, H.; Yamamura, M.; Hioki, K.; Yamamoto, M. Endocrine cells and prognosis in patients with colorectal carcinoma. Cancer 1992, 69, 2641–2646. [Google Scholar] [CrossRef]
- Jesinghaus, M.; Schmitt, M.; Lang, C.; Reiser, M.; Scheiter, A.; Konukiewitz, B.; Steiger, K.; Silva, M.; Tschurtschenthaler, M.; Lange, S.; et al. Morphology Matters: A Critical Reappraisal of the Clinical Relevance of Morphologic Criteria From the 2019 WHO Classification in a Large Colorectal Cancer Cohort Comprising 1004 Cases. Am. J. Surg. Pathol. 2021, 45, 969–978. [Google Scholar] [CrossRef]
- Watanabe, J.; Suwa, Y.; Ota, M.; Ishibe, A.; Masui, H.; Nagahori, K.; Tsuura, Y.; Endo, I. Clinicopathological and Prognostic Evaluations of Mixed Adenoneuroendocrine Carcinoma of the Colon and Rectum: A Case-Matched Study. Dis. Colon Rectum. 2016, 59, 1160–1167. [Google Scholar] [CrossRef]
- Gaspar, R.; Santos-Antunes, J.; Marques, M.; Liberal, R.; Melo, D.; Pereira, P.; Lopes, S.; Macedo, G. Mixed Adenoneuroendocrine Tumor of the Rectum in an Ulcerative Colitis Patient. GE Port. J. Gastroenterol. 2019, 26, 125–127. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Jiang, M.; Tan, Y.; Li, X.; Fu, J.; Hu, H.; Ye, X.; Cao, Y.; Xu, J.; Yuan, Y. Clinicopathological Features and Prognostic Factors of Colorectal Neuroendocrine Neoplasms. Gastroenterol. Res. Pract. 2017, 2017, 4206172. [Google Scholar] [CrossRef]
- Susini, C.; Buscail, L. Rationale for the use of somatostatin analogs as antitumor agents. Ann. Oncol. 2006, 17, 1733–1742. [Google Scholar] [CrossRef]
- Theodoropoulou, M.; Stalla, G.K. Somatostatin receptors: From signaling to clinical practice. Front. Neuroendocrinol. 2013, 34, 228–252. [Google Scholar] [CrossRef]
- Payer, J.; Huorka, M.; Duris, I.; Mikulecky, M.; Kratochvílová, H.; Ondrejka, P.; Lukác, L. Plasma somatostatin levels in ulcerative colitis. Hepatogastroenterology 1994, 41, 552–553. [Google Scholar]
- Kasprzak, A. Somatostatin and Its Receptor System in Colorectal Cancer. Biomedicines 2021, 9, 1743. [Google Scholar] [CrossRef]
- de Bruïne, A.P.; Dinjens, W.N.; Pijls, M.M.; vd Linden, E.P.; Rousch, M.J.; Moerkerk, P.T.; de Goeij, A.F.; Bosman, F.T. NCI-H716 cells as a model for endocrine differentiation in colorectal cancer. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1992, 62, 311–320. [Google Scholar] [CrossRef]
- El-Salhy, M.; Mahdavi, J.; Norrgård, O. Colonic endocrine cells in patients with carcinoma of the colon. Eur. J. Gastroenterol. Hepatol. 1998, 10, 517–522. [Google Scholar] [CrossRef]
- Sereti, E.; Gavriil, A.; Agnantis, N.; Golematis, V.C.; Voloudakis-Baltatzis, I.E. Immunoelectron study of somatostatin, gastrin and glucagon in human colorectal adenocarcinomas and liver metastases. Anticancer Res. 2002, 22, 2117–2123. [Google Scholar]
- Seretis, E.C.; Agnantis, N.J.; Golematis, V.C.; Voloudakis-Balatzis, I.E. Electron immunocytochemical demonstration of serotonin, vasoactive intestinal polypeptide, bombesin, somatostatin and glucagon in mirror biopsies from primary colorectal adenocarcinoma. J. Exp. Clin. Cancer Res. 2004, 23, 477–484. [Google Scholar]
- Leiszter, K.; Sipos, F.; Galamb, O.; Krenács, T.; Veres, G.; Wichmann, B.; Fűri, I.; Kalmár, A.; Patai, Á.V.; Tóth, K.; et al. Promoter hypermethylation-related reduced somatostatin production promotes uncontrolled cell proliferation in colorectal cancer. PLoS ONE 2015, 10, e0118332. [Google Scholar] [CrossRef]
- Seretis, E.; Konstantinidou, A.; Arnogiannakis, N.; Xinopoulos, D.; Voloudakis-Baltatzis, I.E. Mucinous colorectal adenocarcinoma with signet-ring cells: Immunohistochemical and ultrastructural study. Ultrastruct. Pathol. 2010, 34, 337–343. [Google Scholar] [CrossRef]
- Sitohy, B.; El-Salhy, M. Colonic endocrine cells in rats with chemically induced colon carcinoma. Histol. Histopathol. 2001, 16, 833–838. [Google Scholar] [CrossRef]
- Hameed, Y.; Ahmad, M.; Ejaz, S.; Liang, S. Identification of Key Biomarkers for the Future Applications in Diagnostics and Targeted Therapy of Colorectal Cancer. Curr. Mol. Med. 2022, 22, 31–37. [Google Scholar] [CrossRef]
- Iftikhar, S.Y.; Watson, S.A.; Morris, D.L. The effect of long acting somatostatin analogue SMS 201.995 therapy on tumour kinetic measurements and serum tumour marker concentrations in primary rectal cancer. Br. J. Cancer 1991, 63, 971–974. [Google Scholar] [CrossRef][Green Version]
- Stewart, G.J.; Connor, J.L.; Lawson, J.A.; Preketes, A.; King, J.; Morris, D.L. Octreotide reduces the kinetic index, proliferating cell nuclear antigen-maximum proliferative index, in patients with colorectal cancer. Cancer 1995, 76, 572–578. [Google Scholar] [CrossRef]
- Pollak, M.N.; Polychronakos, C.; Guyda, H. Somatostatin analogue SMS 201-995 reduces serum IGF-I levels in patients with neoplasms potentially dependent on IGF-I. Anticancer Res. 1989, 9, 889–891. [Google Scholar]
- Klijn, J.G.; Hoff, A.M.; Planting, A.S.; Verweij, J.; Kok, T.; Lamberts, S.W.; Portengen, H.; Foekens, J.A. Treatment of patients with metastatic pancreatic and gastrointestinal tumours with the somatostatin analogue Sandostatin: A phase II study including endocrine effects. Br. J. Cancer 1990, 62, 627–630. [Google Scholar] [CrossRef]
- Di Leo, A.; Bajetta, E.; Ferrari, L.; Biganzoli, L.; Mariani, L.; Carnaghi, C.; Camerini, E.; Buzzoni, R.; Ruiz, J.M. A dose-finding study of lanreotide (a somatostatin analog) in patients with colorectal carcinoma. Cancer 1996, 78, 35–42. [Google Scholar] [CrossRef]
- Cascinu, S.; Del Ferro, E.; Grianti, C.; Ligi, M.; Ghiselli, R.; Foglietti, G.; Saba, V.; Lungarotti, F.; Catalano, G. Inhibition of tumor cell kinetics and serum insulin growth factor I levels by octreotide in colorectal cancer patients. Gastroenterology 1997, 113, 767–772. [Google Scholar] [CrossRef]
- Wu, P.; Mao, J.D.; Yan, J.Y.; Rui, J.; Zhao, Y.C.; Li, X.H.; Xu, G.Q. Correlation between the expressions of gastrin, somatostatin and cyclin and cyclin-depend kinase in colorectal cancer. World J. Gastroenterol. 2005, 11, 7211–7217. [Google Scholar] [CrossRef]
- Fogh, J.; Trempe, G. New human tumor cell lines. In Human Tumor Cells In Vitro; Fogh, J., Ed.; Springer: Boston, MA, USA, 1975; pp. 115–159. [Google Scholar]
- Staykova, S.T.; Wesselinova, D.W.; Vezenkov, L.T.; Naydenova, E.D. Synthesis and in vitro antitumor activity of new octapeptide analogs of somatostatin containing unnatural amino acids. Amino Acids. 2015, 47, 1007–1013. [Google Scholar] [CrossRef]
- Ahmed, D.; Eide, P.W.; Eilertsen, I.A.; Danielsen, S.A.; Eknæs, M.; Hektoen, M.; Lind, G.E.; Lothe, R.A. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2013, 2, e71. [Google Scholar] [CrossRef]
- Olejniczak, A.; Szaryńska, M.; Kmieć, Z. In vitro characterization of spheres derived from colorectal cancer cell lines. Int. J. Oncol. 2018, 52, 599–612. [Google Scholar] [CrossRef]
- Brattain, M.G.; Fine, W.D.; Khaled, F.M.; Thompson, J.; Brattain, D.E. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res. 1981, 41, 1751–1756. [Google Scholar]
- Smith, J.P.; Solomon, T.E. Effects of gastrin, proglumide, and somatostatin on growth of human colon cancer. Gastroenterology 1988, 95, 1541–1548. [Google Scholar] [CrossRef]
- Björk, J.; Nilsson, J.; Hultcrantz, R.; Johansson, C. Growth-regulatory effects of sensory neuropeptides, epidermal growth factor, insulin, and somatostatin on the non-transformed intestinal epithelial cell line IEC-6 and the colon cancer cell line HT 29. Scand. J. Gastroenterol. 1993, 28, 879–884. [Google Scholar] [CrossRef]
- Radulovic, S.; Schally, A.V.; Reile, H.; Halmos, G.; Szepeshazi, K.; Groot, K.; Milovanovic, S.; Miller, G.; Yano, T. Inhibitory effects of antagonists of bombesin/gastrin releasing peptide (GRP) and somatostatin analog (RC-160) on growth of HT-29 human colon cancers in nude mice. Acta Oncol. 1994, 33, 693–701. [Google Scholar] [CrossRef]
- Tóvári, J.; Szende, B.; Bocsi, J.; Falaschi, A.; Simoncsits, A.; Pongor, S.; Erchegyi, J.; Steták, A.; Kéri, G. A somatostatin analogue induces translocation of Ku 86 autoantigen from the cytosol to the nucleus in colon tumour cells. Cell Signal. 1998, 10, 277–282. [Google Scholar] [CrossRef]
- Tejeda, M.; Gaál, D.; Hullán, L.; Hegymegi-Barakonyi, B.; Kéri, G. Evaluation of the antitumor efficacy of the somatostatin structural derivative TT-232 on different tumor models. Anticancer Res. 2006, 26, 3477–3483. [Google Scholar]
- Staykova, S.; Naydenova, E.; Wesselinova, D.; Kalistratova, A.; Vezenkov, L. Synthesis and in vitro study of the anticancer activity of new analogs of octreotide. Protein Pept. Lett. 2012, 19, 1257–1262. [Google Scholar] [CrossRef]
- Ayiomamitis, G.D.; Notas, G.; Zaravinos, A.; Drygiannakis, I.; Georgiadou, M.; Sfakianaki, O.; Mastrodimou, N.; Thermos, K.; Kouroumalis, E. Effects of octreotide and insulin on colon cancer cellular proliferation and correlation with hTERT activity. Oncoscience 2014, 1, 457–467. [Google Scholar] [CrossRef][Green Version]
- Naydenova, E.; Wesselinova, D.; Staykova, S.; Danalev, D.; Dzimbova, T. Synthesis, in vitro biological activity and docking of new analogs of BIM-23052 containing unnatural amino acids. Amino Acids. 2019, 51, 1247–1257. [Google Scholar] [CrossRef]
- Kéri, G.; Erchegyi, J.; Horváth, A.; Mezõ, I.; Idei, M.; Vántus, T.; Balogh, A.; Vadász, Z.; Bökönyi, G.; Seprõdi, J.; et al. A tumor-selective somatostatin analog (TT-232) with strong in vitro and in vivo antitumor activity. Proc. Natl. Acad. Sci. USA 1996, 93, 12513–12518. [Google Scholar] [CrossRef]
- Dy, D.Y.; Whitehead, R.H.; Morris, D.L. SMS 201.995 inhibits in vitro and in vivo growth of human colon cancer. Cancer Res. 1992, 52, 917–923. [Google Scholar]
- di Paolo, A.; Bocci, G.; Innocenti, F.; Agen, C.; Nardini, D.; Danesi, R.; del Tacca, M. Inhibitory effect of the somatostatin analogue SMS 201-995 and cytokines on the proliferation of human colon adenocarcinoma cell lines. Pharmacol. Res. 1995, 32, 135–139. [Google Scholar] [CrossRef]
- Vántus, T.; Csermely, P.; Teplán, I.; Kéri, G. The tumor-selective somatostatin analog, TT2-32 induces a biphasic activation of phosphotyrosine phosphatase activity in human colon tumor cell line, SW620. Tumour Biol. 1995, 16, 261–267. [Google Scholar]
- Hohla, F.; Buchholz, S.; Schally, A.V.; Krishan, A.; Rick, F.G.; Szalontay, L.; Papadia, A.; Halmos, G.; Koster, F.; Aigner, E.; et al. Targeted cytotoxic somatostatin analog AN-162 inhibits growth of human colon carcinomas and increases sensitivity of doxorubicin resistant murine leukemia cells. Cancer Lett. 2010, 294, 35–42. [Google Scholar] [CrossRef]
- Qin, Y.; Schally, A.V.; Willems, G. Somatostatin analogue RC-160 inhibits the growth of transplanted colon cancer in rats. Int. J. Cancer. 1991, 47, 765–770. [Google Scholar] [CrossRef]
- Romani, R.; Morris, D.L. SMS 201.995 (Sandostatin) enhances in-vitro effects of 5-fluorouracil in colorectal cancer. Eur. J. Surg. Oncol. 1995, 21, 27–32. [Google Scholar] [CrossRef]
- Ogasawara, M.; Murata, J.; Ayukawa, K.; Saiki, I. Differential effect of intestinal neuropeptides on invasion and migration of colon carcinoma cells in vitro. Cancer Lett. 1997, 119, 125–130. [Google Scholar] [CrossRef]
- Chen, J.S.; Liang, Q.M.; Li, H.S.; Yang, J.; Wang, S.; Long, J.W. Octreotide inhibits growth of colonic cancer SW480 cells by modulating the Wnt/P-catenin pathway. Pharmazie 2009, 64, 126–131. [Google Scholar]
- Szepeshazi, K.; Schally, A.V.; Halmos, G.; Armatis, P.; Hebert, F.; Sun, B.; Feil, A.; Kiaris, H.; Nagy, A. Targeted cytotoxic somatostatin analogue AN-238 inhibits somatostatin receptor-positive experimental colon cancers independently of their p53 status. Cancer Res. 2002, 62, 781–788. [Google Scholar]
- Tjomsland, V.; El-Salhy, M. Anti-tumour effects of triple therapy with octreotide, galanin and serotonin in comparison with those of 5-fluorouracil/leukovorin on human colon cancer. Int. J. Oncol. 2005, 27, 427–432. [Google Scholar] [CrossRef]
- El-Salhy, M. Effects of triple therapy with octreotide, galanin and serotonin on a human colon cancer cell line. Oncol. Rep. 2005, 13, 45–49. [Google Scholar] [CrossRef]
- Szegedi, Z.; Takács, J.; Szende, B.; Vadász, Z.; Horváth, A.; Gulyás, E.; Tóth, G.; Peták, I.; Bocsi, J.; Kéri, G. A specifically radiolabeled somatostatin analog with strong antitumor activity induces apoptosis and accumulates in the cytosol and the nucleus of HT29 human colon carcinoma cells. Endocrine 1999, 10, 25–34. [Google Scholar] [CrossRef]
- Colucci, R.; Blandizzi, C.; Ghisu, N.; Florio, T.; Del Tacca, M. Somatostatin inhibits colon cancer cell growth through cyclooxygenase-2 downregulation. Br. J. Pharmacol. 2008, 155, 198–209. [Google Scholar] [CrossRef]
- Massari, D.; Trobonjac, Z.; Rukavina, D.; Radosević-Stasić, B. SMS 201-995 enhances S-phase block induced by 5-fluorouracil in a human colorectal cancer cell line. Anticancer Drugs. 2005, 16, 989–996. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Ibrahim, M.A.; Amin, M.A.; Maswadeh, H.; Alwehaibi, M.N.; Al-Harbi, S.N.; Alharbi, Z.A.; Mohammed, H.A.; Mehany, A.B.M.; Saleem, I. Cetuximab Conjugated with Octreotide and Entrapped Calcium Alginate-beads for Targeting Somatostatin Receptors. Sci. Rep. 2020, 10, 4736. [Google Scholar] [CrossRef]
- Fan, J.; Deng, L.; Peng, Y.; Ding, Y. Combined anti-tumor efficacy of somatostatin fusion protein and vaccinia virus on tumor cells with high expression of somatostatin receptors. Sci. Rep. 2022, 12, 16885. [Google Scholar] [CrossRef]
- den Hertog, J.; Ostman, A.; Böhmer, F.D. Protein tyrosine phosphatases: Regulatory mechanisms. FEBS J. 2008, 275, 831–847. [Google Scholar] [CrossRef]
- Kéri, G.; Mezö, I.; Horváth, A.; Vadász, Z.; Balogh, A.; Idei, M.; Vántus, T.; Teplán, I.; Mák, M.; Horváth, J.; et al. Novel somatostatin analogs with tyrosine kinase inhibitory and antitumor activity. Biochem. Biophys. Res. Commun. 1993, 191, 681–687. [Google Scholar] [CrossRef]
- Kéri, G.; Mezô, I.; Vadász, Z.; Horváth, A.; Idei, M.; Vántus, T.; Balogh, A.; Bökönyi, G.; Bajor, T.; Teplán, I.; et al. Structure-activity relationship studies of novel somatostatin analogs with antitumor activity. Pept. Res. 1993, 6, 281–288. [Google Scholar]
- Wlodarchak, N.; Xing, Y. PP2A as a master regulator of the cell cycle. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 162–184. [Google Scholar] [CrossRef]
- Neel, B.G. Structure and function of SH2-domain containing tyrosine phosphatases. Semin. Cell Biol. 1993, 4, 419–432. [Google Scholar] [CrossRef]
- Bertorelle, R.; Rampazzo, E.; Pucciarelli, S.; Nitti, D.; De Rossi, A. Telomeres, telomerase and colorectal cancer. World J. Gastroenterol. 2014, 20, 1940–1950. [Google Scholar] [CrossRef]
- Le Romancer, M.; Reyl-Desmars, F.; Cherifi, Y.; Pigeon, C.; Bottari, S.; Meyer, O.; Lewin, M.J. The 86-kDa subunit of autoantigen Ku is a somatostatin receptor regulating protein phosphatase-2A activity. J. Biol. Chem. 1994, 269, 17464–17468. [Google Scholar] [CrossRef]
- Pucci, S.; Bonanno, E.; Pichiorri, F.; Mazzarelli, P.; Spagnoli, L.G. The expression and the nuclear activity of the caretaker gene ku86 are modulated by somatostatin. Eur. J. Histochem. 2004, 48, 103–110. [Google Scholar] [CrossRef][Green Version]
- Pucci, S.; Mazzarelli, P.; Sesti, F.; Boothman, D.A.; Spagnoli, L.G. Interleukin-6 affects cell death escaping mechanisms acting on Bax-Ku70-Clusterin interactions in human colon cancer progression. Cell Cycle. 2009, 8, 473–481. [Google Scholar] [CrossRef]
- Saydam, O.; Saydam, N. Deficiency of Ku Induces Host Cell Exploitation in Human Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 651818. [Google Scholar] [CrossRef]
- Dy, D.Y.; Morris, D.L. Somatostatin inhibits both in-vitro and in-vivo carcinoembryonic antigen secretion by human colon cancer. Eur. J. Surg. Oncol. 1993, 19, 168–172. [Google Scholar]
- Mao, J.D.; Wu, P.; Xia, X.H.; Hu, J.Q.; Huang, W.B.; Xu, G.Q. Correlation between expression of gastrin, somatostatin and cell apoptosis regulation gene bcl-2/bax in large intestine carcinoma. World J. Gastroenterol. 2005, 11, 721–725. [Google Scholar] [CrossRef]
- Mao, J.D.; Wu, P.; Yang, Y.L.; Wu, J.; Huang, H. Relationship between expression of gastrin, somatostatin, Fas/FasL and caspases in large intestinal carcinoma. World J. Gastroenterol. 2008, 14, 2802–2809. [Google Scholar] [CrossRef]
- Mełen-Mucha, G.; Winczyk, K.; Pawlikowski, M. Somatostatin analogue octreotide and melatonin inhibit bromodeoxyuridine incorporation into cell nuclei and enhance apoptosis in the transplantable murine colon 38 cancer. Anticancer Res. 1998, 18, 3615–3619. [Google Scholar]
- Wang, S.; Bao, Z.; Liang, Q.M.; Long, J.W.; Xiao, Z.S.; Jiang, Z.J.; Liu, B.; Yang, J.; Long, Z.X. Octreotide stimulates somatostatin receptor-induced apoptosis of SW480 colon cancer cells by activation of glycogen synthase kinase-3β, A Wnt/β-catenin pathway modulator. Hepatogastroenterology 2013, 60, 1639–1646. [Google Scholar]
- El-Salhy, M.; Sitohy, B.; Norrgård, O. Triple therapy with octreotide, galanin, and serotonin reduces the size and blood vessel density and increases apoptosis of a rat colon carcinoma. Regul. Pept. 2003, 111, 145–152. [Google Scholar] [CrossRef]
- El-Salhy, M. Comparison between triple therapy with octreotide, galanin and serotonin, 5-fluorouracil/leucovorin, and sequential treatment with both, on human colon cancer. Oncol. Rep. 2004, 11, 1161–1168. [Google Scholar] [CrossRef]
- El-Salhy, M.; Dennerqvist, V. Effects of triple therapy with octreotide, galanin and serotonin on liver metastasis of human colon cancer in xenografts. Oncol. Rep. 2004, 11, 1177–1182. [Google Scholar] [CrossRef]
- El-Salhy, M. Triple treatment with octreotide, galanin and serotonin is a promising therapy for colorectal cancer. Curr. Pharm. Des. 2005, 11, 2107–2117. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hilding, L.; Royson, H.; Tjomsland, V. Comparison between triple therapy with octreotide, galanin and serotonin vs. irinotecan or oxaliplatin in combination with 5-fluorouracil/leukovorin in human colon cancer. Int. J. Oncol. 2005, 27, 687–691. [Google Scholar]
- Jaggi, M.; Prasad, S.; Singh, A.T.; Praveen, R.; Dutt, S.; Mathur, A.; Sharma, R.; Gupta, N.; Ahuja, R.; Mukherjee, R.; et al. Anticancer activity of a peptide combination in gastrointestinal cancers targeting multiple neuropeptide receptors. Investig. New Drugs. 2008, 26, 489–504. [Google Scholar] [CrossRef]
- Singh, A.T.; Jaggi, M.; Prasad, S.; Dutt, S.; Singh, G.; Datta, K.; Rajendran, P.; Sanna, V.K.; Mukherjee, R.; Burman, A.C. Modulation of key signal transduction molecules by a novel peptide combination effective for the treatment of gastrointestinal carcinomas. Investig. New Drugs. 2008, 26, 505–516. [Google Scholar] [CrossRef]
- Danesi, R.; Agen, C.; Benelli, U.; Paolo, A.D.; Nardini, D.; Bocci, G.; Basolo, F.; Campagni, A.; Tacca, M.D. Inhibition of experimental angiogenesis by the somatostatin analogue octreotide acetate (SMS 201-995). Clin. Cancer Res. 1997, 3, 265–272. [Google Scholar]
- García de la Torre, N.; Wass, J.A.; Turner, H.E. Antiangiogenic effects of somatostatin analogues. Clin. Endocrinol. 2002, 57, 425–441. [Google Scholar] [CrossRef]
- Florio, T.; Morini, M.; Villa, V.; Arena, S.; Corsaro, A.; Thellung, S.; Culler, M.D.; Pfeffer, U.; Noonan, D.M.; Schettini, G.; et al. Somatostatin inhibits tumor angiogenesis and growth via somatostatin receptor-3-mediated regulation of endothelial nitric oxide synthase and mitogen-activated protein kinase activities. Endocrinology 2003, 144, 1574–1584. [Google Scholar] [CrossRef]
- Dasgupta, P. Somatostatin analogues: Multiple roles in cellular proliferation, neoplasia, and angiogenesis. Pharmacol. Ther. 2004, 102, 61–85. [Google Scholar] [CrossRef]
- Reubi, J.C.; Horisberger, U.; Laissue, J. High density of somatostatin receptors in veins surrounding human cancer tissue: Role in tumor-host interaction? Int. J. Cancer 1994, 56, 681–688. [Google Scholar] [CrossRef]
- Cascinu, S.; Del Ferro, E.; Ligi, M.; Staccioli, M.P.; Giordani, P.; Catalano, V.; Agostinelli, R.; Muretto, P.; Catalano, G. Inhibition of vascular endothelial growth factor by octreotide in colorectal cancer patients. Cancer Investig. 2001, 19, 8–12. [Google Scholar] [CrossRef]
- Rosiek, V.; Janas, K. Assessment of VEGF and VEGF R1 serum levels in patients with neuroendocrine neoplasms before and after treatment with first-generation somatostatin analogues. Endokrynol. Pol. 2022, 73, 612–618. [Google Scholar] [CrossRef]
- Koizumi, M.; Onda, M.; Tanaka, N.; Seya, T.; Yamada, T.; Takahashi, Y. Antiangiogenic effect of octreotide inhibits the growth of human rectal neuroendocrine carcinoma. Digestion 2002, 65, 200–206. [Google Scholar] [CrossRef]
- Pawlik, W.W.; Gustaw, P.; Czarnobilski, K.; Sendur, R.; Konturek, S.J. Effects of somatostatin on intestinal circulation and oxygen consumption. Acta Physiol. Hung. 1989, 74, 277–283. [Google Scholar]
- Dézsi, L.; Szentiványi, M., Jr.; Dörnyei, G.; Löwenstein, L.; Faragó, M.; Tulassay, T.; Monos, E. Regional differences in nitric oxide-dependent vascular responses to somatostatin. Physiol. Res. 1996, 45, 291–296. [Google Scholar]
- Lauder, H.; Sellers, L.A.; Fan, T.P.; Feniuk, W.; Humphrey, P.P. Somatostatin sst5 inhibition of receptor mediated regeneration of rat aortic vascular smooth muscle cells. Br. J. Pharmacol. 1997, 122, 663–670. [Google Scholar] [CrossRef]
- Schiller, N.K.; Timothy, A.M.; Aurora, H.S.; Chen, I.L.; Coy, D.H.; Murphy, W.A.; Akers, D.L.; Fonseca, V.A.; Kadowitz, P.J.; McNamara, D.B. A selective somatostatin type-2 receptor agonist inhibits neointimal thickening and enhances endothelial cell growth and morphology following aortic balloon injury in the rabbit. Mol. Cell. Biochem. 2002, 240, 31–37. [Google Scholar] [CrossRef]
- Curtis, S.B.; Hewitt, J.; Yakubovitz, S.; Anzarut, A.; Hsiang, Y.N.; Buchan, A.M. Somatostatin receptor subtype expression and function in human vascular tissue. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1815–H1822. [Google Scholar] [CrossRef]
- Watson, J.C.; Balster, D.A.; Gebhardt, B.M.; O’Dorisio, T.M.; O’Dorisio, M.S.; Espenan, G.D.; Drouant, G.J.; Woltering, E.A. Growing vascular endothelial cells express somatostatin subtype 2 receptors. Br. J. Cancer 2001, 85, 266–272. [Google Scholar] [CrossRef]
- Dal Monte, M.; Martini, D.; Ristori, C.; Azara, D.; Armani, C.; Balbarini, A.; Bagnoli, P. Hypoxia effects on proangiogenic factors in human umbilical vein endothelial cells: Functional role of the peptide somatostatin. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 593–612. [Google Scholar] [CrossRef]
- Walter, T.; Hommell-Fontaine, J.; Gouysse, G.; Pourreyron, C.; Nejjari, M.; Villaume, K.; Causeret, S.; Hervieu, V.; Poncet, G.; Roche, C.; et al. Effects of somatostatin and octreotide on the interactions between neoplastic gastroenteropancreatic endocrine cells and endothelial cells: A comparison between in vitro and in vivo properties. Neuroendocrinology 2011, 94, 200–208. [Google Scholar] [CrossRef]
- Aslam, M.; Idrees, H.; Ferdinandy, P.; Helyes, Z.; Hamm, C.; Schulz, R. Somatostatin Primes Endothelial Cells for Agonist-Induced Hyperpermeability and Angiogenesis In Vitro. Int. J. Mol. Sci. 2022, 23, 3098. [Google Scholar] [CrossRef]
- Rizzo, A.; Pallone, F.; Monteleone, G.; Fantini, M.C. Intestinal inflammation and colorectal cancer: A double-edged sword? World J. Gastroenterol. 2011, 17, 3092–3100. [Google Scholar]
- Lasry, A.; Zinger, A.; Ben-Neriah, Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef]
- Yu, X.; Yang, X.; Nie, H.; Jiang, W.; He, X.; Ou, C. Immunological role and prognostic value of somatostatin receptor family members in colon adenocarcinoma. Front. Pharmacol. 2023, 14, 1255809. [Google Scholar] [CrossRef]
- van Bergeijk, J.D.; Wilson, J.H. Somatostatin in inflammatory bowel disease. Mediat. Inflamm. 1997, 6, 303–309. [Google Scholar] [CrossRef]
- ten Bokum, A.M.; Hofland, L.J.; van Hagen, P.M. Somatostatin and somatostatin receptors in the immune system: A review. Eur. Cytokine Netw. 2000, 11, 161–176. [Google Scholar]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef]
- La Salvia, A.; Espinosa-Olarte, P.; Riesco-Martinez, M.D.C.; Anton-Pascual, B.; Garcia-Carbonero, R. Targeted Cancer Therapy: What’s New in the Field of Neuroendocrine Neoplasms? Cancers 2021, 13, 1701. [Google Scholar] [CrossRef]
- Levite, M. Neuropeptides, by direct interaction with T cells, induce cytokine secretion and break the commitment to a distinct T helper phenotype. Proc. Natl. Acad. Sci. USA 1998, 95, 12544–12549. [Google Scholar] [CrossRef]
- Liu, L.; Tan, Q.; Hu, B.; Wu, H.; Wang, C.; Tang, C. Somatostatin inhibits the production of interferon-γ by intestinal epithelial cells during intestinal ischemia-reperfusion in macaques. Dig. Dis. Sci. 2014, 59, 2423–2432. [Google Scholar] [CrossRef]
- Georgiadou, M.; Notas, G.; Xidakis, C.; Drygiannakis, I.; Sfakianaki, O.; Klironomos, S.; Valatas, V.; Kouroumalis, E. TNF receptors in Kupffer cells. J. Recept. Signal Transduct. Res. 2011, 31, 291–298. [Google Scholar] [CrossRef]
- Berndt, U.; Philipsen, L.; Bartsch, S.; Wiedenmann, B.; Baumgart, D.C.; Hämmerle, M.; Sturm, A. Systematic high-content proteomic analysis reveals substantial immunologic changes in colorectal cancer. Cancer Res. 2008, 68, 880–888. [Google Scholar] [CrossRef]
- El-Salhy, M.; Solomon, T.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. Gastrointestinal neuroendocrine peptides/amines in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 5068–5085. [Google Scholar] [CrossRef]
- Kim, E.R.; Chang, D.K. Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 2014, 20, 9872–9881. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, W.; Li, Y.; Cong, Y. Enteroendocrine Cells: Sensing Gut Microbiota and Regulating Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2020, 26, 11–20. [Google Scholar] [CrossRef]
- Koch, T.R.; Carney, J.A.; Morris, V.A.; Go, V.L. Somatostatin in the idiopathic inflammatory bowel diseases. Dis. Colon Rectum. 1988, 31, 198–203. [Google Scholar] [CrossRef]
- Watanabe, T.; Kubota, Y.; Sawada, T.; Muto, T. Distribution and quantification of somatostatin in inflammatory disease. Dis. Colon Rectum. 1992, 35, 488–494. [Google Scholar] [CrossRef]
- De Fontgalland, D.; Brookes, S.J.; Gibbins, I.; Sia, T.C.; Wattchow, D.A. The neurochemical changes in the innervation of human colonic mesenteric and submucosal blood vessels in ulcerative colitis and Crohn’s disease. Neurogastroenterol. Motil. 2014, 26, 731–744. [Google Scholar] [CrossRef]
- Binimelis, J.; Webb, S.M.; Monés, J.; Serrano, J.; Casamitjana, R.; Elena, M.; Peinado, M.A.; Vilardell, F.; De Leiva, A. Circulating immunoreactive somatostatin in gastrointestinal diseases. Decrease after vagotomy and enhancement in active ulcerative colitis, irritable bowel syndrome, and duodenal ulcer. Scand. J. Gastroenterol. 1987, 22, 931–937. [Google Scholar] [CrossRef]
- Kidd, M.; Gustafsson, B.I.; Drozdov, I.; Modlin, I.M. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol. Motil. 2009, 21, 439–450. [Google Scholar] [CrossRef]
- Chowers, Y.; Cahalon, L.; Lahav, M.; Schor, H.; Tal, R.; Bar-Meir, S.; Levite, M. Somatostatin through its specific receptor inhibits spontaneous and TNF-alpha- and bacteria-induced IL-8 and IL-1 beta secretion from intestinal epithelial cells. J. Immunol. 2000, 165, 2955–2961. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G. Changes in enteroendocrine and immune cells following colitis induction by TNBS in rats. Mol. Med. Rep. 2016, 14, 4967–4974. [Google Scholar] [CrossRef]
- Lamrani, A.; Tulliez, M.; Chauvelot-Moachon, L.; Chaussade, S.; Mauprivez, C.; Hagnéré, A.M.; Vidon, N. Effects of octreotide treatment on early TNF-alpha production and localization in experimental chronic colitis. Aliment. Pharmacol. Ther. 1999, 13, 583–594. [Google Scholar] [CrossRef]
- Eliakim, R.; Karmeli, F.; Okon, E.; Rachmilewitz, D. Octreotide effectively decreases mucosal damage in experimental colitis. Gut 1993, 34, 264–269. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H. Abnormalities in endocrine and immune cells are correlated in dextran-sulfate-sodium-induced colitis in rats. Mol. Med. Rep. 2017, 15, 12–20. [Google Scholar] [CrossRef]
- Lei, S.; Cheng, T.; Guo, Y.; Li, C.; Zhang, W.; Zhi, F. Somatostatin ameliorates lipopolysaccharide-induced tight junction damage via the ERK-MAPK pathway in Caco2 cells. Eur. J. Cell Biol. 2014, 93, 299–307. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Xu, H.; Tao, L.; Lu, J.; Cai, L.; Wang, C. Somatostatin regulates tight junction proteins expression in colitis mice. Int. J. Clin. Exp. Pathol. 2014, 7, 2153–2162. [Google Scholar]
- Cai, L.; Li, X.; Geng, C.; Lei, X.; Wang, C. Molecular mechanisms of somatostatin-mediated intestinal epithelial barrier function restoration by upregulating claudin-4 in mice with DSS-induced colitis. Am. J. Physiol. Cell Physiol. 2018, 315, C527–C536. [Google Scholar] [CrossRef]
- Van Raay, T.; Allen-Vercoe, E. Microbial Interactions and Interventions in Colorectal Cancer. Microbiol. Spectr. 2017, 5, 99–130. [Google Scholar] [CrossRef]
- Wieczorska, K.; Stolarek, M.; Stec, R. The Role of the Gut Microbiome in Colorectal Cancer: Where Are We? Where Are We Going? Clin. Color. Cancer 2020, 19, 5–12. [Google Scholar] [CrossRef]
- Xie, X.; Geng, C.; Li, X.; Liao, J.; Li, Y.; Guo, Y.; Wang, C. Roles of gastrointestinal polypeptides in intestinal barrier regulation. Peptides 2022, 151, 170753. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Pina-Vaz, C.; Baltazar, F. Microbes and Cancer: Friends or Faux? Int. J. Mol. Sci. 2020, 21, 3115. [Google Scholar] [CrossRef]
- Perillo, F.; Amoroso, C.; Strati, F.; Giuffrè, M.R.; Díaz-Basabe, A.; Lattanzi, G.; Facciotti, F. Gut Microbiota Manipulation as a Tool for Colorectal Cancer Management: Recent Advances in Its Use for Therapeutic Purposes. Int. J. Mol. Sci. 2020, 21, 5389. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Vetrano, S.; Danese, S. Colitis, microbiota, and colon cancer: An infernal triangle. Gastroenterology 2013, 144, 461–463. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667, Erratum in PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Akin, H.; Tözün, N. Diet, microbiota, and colorectal cancer. J. Clin. Gastroenterol. 2014, 48 (Suppl. S1), S67–S69. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Gu, J. Clinical Implications of the Associations Between Intestinal Microbiome and Colorectal Cancer Progression. Cancer Manag. Res. 2020, 12, 4117–4128. [Google Scholar] [CrossRef]
- Abed, J.; Emgård, J.E.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zheng, X.; Ren, L.; Yang, Y.; Li, W.; Fu, W.; Wang, J.; Du, G. Tumorigenic bacteria in colorectal cancer: Mechanisms and treatments. Cancer Biol. Med. 2021, 19, 147–162. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 615056. [Google Scholar] [CrossRef]
- Cuevas-Ramos, G.; Petit, C.R.; Marcq, I.; Boury, M.; Oswald, E.; Nougayrède, J.P. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl. Acad. Sci. USA 2010, 107, 11537–11542. [Google Scholar] [CrossRef]
- Faïs, T.; Delmas, J.; Barnich, N.; Bonnet, R.; Dalmasso, G. Colibactin: More Than a New Bacterial Toxin. Toxins 2018, 10, 151. [Google Scholar] [CrossRef]
- Prorok-Hamon, M.; Friswell, M.K.; Alswied, A.; Roberts, C.L.; Song, F.; Flanagan, P.K.; Knight, P.; Codling, C.; Marchesi, J.R.; Winstanley, C.; et al. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut 2014, 63, 761–770. [Google Scholar] [CrossRef]
- Kawanishi, M.; Hisatomi, Y.; Oda, Y.; Shimohara, C.; Tsunematsu, Y.; Sato, M.; Hirayama, Y.; Miyoshi, N.; Iwashita, Y.; Yoshikawa, Y.; et al. In vitro genotoxicity analyses of colibactin-producing E. coli isolated from a Japanese colorectal cancer patient. J. Toxicol. Sci. 2019, 44, 871–876. [Google Scholar] [CrossRef]
- Bertocchi, A.; Carloni, S.; Ravenda, P.S.; Bertalot, G.; Spadoni, I.; Lo Cascio, A.; Gandini, S.; Lizier, M.; Braga, D.; Asnicar, F.; et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell 2021, 39, 708–724.e11. [Google Scholar] [CrossRef]
- Long, S.; Yang, Y.; Shen, C.; Wang, Y.; Deng, A.; Qin, Q.; Qiao, L. Metaproteomics characterizes human gut microbiome function in colorectal cancer. NPJ Biofilms Microbiomes 2020, 6, 14. [Google Scholar] [CrossRef]
- Liu, N.N.; Jiao, N.; Tan, J.C.; Wang, Z.; Wu, D.; Wang, A.J.; Chen, J.; Tao, L.; Zhou, C.; Fang, W.; et al. Multi-kingdom microbiota analyses identify bacterial-fungal interactions and biomarkers of colorectal cancer across cohorts. Nat. Microbiol. 2022, 7, 238–250. [Google Scholar] [CrossRef]
- Lichtman, S.M. Bacterial [correction of baterial] translocation in humans. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 1–10. [Google Scholar] [CrossRef]
- Türkçapar, A.G.; Ersöz, S.; Gür, U.; Yerdel, M.A.; Karaaslan, A.; Erden, E.; Kuterdem, E. The effect of octreotid on bacterial translocation from the gut. An experimental study. Int. Surg. 1995, 80, 264–266. [Google Scholar]
- Tocchi, A.; Costa, G.; Lepre, L.; Mazzoni, G.; Liotta, G.; Agostini, N.; Miccini, M.; Cassini, D. The influence of somatostatin on bacterial translocation. Panminerva Med. 2001, 43, 11–14. [Google Scholar]
- Jensen, E.A.; Young, J.A.; Mathes, S.C.; List, E.O.; Carroll, R.K.; Kuhn, J.; Onusko, M.; Kopchick, J.J.; Murphy, E.R.; Berryman, D.E. Crosstalk between the growth hormone/insulin-like growth factor-1 axis and the gut microbiome: A new frontier for microbial endocrinology. Growth Horm. IGF Res. 2020, 53, 101333. [Google Scholar] [CrossRef]
- Dörffel, Y.; Swidsinski, A.; Loening-Baucke, V.; Wiedenmann, B.; Pavel, M. Common biostructure of the colonic microbiota in neuroendocrine tumors and Crohn’s disease and the effect of therapy. Inflamm. Bowel Dis. 2012, 18, 1663–1671. [Google Scholar] [CrossRef]
- LeRoith, D.; Pickens, W.; Crosby, L.K.; Berelowitz, M.; Holtgrefe, M.; Shiloach, J. Evidence for multiple molecular weight forms of somatostatin-like material in Escherichia coli. Biochim. Biophys. Acta 1985, 838, 335–342. [Google Scholar] [CrossRef]
- LeRoith, D.; Pickens, W.; Vinik, A.I.; Shiloach, J. Bacillus subtilis contains multiple forms of somatostatin-like material. Biochem. Biophys. Res. Commun. 1985, 127, 713–719. [Google Scholar] [CrossRef]
- Green, R.; Schaber, M.D.; Shields, D.; Kramer, R. Secretion of somatostatin by Saccharomyces cerevisiae. Correct processing of an alpha-factor-somatostatin hybrid. J. Biol. Chem. 1986, 261, 7558–7565. [Google Scholar] [CrossRef]
- Zeng, X.; Li, X.; Yue, Y.; Wang, X.; Chen, H.; Gu, Y.; Jia, H.; He, Y.; Yuan, Y.; Yue, T. Ameliorative Effect of Saccharomyces cerevisiae JKSP39 on Fusobacterium nucleatum and Dextran Sulfate Sodium-Induced Colitis Mouse Model. J. Agric. Food Chem. 2022, 70, 14179–14192. [Google Scholar] [CrossRef]
- Li, J.Q.; Li, J.L.; Xie, Y.H.; Wang, Y.; Shen, X.N.; Qian, Y.; Han, J.X.; Chen, Y.X.; Fang, J.Y. Saccharomyces cerevisiae may serve as a probiotic in colorectal cancer by promoting cancer cell apoptosis. J. Dig. Dis. 2020, 21, 571–582. [Google Scholar] [CrossRef]
- Vaiopoulos, A.G.; Athanasoula, K.C.; Papavassiliou, A.G. Epigenetic modifications in colorectal cancer: Molecular insights and therapeutic challenges. Biochim. Biophys. Acta 2014, 1842, 971–980. [Google Scholar] [CrossRef]
- Ma, Z.; Williams, M.; Cheng, Y.Y.; Leung, W.K. Roles of Methylated DNA Biomarkers in Patients with Colorectal Cancer. Dis. Markers 2019, 2019, 2673543. [Google Scholar] [CrossRef]
- Jung, G.; Hernández-Illán, E.; Moreira, L.; Balaguer, F.; Goel, A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 111–130. [Google Scholar] [CrossRef]
- SST and SST1-5. Available online: https://www.genecards.org (accessed on 29 February 2024).
- Mori, Y.; Cai, K.; Cheng, Y.; Wang, S.; Paun, B.; Hamilton, J.P.; Jin, Z.; Sato, F.; Berki, A.T.; Kan, T.; et al. A genome-wide search identifies epigenetic silencing of somatostatin, tachykinin-1, and 5 other genes in colon cancer. Gastroenterology 2006, 131, 797–808, Erratum in Gastroenterology 2006, 131, 1659. [Google Scholar] [CrossRef]
- Liu, Y.; Chew, M.H.; Tham, C.K.; Tang, C.L.; Ong, S.Y.; Zhao, Y. Methylation of serum SST gene is an independent prognostic marker in colorectal cancer. Am. J. Cancer Res. 2016, 6, 2098–2108. [Google Scholar]
- Liu, J.; Li, H.; Sun, L.; Wang, Z.; Xing, C.; Yuan, Y. Aberrantly methylated-differentially expressed genes and pathways in colorectal cancer. Cancer Cell Int. 2017, 17, 75. [Google Scholar] [CrossRef]
- Klomp, M.J.; Dalm, S.U.; de Jong, M.; Feelders, R.A.; Hofland, J.; Hofland, L.J. Epigenetic regulation of somatostatin and somatostatin receptors in neuroendocrine tumors and other types of cancer. Rev. Endocr. Metab. Disord. 2021, 22, 495–510. [Google Scholar] [CrossRef]
- Tham, C.; Chew, M.; Soong, R.; Lim, J.; Ang, M.; Tang, C.; Zhao, Y.; Ong, S.Y.; Liu, Y. Postoperative serum methylation levels of TAC1 and SEPT9 are independent predictors of recurrence and survival of patients with colorectal cancer. Cancer 2014, 120, 3131–3141. [Google Scholar] [CrossRef]
- Dai, X.; Sun, X.; Wu, Y.; Lv, Z.; Yu, Z.; Yuan, Y.; Sun, L. Site-Specific Hypermethylation of SST 1stExon as a Biomarker for Predicting the Risk of Gastrointestinal Tract Cancers. Dis. Markers. 2022, 2022, 4570290. [Google Scholar] [CrossRef]
- Harris, A.G. Somatostatin and somatostatin analogues: Pharmacokinetics and pharmacodynamic effects. Gut 1994, 35 (Suppl. S3), S1–S4. [Google Scholar] [CrossRef]
- Pradayrol, L.; Jörnvall, H.; Mutt, V.; Ribet, A. N-terminally extended somatostatin: The primary structure of somatostatin-28. FEBS Lett. 1980, 109, 55–58. [Google Scholar] [CrossRef]
- Martinez, V. Somatostatin. In Handbook of Biologically Active Peptides; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1320–1329. [Google Scholar] [CrossRef]
- Marbach, P.; Briner, U.; Lemaire, M.; Schweitzer, A.; Terasaki, T. From somatostatin to Sandostatin: Pharmacodynamics and pharmacokinetics. Digestion 1993, 54 (Suppl. S1), 9–13. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Buonaguro, L.; Tornesello, M.L.; Buonaguro, F.M. New Insights in the Design of Bioactive Peptides and Chelating Agents for Imaging and Therapy in Oncology. Molecules 2017, 22, 1282. [Google Scholar] [CrossRef]
- Bauer, W.; Briner, U.; Doepfner, W.; Haller, R.; Huguenin, R.; Marbach, P.; Petcher, T.J.; Pless, J. SMS 201–995: A very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982, 31, 1133–1140. [Google Scholar] [CrossRef]
- Weckbecker, G.; Raulf, F.; Stolz, B.; Bruns, C. Somatostatin analogs for diagnosis and treatment of cancer. Pharmacol. Ther. 1993, 60, 245–264. [Google Scholar] [CrossRef]
- Chanson, P.; Timsit, J.; Harris, A.G. Clinical pharmacokinetics of octreotide. Therapeutic applications in patients with pituitary tumours. Clin. Pharmacokinet. 1993, 25, 375–391. [Google Scholar] [CrossRef]
- Kuhn, J.M.; Legrand, A.; Ruiz, J.M.; Obach, R.; De Ronzan, J.; Thomas, F. Pharmacokinetic and pharmacodynamic properties of a long-acting formulation of the new somatostatin analogue, lanreotide, in normal healthy volunteers. Br. J. Clin. Pharmacol. 1994, 38, 213–219. [Google Scholar] [CrossRef]
- Bruns, C.; Lewis, I.; Briner, U.; Meno-Tetang, G.; Weckbecker, G. SOM230: A novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 2002, 146, 707–716. [Google Scholar] [CrossRef]
- Yordanova, A.; Biersack, H.J.; Ahmadzadehfar, H. Advances in Molecular Imaging and Radionuclide Therapy of Neuroendocrine Tumors. J. Clin. Med. 2020, 9, 3679. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Novel therapeutics for patients with well-differentiated gastroenteropancreatic neuroendocrine tumors. Ther. Adv. Med. Oncol. 2021, 13, 17588359211018047. [Google Scholar] [CrossRef]
- Laskaratos, F.M.; Armeni, E.; Shah, H.; Megapanou, M.; Papantoniou, D.; Hayes, A.R.; Navalkissoor, S.; Gnanasegaran, G.; von Stempel, C.; Phillips, E.; et al. Predictors of antiproliferative effect of lanreotide autogel in advanced gastroenteropancreatic neuroendocrine neoplasms. Endocrine 2020, 67, 233–242. [Google Scholar] [CrossRef]
- Faggiano, A. Long-acting somatostatin analogs and well differentiated neuroendocrine tumors: A 20-year-old story. J. Endocrinol. Investig. 2023, 47, 35–46. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: The CLARINET open-label extension study. Endocr. Relat. Cancer 2016, 23, 191–199. [Google Scholar] [CrossRef]
- Michael, M.; Garcia-Carbonero, R.; Weber, M.M.; Lombard-Bohas, C.; Toumpanakis, C.; Hicks, R.J. The Antiproliferative Role of Lanreotide in Controlling Growth of Neuroendocrine Tumors: A Systematic Review. Oncologist 2017, 22, 272–285. [Google Scholar] [CrossRef]
- Lepage, C.; Phelip, J.M.; Lievre, A.; Le-Malicot, K.; Dahan, L.; Tougeron, D.; Toumpanakis, C.; Di-Fiore, F.; Lombard-Bohas, C.; Borbath, I.; et al. Lanreotide as maintenance therapy after first-line treatment in patients with non-resectable duodeno-pancreatic neuroendocrine tumours: An international double-blind, placebo-controlled randomised phase II trial—Prodige 31 REMINET: An FFCD study. Eur. J. Cancer 2022, 175, 31–40. [Google Scholar] [CrossRef]
- Anthony, L.; Vinik, A.I. Evaluating the characteristics and the management of patients with neuroendocrine tumors receiving octreotide LAR during a 6-year period. Pancreas 2011, 40, 987–994. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Jin, K.; Fang, C.; Lin, Y.; Xue, L.; Feng, S.; Zhou, Z.; Shao, C.; Chen, M.; et al. Somatostatin receptor expression indicates improved prognosis in gastroenteropancreatic neuroendocrine neoplasm, and octreotide long-acting release is effective and safe in Chinese patients with advanced gastroenteropancreatic neuroendocrine tumors. Oncol. Lett. 2017, 13, 1165–1174. [Google Scholar] [CrossRef]
- Cantone, M.C.; Dicitore, A.; Vitale, G. Somatostatin-Dopamine Chimeric Molecules in Neuroendocrine Neoplasms. J. Clin. Med. 2021, 10, 501. [Google Scholar] [CrossRef]
- Mandriani, B.; Pellè, E.; Mannavola, F.; Palazzo, A.; Marsano, R.M.; Ingravallo, G.; Cazzato, G.; Ramello, M.C.; Porta, C.; Strosberg, J.; et al. Development of anti-somatostatin receptors CAR T cells for treatment of neuroendocrine tumors. J. Immunother. Cancer 2022, 10, e004854. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Koch, T.; Schröder, H.; Klutzny, M.; Kirscht, S.; Kreienkamp, H.J.; Höllt, V.; Schulz, S. Homo- and heterodimerization of somatostatin receptor subtypes. Inactivation of sst(3) receptor function by heterodimerization with sst(2A). J. Biol. Chem. 2001, 276, 14027–14036. [Google Scholar] [CrossRef]
- Goldberg, R.M.; Moertel, C.G.; Wieand, H.S.; Krook, J.E.; Schutt, A.J.; Veeder, M.H.; Mailliard, J.A.; Dalton, R.J. A phase III evaluation of a somatostatin analogue (octreotide) in the treatment of patients with asymptomatic advanced colon carcinoma. North Central Cancer Treatment Group and the Mayo Clinic. Cancer 1995, 76, 961–966. [Google Scholar] [CrossRef]
- Cascinu, S.; Del Ferro, E.; Catalano, G. A randomised trial of octreotide vs best supportive care only in advanced gastrointestinal cancer patients refractory to chemotherapy. Br. J. Cancer 1995, 71, 97–101. [Google Scholar] [CrossRef][Green Version]
- Cascinu, S.; Catalano, V.; Giordani, P.; Baldelli, A.M.; Agostinelli, R.; Catalano, G. Gastrointestinal cancer refractory to chemotherapy: A role for octreotide? Chemotherapy 2001, 47 (Suppl. S2), 127–133. [Google Scholar] [CrossRef]
- Savage, A.P.; Calam, J.; Wood, C.B.; Bloom, S.R. SMS 201-995 treatment and advanced intestinal cancer: A pilot study. Aliment. Pharmacol. Ther. 1987, 1, 133–139. [Google Scholar] [CrossRef]
- Periferakis, A.; Tsigas, G.; Periferakis, A.T.; Badarau, I.A.; Scheau, A.E.; Tampa, M.; Georgescu, S.R.; Didilescu, A.C.; Scheau, C.; Caruntu, C. Antitumoral and Anti-inflammatory Roles of Somatostatin and Its Analogs in Hepatocellular Carcinoma. Anal. Cell. Pathol. 2021, 2021, 1840069. [Google Scholar] [CrossRef]
- Setyono-Han, B.; Henkelman, M.S.; Foekens, J.A.; Klijn, G.M. Direct inhibitory effects of somatostatin (analogues) on the growth of human breast cancer cells. Cancer Res. 1987, 47, 1566–1570. [Google Scholar]
- Sharma, K.; Srikant, C.B. Induction of wild-type p53, Bax, and acidic endonuclease during somatostatin-signaled apoptosis in MCF-7 human breast cancer cells. Int. J. Cancer 1998, 76, 259–266. [Google Scholar] [CrossRef]
- Huang, C.M.; Wu, Y.T.; Chen, S.T. Targeting delivery of paclitaxel into tumor cells via somatostatin receptor endocytosis. Chem. Biol. 2000, 7, 453–461. [Google Scholar] [CrossRef]
- Ju, R.J.; Cheng, L.; Peng, X.M.; Wang, T.; Li, C.Q.; Song, X.L.; Liu, S.; Chao, J.P.; Li, X.T. Octreotide-modified liposomes containing daunorubicin and dihydroartemisinin for treatment of invasive breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S1), 616–628. [Google Scholar] [CrossRef]
- Bharti, R.; Dey, G.; Banerjee, I.; Dey, K.K.; Parida, S.; Kumar, B.N.; Das, C.K.; Pal, I.; Mukherjee, M.; Misra, M.; et al. Somatostatin receptor targeted liposomes with Diacerein inhibit IL-6 for breast cancer therapy. Cancer Lett. 2017, 388, 292–302. [Google Scholar] [CrossRef]
- Watt, H.L.; Kharmate, G.D.; Kumar, U. Somatostatin receptors 1 and 5 heterodimerize with epidermal growth factor receptor: Agonist-dependent modulation of the downstream MAPK signalling pathway in breast cancer cells. Cell. Signal. 2009, 21, 428–439. [Google Scholar] [CrossRef]
- War, S.A.; Kim, B.; Kumar, U. Human somatostatin receptor-3 distinctively induces apoptosis in MCF-7 and cell cycle arrest in MDA-MB-231 breast cancer cells. Mol. Cell. Endocrinol. 2015, 413, 129–144. [Google Scholar] [CrossRef]
- Canobbio, L.; Cannata, D.; Miglietta, L.; Boccardo, F. Somatuline (BIM 23014) and tamoxifen treatment of postmenopausal breast cancer patients: Clinical activity and effect on insulin-like growth factor-I (IGF-I) levels. Anticancer Res. 1995, 15, 2687–2690. [Google Scholar]
- O’Byrne, K.J.; Dobbs, N.; Propper, D.J.; Braybrooke, J.P.; Koukourakis, M.I.; Mitchell, K.; Woodhull, J.; Talbot, D.C.; Schally, A.V.; Harris, A.L. Phase II study of RC-160 (vapreotide), an octapeptide analogue of somatostatin, in the treatment of metastatic breast cancer. Br. J. Cancer 1999, 79, 1413–1418. [Google Scholar] [CrossRef]
- Di Leo, A.; Ferrari, L.; Bajetta, E.; Bartoli, C.; Vicario, G.; Moglia, D.; Miceli, R.; Callegari, M.; Bono, A. Biological and clinical evaluation of lanreotide (BIM 23014), a somatostatin analogue, in the treatment of advanced breast cancer. A pilot study by the I.T.M.O. Group. Italian Trials in Medical Oncology. Breast Cancer Res. Treat. 1995, 34, 237–244. [Google Scholar] [CrossRef]
- Pritchard, K.I.; Shepherd, L.E.; Chapman, J.A.; Norris, B.D.; Cantin, J.; Goss, P.E.; Dent, S.F.; Walde, D.; Vandenberg, T.A.; Findlay, B.; et al. Randomized trial of tamoxifen versus combined tamoxifen and octreotide LAR Therapy in the adjuvant treatment of early-stage breast cancer in postmenopausal women: NCIC CTG MA.14. J. Clin. Oncol. 2011, 29, 3869–3876. [Google Scholar] [CrossRef]
- Pinski, J.; Schally, A.V.; Halmos, G.; Szepeshazi, K. Effect of somatostatin analog RC-160 and bombesin/gastrin releasing peptide antagonist RC-3095 on growth of PC-3 human prostate-cancer xenografts in nude mice. Int. J. Cancer. 1993, 55, 963–967. [Google Scholar] [CrossRef]
- Brevini, T.A.; Bianchi, R.; Motta, M. Direct inhibitory effect of somatostatin on the growth of the human prostatic cancer cell line LNCaP: Possible mechanism of action. J. Clin. Endocrinol. Metab. 1993, 77, 626–631. [Google Scholar] [CrossRef]
- Plonowski, A.; Schally, A.V.; Nagy, A.; Sun, B.; Szepeshazi, K. Inhibition of PC-3 human androgen-independent prostate cancer and its metastases by cytotoxic somatostatin analogue AN-238. Cancer Res. 1999, 59, 1947–1953. [Google Scholar]
- Bogden, A.E.; Taylor, J.E.; Moreau, J.P.; Coy, D.H. Treatment of R-3327 prostate tumors with a somatostatin analogue (somatuline) as adjuvant therapy following surgical castration. Cancer Res. 1990, 50, 2646–2650. [Google Scholar]
- Schally, A.V.; Redding, T.W. Somatostatin analogs as adjuncts to agonists of luteinizing hormone-releasing hormone in the treatment of experimental prostate cancer. Proc. Natl. Acad. Sci. USA 1987, 84, 7275–7279. [Google Scholar] [CrossRef]
- Zalatnai, A.; Paz-Bouza, J.I.; Redding, T.W.; Schally, A.V. Histologic changes in the rat prostate cancer model after treatment with somatostatin analogs and D-Trp-6-LH-RH. Prostate 1988, 12, 85–98. [Google Scholar] [CrossRef]
- Logothetis, C.J.; Hossan, E.A.; Smith, T.L. SMS 201-995 in the treatment of refractory prostatic carcinoma. Anticancer Res. 1994, 14, 2731–2734. [Google Scholar]
- Maulard, C.; Richaud, P.; Droz, J.P.; Jessueld, D.; Dufour-Esquerré, F.; Housset, M. Phase I–II study of the somatostatin analogue lanreotide in hormone-refractory prostate cancer. Cancer Chemother. Pharmacol. 1995, 36, 259–262. [Google Scholar] [CrossRef]
- Figg, W.D.; Thibault, A.; Cooper, M.R.; Reid, R.; Headlee, D.; Dawson, N.; Kohler, D.R.; Reed, E.; Sartor, O. A phase I study of the somatostatin analogue somatuline in patients with metastatic hormone-refractory prostate cancer. Cancer 1995, 75, 2159–2164. [Google Scholar] [CrossRef]
- Mitsogiannis, I.C.; Skolarikos, A.; Deliveliotis, C. Somatostatin analog lanreotide in the treatment of castration-resistant prostate cancer (CRPC). Expert Opin. Pharmacother. 2009, 10, 493–501. [Google Scholar] [CrossRef]
- O’Byrne, K.J.; Schally, A.V.; Thomas, A.; Carney, D.N.; Steward, W.P. Somatostatin, its receptors and analogs, in lung cancer. Chemotherapy 2001, 47 (Suppl. S2), 78–108. [Google Scholar] [CrossRef]
- van Hoek, M.; Hofland, L.J.; de Rijke, Y.B.; van Nederveen, F.H.; de Krijger, R.R.; van Koetsveld, P.M.; Lamberts, S.W.; van der Lely, A.J.; de Herder, W.W.; Feelders, R.A. Effects of somatostatin analogs on a growth hormone-releasing hormone secreting bronchial carcinoid, in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 2009, 94, 428–433. [Google Scholar] [CrossRef]
- Tartarone, A.; Lerose, R.; Aieta, M. Somatostatin Analog Therapy in Small Cell Lung Cancer. Semin. Nucl. Med. 2016, 46, 239–242. [Google Scholar] [CrossRef]
- Macaulay, V.M.; Smith, I.E.; Everard, M.J.; Teale, J.D.; Reubi, J.C.; Millar, J.L. Experimental and clinical studies with somatostatin analogue octreotide in small cell lung cancer. Br. J. Cancer 1991, 64, 451–456. [Google Scholar] [CrossRef][Green Version]
- Stumpf, C.; Kaemmerer, D.; Neubauer, E.; Sänger, J.; Schulz, S.; Lupp, A. Somatostatin and CXCR4 expression patterns in adenocarcinoma and squamous cell carcinoma of the lung relative to small cell lung cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 1921–1932. [Google Scholar] [CrossRef]
- Zarogoulidis, K.; Eleftheriadou, E.; Kontakiotis, T.; Gerasimou, G.; Zarogoulidis, P.; Sapardanis, I.; Galaktidou, G.; Sakkas, L.; Gotzamani-Psarrakou, A.; Karatzas, N. Long acting somatostatin analogues in combination to antineoplastic agents in the treatment of small cell lung cancer patients. Lung Cancer 2012, 76, 84–88. [Google Scholar] [CrossRef]
- Santo, A.; Pilotto, S.; Galetta, D.; Grossi, F.; Fasola, G.; Romano, G.; Bonanno, L.; Bearz, A.; Papi, M.; Roca, E.; et al. Maintenance with lanreotide in small-cell lung cancer expressing somatostatine receptors: A multicenter, randomized, phase 3 trial. Lung Cancer 2019, 134, 121–126. [Google Scholar] [CrossRef]
- Liu, H.L.; Huo, L.; Wang, L. Octreotide inhibits proliferation and induces apoptosis of hepatocellular carcinoma cells. Acta Pharmacol. Sin. 2004, 25, 1380–1386. [Google Scholar]
- Yuen, M.F.; Poon, R.T.; Lai, C.L.; Fan, S.T.; Lo, C.M.; Wong, K.W.; Wong, W.M.; Wong, B.C. A randomized placebo-controlled study of long-acting octreotide for the treatment of advanced hepatocellular carcinoma. Hepatology 2002, 36, 687–691, Erratum in Hepatology 2003, 37, 489. [Google Scholar] [CrossRef]
- Dimitroulopoulos, D.; Xinopoulos, D.; Tsamakidis, K.; Zisimopoulos, A.; Andriotis, E.; Panagiotakos, D.; Fotopoulou, A.; Chrysohoou, C.; Bazinis, A.; Daskalopoulou, D.; et al. Long acting octreotide in the treatment of advanced hepatocellular cancer and overexpression of somatostatin receptors: Randomized placebo-controlled trial. World J. Gastroenterol. 2007, 13, 3164–3170. [Google Scholar] [CrossRef]
- Hua, Y.P.; Yin, X.Y.; Peng, B.G.; Li, S.Q.; Lai, J.M.; Liang, H.Z.; Liang, L.J. Mechanisms and influence of octreotide-induced regulation of somatostatin receptor 2 on hepatocellular carcinoma. Chemotherapy 2009, 55, 312–320. [Google Scholar] [CrossRef]
- Tsagarakis, N.J.; Drygiannakis, I.; Batistakis, A.G.; Kolios, G.; Kouroumalis, E.A. Octreotide induces caspase activation and apoptosis in human hepatoma HepG2 cells. World J. Gastroenterol. 2011, 17, 313–321. [Google Scholar] [CrossRef]
- Raderer, M.; Hejna, M.H.; Muller, C.; Kornek, G.V.; Kurtaran, A.; Virgolini, I.; Fiebieger, W.; Hamilton, G.; Scheithauer, W. Treatment of hepatocellular cancer with the long acting somatostatin analog lanreotide in vitro and in vivo. Int. J. Oncol. 2000, 16, 1197–1201. [Google Scholar] [CrossRef]
- Kouroumalis, E.; Skordilis, P.; Thermos, K.; Vasilaki, A.; Moschandrea, J.; Manousos, O.N. Treatment of hepatocellular carcinoma with octreotide: A randomised controlled study. Gut 1998, 42, 442–447. [Google Scholar] [CrossRef][Green Version]
- Becker, G.; Allgaier, H.P.; Olschewski, M.; Zähringer, A.; Blum, H.E.; HECTOR Study Group. Long-acting octreotide versus placebo for treatment of advanced HCC: A randomized controlled double-blind study. Hepatology 2007, 45, 9–15. [Google Scholar] [CrossRef]
- Barbare, J.C.; Bouché, O.; Bonnetain, F.; Dahan, L.; Lombard-Bohas, C.; Faroux, R.; Raoul, J.L.; Cattan, S.; Lemoine, A.; Blanc, J.F.; et al. Treatment of advanced hepatocellular carcinoma with long-acting octreotide: A phase III multicentre, randomised, double blind placebo-controlled study. Eur. J. Cancer 2009, 45, 1788–1797. [Google Scholar] [CrossRef]
- Pan, D.Y.; Qiao, J.G.; Chen, J.W.; Huo, Y.C.; Zhou, Y.K.; Shi, H.A. Tamoxifen combined with octreotide or regular chemotherapeutic agents in treatment of primary liver cancer: A randomized controlled trial. Hepatobiliary Pancreat. Dis. Int. 2003, 2, 211–215. [Google Scholar]
- Verset, G.; Verslype, C.; Reynaert, H.; Borbath, I.; Langlet, P.; Vandebroek, A.; Peeters, M.; Houbiers, G.; Francque, S.; Arvanitakis, M.; et al. Efficacy of the combination of long-acting release octreotide and tamoxifen in patients with advanced hepatocellular carcinoma: A randomised multicentre phase III study. Br. J. Cancer 2007, 97, 582–588. [Google Scholar] [CrossRef]
- Treiber, G.; Wex, T.; Röcken, C.; Fostitsch, P.; Malfertheiner, P. Impact of biomarkers on disease survival and progression in patients treated with octreotide for advanced hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2006, 132, 699–708. [Google Scholar] [CrossRef]
- Tong, H.; Wei, B.; Chen, S.; Xie, Y.M.; Zhang, M.G.; Zhang, L.H.; Huang, Z.Y.; Tang, C.W. Adjuvant celecoxib and lanreotide following transarterial chemoembolisation for unresectable hepatocellular carcinoma: A randomized pilot study. Oncotarget 2017, 8, 48303–48312. [Google Scholar] [CrossRef]
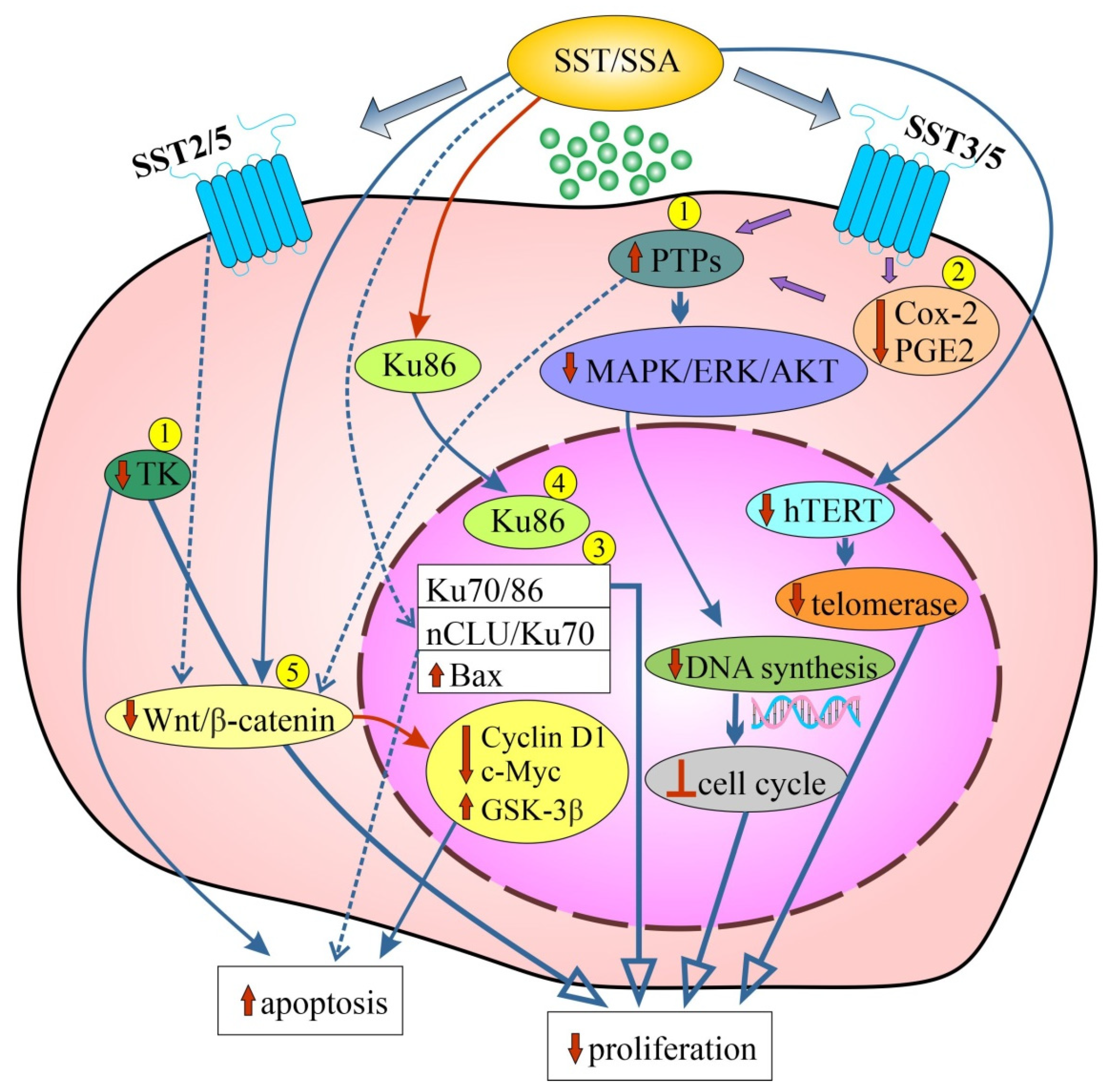
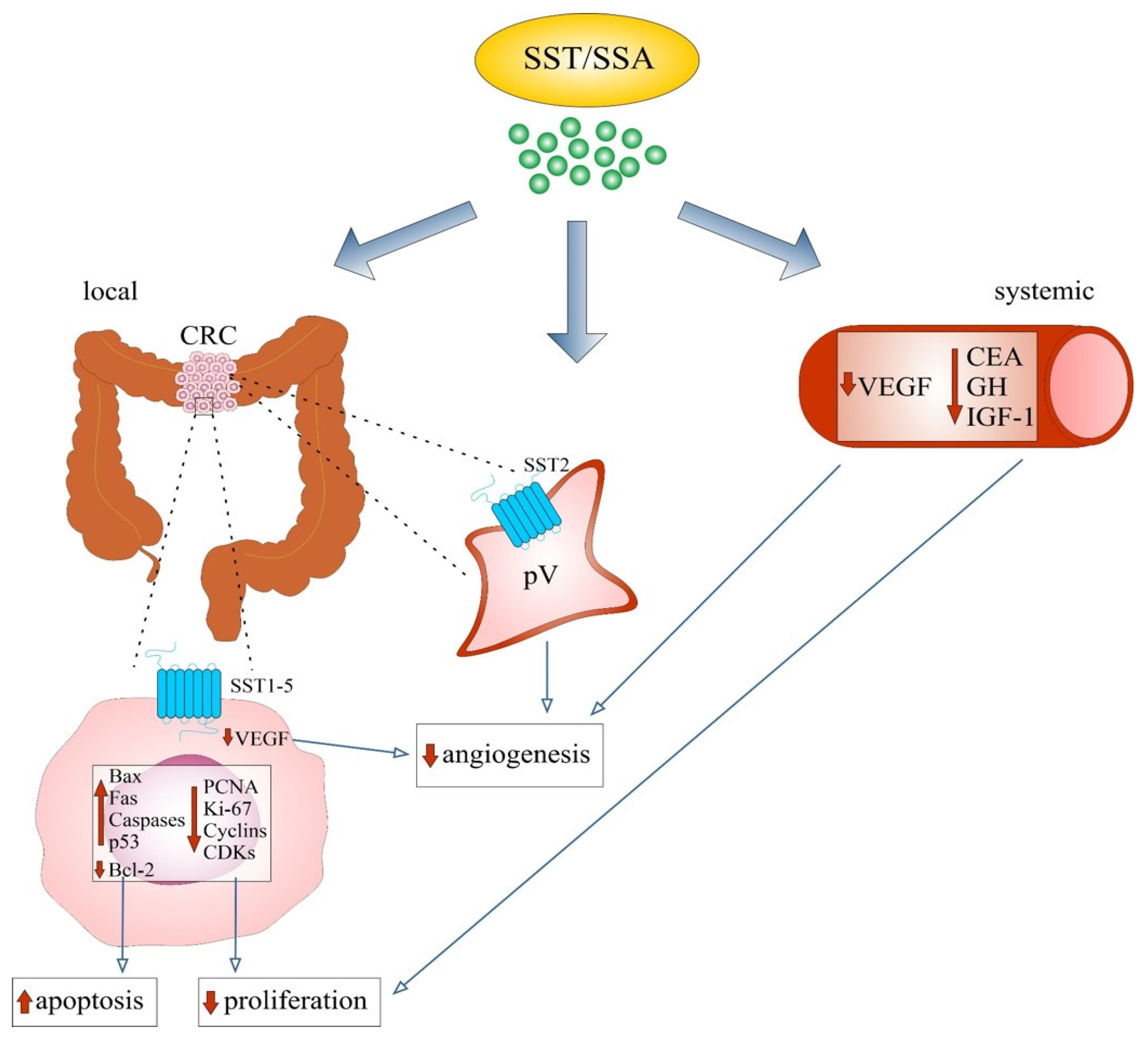
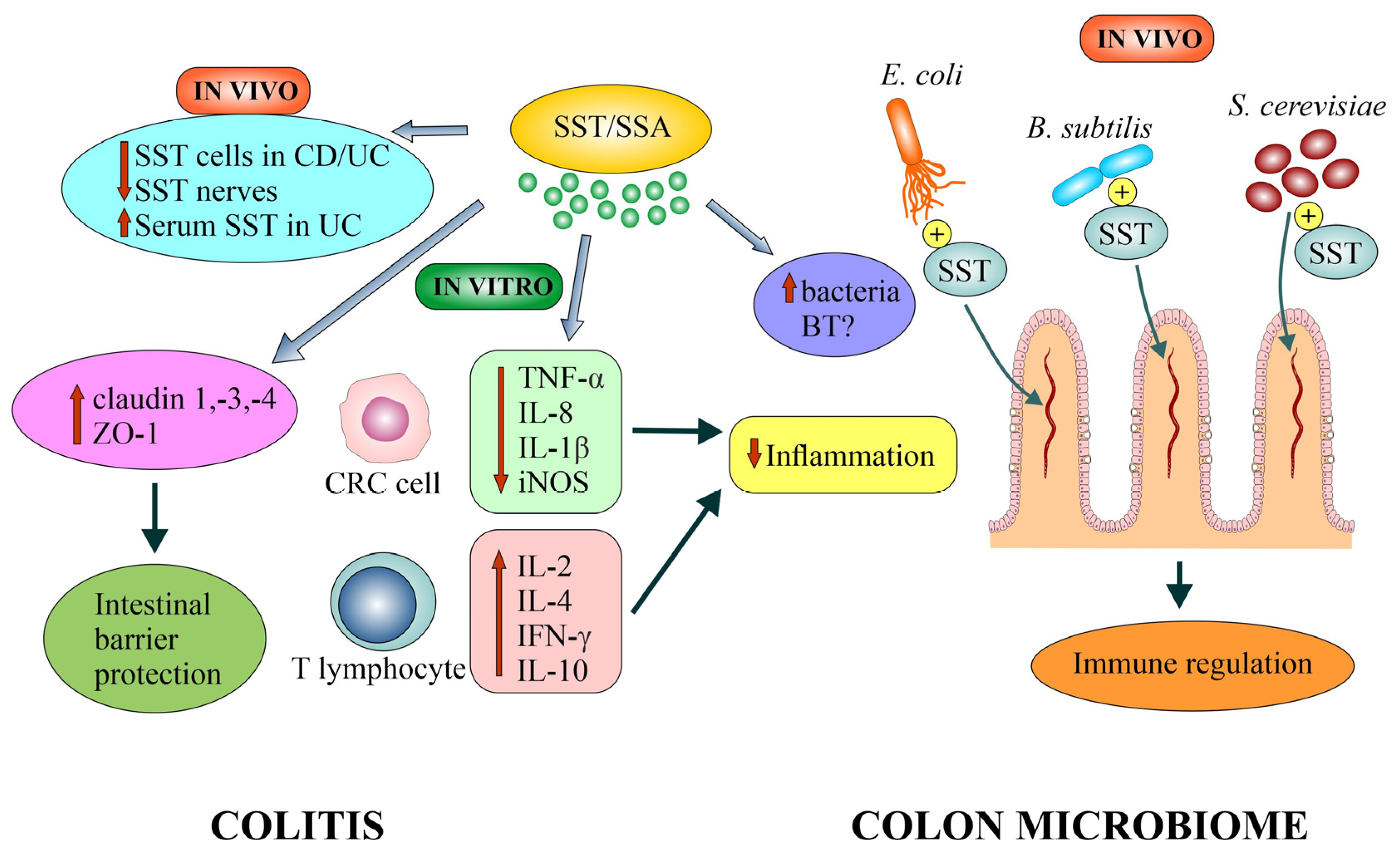
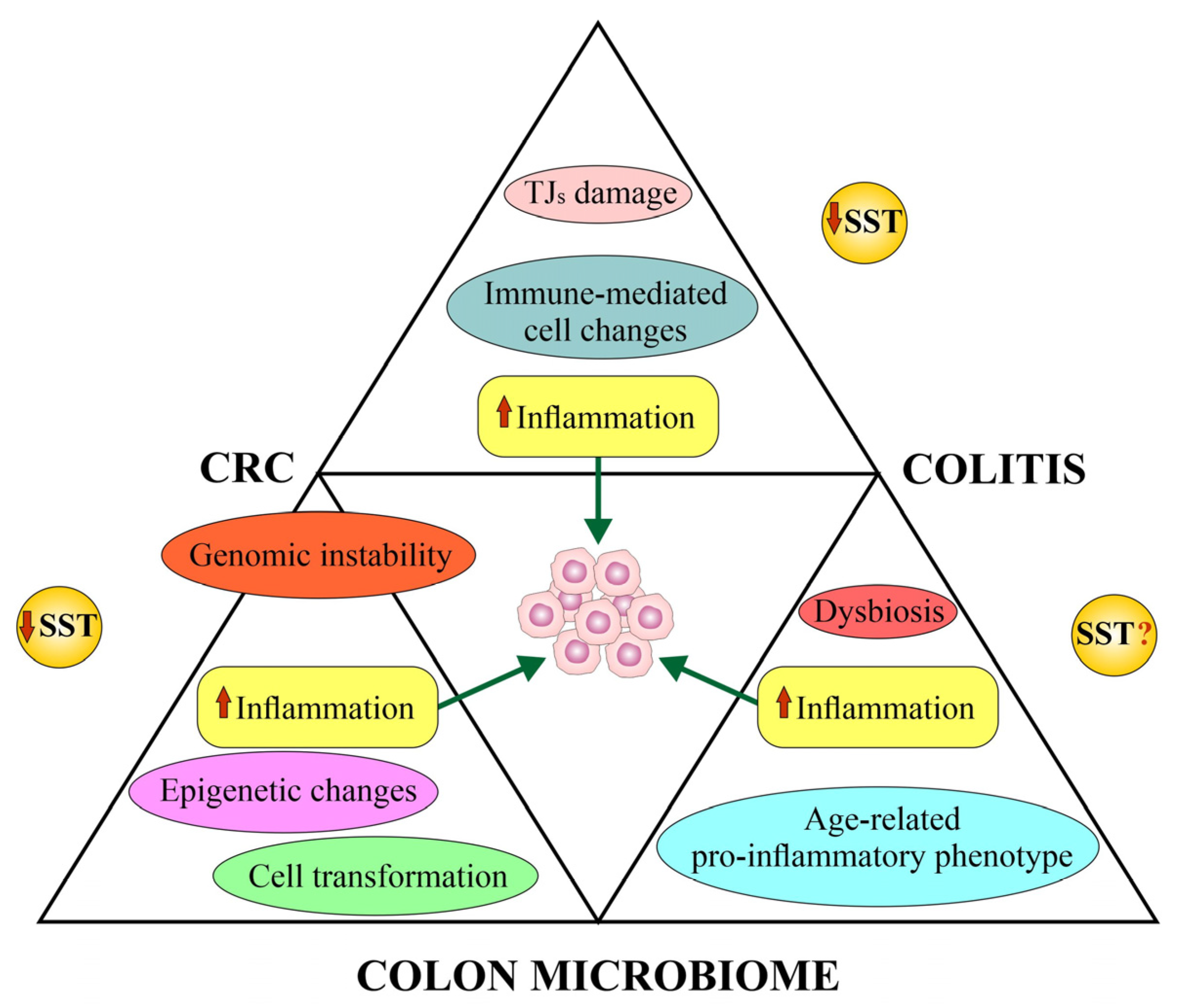
| Cell Line/Animal Model | SSA Type/Methods | Findings | Antitumor Activity | Ref. No. |
|---|---|---|---|---|
| CX1, X56 and HT-29; nude mice xenografts (CX1 and X56 cells) | SST-14 | (i) Ʇ tumor growth in CX1 in vivo and HT-29 in vitro (but not X56 cells); (ii) Ʇ the gastrin-induced growth | ↓ Cell proliferation Ʇ Tumor growth | [113] |
| DHD/K12 rat colon cancer cells and implanted tumors | SST-14 and RC-160 | (i) Ʇ tumor growth in vivo; (ii) ↓ LI by 35%; (iii) ↓ total protein/DNA in the tumors by 70.1%/68.7%, respectively | [126] | |
| LIM 1215, LIM 1863, LIM 2405, LIM 2412; LIM 2412 and LIM 2405; xenografts in nude mice | SMS 201-995 | Ʇ both in vitro and in vivo growth of colon cancer | [122] | |
| LIM 2412 and LoVo; LIM 2412 xenografts | SMS 201-995 | (i) a 13% ↓ in CEA (LoVo); (ii) a direct correlation between the mean volume of the xenografts and serum CEA level; (iii) Ʇ xenograft growth correlated with a ↓ serum CEA | [148] | |
| HT-29 and IEC-6 | SST-14 | Ʇ HT 29 cell growth (only in the presence of serum) | [114] | |
| HT-29, SW620 and other cell line (MCF-7) | TT-232 | (i) strong tyrosine kinase inhibitory effect; (ii) Ʇ proliferation (~70%) in both CRC cell lines | [139] | |
| HT-29 and nude mice bearing xenografts | RC-160 | (i) ↓ tumor growth; (ii) specific binding sites of SST, bombesin and EGF on intact HT-29 cells or on HT-29 xenografts | [115] | |
| SW480 and SW620 | SMS 201-995 | (i) Ʇ cell proliferation vs. untreated cultures; (ii) after OCT 10−8 M: ↓ the mitogenic effect of EGF on SW480 vs. cells exposed to EGF alone; (iii) ↑ the effect on cell growth by its combination with cytokines (IL-2 and IFN-γ) in SW620 | [123] | |
| C170 and LIM 1215 | SMS 201-995 (Sandostatin) alone and in combination with 5-FU | (i) SST alone with minimal inhibitory effects on cell growth; (ii) after 5-FU alone: Ʇ as low as 39.6% of C; (iii) after 5-FU + SST: a 10–30% Ʇ vs. 5-FU alone | [127] | |
| SW620 | TT-232 | (i) a strong Ʇ of cell proliferation; (ii) a rapid and sustained ↑ PTP (5–30 min) | [124] | |
| HT-29, SW620, Colo205 and many other cell lines; transplanted Colon 26 tumor | TT-232 | (i) Ʇ cell proliferation >50% in Colo205; (ii) Ʇ tumor growth (70%); (iii) ↑ AI in HT-29 (7×); (iv) Ʇ tyrosine kinase (75%) in SW620 which correlated with the antiproliferative and pro-apoptotic effect | [121] | |
| HT-29 | Sandostatin and TT-232 | (i) after TT-232: ↓ 59 ± 6% in cell no., after Sandostatin: ↓ 21 ± 12%; (ii) ↓ p86 Ku in cytoplasm at the first 4 h, and ↑ in the nucleus at 1 h followed by a ↓ at 4 h | [116] | |
| HCT-116 and LoVo expressing wtp53; HCT-15 and HT-29 with mp53; nude mice model | AN-238, consisting of 2-pyrrolinodoxorubicin (AN-201) linked to octapeptide SST carrier RC-121; DOX treatment | (i) functional SSTRs on HCT-116, HCT-15 and HT-29; (ii) ↑ p53 activity; (iii) AN-238, AN-201 and DOX equally effective on HCT-116 tumors; (iv) after AN-238: Ʇ growth of HCT-15 and HT-29 cancers | [130] | |
| Caco-2 | SST | (i) Ʇ cell growth and modulation of the activation of Ku70/86 heterodimer and the Ku86 levels in the nucleus by ↑ its mRNA level | [145] | |
| C-26 and HT-29 in xenografted mice | TT-232 | in xenografts max tumor Ʇ—27%; in C-26 −75% | [117] | |
| Caco-2, HT-29 and HCT-116 | SST-14; colorimetric assay; BrDU assay; EIA for PGE2; COX-2 mRNA silencing; RT-PCR, WB for SSTRs, COX isoforms, p-ERK-1/ERK-2 and p-AKT | (i) (+) COX-2 and SST3/4/5 in HT-29 and Cox-2 and SST3/5 in Caco-2; (+) SST2/3/5 in HCT-116; (ii) Ʇ basal COX-2, ꞱPGE2, ꞱDNA synthesis and growth in Caco-2 and HT-29 via SST3 or SST5; (iii) ↓ phosphorylation status of ERK-1/ERK-2 in Caco-2; (iv) ↓ constitutive COX-2 via SST-mediated activation of PTP leading to ꞱMAPK pathway | [134] | |
| HT-29 and other cell lines (MDA-MB-23, HepG2, HeLa, Lep-3) | SMS 201-995 and other modified analogues | (i) all compounds with different concentration-dependent antiproliferative effects against all cell lines except Lep-3 after 24 h | [118] | |
| Caco-2 and HT-29 | OCT with or without the trophic effect of insulin treatment; MTT; TRAP; IHC; RT-PCR | (i) (+) SST1/2A/2B/3/4/5 in both lines; (ii) ↓ proliferation of both lines in a time and dose-dependent manner; (iii) ↓ telomerase activity in serum-free cultured medium and ↑ telomerase in the presence of 10% FBS (Caco-2); ↑ telomerase in both conditions in HT-29 | [119] | |
| HT-29 and other cell lines (MDA-MB-23, HepG2, HeLa, Lep-3) | Several modified octapeptide analogues of SST containing unnatural AA | (i) concentration-dependent antiproliferative effect after 24 h; (ii) the compound 4C (Orn5, Aib6) the most pronounced antiproliferative effects on HT-29 | [109] | |
| HT-29, SW480, LoVo and two cell lines to verify NE cell marker expression (DiFi and Colo320) | Exogenous SST and SST inhibitor cycloSST; ALDEFLUOR assay; FC; RT-PCR; crypt isolation | (i) after SST: Ʇproliferation but not ALDH+ population size or viability; (ii) co-cultured with SST1+ cells: Ʇ sphere-formation and Ʇcell proliferation of ALDH+ cells | [37] | |
| HT-29 and other cell lines (MDA-MB-23, HepG2, HeLa, Lip-3) | A series of new analogues of BIM-23052, a linear SST analogue | (i) different concentration-dependent antiproliferative effect on cells after 24 h; (ii) all compounds bind well to SSTRs with preference to SST3 and SST5 | [120] | |
| HCT-116, HepG-2 and MCF-7 | conjugated CTX-OCT loaded onto Ca-alginate-beads; DSC; FTIR; SEM; UV spectroscopy; cytotoxicity assay | CTX-OCT-Alg beads with gastro-resistant activity and efficiently deliver anti-cancer drugs to the higher pH environments of the colon with > antiproliferative activity vs. free drug | [136] | |
| HCT-116, CT26 and others (Vero and BSC-40, HEK-293, B16, LO2, A549; U-2 OS, HeLa, SMMC-7721, SGC-790, MDA-MB-231 and MCF-7); BALB/c-nu mice, C57BL/6 and BALB/c mice with tumor cells | vaccinia VG9/(SST-14)2-HSA recombinant constructed by replacing SST fusion gene into TK locus of attenuated VG9 strain via homologous recombination | (i) a combined antitumor effect on SSTR+ cells; (ii) complete Ʇtumor in 3/10 mice after vaccinia VG9/TK− or VG9/(SST-14)2-HSA, and ↑ survival of all mice in both groups; (iii) VG9/(SST-14)2-HSA is more effective in ↑ survival vs. VG9/TK; (iv) the oncolytic activity of vaccinia viruses not high enough in HCT-116; CT26 sensitive to all 3 viruses | [137] | |
| SW620, HT-29, Colo205 and 17 other tumor cell lines; Colon 26 tumor fragments transplanted into BALB/C inbred mice | TT-232 | (i) a 70% Ʇ tumor growth of Colon 26 tumor; (ii) ↑ apoptosis in HT-29 and SW620 (7× ↑ AI in HT-29 cells); (iii) Ʇ tyrosine kinase (75%) after 24 h in SW620, correlated well with ↓ proliferation and ↑ apoptosis; (iv) p53-independent apoptotic effect | ↓ Cell proliferation Ʇ Tumor growth ↑ Cell apoptosis | [121] |
| transplantable murine colon 38 cancer | OCT (SMS, Sandostatin) and Mel; BrDU incorporation, weight of tumors; TUNEL; AI, LI | (i) after both SMS and Mel: ↓ LI and ↑ AI; (ii) AI in the group treated jointly with SMS and Mel < in groups treated separately with SMS or Mel; (iii) proliferation/apoptosis ratio < in the group treated with SMS or Mel | [151] | |
| HT-29 | 3H-labeled heptapeptide somatostatin analogue TT-232 | (i) (+) membranous and nuclear expression; (ii) low-affinity SSTRs in such cells, which might mediate the apoptosis-inducing effect | [133] | |
| HT-29, HCT-15, HCT-116 and P388/R84; nude mice with tumor transplantation | AN-162, (DOX conjugated to SST carrier RC-121); RT-PCR | (i) (+) mRNA SST and high-affinity binding sites for SST in all cell lines; (ii) Ʇ HCT-116 and P388/R84 in S/G2 phase and ↑no. of apoptotic cells; (ii) ↓ volume of xenografts > its unconjugated components | [125] | |
| WiDr with mp53 | SMS 201-995 alone or in combination with 5-FU | (i) ↑ apoptosis, ↑ the % of cells with subdiploid DNA content; (ii) ↓ G0/G1 phase cells by 22.9% and 14.3% and G2/M by 14.3%; (iii) ↑ of 5-FU-induced S-phase Ʇ by a further 7.9%/12.9%/42.1% at 24/36/72 h, respectively | [135] | |
| SW480 | OCT; MTT and flow cytometric assays; microarray; WB | (i) Ʇ growth, ↑ apoptosis and arrested the G1 phase cells in a dose-dependent manner; (ii) ↑ 13 genes and ↓ 17 genes in Wnt/β–catenin pathway; (iii) ↑ pβ-catenin | [129] | |
| Caco-2 | SST; WB for Ku70, Ku86 and CLU; confocal microscopy; RT-PCR for Ku86 | (i) ↑ Ku86 after 4 h; (ii) ↑ nCLU and ↑ Bax; (iii) ↑ binding between Ku70 and Ku86; (iv) ↑ the release of Bax from the Ku70/nCLU complex; (v) Ʇ proliferation after 24 h; (vi) restore apoptosis | [146] | |
| SW480 | OCT; apoptosis-DNA ladder assay; WB; RT-PCR; IHC | (i) ↑ SST2/SST5-induced apoptosis; (ii) ↑ accumulation of β-catenin in plasmalemma; (iii) Ʇ TCF-dependent transcription, and ↓ cyclin D1 and c-Myc; (iv) role in GSK-3β activation | [152] | |
| Caco-2 | OCT; FC and Sub-G1 fraction detection | (i) ↑ the proportion of apoptotic cells and ↓ cell proliferation; (ii) ↑ DNA fragmentation | [97] | |
| A rat colonic ac implanted in female C57BL/6JBom-nu mice | OCT + galanin + serotonin | (i) ↓ the tumor volume, wet weight and relative volume density of BVs vs. C; (ii) ↑ AI in mice | ↓ Cell proliferation ↑ Apoptosis ↓ Angiogenesis | [153] |
| human colon cancer cells injected in nude mice | OCT + galanin + serotonin vs. LV/5-FU | (i) ↓ the PI and the no. of tumor BVs; (ii) ↑ AI in the mice treated with both LV/FU-triple therapy and with triple therapy only vs. LV/FU-treated mice | [154] | |
| SW620 cells implanted of the female nude mice (C57BL/6JBom-nu) | OCT + galanin + serotonin | (i) ↓ tumor volume, wet weight, PI and no. of tumor BVs in the treated animals; (ii) ↑ AI in the treated mice | [155] | |
| human colon cancer cells injected in nude mice | OCT + galanin + serotonin; IHC, TUNEL; computed image analysis | (i) ↓ PI and the no. of tumor BVs in the mice; (ii) ↑ AI in the treated mice | [156] | |
| human colon cancer cells injected into nude mice | OCT + galanin + serotonin vs. 5-FU/LV-irinotecan vs. 5-FU/LV-oxaliplatin | (i) no difference between the 3 groups regarding tumor proliferation, apoptosis, BVs density, EGF and VEGF expression | [157] | |
| human colon cancer cells injected in nude mice; IHC; TUNEL; computed image analysis | OCT + galanin + serotonin vs. 5-FU/LV | (i) no difference between tumors treated with 5-FU/LV or triple therapy regarding the volume and weights of the tumors, apoptotis, proliferation, VEGF indices, BVs density; (ii) ↓ LI of EGF in the tumors treated with triple therapy vs. 5-FU/LV | [131] | |
| Colo205 and HT-29 | Four component peptides of DRF 7295 (VIP + bombesin + SP + SST) | (i) ↓ cAMP; (ii) ↓ EGF-dependent proliferation and the pMAPK (pERK1/2); (iii) ↑ p53 and ↓ Bcl-2 levels (in Colo205); (iv) Ʇ VEGF secretion and ↑ caspase-3 (in HT-29); (v) ↓ capillary tube like formation in ECs | [159] |
| Antitumor Effect | Material (No. of Cases) and Methods (SST/SSA, Techniques) | Findings | Mechanism of Antitumor Activity/Clinical Significance | Ref. No. |
|---|---|---|---|---|
| ↓ proliferation | Non-endocrine solid tumors (8); SMS 201-995 | ↓ in basal and arginine-stimulated sGH and sIGF-1 | ↓ sGH and ↓ sIGF-1 levels | [103] |
| CRC (16); Sandostatin; a phase II study | (i) SD in 4 pts for 3–9 months; (ii) median survival—8 months; (iii) subjective improvement with a ↓ in pain | (i) ↑ Survival; (ii) disease stabilization | [104] | |
| RC (12); SMS 201-995; IHC | (i) ↓ Ki-67 tissue expression in 33% of pts; (ii) ↓ CEA in 50% of pts | ↓ Ki-67 tissue expression | [101] | |
| CRC (25) and C (16); Sandostatin; IHC | ↓ PCNA-MPI in 6/10 treated pts | ↓ PCNA tissue expression | [102] | |
| CRC (24); LAN; ELISA | the highest doses seemed to maintain ↓ serum IGF-1; with the lowest doses, a “rebound” IGF-1 levels during therapy | No antitumor activity or tumor marker reduction | [105] | |
| CRC (75); OCT; [3H]thymidine LI; FC; ELISA | (i) ↓ in the mean % of the S-phase fraction; (ii) ↓ sIGF-1; (iii) EGF and GH levels not affected | (i) Ʇ Cell cycle; (ii) ↓ sIGF-1 | [106] | |
| CRC (12) and C (12); IHC; computer image analysis | (i) ↓ SST(+) cells and CSI in CRC vs. C; (ii) the nuclear volume of these cells did not differ vs. C | (i) ↓ Colonic content of SST in CRC vs. C; (ii) ↓ secretory activity (no antitumor activity) | [94] | |
| CRC (35) with LM (25); iEM; IHC | (i) ↑ SST in well-differentiated vs. poorly differentiated tumors | ↓ SST correlated with poor grading and poor prognosis (no antitumor activity) | [95] | |
| CRC mirror biopsies (90); iEM | ↓ SST tissue expression | ↓ SST and ↑ or ectopic expression of other NPs may indicate the preneoplastic nature of the tissues | [96] | |
| CRC (79); IHC | (i) cyclin E LE > in low SST group vs. high and middle groups; (ii) CDK2 LE > in low SST group vs. high SST group; (iii) (+) correlation between the integral ratio of gastrin/SST and the cyclins (D1, E, A) and CDK2, CDK4 expression | (i) Abnormal tissue expression of cyclins and CDKs; (ii) the regulatory site of SST may be at the entrance of S phase | [107] | |
| CRC (34) and C (6/41) (children/adults) (TMA); CRC (13) and C (14/20) (IHC); CRC (12) and C (12/12) (qRT-PCR; IHC) | (i) ↓ SST mRNA in CRC vs. C (adults); (ii) ↑ ratio of SST(+) cells in children vs. CRC | SST gene promoter hypermethylation | [97] | |
| C (5), and matched CRC (5); qRT-PCR | (+) in all the C; (−) in matching CRC samples | Monitoring the rate of NCs maturation and SCs quiescence | [37] | |
| ↑ apoptosis | CRC (53) and tumor-neighboring mucosa with hyperplasia; IHC | (i) (+) SST in 84.6% CRC vs. 88.5% tumor-neighboring mucosa; (ii) SST coexpression with Bcl-2 | Bcl-2 expression | [33] |
| CRC (62); IHC | (i) LE of Bax in SST high and intermediate groups > low expression group; (ii) LE of Bcl-2 in SST high and intermediate expression groups < low expression group | Bax and Bcl-2 expression | [149] | |
| CRC (79); IHC; nested RT-PCR | (i) (+) correlation between SST mRNA and protein expression; (ii) AI in SST high and moderate expression groups > low expression groups; (iii) (+) LE of Fas, caspases 8/3 in SST high and moderate expression groups > low expression group | Fas, caspase 8 and caspase 3 expression | [150] | |
| ↓ angiogenesis | CRC (35); OCT; IHC; ELISA | (i) ↓ VEGF (t/s level); (ii) (+) correlation between t/s VEGF expression | ↓ VEGF | [165] |
| Model of the Study | Material (No. of Cases) and Methods | Findings | Potential Role in IBD | Ref. No. |
|---|---|---|---|---|
| In vivo (Human) | Various GIT diseases (including UC), C; RIA | a postprandial ↑ SST in all pts and age-matched C, especially ↑↑ in pts with active UC (176 ± 17 pg/mL), IBS (194.4 ± 20.4 pg/mL) and duodenal ulcer (159 ± 20 pg/mL) | The variations in circulating IR SST concentrations may be of pathophysiologic importance | [194] |
| Idiopathic IBD (UC, CD) and C; RIA | (i) SST-28 the major IR species; (ii) ↓ SST in the mucosa-submucosa of the descending colon in UC and in CD vs. C; (iii) SST levels: in severe < minimal colitis | Consistent with morphologic studies, which have suggested ↓ of EECs in UC | [191] | |
| CD (25); UC (25); CRC (25); IHC | (i) ↓ SST cells in CD and UC; (ii) the distal colon tended to contain >IR cells than the proximal colon did; (iii) ↓ (+) EECs in IBD vs. CRC; (iv) inverse correlation between (+) cells and the degree of inflammation in CD; (iv) ↓ (+) ganglion cells in CD | The decrease in SST-containing cells rather secondary to inflammation, however, may have some role in the pathogenesis of IBD | [192] | |
| UC, C; RIA | ↑24 h amplitude, a ↑ average level and a longer peak level phase of plasma SST in UC vs. C | Potential defensive role of SST in IBD | [91] | |
| CD tissue (9); LAN; ELISA; ECs isolation and culture; FACS sorting; RT-PCR | after LAN: Ʇ IL-1β-stimulated 5-HT secretion in normal and Crohn’s-derived ECs | Inflammatory ECs are more sensitive to cytokine-mediated activation and have intact inhibitory mechanism via SSTR | [195] | |
| UC (5), CD (6), C; IHC for SST and other markers of innervation | ↓ SST(+) nerve fibers surrounding submucosal arteries, from 22% to 1% (UC) and 2% (CD), but not perivascular mesenteric nerve fibers | Changes in the perivascular nerves may be responsible for the congestion, ulcerations and pain | [193] | |
| In vivo (Animal) | male rats; acetic acid colitis; histology; RIA; OCT s.c. (10 μg/rat) | after OCT: (i) ↓ in mucosal damage; (ii) ↓ PAF, leukotriene B4 and VIP concentrations | Possible role of SST in the pathogenesis of colitis; the mechanism of OCT action—not determined | [199] |
| male Wistar rats; TNBS colitis and C; OCT s.c. (2 × 10 μg × day/rat); IHC; WB; enzyme activities; culture ex vivo; ELISA | (i) max TNF-α produced at the 8th h, correlated with intense immunostaining of the external muscle layer; (ii) after OCT: ↓ TNF-α expression (staining and activity) and iNOS activity; (iii) ↓ submucosal MA TNF-α (+) and colonic production of IL-1β and IFN-γ | TNF-α regulation by OCT suggests that this drug might exert anti-inflammatory activities via smooth muscle cells | [198] | |
| old female C57BL/6 mice with C. rodentium (CR)- and DSS colitis; OCT; IHC; RIA | after OCT: (i) in CR colitis: ↑claudin-1 and claudin-3 expression vs. untreated CR infected mice; (ii) in DSS colitis: ↑claudin-3 expression vs. untreated DSS colitis mice | SST may play role in intestinal barrier protection by modulating TJ proteins expression | [202] | |
| male rats (30); TNBS colitis and C; IHC | (i) ↑ the densities of SST cells in TNBS vs. C group; (ii) ↑ the densities of mucosal leukocytes, B/T lymphocytes, T lymphocytes, B lymphocytes, MA/monocytes and mast cells in the TNBS group vs. C; (iii) (+) correlation between no. of specific immune cells and SST cells | Possible interactions between intestinal hormones (including SST) and immune cells | [197] | |
| male rats (40); TNBS colitis and C; IHC | ↑SST cells in the TNBS group vs. control group | Potential effects of signaling substances produced during inflammation on hormone expression, resulting in abnormalities in EECs and SCs and their progenitors | [48] | |
| male rats (24); DSS colitis and C; IHC | (i) ↓ the densities of SST cells in DSS vs. C group; (ii) ↑ the densities of mucosal leukocytes, B/T lymphocytes, T lymphocytes, B lymphocytes, MA/monocytes and mast cells in the DSS group vs. C; (iii) (−) correlation between SST cells and no. of all types of immune cells | Possible interaction between intestinal hormones (including SST) and the immune cells | [200] | |
| wt C57BL/6 mice; DSS colitis; OCT; histology; EM; FITC: BT detection; IF | after OCT: (i) improvement of clinical symptoms and histopathology scores; (ii) ↓ epithelial barrier dysfunction and restores TJ complex; (iii) ↑ claudin-4 expression | The protective effect of SST is achieved by ↑ claudin-4 expression | [203] | |
| In vitro | Caco-2 and HT-29; SST/OCT and cycloSST; ELISA; RNA extraction and RNA protection assay; MTT | after SST: (i) Ʇ the spontaneous and TNF-α-induced secretion of IL-8 and IL-1β mRNAs in dose-dependent manner, reaching >90% Ʇ at 3 nM; (ii) abrogation of the ↑ secretion of IL-8 and IL-1β after invasion by Salmonella; (iii) via SST2 and SST5 similar impact on the secretion of IL-8 and IL-1β; (iv) cycloSST completely Ʇ the SST- and OCT-induced ↓ effects; (v) no effect in cell viability | SST/SSAs are responsible for regulating the mucosal inflammatory response of intestinal epithelial cells following physiological and pathophysiological stimuli, including bacterial invasion | [196] |
| Caco-2; CCK; FITC and PI; IF; TER; RT-PCR; SST-14 (1 nM) | after SST: (i) improvement of the barrier dysfunction and ↑ expression of occludin and ZO-1; (ii) ↓ the redistribution of TJ proteins due to LPS stimulation; (iii) ↓ the LPS-induced phosphorylation of ERK1/2; (iv) ↓ mRNA of SST5 increased by LPS | SST protects the Caco2 monolayer barrier against LPS-induced TJ breakdown by ↓the activation of the ERK/MAPK pathway and suppressing the activation of SST5 | [201] | |
| Caco-2; EPEC and TNF-α; OCT (1 μM); WB | after OCT: (i) ↑ claudin-1 and ↑ claudin-3 expression in EPEC-infected cells vs. untreated cells; (ii) in cells exposed to TNF-α: ↑ claudin-3 expression vs. untreated cells | SST may play role in intestinal barrier protection by modulating TJ proteins expression | [202] | |
| Caco-2 pretreated with TNF-α; SST (1 μM); SSTR agonist; TER; RT-PCR; WB | after SST: (i) ↑ claudin-4 expression via SSTR5 in TNF-α intervened cells; (ii) ↓ the phosphorylation levels of p38 and ERK1/2 to the basal level vs. C | The protective effect of SST followed activation of SST5 and subsequent Ʇ of the ERK1/2 MAPK pathway | [203] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak, A.; Geltz, A. The State-of-the-Art Mechanisms and Antitumor Effects of Somatostatin in Colorectal Cancer: A Review. Biomedicines 2024, 12, 578. https://doi.org/10.3390/biomedicines12030578
Kasprzak A, Geltz A. The State-of-the-Art Mechanisms and Antitumor Effects of Somatostatin in Colorectal Cancer: A Review. Biomedicines. 2024; 12(3):578. https://doi.org/10.3390/biomedicines12030578
Chicago/Turabian StyleKasprzak, Aldona, and Agnieszka Geltz. 2024. "The State-of-the-Art Mechanisms and Antitumor Effects of Somatostatin in Colorectal Cancer: A Review" Biomedicines 12, no. 3: 578. https://doi.org/10.3390/biomedicines12030578
APA StyleKasprzak, A., & Geltz, A. (2024). The State-of-the-Art Mechanisms and Antitumor Effects of Somatostatin in Colorectal Cancer: A Review. Biomedicines, 12(3), 578. https://doi.org/10.3390/biomedicines12030578






