Angiographic Predictors for Repeated Revascularization in Patients with Intermediate Coronary Lesions
Abstract
1. Introduction
2. Study Population and Methods
2.1. Study Population
2.2. Angiographic Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics (Table 1)
| Variables | TLR (−) n = 302 (69.1%) | TLR (+) n = 135 (30.9%) | Total n = 437 (100%) | p-Value |
|---|---|---|---|---|
| FU duration, month | 33.0 ± 32.0 | 36.8 ± 32.0 | 34.2 ± 32.0 | 0.254 |
| Age, years | 63.4 ± 11.0 | 62.6 ± 10.5 | 63.2 ± 10.8 | 0.443 |
| Men, n (%) | 213 (70.5) | 96 (71.1) | 309 (70.7) | 0.902 |
| Hypertension, n (%) | 207 (68.5) | 87 (64.4) | 294 (67.3) | 0.399 |
| Diabetes mellitus, n (%) | 128 (42.4) | 40 (29.6) | 168 (38.4) | 0.011 |
| Smoking, n (%) | 74 (30.8) | 34 (30.6) | 108 (30.8) | 0.969 |
| Diagnosis, n (%) | ||||
| Stable angina | 165 (54.6) | 66 (48.9) | 231 (52.9) | 0.266 |
| ACS | 122 (40.4) | 65 (48.1) | 187 (42.8) | 0.130 |
| Lipid profile | ||||
| Total cholesterol, mg/dL | 166.7 ± 47.0 | 181.2 ± 47.9 | 171.3 ± 47.7 | 0.004 |
| Triglyceride, mg/dL | 163.1 ± 131.9 | 169.6 ± 99.5 | 165.1 ± 122.6 | 0.614 |
| HDL cholesterol, mg/dL | 45.9 ± 35.1 | 44.3 ± 24.4 | 45.4 ± 32.1 | 0.647 |
| LDL cholesterol, mg/dL | 76.9 ± 28.3 | 80.7 ± 28.7 | 114.7 ± 37.6 | 0.004 |
| Creatinine, mg/dL | 1.31 ± 1.62 | 1.21 ± 1.53 | 1.28 ± 1.59 | 0.545 |
| eGFR, mL/min/1.73 m2 | 77.1 ± 26.6 | 79.1 ± 22.9 | 77.7 ± 25.5 | 0.452 |
| Fasting glucose, mg/dL | 134.8 ± 65.7 | 127.1 ± 57.3 | 132.4 ± 63.2 | 0.244 |
| WBC, /μL | 8329.4 ± 3428.9 | 7655.4 ± 2426.7 | 8122.3 ± 3167.5 | 0.020 |
| Hb, g/dL | 13.2 ± 2.1 | 13.8 ± 3.6 | 13.4 ± 2.7 | 0.037 |
| HbA1C, % | 7.3 ± 4.9 | 6.7 ± 1.3 | 7.1 ± 4.2 | 0.301 |
| Hs C-reactive protein, mg/L | 0.86 ± 6.20 | 0.42 ± 1.05 | 0.73 ± 5.24 | 0.482 |
| Ejection fraction, % | 61.6 ± 11.4 | 63.1 ± 9.8 | 62.1 ± 10.9 | 0.192 |
| Multi-vessel disease, n (%) | 173 (57.3) | 87 (64.4) | 260 (59.5) | 0.159 |
| LM disease, n (%) | 14 (4.6) | 6 (4.4) | 20 (4.6) | 0.930 |
| Previous PCI, n (%) | 33 (10.9) | 16 (11.9) | 49 (11.2) | 0.777 |
| Previous CABG, n (%) | 2 (0.7) | 1 (0.7) | 3 (0.7) | 1.000 |
| Initial PCI site | 287 | 123 | 410 | 0.896 |
| LM, n (%) | 9 (3.1) | 1 (0.8) | 10 (2.4) | |
| LAD, n (%) | 130 (45.3) | 55 (44.7) | 185 (45.1) | |
| LCX, n (%) | 66 (23.0) | 30 (24.4) | 96 (23.4) | |
| RCA, n (%) | 82 (28.6) | 37 (30.1) | 119 (29.0) | |
| Medication | ||||
| Aspirin | 300 (99.3) | 135 (100.0) | 435 (99.5) | 1.000 |
| P2Y12 inhibitor | 0.402 | |||
| Clopidogrel | 226 (74.8) | 104 (77.0) | 330 (75.5) | |
| Ticagrelor | 29 (9.6) | 16 (11.9) | 45 (10.3) | |
| Anticoagulation | 0.427 | |||
| Warfarin | 1 (0.3) | 0 (0.0) | 1 (0.2) | |
| DOAC | 5 (1.7) | 0 (0.0) | 5 (1.1) | |
| RASI | 0.032 | |||
| ACEi | 18 (6.0) | 16 (11.9) | 34 (7.8) | |
| ARB | 180 (59.6) | 85 (63.0) | 265 (60.6) | |
| BB | 184 (60.9) | 100 (74.1) | 284 (65.0) | 0.008 |
| Statin | 284 (94.0) | 123 (91.1) | 407 (93.1) | 0.263 |
3.2. Coronary Angiographic Findings (Table 2)
| Variables | TLR (−) 470 (75.0%) | TLR (+) 157 (25.0%) | Total 627 (100%) | p-Value |
|---|---|---|---|---|
| IL location | 0.183 | |||
| LAD, n (%) | 164 (34.9) | 63 (40.1) | 227 (36.2) | |
| LCX, n (%) | 100 (21.3) | 35 (22.3) | 135 (21.5) | |
| RCA, n (%) | 203 (43.2) | 56 (35.7) | 259 (41.3) | |
| LM, n (%) | 3 (0.6) | 3 (1.9) | 6 (1.0) | |
| IL site | 0.494 | |||
| Ostium, n (%) | 9 (1.9) | 5 (3.2) | 14 (2.2) | |
| Proximal, n (%) | 144 (30.6) | 55 (35.0) | 199 (31.7) | |
| Middle, n (%) | 247 (52.6) | 78 (49.7) | 325 (51.8) | |
| Distal, n (%) | 70 (14.9) | 19 (12.1) | 89 (14.2) | |
| Percent diameter stenosis (%) | 44.2 ± 12.2 | 47.3 ± 13.5 | 44.9 ± 12.6 | 0.006 |
| % DS ≥ 60%, n (%) | 89 (18.9%) | 46 (29.3) | 135 (21.5) | |
| Lesion length, mm | 18.4 ± 11.1 | 19.3 ± 10.9 | 18.6 ± 11.1 | 0.367 |
| IL angiographic characteristics | ||||
| Branch | 325 (69.1) | 124 (79.0) | 449 (71.6) | 0.018 |
| Irregularity | 63 (13.4) | 28 (17.8) | 91 (14.5) | 0.172 |
| Tortuosity | 143 (30.4) | 44 (28.0) | 187 (29.8) | 0.569 |
| Ulcer/erosion rupture | 23 (4.9) | 12 (7.6) | 35 (5.6) | 0.194 |
| Calcium | 106 (22.6) | 37 (23.6) | 143 (22.8) | 0.793 |
| Haziness | 12 (2.6) | 15 (4.3) | 27 (4.3) | <0.001 |
| FU diagnosis | ||||
| SAP | 278 (59.8) | 81 (52.3) | 359 (57.9) | 0.100 |
| ACS | 171 (36.8) | 74 (47.7) | 245 (39.5) | 0.016 |
| FU percent diameter stenosis | 45.0 ± 15.7 | 85.8 ± 14.6 | 55.2 ± 23.5 | <0.001 |
| FU treatment | <0.001 | |||
| Medication only | 470 | 0 (0.0) | ||
| +POBA | 2 (0.5) | |||
| +Stent | 154 (98.1) | |||
| +CABG | 1 (0.6) |
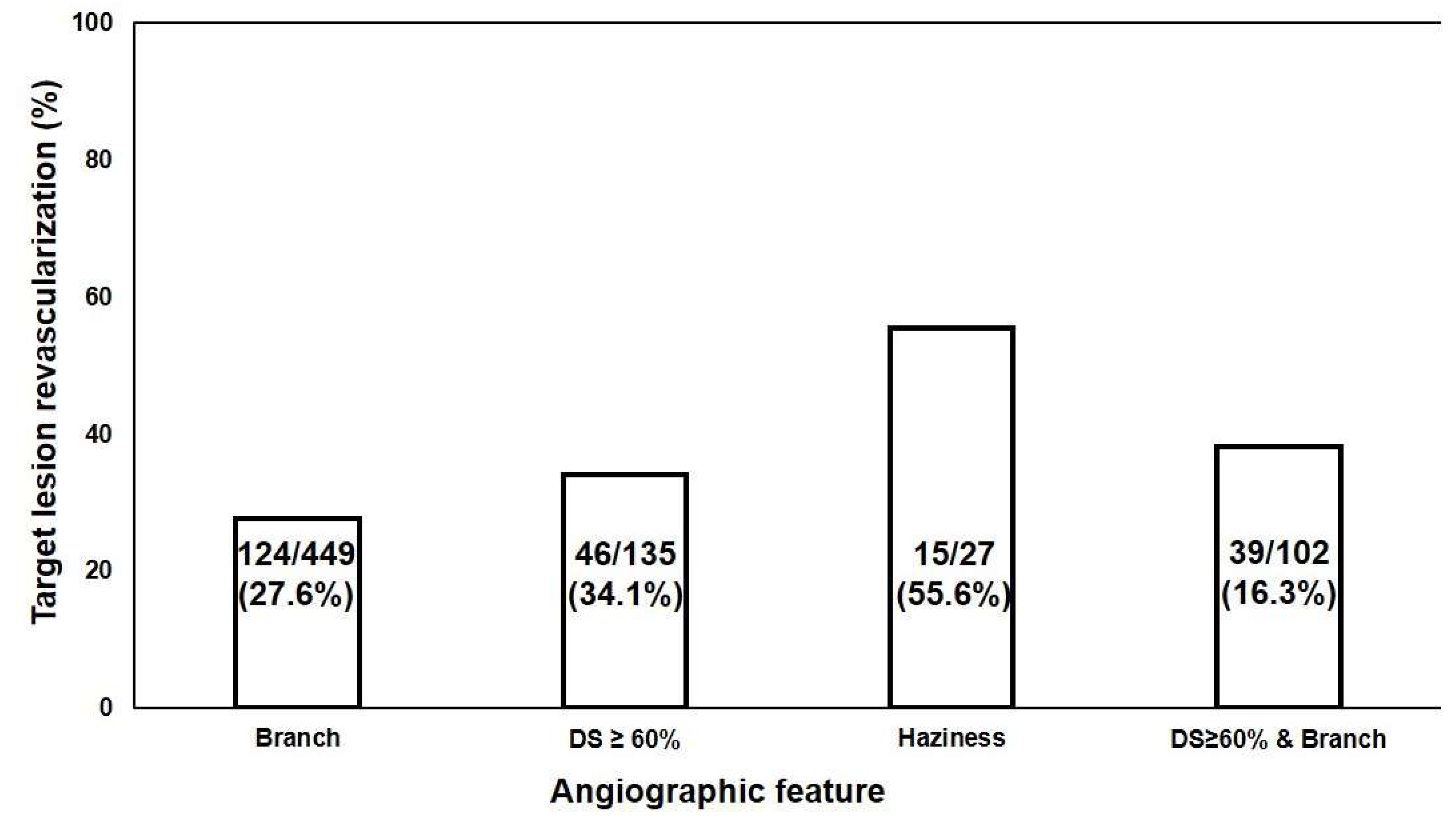
3.3. Angiographic Predictors of IL for TLR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hahn, J.-Y.; Choi, S.-H.; Jeong, J.-O.; Song, Y.B.; Choi, J.-H.; Park, Y.H.; Chun, W.J.; Oh, J.H.; Cho, D.K.; Lim, S.-H.; et al. Conservative versus aggressive treatment strategy with angiographic guidance alone in patients with intermediate coronary lesions: The SMART-CASE randomized, non-inferiority trial. Int. J. Cardiol. 2017, 240, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, J.A.; Tannenbaum, M.A.; Alexopoulos, D.; Hjemdahl-Monsen, C.E.; Leavy, J.; Weiss, M.; Borrico, S.; Gorlin, R.; Fuster, V. Angiographic progression of coronary artery disease and the development of myocardial infarction. J. Am. Coll. Cardiol. 1988, 12, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.; van Schaardenburgh, P.; Manoharan, G.; Boersma, E.; Bech, J.-W.; van’t Veer, M.; Bär, F.; Hoorntje, J.; Koolen, J.; Wijns, W. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J. Am. Coll. Cardiol. 2007, 49, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-H.; Corban, M.T.; Seo, Y.-H.; Kim, T.; Lee, G.; Kwon, T.-G.; Kim, K.-H.; Song, I.-G.; Lee, M.-S.; Rihal, C.S.; et al. Ten-year clinical outcomes of an intermediate coronary lesion; prognosis and predictors of major adverse cardiovascular events. Int. J. Cardiol. 2020, 299, 26–30. [Google Scholar] [CrossRef]
- Kim, Y.K.; Jang, C.W.; Kwon, S.H.; Kim, J.H.; Lerman, A.; Bae, J.-H. Ten-year clinical outcomes in patients with intermediate coronary stenosis according to the combined culprit lesion. Clin. Cardiol. 2021, 44, 1161–1168. [Google Scholar] [CrossRef]
- Pijls Nico, H.J.; Fearon William, F.; Tonino Pim, A.L.; Siebert, U.; Ikeno, F.; Bornschein, B.; van’t Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; et al. Fractional Flow Reserve Versus Angiography for Guiding Percutaneous Coronary Intervention in Patients with Multivessel Coronary Artery Disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J. Am. Coll. Cardiol. 2010, 56, 177–184. [Google Scholar] [CrossRef]
- Tonino, P.A.L.; Fearon, W.F.; De Bruyne, B.; Oldroyd, K.G.; Leesar, M.A.; Ver Lee, P.N.; MacCarthy, P.A.; van’t Veer, M.; Pijls, N.H.J. Angiographic Versus Functional Severity of Coronary Artery Stenoses in the FAME Study: Fractional Flow Reserve Versus Angiography in Multivessel Evaluation. J. Am. Coll. Cardiol. 2010, 55, 2816–2821. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef]
- Xaplanteris, P.; Fournier, S.; Pijls, N.H.; Fearon, W.F.; Barbato, E.; Tonino, P.A.; Engstrøm, T.; Kääb, S.; Dambrink, J.-H.; Rioufol, G.; et al. Five-year outcomes with PCI guided by fractional flow reserve. N. Engl. J. Med. 2018, 379, 250–259. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018, 40, 87–165. [Google Scholar] [CrossRef]
- Belle, E.V.; Rioufol, G.; Pouillot, C.; Cuisset, T.; Bougrini, K.; Teiger, E.; Champagne, S.; Belle, L.; Barreau, D.; Hanssen, M.; et al. Outcome Impact of Coronary Revascularization Strategy Reclassification with Fractional Flow Reserve at Time of Diagnostic Angiography. Circulation 2014, 129, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Curzen, N.; Rana, O.; Nicholas, Z.; Golledge, P.; Zaman, A.; Oldroyd, K.; Hanratty, C.; Banning, A.; Wheatcroft, S.; Hobson, A. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain? The RIPCORD study. Circ. Cardiovasc. Interv. 2014, 7, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Tebaldi, M.; Biscaglia, S.; Fineschi, M.; Manari, A.; Menozzi, M.; Secco, G.G.; Di Lorenzo, E.; D’Ascenzo, F.; Fabbian, F.; Tumscitz, C.; et al. Fractional Flow Reserve Evaluation and Chronic Kidney Disease: Analysis From a Multicenter Italian Registry (the FREAK Study). Catheter. Cardiovasc. Interv. 2016, 88, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, P.B.; Prasad, A.; Honeycutt, E.; Wang, T.Y.; Messenger, J.C. Contemporary Patterns of Fractional Flow Reserve and Intravascular Ultrasound Use Among Patients Undergoing Percutaneous Coronary Intervention in the United States: Insights From the National Cardiovascular Data Registry. J. Am. Coll. Cardiol. 2012, 60, 2337–2339. [Google Scholar] [CrossRef]
- Ghanem, F.; Alshami, M.; Aburumman, H.; Jbara, M.H.; Ramu, V.K.; Gajjar, B.; Altibi, A.; Mkhaimer, Y.; Alhuneafat, L.; Patel, J.B. Abstract 15572: Utility Trends and Outcomes of Fractional Flow Reserve (FFR) in Diagnostic Coronary Angiograms for Patients with Stable Angina: National Readmission Database (2016–2019). Circulation 2022, 146, A15572. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Mohananey, D.; Razzouk, L.; Weisz, G.; Slater, J.N. Impact and trends of intravascular imaging in diagnostic coronary angiography and percutaneous coronary intervention in inpatients in the United States. Catheter. Cardiovasc. Interv. 2018, 92, E410–E415. [Google Scholar] [CrossRef]
- de la Torre Hernandez, J.M.; Hernández Hernandez, F.; Alfonso, F.; Rumoroso, J.R.; Lopez-Palop, R.; Sadaba, M.; Carrillo, P.; Rondan, J.; Lozano, I.; Ruiz Nodar, J.M.; et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions: Results from the multicenter LITRO study. J. Am. Coll. Cardiol. 2011, 58, 351–358. [Google Scholar] [CrossRef]
- Fassa, A.-A.; Wagatsuma, K.; Higano, S.T.; Mathew, V.; Barsness, G.W.; Lennon, R.J.; Holmes, D.R.; Lerman, A. Intravascular ultrasound-guided treatment for angiographically indeterminate left main coronary artery disease. J. Am. Coll. Cardiol. 2005, 45, 204–211. [Google Scholar] [CrossRef]
- Li, J.; Montarello, N.J.; Hoogendoorn, A.; Verjans, J.W.; Bursill, C.A.; Peter, K.; Nicholls, S.J.; McLaughlin, R.A.; Psaltis, P.J. Multimodality Intravascular Imaging of High-Risk Coronary Plaque. JACC Cardiovasc. Imaging 2022, 15, 145–159. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A Prospective Natural-History Study of Coronary Atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef]
- Christou, M.A.; Siontis, G.C.; Katritsis, D.G.; Ioannidis, J.P. Meta-analysis of fractional flow reserve versus quantitative coronary angiography and noninvasive imaging for evaluation of myocardial ischemia. Am. J. Cardiol. 2007, 99, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Donohue, T.J.; Younis, L.T.; Bach, R.G.; Aguirre, F.V.; Wittry, M.D.; Goodgold, H.M.; Chaitman, B.R.; Kern, M.J. Correlation of pharmacological 99mTc-sestamibi myocardial perfusion imaging with poststenotic coronary flow reserve in patients with angiographically intermediate coronary artery stenoses. Circulation 1994, 89, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Arbab-Zadeh, A.; Fuster, V. The Myth of the “Vulnerable Plaque”: Transitioning From a Focus on Individual Lesions to Atherosclerotic Disease Burden for Coronary Artery Disease Risk Assessment. J. Am. Coll. Cardiol. 2015, 65, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Tomaniak, M.; Katagiri, Y.; Modolo, R.; de Silva, R.; Khamis, R.Y.; Bourantas, C.V.; Torii, R.; Wentzel, J.J.; Gijsen, F.J.H.; van Soest, G.; et al. Vulnerable plaques and patients: State-of-the-art. Eur. Heart J. 2020, 41, 2997–3004. [Google Scholar] [CrossRef]
- Ali, Z.A.; Karimi Galougahi, K.; Maehara, A.; Shlofmitz, R.A.; Ben-Yehuda, O.; Mintz, G.S.; Stone, G.W. Intracoronary optical coherence tomography 2018: Current status and future directions. JACC Cardiovasc. Interv. 2017, 10, 2473–2487. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Kan, J.; Ge, Z.; Han, L.; Lu, S.; Tian, N.; Lin, S.; Lu, Q.; Wu, X.; et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. J. Am. Coll. Cardiol. 2018, 72, 3126–3137. [Google Scholar] [CrossRef]
- Dai, J.; Xing, L.; Jia, H.; Zhu, Y.; Zhang, S.; Hu, S.; Lin, L.; Ma, L.; Liu, H.; Xu, M.; et al. In vivo predictors of plaque erosion in patients with ST-segment elevation myocardial infarction: A clinical, angiographical, and intravascular optical coherence tomography study. Eur. Heart J. 2018, 39, 2077–2085. [Google Scholar] [CrossRef]
- Pu, J.; Mintz, G.S.; Biro, S.; Lee, J.-B.; Sum, S.T.; Madden, S.P.; Burke, A.P.; Zhang, P.; He, B.; Goldstein, J.A.; et al. Insights Into Echo-Attenuated Plaques, Echolucent Plaques, and Plaques with Spotty Calcification: Novel Findings From Comparisons Among Intravascular Ultrasound, Near-Infrared Spectroscopy, and Pathological Histology in 2294 Human Coronary Artery Segments. J. Am. Coll. Cardiol. 2014, 63, 2220–2233. [Google Scholar] [CrossRef]
- Giroud, D.; Li, J.M.; Urban, P.; Meier, B.; Rutishauser, W. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am. J. Cardiol. 1992, 69, 729–732. [Google Scholar] [CrossRef]
- Saw, J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter. Cardiovasc. Interv. 2014, 84, 1115–1122. [Google Scholar] [CrossRef]
- Block, P.C.; Myler, R.K.; Stertzer, S.; Fallon, J.T. Morphology after transluminal angioplasty in human beings. N. Engl. J. Med. 1981, 305, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Naruko, T.; Ueda, M.; Becker, A.E.; Tojo, O.; Teragaki, M.; Takeuchi, K.; Takeda, T. Angiographic-pathologic correlations after elective percutaneous transluminal coronary angioplasty. Circulation 1993, 88, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-J.; Nakano, M.; Virmani, R.; Song, H.-G.; Ahn, J.-M.; Kim, W.-J.; Lee, J.-Y.; Park, D.-W.; Lee, S.-W.; Kim, Y.-H.; et al. OCT Findings in Patients with Recanalization of Organized Thrombi in Coronary Arteries. JACC Cardiovasc. Imaging 2012, 5, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Ziada, K.M.; Tuzcu, E.M.; De Franco, A.C.; Kim, M.H.; Raymond, R.E.; Franco, I.; Whitlow, P.L.; Ellis, S.G.; Nissen, S.E. Intravascular Ultrasound Assessment of the Prevalence and Causes of Angiographic “Haziness” Following High-Pressure Coronary Stenting. Am. J. Cardiol. 1997, 80, 116–121. [Google Scholar] [CrossRef]
- Vijayvergiya, R.; Krishnappa, D.; Kasinadhuni, G.; Gupta, A.; Panda, P.; Ratheesh, K.J. Coronary dissection or a recanalized thrombus? Optical coherence tomography has the answer. IHJ Cardiovasc. Case Rep. (CVCR) 2018, 2, 6–8. [Google Scholar] [CrossRef]
- Chien, S. Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H1209–H1224. [Google Scholar] [CrossRef]
- Molony, D.S.; Timmins, L.H.; Hung, O.Y.; Rasoul-Arzrumly, E.; Samady, H.; Giddens, D.P. An assessment of intra-patient variability on observed relationships between wall shear stress and plaque progression in coronary arteries. Biomed. Eng. Online 2015, 14, S2. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, J.M.; Yoon, A.H.; Chang, K.; Lee, S.-W. Perspectives in Predicting Rapid Plaque Progression and Future Coronary Events Using Comprehensive Plaque and Hemodynamic Assessment. J. Cardiovasc. Interv. 2023, 2, 77. [Google Scholar] [CrossRef]


| Total | ||||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| % DS ≥ 60% | 1.023 | 1.011–1.035 | <0.001 | 1.025 | 1.013–1.037 | <0.001 |
| Haziness | 1.855 | 1.087–3.165 | 0.023 | 2.126 | 1.240–3.644 | 0.006 |
| Initial ACS | 1.327 | 0.967–1.822 | 0.080 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-K.; Kwon, S.-H.; Seo, Y.-H.; Kim, K.-H.; Kwon, T.-G.; Bae, J.-H. Angiographic Predictors for Repeated Revascularization in Patients with Intermediate Coronary Lesions. Biomedicines 2024, 12, 2825. https://doi.org/10.3390/biomedicines12122825
Kim Y-K, Kwon S-H, Seo Y-H, Kim K-H, Kwon T-G, Bae J-H. Angiographic Predictors for Repeated Revascularization in Patients with Intermediate Coronary Lesions. Biomedicines. 2024; 12(12):2825. https://doi.org/10.3390/biomedicines12122825
Chicago/Turabian StyleKim, Yong-Kyun, Soon-Ho Kwon, Young-Hoon Seo, Ki-Hong Kim, Taek-Geun Kwon, and Jang-Ho Bae. 2024. "Angiographic Predictors for Repeated Revascularization in Patients with Intermediate Coronary Lesions" Biomedicines 12, no. 12: 2825. https://doi.org/10.3390/biomedicines12122825
APA StyleKim, Y.-K., Kwon, S.-H., Seo, Y.-H., Kim, K.-H., Kwon, T.-G., & Bae, J.-H. (2024). Angiographic Predictors for Repeated Revascularization in Patients with Intermediate Coronary Lesions. Biomedicines, 12(12), 2825. https://doi.org/10.3390/biomedicines12122825







